Abstract
Organic anion transporter 3 (OAT3) plays a vital role in removing a broad variety of anionic drugs from kidney, thus avoiding their possible toxicity in the body. In the current study, we investigated the role of insulin-like growth factor 1 (IGF-1) in the regulation of OAT3. We showed that IGF-1 induced a dose- and time-dependent increase in OAT3 transport activity, which correlated well with an increase in OAT3 expression. The IGF-1-induced increase in OAT3 expression was blocked by protein kinase A (PKA) inhibitor H89. Moreover, IGF-1 induced an increase in OAT3 phosphorylation, which was also blocked by H89. These data suggest that the IGF-1 modulation of OAT3 occurred through PKA signaling pathway. To further confirm the involvement of PKA, we treated OAT3-expressing cells with PKA activator Bt2-cAMP, followed by examining OAT activity and phosphorylation. We showed that OAT3 activity and phosphorylation were much enhanced in Bt2-cAMP-treated cells as compared to that in control cells. Finally, linsitinib, an anticancer drug that blocks the IGF-1 receptor, abrogated IGF-1-stimulated OAT3 transport activity. In conclusion, our study demonstrated that IGF-1 regulates OAT3 expression and transport activity through PKA signaling pathway, possibly by phosphorylating the transporter.
Key words: Organic anion transporter, Drug transport, Regulation, IGF-1, PKA, Phosphorylation
Graphical abstract
IGF-1/PKA signaling pathway regulates OAT3 phosphorylation, expression and transport activity.
1. Introduction
Organic anion transporter 3 (OAT3) is a member of the organic anion transporter family, which plays vital parts in the removal of many drugs from the kidney, such as anti-viral drugs, anti-tumor therapeutics, antibiotics, antihypertensive and anti-inflammatory drugs, and thereby avoiding their possible toxicity in the body1, 2, 3, 4, 5, 6.
A biology model of transporters called remote sensing and signaling has recently attracted a lot attention7, 8, 9. In this model, OATs play a key role in interorgan communication and in regulating local and whole-body homeostasis. The intercellular and inter-organ communication is carried out by hormones, small molecules and cell signaling. Hormones produced in the original organ under stimuli and released into blood stream regulate target organ transporters through activating cell signaling.
Hormones regulate OATs through the activation of various protein kinases. A protein kinase modifies its substrate molecules, typically proteins, by chemically conjugating phosphate groups to them, a post-translational process called phosphorylation. Phosphorylation frequently results in a functional change of the target protein by changing its three-dimensional conformation, activity, cellular distribution, protein stability or its interaction with other proteins. Protein kinases regulate various membrane proteins including channels, transporters and receptors. For example, parathyroid hormone down-regulates NaPi-IIc transporter involving protein kinase C (PKC)-induced phosphorylation of the transporter10. Glucose transporter 4 is up-regulated by PKC-induced phosphorylation11, and PMA, a PKC activator, increases P-glycoprotein (P-gp) phosphorylation, which is correlated with increased P-gp transporter activity12.
Previous studies from our laboratory demonstrated that OAT transport activity is subjected to the regulation by several physiological stimuli such as angiotensin II, peptide hormone bradykinin, and progesterone13, 14, 15. We showed that these hormones down-regulate OAT activity through the activation of PKC. Interestingly, our laboratory demonstrated that activation of PKC inhibits OAT transport activity without directly phosphorylating the transporter itself16.
IGF-1 is produced primarily by the liver under the stimulation of growth hormone (GH) and plays significantly roles in growth, development, and metabolism17, 18, 19. IGF-1 exerts its effect on its substrates through IGF-1 receptor. IGF-1 is involved in various renal physiological processes including renal development, glomerular functions, and tubular handling. In addition, GH/IGF-1 axis contributes to various kidney diseases including renal cancer, acute kidney failure, diabetic nephropathy and polycystic kidney disease17, 20.
PKA, also known as cAMP-dependent protein kinase, exists in a physiological tetrameric complex which consists of two regulatory subunits and two catalytic subunits21. PKA is one of the most widely studied protein kinases and is activated following the release of the catalytic subunits in response to the second messenger cAMP22. We previously demonstrated that activation of PKA by Bt2-cAMP enhanced OAT3 transport activity, stimulated SUMOylation and suppressed ubiquitination23. IGF-1 regulates physiological and pathological processes through various signaling pathways including the activation of PKA. For example, IGF-1 enhanced cell survival via protein kinase A pathway24. IGF-1/PKA pathway plays a vital role in regulating stem cell protection, self-renew Guofengal, and regeneration25, and IGF-1 stimulated OAT3 SUMOylation involving PKA signaling23.
The abnormalities in the IGF-1 have been reported to be related to the development of several diseases, such as Laron syndrome and acromegaly26, 27. Mecasermin, a synthetic analog of IGF-1, has been used to treat patients with growth failure and short stature caused by IGF-1 deficiency28. IGF-1 receptor is a transmembrane protein activated by IGF-1 binding. The mutation of IGF-1 receptor causes pre and postnatal growth retardation29.
In the current study, we investigated the effect of IGF-1 on OAT3 phosphorylation, expression and transport activity as well as its downstream signaling pathway.
2. Materials and methods
2.1. Materials
COS-7 cells were obtained from ATCC (Manassas, VA, USA). [3H]-labeled estrone sulfate (ES) was obtained from PerkinElmer (Waltham, MA, USA). Membrane-impermeable biotinylation reagents, Sulfo-NHS-SS-biotin, streptavidin agarose beads, and protein G agarose beads were purchased from Pierce (Rockford, IL, USA). Mouse anti-myc antibody (9E10) was obtained from Roche (Indianapolis, IN, USA). Mouse anti-E-cadherin, anti-GAPDH and anti-phospho-Ser/Thr antibodies were obtained from Abcam (Cambridge, MA, USA). Horseradish peroxidase-conjugated anti-mouse antibody was bought from Santa Cruz (Santa Cruz, CA, USA). Dibutyryl cyclic-AMP sodium salt (Bt2-cAMP), H89 dihydrochloride hydrate (H89), insulin-like growth factor-I human (IGF-1) and anti-myc agarose affinity gel were bought from Sigma–Aldrich (St. Louis, MO, USA). IGF-1 receptor inhibitor, linsitinib, was purchased from Selleck Chemicals (Houston, TX, USA).
2.2. Cell culture
Parental COS-7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Corning, Corning, NY, USA) containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA) at 37 °C with 5% CO2. Cells stably expressing human OAT3 (hOAT3) were established in our laboratory as previously described14, 30. Cells stably expressing hOAT3 were maintained in DMEM containing 0.2 mg/mL G418 (Invitrogen, Carlsbad, CA, USA) and 10% fetal bovine serum.
2.3. Transport measurements
The transport activity of OAT3 was determined by measuring [3H]-ES uptake into stable OAT3-expressing COS-7 cells. The uptake solution consisted of phosphate-buffered saline (pH 7.4) with 1 mmol/L CaCl2, 1 mmol/L MgCl2 (PBS/CM) and [3H]-ES (300 nmol/L). The uptake solution was added to the cells. After an indicated period of time, uptake was ended by aspirating the uptake solution and rapidly washing the cells with ice-cold PBS solution. The cells were then lysed in 0.2 mol/L NaOH, neutralized in 0.2 mol/L HCl, and collected for liquid scintillation counting by using a liquid scintillation counter (Beckman LSC LS6500). Uptake activity was expressed as the percentage of the uptake value measured in control cells.
2.4. Cell surface biotinylation
The amount of OAT3 at the cell surface was determined using the membrane-impermeable biotinylation reagent, Sulfo-NHS-SS-biotin, as described in our previous publications9, 26. The cells in culture were washed two times by PBS/CM pH 8.0 and incubated with Sulfo-NHS-SS-biotin (0.5 mg/mL in PBS/CM, pH 8.0) for two consecutive 20 min. After biotinylation, the cells were washed and quenched with 100 mmol/L glycine to remove the unreacted Sulfo-NHS-SS-biotin. Then the cells were lysed on ice for 45 min and centrifuged at 16,000×g at 4 °C. The supernatant of cell lysates was added to 40 μL of streptavidin-agarose beads to pull down the cell membrane proteins. Cell surface OAT3 protein was detected by SDS-PAGE and immunoblotting using an anti-myc antibody (9E10) (myc was tagged to OAT3 for immune detection).
2.5. Protein phosphorylation
Cells were lysed with lysis buffer. Protein concentration for each sample was measured and same amount of proteins were precleared at 4 °C for 2 h and incubated with anti-myc agarose affinity gel (Sigma–Aldrich) at 4 °C overnight. On next day the beads carrying immunoprecipitated proteins were washed with lysis buffer three times, denatured with urea denature buffer containing β-mercaptoethanol, and analyzed by SDS-PAGE and immunoblotting with anti-phospho-Ser/Thr antibody.
2.6. Electrophoresis and immunoblotting
We followed the procedure previously established in our laboratory31, 32. Protein samples were separated on 7.5% SDS-PAGE mini-gels (Bio-Rad, Hercules, CA, USA) and electroblotted onto PVDF membranes (Invitrogen). The membranes were treated with 5% nonfat dry milk in PBST (0.05% Tween-20 in PBS) for 1 h at room temperature and incubated overnight at 4 °C with appropriate primary antibodies. Then the blots were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, followed by detection with SuperSignal West Dura Extended Duration Substrate kit (Pierce). FluorChem 8800 imaging system (Alpha Innotech Corp., San Leandro, CA, USA) was used to quantify the non-saturated, immunoreactive protein bands.
2.7. Data analysis
Each experiment was repeated a minimum of three times. The statistical analysis was from multiple experiments. Between two groups, statistical analysis was performed using Student's paired t-tests. Among multiple treatments, one-way ANOVA was applied by using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). A P-value of <0.05 was considered significant.
3. Results
3.1. Effect of IGF-1 on OAT3 transport activity
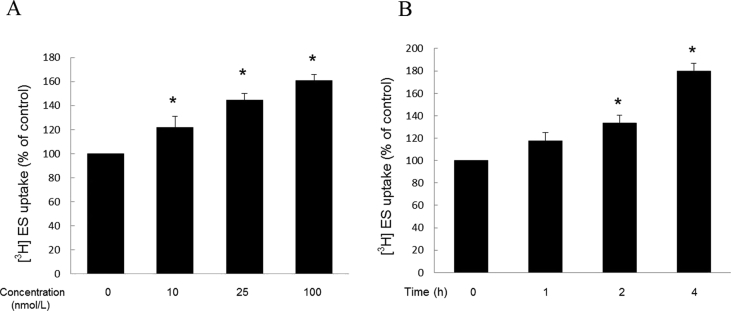
To explore the role of IGF-1 in OAT3 transport function, we treated OAT3-expressing COS-7 cells with IGF-1 for 4 h, then measured the OAT3-mediated uptake of [3H]-ES, a prototypical substrate for OAT3. As shown in Fig. 1A, IGF-1 induced a dose-dependent increase in the ES uptake. When compared with non-treated group, treatment with 100 nmol/L IGF-1 resulted in a significant increase of 60% in the ES uptake. OAT3-expressing COS-7 cells were also treated with IGF-1 for various time periods. As seen in Fig. 1B and 100 nmol/L IGF-1 increased the ES uptake in a time-dependent manner, with ∼15%, 30% and 75% increase at treatment time points of 1, 2, and 4 h.
Figure 1.
Effect of IGF-1 on OAT3 transport activity. (A) OAT3-expressing cells were treated with IGF-1 at various doses for 4 h. 4-min uptake of [3H]-estrone sulfate (ES, 0.3 μmol/L) was then measured. Transport activity was expressed as percentage of the uptake in control cells. (mock cells: ∼400 CPM and OAT3-expressing cells without treatment: ∼4000 CPM). Values are mean ± SD (n = 3); *P < 0.05. (B) OAT3-expressing cells were treated with 100 nmol/L IGF-1 for various time points. 4-min uptake of [3H]-estrone sulfate (ES, 0.3 μmol/L) was then measured. Transport activity was expressed as percentage of the uptake in control cells. Values are mean ± SD (n = 3); *P < 0.05. Statistical analysis was performed using one-way ANOVA by using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA).
3.2. Effect of IGF-1 on OAT3 expression
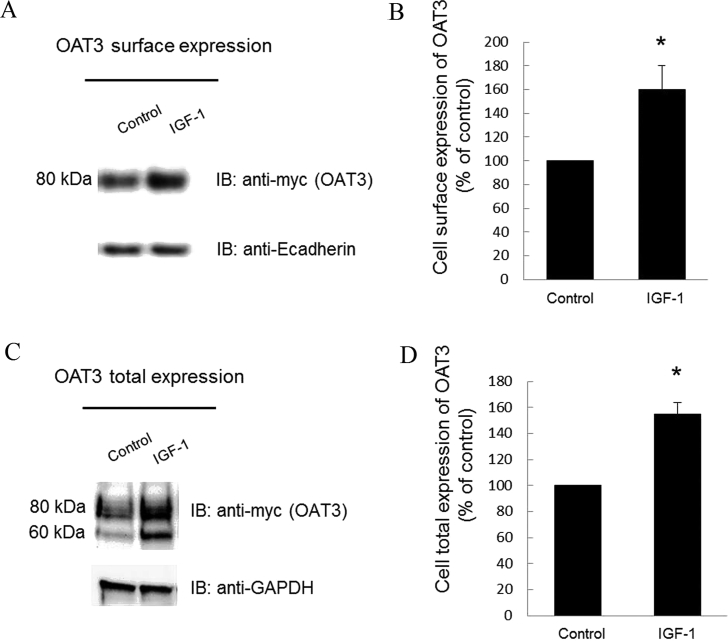
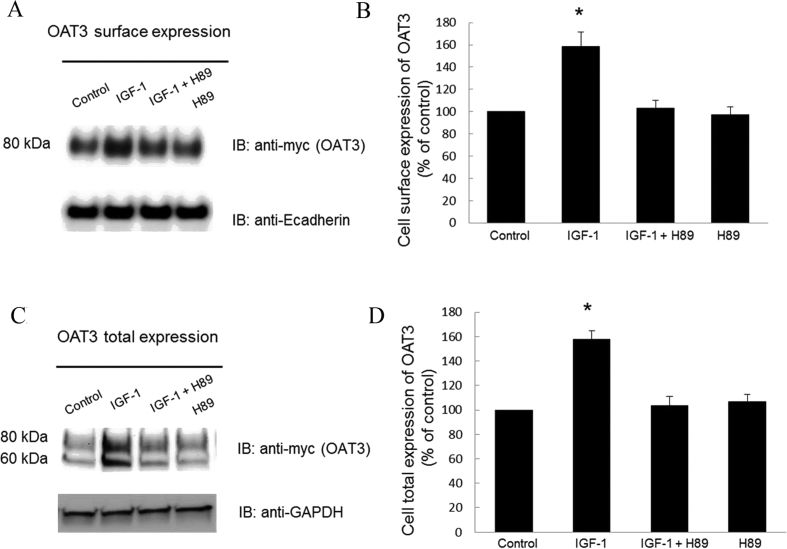
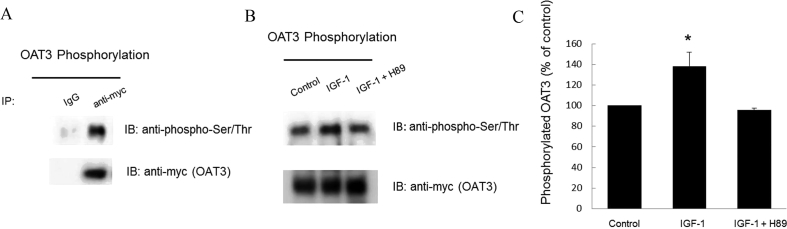
The change in transport activity of OAT3 may reflect a change in its three-dimensional structure or in its level of expression. We therefore examined the effect of IGF-1 on OAT3 expression. Our results revealed that treatment of OAT3-expressing cells with IGF-1 led to an increase of OAT3 expression both at the cell surface (Fig. 2A top panel and 2B), and in its total cell extract (Fig. 2C top panel and 2D). Such a change in OAT3 expression was not because of the overall disturbance of membrane and cellular proteins since the expression of plasma membrane protein marker E-cadherin and cellular protein marker GAPDH were not affected under these situations (Fig. 2A, bottom panel and Fig. 2C, bottom panel). OAT3 at the cell surface displayed a single band at 80 kDa (Fig. 2A, top panel), whereas OAT3 showed two bands at 60 and 80 kDa in total cell extracts (Fig.2C, top panel). Our laboratory previously illustrated that OAT undergoes glycosylation as a maturation process in the endoplasmic reticulum (ER)–Golgi complex33, 34. The immature form is a non-glycosylated form of 60 kDa, which matures in ER–Golgi complex to a glycosylated form of 80 kDa. Only the mature form (80 kDa) can target to cell surface. Furthermore, IGF-1-induced increase in OAT3 expression was blocked by PKA inhibitor H89 (Fig. 3), suggesting that IGF-1 modulates OAT3 through PKA signaling pathway.
Figure 2.
Effect of IGF-1 on OAT3 expression. (A) Cell surface expression of OAT3. Top panel: OAT3-expressing cells were treated with IGF-1 (100 nmol/L, 4 h). Biotinylated cell surface proteins were separated with streptavidin beads, followed by immunoblotting (IB) with anti-myc antibody (OAT3 was tagged with epitope myc for immunodetection). Bottom panel: The identical blot of the top panel was re-probed with anti-E-cadherin antibody. E-cadherin is a marker for cell membrane proteins. (B) Densitometry analysis of blot results from Fig. 2A, top panel as well as from other experiments. The values are mean ± SD (n = 3); *P < 0.05. (C) Total protein expression of OAT3. Top panel: OAT3-expressing cells were treated with IGF-1 (100 nmol/L, 4 h). After treatment, cells were lysed, followed by immunoblotting with anti-myc antibody. Bottom panel: The identical blot of the top panel was re-probed with anti-GAPDH antibody. GAPDH is a marker for total cell proteins. (D) Densitometry analysis of blot results from Fig. 2C, top panel as well as from other experiments. The values are mean ± SD (n = 3); *P < 0.05. Statistical analysis was performed using Student's paired t-tests.
Figure 3.
IGF-1 regulates OAT3 expression through PKA pathway. (A) Top panel: OAT3-expressing cells were treated with IGF-1 (100 nmol/L) with or without PKA inhibitor H89 (10 μmol/L) for 4 h. Cells were labeled with membrane impermeable biotin. Biotinylated cell surface proteins were separated with streptavidin beads, followed by immunoblotting (IB) with anti-myc antibody (OAT3 was tagged with epitope myc for immunodetection). Bottom panel: The identical blot of the top panel was re-probed with anti-E-cadherin antibody. E-cadherin is a marker for cell membrane proteins. (B) Densitometry analysis of blot results from Fig. 3A top panel as well as from other experiments. The values are mean ± SD (n = 3); *P < 0.05. (C). Top panel: OAT3-expressing cells were treated with IGF-1 (100 nmol/L) with or without H89 (10 μmol/L) for 4 h. The cells were collected and lysed, followed by immunoblotting with anti-myc antibody. Bottom panel: The identical blot of the top panel was re-probed with anti-GAPDH antibody. GAPDH is a marker for total cell proteins. (D). Densitometry analyses of blot results from Fig. 3C top panel as well as from other experiments. The values are mean ± SD (n = 3); *P < 0.05. Statistical analysis was performed using one-way ANOVA by using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA).
3.3. Effect of IGF-1 on OAT3 phosphorylation
As protein kinases regulate their substrate proteins, by phosphorylating these substrates, IGF-1 may regulate OAT3 through PKA-dependent OAT3 phosphorylation. We therefore examined OAT3 phosphorylation in IGF-1-treated cells (Fig. 4). OAT3 was immunoprecipitated (IP) with anti-myc antibody affinity gel (OAT3 was tagged with epitope myc for immunodetection) or with control IgG–agarose (as negative control), followed by immunoblotting with anti-phospho-Ser/Thr antibody. As shown in Fig. 4A, top panel, phosphorylated OAT3 was only detected in sample that was immunoprecipitated with anti-myc antibody but not in sample that was immunoprecipitated with negative control IgG, demonstrating the specificity of the phosphorylation band for OAT3. In Fig. 4B, we showed that IGF-1 significantly enhanced OAT3 phosphorylation as compared to that in control cells, and such enhancement of OAT3 phosphorylation was blocked by PKA inhibitor H89, once again indicating that IGF-1 modulates OAT3 through PKA signaling pathway.
Figure 4.
IGF-1 regulates OAT3 phosphorylation through PKA pathway. (A) Top panel: OAT3-expressing cells were lysed, pre-cleared and immunoprecipitated with control IgG-agarose (as negative control) or anti-myc agarose affinity gel, followed by immunoblotting (IB) with anti-phospho-Ser/Thr antibody. Bottom panel: The identical blot of the top panel was re-probed with anti-myc antibody to determine the amount of OAT3 immunoprecipitated. (B) Phosphorylation of OAT3. Top panel: OAT3-expressing cells were treated with IGF-1 (100 nmol/L) with or without PKA inhibitor H89 (10 μmol/L) for 4 h. After treatment, cells were lysed, pre-cleared and immunoprecipitated with anti-myc agarose affinity gel, followed by immunoblotting (IB) with anti-phospho-Ser/Thr antibody. Bottom panel: The identical blot of the top panel was re-probed with anti-myc antibody to determine the amount of OAT3 immunoprecipitated. (C) Densitometry analysis of blot results from Fig. 4B, top panel as well as from other experiments. The values are mean ± SD (n = 3); *P < 0.05. Statistical analysis was performed using one-way ANOVA by using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA).
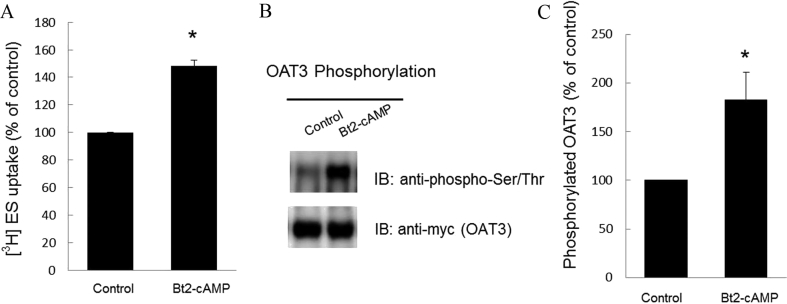
3.4. Effect of PKA activator Bt2-cAMP on OAT3 transport activity and phosphorylation
To confirm the direct involvement of PKA in OAT3 function and phosphorylation, we treated OAT3-expressing cells with PKA activator Bt2-cAMP, followed by the measurement of OAT3-mediated uptake of [3H]-estrone sulfate (ES) and OAT3 phosphorylation. Our results revealed that OAT3 transport activity (Fig. 5A) and phosphorylation (Fig. 5B, top panel) was much more enhanced in Bt2-cAMP-treated cells as compared to that in control cells. The change in OAT3 phosphorylation (Fig. 5B, top panel) was not due to the difference in the amount of OAT3 immunoprecipitated because similar amount of OAT3 was pulled down in all samples (Fig. 5B, bottom panel).
Figure 5.
PKA activator Bt2-cAMP up-regulates OAT3 transport function and phosphorylation. (A) OAT3-expressing cells were treated with Bt2-cAMP (5 nmol/L) for 2 h. 4-min uptake of [3H]-estrone sulfate (ES, 300 nmol/L) was then measured. Transport activity was expressed as percentage of the uptake in control cells. The data corresponded to the uptake of OAT3-expressing cells minus uptake of untreated parental cells. Values are mean ± SD (n = 3); *P < 0.05. (B) Phosphorylation of OAT3. Top panel: OAT3-expressing cells were treated with Bt2-cAMP (5 nmol/L) for 2 h. After treatment, cells were lysed, pre-cleared and immunoprecipitated with anti-myc agarose affinity gel, followed by immunoblotting (IB) with anti-phospho-Ser/Thr antibody. Bottom panel: The identical blot of the top panel was re-probed with anti-myc antibody to determine the amount of OAT3 immunoprecipitated. (C) Densitometry analysis of blot results from Fig. 5B top panel as well as from other experiments. The values are mean ± SD (n = 3); *P < 0.05. Statistical analysis was performed using Student's paired t-tests.
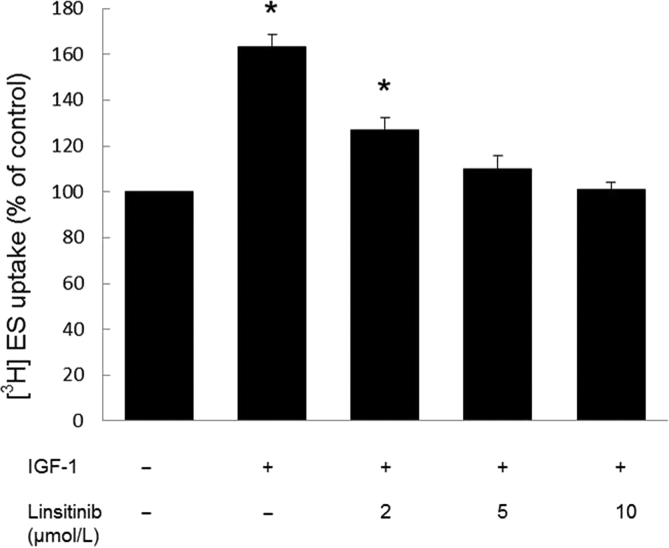
3.5. Effect of linsitinib on OAT3 transport activity
Linsitinib is an anti-cancer drug and an inhibitor for IGF-1 receptor, which has been investigated in a phase III clinical trial. We treated OAT3-expressing cells with IGF-1 in the presence and absence of linsitinib, then measured OAT3-mediated uptake of [3H]-estrone sulfate (ES). Our results (Fig. 6) showed that IGF-1 significantly stimulated OAT3 transport activity, and such stimulation was blocked by linsitinib in a dose-dependent manner, suggesting that IGF-1 and linsitinib have antagonistic roles in the regulation of OAT3 transport activity.
Figure 6.
Linsitinib blocked IGF-1-stimulated OAT3 transport activity. OAT3-expressing cells were treated with IGF-1 (100 nmol/L) and with various concentrations of linsitinib for 4 h. 4-min uptake of [3H]-estrone sulfate (ES, 300 nmol/L) was then measured. Transport activity was expressed as percentage of the uptake in control cells. The data corresponded to the uptake of OAT3-expressing cells minus uptake of untreated parental cells. Values are mean ± SD (n = 3); *P < 0.05. Statistical analysis was performed using one-way ANOVA by using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA).
4. Discussion
Active transport of organic anion carried out by organic anion transporters (OATs) is a major determining factor of the outcomes of therapeutic and toxic chemicals1, 2, 3, 4, 5, 6. Thus, it is of clinical and pharmacological importance to understand the mechanisms governing OAT regulation. Our current study investigated the regulatory mechanism of OAT3 by IGF-1 and revealed that this hormone modulates OAT3 expression and transport activity possibly through PKA-dependent phosphorylation of the transporter.
Our current work was conducted in COS-7 cells, an excellent cell model for investigating organic anion transporters and other renal transporters31, 35, 36, 37, 38. COS-7 cells are derived from kidney tissue of the African green monkey. Many features of OATs in COS-7 cells are consistent with those observed in other systems such as animal models39. Therefore, studies conducted in COS-7 cells will pave the way for future work exploring the mechanisms in native epithelia.
Protein kinases exert their roles through phosphorylating their substrate proteins. Introducing a negatively charged phosphate group to a substrate protein (phosphorylation) may change the three-dimensional conformation of the substrate, its activity, cellular distribution, stability or the interaction of the substrate protein with its interacting partner. Many membrane proteins such as organic cation transporter type 1, glucose transporter GLUT1 and dopamine transporter are regulated by PKC-dependent phosphorylation40, 41, 42. Previous studies from our lab demonstrated that OAT transport activity is subjected to the down-regulation by several physiological stimuli through the activation of PKC13, 14, 15. Among those physiological stimuli are angiotensin II, peptide hormone bradykinin, and progesterone. We established that activation PKC accelerates the rate of OAT internalization from cell surface to intracellular endosomes and the rate of OAT degradation, a process catalyzed by an ubiquitin ligase Nedd4-231, 35, 43.
Interestingly through the course of our investigation, we demonstrated that activation of PKC down-regulates OAT expression and transport activity without directly phosphorylating the transporters. Site-directed mutagenesis of potential PKC sites by Wolff et al.44 further confirmed our observation. In contrast to peptide hormone bradykinin/PKC and angiotensin II/PKC pathways, our interesting results in the current study showed that IGF-1/PKA pathway did involve direct phosphorylation of OAT3. We showed that both IGF-1 and activation of PKA induced significant increase in OAT3 phosphorylation (Figure 4, Figure 5), which correlated well with an increase in OAT3 expression and transport activity (Figure 1, Figure 5). Adding a phosphate group on a target protein may change protein's structural properties, its stability and dynamics45, 46, 47. Furthermore, the cross-talk among various post-translational modifications occurs when the phosphorylation modification on the target protein influences other modifications including SUMOylation and ubiquitination. It has been reported that phosphorylation of the protein could stimulate or inhibit its SUMOylation48, 49, 50, 51. In addition, the interplay between phosphorylation and ubiquitination has also been reported46, 47, 52. Recently, we demonstrated that activation of PKA stimulates SUMOylation and inhibits ubiquitination of OAT323, and ubiquitination of OAT3 leads to the internalization of OAT3 from cell surface to intracellular compartment and subsequent degradation. Therefore, it would be an interesting future direction for us to explore whether OAT3 phosphorylation promotes OAT3 SUMOylation, which in turn, suppresses OAT3 ubiquitination. As a result, the ubiquitination-dependent degradation is decreased and the stability of OAT3 is enhanced. Furthermore, the phosphorylation of OAT3 may not only increase its protein level but may also change its three-dimensional conformation, which leads to the change of the binding affinity of the transporter to its substrates.
Most protein substrates for PKA bear the consensus motif, R/K-R/K-X-S/T-ψ (where R is arginine, K is lysine, X is any amino acid, S is serine, T is threonine and ψ is a hydrophobic residue53. Using program NetPhos3.1 to predict phosphorylation sites, we identified several intracellular locations that bear the sequences for PKA phosphorylation. On the other hand, PKA phosphorylation can also occur at residues outside conventional motifs/sequences and the presence of conventional motifs does not guarantee the phosphorylation. In addition, it could not be ruled out that PKA may activate some other protein kinases that possibly phosphorylate OAT3. For example, PKB can be activated by PKA through a PI3-kinase-independent pathway54, 55 and AMP-activated protein kinase (AMPK) also can be a substrate activated by PKA56. The mapping of PKA-dependent phosphorylation sites on OAT3 is currently underway in our laboratory.
IGF-1 binds to its cell surface receptor to initiate the cell signaling. The inabilities to produce or to respond to IGF-1 cause several diseases and symptoms. For example, patients with Laron dwarfism showed severely low levels of IGF-1 caused by the lack of IGF-1 synthesis. The homozygous mutation of the IGF1 receptor is related with intrauterine growth retardation, insulin resistance and dysmorphism57. And the heterozygous mutation of IGF-1 receptor is also associated with intrauterine and postnatal growth retardation58. In addition, the heterozygous mutation of IGF-1 receptor was indicated to increase resistance to oxidative stress and lifespan in mice59.
IGF-1 signaling pathway is essential for cell growth, proliferation and survival and also plays an important role in development and sustainability of malignant tumors including breast cancer, sarcoma and lung cancer60, 61, 62, 63, 64. Thus manipulation of IGF-1 signaling system is a very promising strategy for novel anti-cancer therapeutics and IGF-1 receptor blockade has been identified as the target for cancer treatment65, 66, 67, 68. Numerous IGF-1 receptor inhibitors are at different stages of clinic trials and they can be classified into three groups including monoclonal antibody, ligand inhibitors and tyrosine kinase inhibitors. Linsitinib is a selective and potent IGF-1 receptor inhibitor and has been investigated in a phase III clinical trial for adrenocortical carcinoma69. Furthermore, the linsitinib clinical trials in different phases are initiated for multiple cancers including ovarian cancer, myeloma and prostate cancer70, 71, 72. In our studies, linsitinib significantly blocked stimulatory effect of IGF-1 on OAT3 transport activity in a dose dependent manner. Therefore, OAT transport activity should be taken into consideration in cancer patients treated with linsitinib.
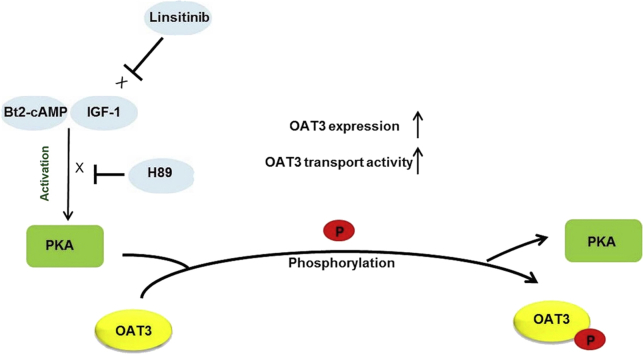
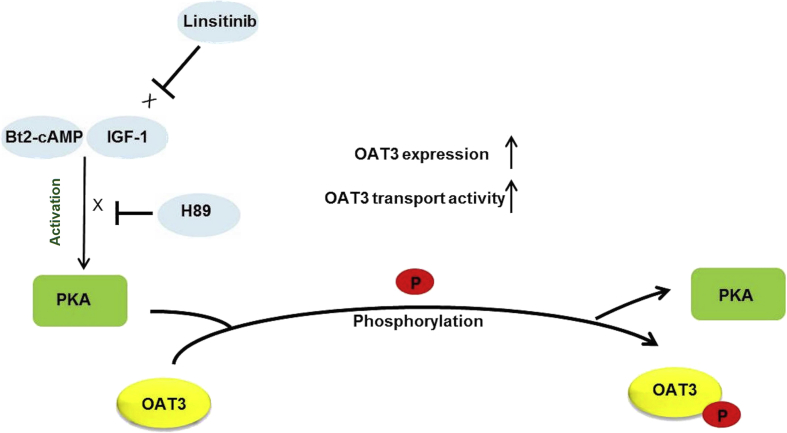
Our discovery about the IGF-1 modulation of OAT3 via PKA signaling supports a remote sensing and signaling model for transporters5, 9, 73. In such model, transporters in different tissues form networks and are regulated by hormones and growth factors, thereby efficiently talk among one another. In doing so, these transporters coordinately keep the balance among multiple organs and thus system homeostasis. Hormones/growth factors, produced from one organ under the influence of stimuli/environmental alterations, get into the blood stream, and then arrive at the target organs and apply their regulatory roles on transporters via cell signaling. Aligning with this model, our results support that IGF-1, which is formed mostly by the liver under stimuli, arrives at the kidney via blood stream, and then binds to its receptors and stimulates OAT3 expression and activity through PKA signaling (Fig. 7).
Figure 7.
IGF-1/PKA signaling pathway regulates OAT3 phosphorylation, expression and transport activity. P: phosphate group.
5. Conclusions
Our study demonstrated that IGF-1 regulates OAT3 expression and transport activity through PKA signaling pathway, possibly by phosphorylating the transporter.
Acknowledgments
This work was supported by grants (to Dr. Guofeng You) from National Institute of General Medical Sciences (R01-GM079123 and R01-GM097000, USA).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Author Contributions
Jinghui Zhang: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization. Zhou Yu: Validation, Formal analysis, Investigation, Writing - Review & Editing, Visualization. Guofeng You: Conceptualization, Methodology, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Funding acquisition.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.You G. Structure, function, and regulation of renal organic anion transporters. Med Res Rev. 2002;22:602–616. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 2.Srimaroeng C., Perry J.L., Pritchard J.B. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008;38:889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantzler W.H., Wright S.H. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta. 2003;1618:185–193. doi: 10.1016/j.bbamem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 4.VanWert A.L., Gionfriddo M.R., Sweet D.H. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31:1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 5.Ahn S.Y., Nigam S.K. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol. 2009;76:481–490. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terada T., Inui K. Gene expression and regulation of drug transporters in the intestine and kidney. Biochem Pharmacol. 2007;73:440–449. doi: 10.1016/j.bcp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Nigam S.K., Bhatnagar V. The systems biology of uric acid transporters: the role of remote sensing and signaling. Curr Opin Nephrol Hypertens. 2018;27:305–313. doi: 10.1097/MNH.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nigam S.K. The SLC22 transporter family: a paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu Rev Pharmacol Toxicol. 2018;58:663–687. doi: 10.1146/annurev-pharmtox-010617-052713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigam S.K., Bush K.T., Martovetsky G., Ahn S.Y., Liu H.C., Richard E. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev. 2015;95:83–123. doi: 10.1152/physrev.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii T., Segawa H., Hanazaki A., Nishiguchi S., Minoshima S., Ohi A. Role of the putative PKC phosphorylation sites of the type IIc sodium-dependent phosphate transporter in parathyroid hormone regulation. Clin Exp Nephrol. 2019 doi: 10.1007/s10157-019-01725-6. Available from: [DOI] [PubMed] [Google Scholar]
- 11.Huang W., Zeng J., Liu Z., Su M., Li Q., Zhu B. Acetylshikonin stimulates glucose uptake in L6 myotubes via a PLC-β3/PKCδ-dependent pathway. Biomed Pharmacother. 2019;112:108588. doi: 10.1016/j.biopha.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 12.Chambers T.C., McAvoy E.M., Jacobs J.W., Eilon G. Protein kinase C phosphorylates P-glycoprotein in multidrug resistant human KB carcinoma cells. J Biol Chem. 1990;265:7679–7686. [PubMed] [Google Scholar]
- 13.Li S., Duan P., You G. Regulation of human organic anion transporter 3 by peptide hormone bradykinin. J Pharmacol Exp Ther. 2010;333:970–975. doi: 10.1124/jpet.110.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan P., Li S., You G. Angiotensin II inhibits activity of human organic anion transporter 3 through activation of protein kinase Cα: accelerating endocytosis of the transporter. Eur J Pharmacol. 2010;627:49–55. doi: 10.1016/j.ejphar.2009.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S., Duan P., You G. Regulation of human organic anion transporter 1 by Ang II: involvement of protein kinase Cα. Am J Physiol Endocrinol Metab. 2009;296:E378–E383. doi: 10.1152/ajpendo.90713.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You G., Kuze K., Kohanski R.A., Amsler K., Henderson S. Regulation of moat-mediated organic anion transport by okadaic acid and protein kinase C in LLc-PK1 cells. J Biol Chem. 2000;275:10278–10284. doi: 10.1074/jbc.275.14.10278. [DOI] [PubMed] [Google Scholar]
- 17.Bach L.A., Hale L. Insulin-like growth factors and kidney disease. Am J Kidney Dis. 2015;65:327–336. doi: 10.1053/j.ajkd.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Yakar S., Adamo M.L. Insulin-like growth factor 1 physiology: lessons from mouse models. Endocrinol Metab Clin N Am. 2012;41:231–247. doi: 10.1016/j.ecl.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yakar S., Wu Y., Setser J., Rosen C.J. The role of circulating IGF-I: lessons from human and animal models. Endocrine. 2002;19:239–248. doi: 10.1385/ENDO:19:3:239. [DOI] [PubMed] [Google Scholar]
- 20.Kamenicky P., Mazziotti G., Lombes M., Giustina A., Chanson P. Growth hormone, insulin-like growth factor-1, and the kidney: pathophysiological and clinical implications. Endocr Rev. 2014;35:234–281. doi: 10.1210/er.2013-1071. [DOI] [PubMed] [Google Scholar]
- 21.Turnham R.E., Scott J.D. Protein kinase a catalytic subunit isoform PRKACA; history, function and physiology. Gene. 2016;577:101–108. doi: 10.1016/j.gene.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J.H., Han J.S., Kong J., Ji Y., Lv X., Lee J. Protein kinase a subunit balance regulates lipid metabolism in caenorhabditis elegans and mammalian adipocytes. J Biol Chem. 2016;291:20315–20328. doi: 10.1074/jbc.M116.740464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H., Zhang J., You G. Activation of protein kinase a stimulates sumoylation, expression, and transport activity of organic anion transporter 3. AAPS J. 2019;21:30. doi: 10.1208/s12248-019-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramaniam S., Shahani N., Strelau J., Laliberte C., Brandt R., Kaplan D. Insulin-like growth factor 1 inhibits extracellular signal-regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase/protein kinase A/c-Raf pathway. J Neurosci. 2005;25:2838–2852. doi: 10.1523/JNEUROSCI.5060-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng C.W., Adams G.B., Perin L., Wei M., Zhou X., Lam B.S. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giustina A., Chanson P., Kleinberg D., Bronstein M.D., Clemmons D.R., Klibanski A. Expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10:243–248. doi: 10.1038/nrendo.2014.21. [DOI] [PubMed] [Google Scholar]
- 27.Laron Z., Klinger B. Laron syndrome: clinical features, molecular pathology and treatment. Horm Res. 1994;42:198–202. doi: 10.1159/000184193. [DOI] [PubMed] [Google Scholar]
- 28.Keating G.M. Mecasermin. BioDrugs. 2008;22:177–188. doi: 10.2165/00063030-200822030-00004. [DOI] [PubMed] [Google Scholar]
- 29.Walenkamp M.J., Losekoot M., Wit J.M. Molecular IGF-1 and IGF-1 receptor defects: from genetics to clinical management. Endocr Dev. 2013;24:128–137. doi: 10.1159/000342841. [DOI] [PubMed] [Google Scholar]
- 30.Duan P., Li S., Ai N., Hu L., Welsh W.J., You G. Potent inhibitors of human organic anion transporters 1 and 3 from clinical drug libraries: discovery and molecular characterization. Mol Pharm. 2012;9:3340–3346. doi: 10.1021/mp300365t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q., Li S., Patterson C., You G. Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis. Mol Pharmacol. 2013;83:217–224. doi: 10.1124/mol.112.082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J., Liu C., You G. Ag490, a JAK2-specific inhibitor, downregulates the expression and activity of organic anion transporter-3. J Pharmacol Sci. 2018;136:142–148. doi: 10.1016/j.jphs.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F., Xu W., Hong M., Pan Z., Sinko P.J., Ma J. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol. 2005;67:868–876. doi: 10.1124/mol.104.007583. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K., Xu W., Zhou F., You G. Role of glycosylation in the organic anion transporter OAT1. J Biol Chem. 2004;279:14961–14966. doi: 10.1074/jbc.M400197200. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q., Hong M., Duan P., Pan Z., Ma J., You G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem. 2008;283:32570–32579. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhardwaj R.K., Herrera-Ruiz D., Eltoukhy N., Saad M., Knipp G.T. The functional evaluation of human peptide/histidine transporter 1 (hPHT1) in transiently transfected COS-7 cells. Eur J Pharm Sci. 2006;27:533–542. doi: 10.1016/j.ejps.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Segawa H., Kaneko I., Takahashi A., Kuwahata M., Ito M., Ohkido I. Growth-related renal type II Na/Pi cotransporter. J Biol Chem. 2002;277:19665–19672. doi: 10.1074/jbc.M200943200. [DOI] [PubMed] [Google Scholar]
- 38.Goyal S., Vanden Heuvel G., Aronson P.S. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol. 2003;284:F467–F473. doi: 10.1152/ajprenal.00352.2002. [DOI] [PubMed] [Google Scholar]
- 39.Phatchawan A., Chutima S., Varanuj C., Anusorn L. Decreased renal organic anion transporter 3 expression in type 1 diabetic rats. Am J Med Sci. 2014;347:221–227. doi: 10.1097/MAJ.0b013e3182831740. [DOI] [PubMed] [Google Scholar]
- 40.Ciarimboli G., Koepsell H., Iordanova M., Gorboulev V., Durner B., Lang D. Individual PKC-phosphorylation sites in organic cation transporter 1 determine substrate selectivity and transport regulation. J Am Soc Nephrol. 2005;16:1562–1570. doi: 10.1681/ASN.2004040256. [DOI] [PubMed] [Google Scholar]
- 41.Lee E.E., Ma J., Sacharidou A., Mi W., Salato V.K., Nguyen N. A protein kinase C phosphorylation motif in GLUT1 affects glucose transport and is mutated in GLUT1 deficiency syndrome. Mol Cell. 2015;58:845–853. doi: 10.1016/j.molcel.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moritz A.E., Foster J.D., Gorentla B.K., Mazei-Robison M.S., Yang J.W., Sitte H.H. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. J Biol Chem. 2013;288:20–32. doi: 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu D., Zhang J., Zhang Q., Fan Y., Liu C., You G. PKC/Nedd4-2 signaling pathway regulates the cell surface expression of drug transporter hOAT1. Drug Metab Dispos. 2017;45:887–895. doi: 10.1124/dmd.117.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolff N.A., Thies K., Kuhnke N., Reid G., Friedrich B., Lang F. Protein kinase C activation downregulates human organic anion transporter 1-mediated transport through carrier internalization. J Am Soc Nephrol. 2003;14:1959–1968. doi: 10.1097/01.asn.0000079040.55124.25. [DOI] [PubMed] [Google Scholar]
- 45.Coutinho R.A., van Griensven G.J., Moss A. Effects of preventive efforts among homosexual men. AIDS. 1989;3 Suppl 1:S53–S56. doi: 10.1097/00002030-198901001-00008. [DOI] [PubMed] [Google Scholar]
- 46.Tedja R., Roberts C.M., Alvero A.B., Cardenas C., Yang-Hartwich Y., Spadinger S. Protein kinase Cα-mediated phosphorylation of Twist1 at Ser-144 prevents Twist1 ubiquitination and stabilizes it. J Biol Chem. 2019;294:5082–5093. doi: 10.1074/jbc.RA118.005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H., Wang K., Chen S., Sun Q., Zhang Y., Chen L. NFATc1 phosphorylation by DYRK1A increases its protein stability. PLoS One. 2017;12:e0172985. doi: 10.1371/journal.pone.0172985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang S.H., Jaffray E., Senthinathan B., Hay R.T., Sharrocks A.D. SUMO and transcriptional repression: dynamic interactions between the MAP kinase and SUMO pathways. Cell Cycle. 2003;2:528–530. doi: 10.4161/cc.2.6.597. [DOI] [PubMed] [Google Scholar]
- 49.Hietakangas V., Ahlskog J.K., Jakobsson A.M., Hellesuo M., Sahlberg N.M., Holmberg C.I. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol. 2003;23:2953–2968. doi: 10.1128/MCB.23.8.2953-2968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajan S., Dickson L.M., Mathew E., Orr C.M., Ellenbroek J.H., Philipson L.H. Chronic hyperglycemia downregulates GLP-1 receptor signaling in pancreatic β-cells via protein kinase A. Mol Metab. 2015;4:265–276. doi: 10.1016/j.molmet.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spengler M.L., Kurapatwinski K., Black A.R., Azizkhan-Clifford J. SUMO-1 modification of human cytomegalovirus IE1/IE72. J Virol. 2002;76:2990–2996. doi: 10.1128/JVI.76.6.2990-2996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen L.K., Kolch W., Kholodenko B.N. When ubiquitination meets phosphorylation: a systems biology perspective of EGFR/MAPK signalling. Cell Commun Signal. 2013;11:52. doi: 10.1186/1478-811X-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rust H.L., Thompson P.R. Kinase consensus sequences: a breeding ground for crosstalk. ACS Chem Biol. 2011;6:881–892. doi: 10.1021/cb200171d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filippa N., Sable C.L., Filloux C., Hemmings B., Van Obberghen E. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol Cell Biol. 1999;19:4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sable C.L., Filippa N., Hemmings B., Van Obberghen E. cAMP stimulates protein kinase B in a Wortmannin-insensitive manner. FEBS Lett. 1997;409:253–257. doi: 10.1016/s0014-5793(97)00518-8. [DOI] [PubMed] [Google Scholar]
- 56.Medina E.A., Oberheu K., Polusani S.R., Ortega V., Velagaleti G.V., Oyajobi B.O. PKA/AMPK signaling in relation to adiponectin's antiproliferative effect on multiple myeloma cells. Leukemia. 2014;28:2080–2089. doi: 10.1038/leu.2014.112. [DOI] [PubMed] [Google Scholar]
- 57.Gannage-Yared M.H., Klammt J., Chouery E., Corbani S., Megarbane H., Abou Ghoch J. Homozygous mutation of the IGF1 receptor gene in a patient with severe pre- and postnatal growth failure and congenital malformations. Eur J Endocrinol. 2013;168:K1–K7. doi: 10.1530/EJE-12-0701. [DOI] [PubMed] [Google Scholar]
- 58.Wallborn T., Wuller S., Klammt J., Kruis T., Kratzsch J., Schmidt G. A heterozygous mutation of the insulin-like growth factor-I receptor causes retention of the nascent protein in the endoplasmic reticulum and results in intrauterine and postnatal growth retardation. J Clin Endocrinol Metab. 2010;95:2316–2324. doi: 10.1210/jc.2009-2404. [DOI] [PubMed] [Google Scholar]
- 59.Holzenberger M., Dupont J., Ducos B., Leneuve P., Geloen A., Even P.C. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 60.Yakar S., Leroith D., Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407–420. doi: 10.1016/j.cytogfr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Yakar S., Pennisi P., Zhao H., Zhang Y., LeRoith D. Circulating IGF-1 and its role in cancer: lessons from the IGF-1 gene deletion (lid) mouse. Novartis Found Symp. 2004;262:3–9. discussion 9-18, 265-8. [PubMed] [Google Scholar]
- 62.Christopoulos P.F., Msaouel P., Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu Q., Zhou Y., Ying K., Ruan W. IGFBP, a novel target of lung cancer?. Clin Chim Acta. 2017;466:172–177. doi: 10.1016/j.cca.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 64.van Maldegem A.M., Bovee J.V., Peterse E.F., Hogendoorn P.C., Gelderblom H. Ewing sarcoma: the clinical relevance of the insulin-like growth factor 1 and the poly-ADP-ribose-polymerase pathway. Eur J Cancer. 2016;53:171–180. doi: 10.1016/j.ejca.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Beckwith H., Yee D. Minireview: were the IGF signaling inhibitors all bad?. Mol Endocrinol. 2015;29:1549–1557. doi: 10.1210/me.2015-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H.X., Sharon E. IGF-1R as an anti-cancer target—trials and tribulations. Chin J Canc. 2013;32:242–252. doi: 10.5732/cjc.012.10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pillai R.N., Ramalingam S.S. Inhibition of insulin-like growth factor receptor: end of a targeted therapy?. Transl Lung Cancer Res. 2013;2:14–22. doi: 10.3978/j.issn.2218-6751.2012.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iams W.T., Lovly C.M. Molecular pathways: clinical applications and future direction of insulin-like growth factor-1 receptor pathway blockade. Clin Cancer Res. 2015;21:4270–4277. doi: 10.1158/1078-0432.CCR-14-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fassnacht M., Berruti A., Baudin E., Demeure M.J., Gilbert J., Haak H. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16:426–435. doi: 10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]
- 70.Chiappori A.A., Otterson G.A., Dowlati A., Traynor A.M., Horn L., Owonikoko T.K. A randomized phase II study of linsitinib (OSI-906) versus topotecan in patients with relapsed small-cell lung cancer. Oncol. 2016;21:1163–1164. doi: 10.1634/theoncologist.2016-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oza A., Kaye S., Van Tornout J., Sessa C., Gore M., Naumann R.W. Phase 2 study evaluating intermittent and continuous linsitinib and weekly paclitaxel in patients with recurrent platinum resistant ovarian epithelial cancer. Gynecol Oncol. 2018;149:275–282. doi: 10.1016/j.ygyno.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 72.Barata P., Cooney M., Tyler A., Wright J., Dreicer R., Garcia J.A. A phase 2 study of OSI-906 (linsitinib, an insulin-like growth factor receptor-1 inhibitor) in patients with asymptomatic or mildly symptomatic (non-opioid requiring) metastatic castrate resistant prostate cancer (CRPC) Investig New Drugs. 2018;36:451–457. doi: 10.1007/s10637-018-0574-0. [DOI] [PubMed] [Google Scholar]
- 73.Wu W., Dnyanmote A.V., Nigam S.K. Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol Pharmacol. 2011;79:795–805. doi: 10.1124/mol.110.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]