Abstract
Few medications are available for meeting the increasing disease burden of nonalcoholic fatty liver disease (NAFLD) and its progressive stage, nonalcoholic steatohepatitis (NASH). Traditional herbal medicines (THM) have been used for centuries to treat indigenous people with various symptoms but without clarified modern-defined disease types and mechanisms. In modern times, NAFLD was defined as a common chronic disease leading to more studies to understand NAFLD/NASH pathology and progression. THM have garnered increased attention for providing therapeutic candidates for treating NAFLD. In this review, a new model called “multiple organs-multiple hits” is proposed to explain mechanisms of NASH progression. Against this proposed model, the effects and mechanisms of the frequently-studied THM-yielded single anti-NAFLD drug candidates and multiple herb medicines are reviewed, among which silymarin and berberine are already under U.S. FDA-sanctioned phase 4 clinical studies. Furthermore, experimental designs for anti-NAFLD drug discovery from THM in treating NAFLD are discussed. The opportunities and challenges of reverse pharmacology and reverse pharmacokinetic concepts-guided strategies for THM modernization and its global recognition to treat NAFLD are highlighted. Increasing mechanistic evidence is being generated to support the beneficial role of THM in treating NAFLD and anti-NAFLD drug discovery.
Key words: Natural products, Fatty liver, Metabolic syndrome, TCM, NAFLD
Graphical abstract
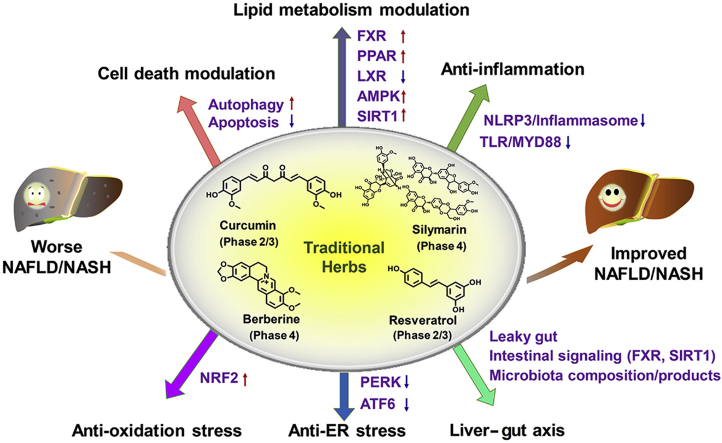
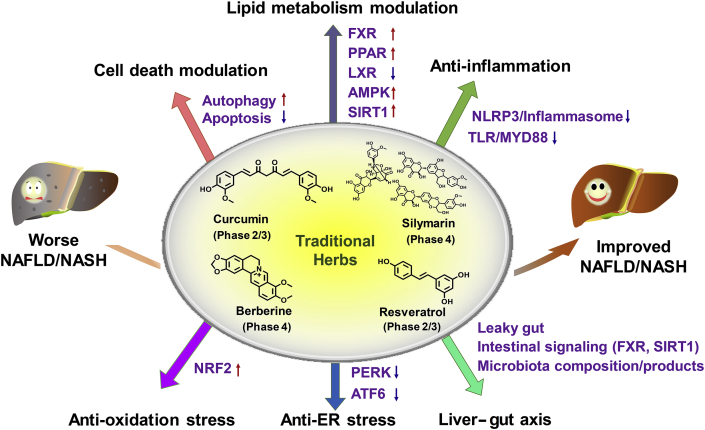
This review summarized the role and mechanisms of frequently studied traditional herbs in treating NAFLD. Four frequently studied traditional herbs (silymarin, berberine, curcumin and resveratrol) that are registered in ClinicalTrials.gov preclinically benefits NAFLD/NASH treatment via various molecular pathways including cell death modulation, lipid metabolism modulation, anti-inflammation, anti-oxidative stress, and liver–gut axis.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is among the most common chronic liver diseases worldwide, and its progressive stage that shows hepatic inflammation and fibrosis is termed as nonalcoholic steatohepatitis (NASH). NASH, in turn can lead to cirrhosis, liver failure and liver cancer1,2. Because of the pandemic spread of obesity, particularly in western countries, the worldwide pooled prevalence of NAFLD continues to increase and is now estimated at 24%3. Notably, 8%–19% of NAFLD patients are found to be lean or non-obese in Asia4,5. NAFLD has become the second leading cause of liver transplantation in the United States5.
Generally, the non-progressive stage of NAFLD is asymptomatic and pharmacologically curable, while the progressive NASH is refractory to treatment. Most market-available drugs, such as vitamin E, only improve hepatic steatosis and inflammation, but have little impact on the progressive fibrosis, during treatment of NAFLD6,7. Diverse clinical trials for testing modern drug candidates of NASH have failed to reach the major endpoint or has limited therapy efficacy, such as obeticholic acid8. Several agents such as nuclear receptor agonists (obeticholic acid, GFT505, elafibranor), insulin sensitizers (glitazones, pioglitazone, metformin) and glucagon-like peptide-1 receptor agonists are still in the drug pipeline for NASH9, 10, 11. It takes up to three years for obtaining outcomes to register promising anti-NASH drugs, and no drugs have been approved by the U.S. Food and Drug Administration (FDA) to treat NASH until now. Currently only weight loss by bariatric surgery treatment or non-pharmacological managements by healthy life/diet style and/or physical activity can be effective12,13. Thus, the development of medicines for treating NAFLD, especially the incurable NASH, is an unmet medical need.
Traditional herb medicines (THM), a predominant source of natural medicines and herbal products, are indispensable sources for developing hepatoprotective drugs. Although there is still no compelling evidence from large-scale randomized controlled trails (RCTs) to support the therapeutic effects of THM, a recent survey showed that 20%–30% of patients used traditional medicine in Indonesia for treating various diseases14 and THM use in some Asian countries has increased in recent years15. Another survey showed a similar percentage of herbal use for treating chronic liver disease as complementary and alternative medicine16,17. In another systematic meta-analysis, traditional Chinese medicine (TCM) decreases alanine aminotransferase (ALT), aspartate aminotransferase (AST) and radiological steatosis and thus benefits the treatment of NAFLD, suggesting TCM have modest benefits in the treatment of NAFLD in 62 RCTs among 25,661 patients from 419 clinical studies18. Thus, prior to the development of conclusive evidence-based effective pharmacological therapies, the clinical use of THM plays a non-negligible role in treating NASH19. The expanding knowledge of THM in benefiting the improvement of metabolic diseases, especially NAFLD and NASH, against the extremely long period of modern drug discovery, has driven studies to pursue the potential efficacious and safe therapies by use of THM, which could be called “a natural combinatorial chemical sample library” gift from ancient practical experiences.
In this review, to better elucidate how THM provides NAFLD/NASH improvement and anti-NASH drug discovery, we first summarized the FDA-sanctioned clinical studies of herbal products. To better understand the effects and mechanisms of THM in anti-NAFLD or drug discovery, we firstly reviewed the updated publications about NAFLD/NASH pathogenesis, and we proposed a new “multiple organs-multiple hits” model for updating the explanation of NAFLD/NASH progression mechanisms. Against the proposed “multiple organs-multiple hits” NAFLD/NASH progression model, the emerging effects and molecular mechanisms/targets for the frequently-studied herbs are reviewed. To benefit the discovery of herb-derived anti-NAFLD drugs, the reverse-pharmacology and reverse-pharmacokinetic concepts for guiding preclinical experimental design, as well as experiment design details including NAFLD model choice, drug dosing method choice, and new technology-derived omics/hypothesis-based mechanism exploration for studying the effects of THM on NAFLD are discussed. This review will update the understanding of NAFLD/NASH pathogenesis mechanisms, effects and mechanisms of anti-NAFLD herbs, and guides the discovery of anti-NAFLD drugs from traditional herbs.
2. THM in this era: source and market, clinical trials, preclinical studies
2.1. THM source, market and modernization
THM, used to treat illness that could date back more than 5000 years, are mainly sourced from TCM, Ayurveda, Japanese and Kampo medicine. They have been used and recorded in their respective ancient medical books by the prior civilizations in countries including such as China, India, Japan, Arabia20. To promote THM modernization, a plan by the Chinese government was launched in 2007 to expand basic and clinical research on TCM21. TCM modernization has achieved significant progress as revealed by the fact that most FDA-sanctioned clinical trials are on TCM-derived drugs (Table 122, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32). However, the modern knowledge for how best to use THM is still limited and no conclusive clinical results are found to support the therapeutic potential of TCM. Further, against the common belief that “natural is safe and better", some THM have been associated with toxicity that indicates a potential risk for use of THM17,33. Thus, more efforts are still needed to fully clarify the mechanisms and targets of THM-derived compounds, including detailed hepatoprotective effects and research strategies, for guiding patients or consumers to better use THM.
Table 1.
Clinical trials for THM in treating NAFLD/NASH registered at ClinicalTrials.gov.
| Study title | Status | Identifier |
|---|---|---|
| Role of pioglitazone and berberine in treatment of NAFLD | Completed, phase 2 | NCT00633282 |
| Efficacy and safety of berberine in NASH22, 23, 24, 25 | Recruiting, phase 4 | NCT03198572 |
| Silymarin in NAFLD | Not recruiting, phase 4 | NCT02973295 |
| Silymarin for the treatment of NAFLD26 | Completed, phase 2 | NCT02006498 |
| Effect of silymarin in patients with NAFLD | Recruiting, N/A | NCT03749070 |
| Phase I trial of silymarin for chronic liver diseases | Completed, phase 1 | NCT00389376 |
| Study to evaluate the effect of RGMA001 on patients with NAFLD | Unknown, N/A | NCT01511523 |
| Phase II trial of silymarin for non-cirrhotic patients with NASH | Completed, phase 2 | NCT00680407 |
| Efficacy of a natural components mixture in the treatment of NAFLD | Completed, N/A | NCT02369536 |
| Resveratrol for the treatment of NAFLD and insulin resistance in overweight adolescents27 | Completed, phase 2/3 | NCT02216552 |
| Long-term investigation of resveratrol on fat metabolism in obese men with NAFLD28,29 | Completed, N/A | NCT01446276 |
| The effects of resveratrol supplement on biochemical factors and hepatic fibrosis in patients with NASH30 | Completed, phase 2/3 | NCT02030977 |
| Resveratrol in patients with NAFLD | Completed, N/A | NCT01464801 |
| Potential beneficial effects of Rresveratrol | Completed, N/A | NCT01150955 |
| Therapeutic effects of compound Zhenzhu Tiaozhi capsules in NAFLD | Recruiting, N/A | NCT03375580 |
| Purified anthocyanin and NAFLD31 | Completed, phase 1 | NCT01940263 |
| Clinical investigation on the effects of bayberry juice treatment in adult subjects with features of fatty liver disease24 | Completed, N/A | NCT01707914 |
| The clinical trial of NAFLD treated by TCM | Completed, phase 1 | NCT01677325 |
| Curcumin supplement in NAFL patients with type 2 diabetes | Completed, phase 2/3 | NCT02908152 |
| The effects of Zataria Multiflora Boiss. (Shirazi's thyme) on NAFLD | Completed, N/A | NCT02983669 |
| Effect of ginger supplement on NAFL | Completed, phase 2/3 | NCT02535195 |
| Effects of ginger on NAFLD in T2DM | Enrolling, phase 1 | NCT02289235 |
| The effect of Protandim on NASH | Completed, N/A | NCT00977730 |
| A study of Siliphos in adults with NASH32 | Completed, phase 2 | NCT00443079 |
| The effect of curcumin on liver fat content in obese subjects | Not recruiting, N/A | NCT03864783 |
A search of key word “NAFLD” or “NASH” in the item “Condition or disease” at https://clinicaltrials.gov/(at July 22, 2019) yielded 737 listed studies, 25 of which were found and listed in this table that are related to traditional herbs.
NAFL, non-alcoholic fatty liver; T2DM, type 2 diabetes mellitus.
N/A, not available.
2.2. THM clinical trials
Although not approved by the FDA, many THM have already been used in the clinic for treating various types of liver diseases including NAFLD/NASH. In this era of “precision medicine” and “evidence-based medicine", developing large-scale RCTs is extremely important and urgently needed to conclusively validate whether a given THM is effective in NAFLD or NASH treatment. A search of ClinicalTrails.gov lists 737 studies for treating NAFLD, among which 25 are traditional herbs (Table 1). More than 20 out of these 25 herbs include or belong to TCM. Seven studies are silymarin related, five for resveratrol, three for curcumin, while two are for berberine.
Among these frequently studied THM, silymarin contains multiple active compounds, while resveratrol, curcumin and berberine are single compounds. Notably, both silymarin and berberine have been registered as phase 4 clinical trials for treating NAFLD or NASH. Limited studies are on ginger and Zhengzhu Tiaozi Capsule. Eight of 25 are used as drugs, while another 17 are used as dietary supplements. As an unmet therapeutic need, anti-NASH drugs have been granted an accelerated access pathway to be approved by the FDA34. The results and clinical trial outcomes are expected to come within 1–3 years for these two promising compounds.
2.3. Hepatoprotective effects and mechanisms of THM in treating NAFLD and NASH
2.3.1. A “multiple organs-multiple hits” model for updating NAFLD pathogenesis and pharmacotherapy targets
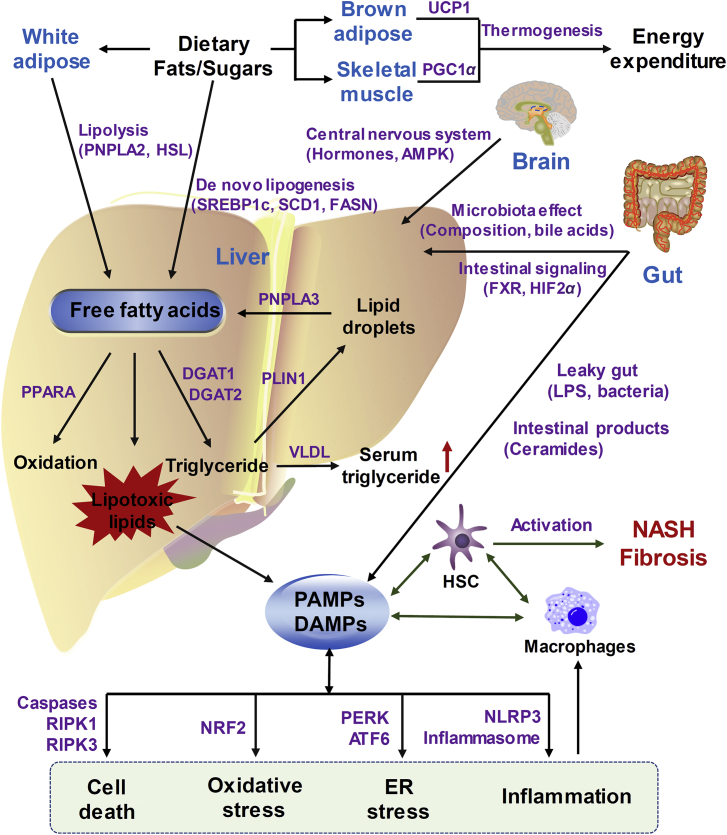
Many recent publications on molecular NAFLD-promoting pathways have updated the original “two-hit” theory (the first hit depends on steatosis and the second hit from other pathogenic factors such as oxidative stress and inflammation), as recently reviewed1,35, 36, 37, 38, 39, 40, 41, 42. Multiple targets by combined coordination among different organs are known to drive hepatic NAFLD progression43. Thus, in this review, by combining previously-proposed NAFLD progression models with recently-updated research results44, 45, 46, 47, 48, a “multiple organs-multiple hits” model is proposed to explain NASH pathogenesis and pharmacotherapy targets (Fig. 1). In this “multiple organs-multiple hits” model, the crosstalk between liver and multiple organs (such as gut, white/brown adipose tissue, skeletal muscle and central nervous system) is described. On one hand, in the liver, free fatty acids from either lipolysis of white adipose tissue or hepatic lipid droplets or de novo lipogenesis from dietary fats/sugars are overloaded. Then, fatty acids could be removed by fatty acid β-oxidation (regulated by peroxisome proliferator-activated receptor alpha (PPARA)-activation) or formation of lipotoxic lipids or triglycerides. When the disposal pathways of over-loaded fatty acids are saturated, fatty acids then form lipotoxic species that could cause cell death, oxidative stress, endoplasmic reticulum (ER) stress and inflammation. On the other hand, non-liver organs and non-liver cells including gut, adipose, skeletal muscle, brain and immune systems also contribute directly or indirectly to NASH progression. All the pathogen-associated molecular patterns (PAMPs) and/or damage associated molecular patterns (DAMPs) generated from fatty acids-overloaded liver, “leaky gut” or inflamed macrophages, together work to activate hepatic stellate cells (HSC) and cause liver fibrosis.
Figure 1.
Proposed “multiple organs-multiple hits” model for explaining the NAFLD/NASH pathogenesis. “Multiple organs-multiple hits” model describes how the crosstalk between liver and other tissues/non-liver cells (gut, brown/white adipose, skeletal muscle, brain and macrophages) promotes NASH progression. Free fatty acids, sourced from either adipose lipolysis or hepatic lipid droplets or de novo lipogenesis from dietary fats/sugars, overload the liver. Then, fatty acids could be degraded by fatty acid β-oxidation and triglyceride secreted from liver to serum, or can be converted to lipotoxic lipids when the disposal pathways of fatty acids are saturated. Lipotoxic lipids could then cause oxidative stress, ER stress, inflammation and possibly cell death. Non-liver organs can also contribute directly or indirectly to NASH progression. During NAFLD progress, changes in gut microbiota composition or intestinal lipids modulation signaling, can yield toxic microbiota products, or even forms leaky gut to release LPS or bacteria, all of which could be PAMPs or DAMPs and enter the liver via the portal vein. All the PAMPs and/or DAMPs generated from liver, gut or macrophages can work together to activate HSC and cause liver fibrosis. Energy balance could be regulated by the central nervous system via food intake or central hormones/signaling, as well as by brown adipose or skeletal muscle that helps burn adipose fat via thermogenesis or energy expenditure, thus indirectly decrease the overloaded burden of hepatic fatty acids and alleviate NAFLD.
2.3.1.1. Gut–liver axis in NAFLD/NASH
Intestine factors including both host and microbiota play a key role in mediating NASH progression via the gut–liver axis as reviewed previously49, 50, 51. Many intestinal mediators produced from either host or microbiota could act as bioactive signaling molecules, PAMPs or DAMPs that are circulated via the portal vein to affect liver function51. For example, genetic disruption of the intestinal farnesoid X receptror (FXR) or FXR inhibition by an antagonist, improves NAFLD and obesity-associated metabolic syndrome via decreasing the circulating ceramides45,52. Obesity or high-fat diet (HFD) feeding induces activation of intestinal hypoxia-inducible factor 2 alpha (HIF2A) that contributes to hepatic steatosis via directly modulating intestinal ceramide metabolism to reduce the ceramide levels44. Numerous studies have revealed the role of microbiota in mediating NAFLD progression, exemplified by the fact that gut microbiota composition was altered in obese rodent animals or human patients46,51, and probiotics used to normalize the microbiota composition, could therapeutically alleviate NAFLD progression in experimental animal models39,53. In addition, increased intestinal permeability (leaky gut) increases exposure of the liver to gut or microbiota-derived bacterial products such as lipopolysaccharides (LPS) that could act as PAMPs or DAMPs to stimulate innate immune systems such as toll-like receptors (TLRs) and NLR family, pyrin domain containing 3 (NLRP3) inflammasome activation49,51.
2.3.1.2. Immune system in NAFLD/NASH
NAFLD is characterized by the hallmarks of inflammation, hepatocyte death and the resulted fibrosis. Obesity, a major cause of NAFLD, is associated with low-grade systemic inflammation through disturbance of the immune system54. The key immune cells including Kupffer cells, dendritic cells, neutrophils, and natural killer cells could promote NAFLD/NASH progression by sensing DAMPs released from damaged hepatocytes or PAMPs derived from other tissues such as intestine (bacterial-produced LPS or other toxins) or adipose tissue (adipokine imbalance)55. The role of Kupffer cells in regulating the progression of NAFLD is well-established, although the role of other immune cells in NAFLD/NASH development is still controversial. Depletion of Kupffer cells improves NAFLD/NASH, supporting a pro-NASH role of Kupffer cells56, while further studies revealed the activated M1 phenotype as pro-inflammatory promotion of NAFLD/NASH progression and the M2 as immunoregulatory that inhibits NAFLD/NASH progression55,57. Thus, innate immune system modulation presents an opportunity for NASH therapy. Notably, immune cells also directly provide novel molecular anti-NASH targets. For example, immune cell-expressed TLRs could sense PAMPs and DAMPs that could mediate NAFLD/NASH progression via PAMPs/DAMPs-TLR-myeloid differentiation primary response 88 (Myd88) pathway41. Immune cells also, at least partially, contribute to NLRP3 inflammasome activation that may mediate NAFLD progression46,58, while NLRP3 inflammasome blockade by NLRP3 inhibitor reduces experimental NASH in mice59, suggesting that NLRP3 inflammasome pathway is an effective druggable target for NASH pharmacotherapy.
2.3.1.3. Adipose and skeletal muscle in NAFLD/NASH
Brown adipose tissue or subcutaneous white adipose tissue “browning” or skeletal muscle could help burn fat via uncoupling protein 1 (UCP1)-dependent thermogenesis or PPARG coactivator 1 alpha (PGC1A)-dependent energy expenditure47,60. White adipose tissue could modulate adipose lipolysis (mainly via hormone-sensitive lipase, HSL)61 and mediate UCP1-independent thermogenesis62. Many genes specifically expressed in adipose or skeletal muscle have the potential to modulate NAFLD development or NASH progression via modulating obesity. For example, adipose-specific SIRT6 ablation sensitizes mice to HFD-induced obesity and hepatic steatosis by inhibiting lipolysis63.
2.3.1.4. Central nervous system in NAFLD/NASH
Central nervous system mediates metabolic syndrome and hepatic lipid/lipoprotein metabolism via various mechanisms64. For example, the central nervous system modulates food intake (appetite)65, regulates gut-nervous system axis66, senses glucagon like peptide-1 (GLP-1)67, and mediates endogenous hormones (such as leptin and insulin) secretion68. For example, central nervous system regulates hepatic lipogenesis, lipid oxidation of brown adipose tissue and thus modulates energy balance via central actions of thyroid hormones through pathway of hypothalamic AMP-activated protein kinase (AMPK)-ER stress-c-Jun N-terminal kinase 1 axis69. Genetic ablation of activating transcription factor 4 and/or 5 in hypothalamic proopiomelanocortin neuron modulates energy expenditure and thus HFD-induced obesity and obesity-associated fatty liver70.
2.3.2. Hepatoprotective effects and mechanisms of traditional herbs in treating NAFLD/NASH
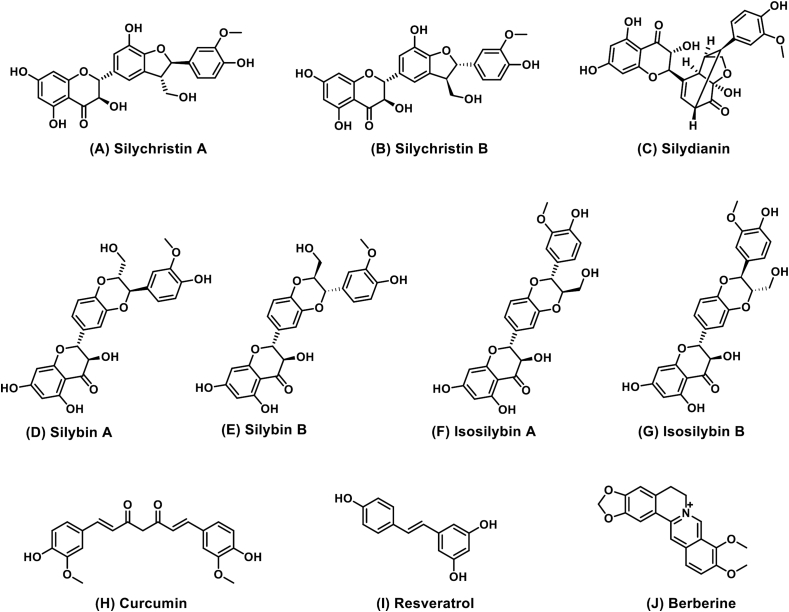
Herbal drug(s) (combination therapies or single drugs) that target one or multiple hits of the “multiple organs-multiple hits” pathogenesis processes could affect NASH. The structures (Fig. 2) and possible molecular hepatoprotective mechanisms (Table 271-122) of the most frequently investigated THM for treating NAFLD and NASH and their are listed below.
Figure 2.
Chemical structures of frequently-examined THM-derived components for the treatment of NAFLD in clinical trials registered at Clinicaltrials.gov. Compounds A–G are hepatoprotective components isolated from silymarin, while curcumin (H), resveratrol (I) and berberine (J) belong to single compound drug.
Table 2.
Molecular mechanisms of four frequently-studied herbs in treating NAFLD.
| Herb | Molecular pathway |
|---|---|
| Resveratrol | SIRT1 activator71, 72, 73 AMPK activator74, 75, 76 Adipose AMPK activation to induce white adipose browning77 NLRP3 antagonism78 Autophagy induction and NFKB1 activity inhibition79,80 Inhibition of LXR-dependent hepatic lipogenesis81 NRF2 activator82,83 Gut AMPK-SIRT1 axis84 Gut–brain neuronal axis84 Modulating gut microbiota composition85, 86, 87 Gut microbiota-derived metabolites88 Protecting “leaky gut”86 |
| Curcumin | NFKB1 inhibition89, 90, 91 Inhibiting apoptosis92 Reducing TIMP1 secretion93 PPARG induction94 Interrupting leptin signaling95,96 Activating NRF2, FXR and LXR97 AMPK activation98 and PPARA activation99 NLRP3-dependent anti-inflammation100 TLR4-MYD88 axis101 Gut microbiota modulation102,103 |
| Berberine | Activate adipose thermogenesis104 Inhibition of NLRP3 and ER stress and autophagy induction26,105,106 NRF2 and PPARG activation107, 108, 109 Systematic AMPK activation104,110,111 Intestinal FXR signaling and bile acids modulation112 Modulating gut microbiota23,113,114 Producing microbiota-derived metabolites115, 116, 117 |
| Silymarin | SIRT1/AMPK activation118 FXR and AMPK activation119,120 NLRP3 signaling antagonism121 Antioxidant that could increase NRF2 translocation121,122 |
Summary of possible molecular pathways that are involved in the hepatoprotective effects for resveratrol, curcumin, berberine, and silymarin.
2.3.2.1. Resveratrol
Resveratrol, a natural polyphenol that activates sirtuin 1 (SIRT1), has demonstrated potent effects in alleviating NAFLD in both rodent and cell models, while its effect in NAFLD patients is still inconclusive123. A clinical study in 24 obese but otherwise healthy men showed that a high dose of resveratrol did not affect body composition, insulin sensitivity, and other inflammatory or metabolic biomarkers124, while other studies in non-obese middle-aged subjects with normal glucose tolerance also failed to benefit from resveratrol supplements125, suggesting that resveratrol has no efficacy in humans that do not have metabolic disease. In contrast, for metabolic disease patients, a study of 50 NAFLD patients revealed that 500 mg of resveratrol treatment for 12 weeks could further benefit NAFLD treatment when compared to lifestyle modification alone, partially by inhibiting inflammation and apoptotic liver injury31. However, other studies revealed no benefit of resveratrol in metabolic disease patients. For example, in one clinical study with 16 obese male NAFLD patients, resveratrol showed no significant metabolic benefit29. Similarly, an updated systematic review and meta-analysis for four RCTs (n = 158 patients) concluded that resveratrol supplementation in NAFLD patients had no efficacy in attenuating NAFLD126. Thus, evidence for the clinical benefit of resveratrol in treating NAFLD is still inconclusive, albeit many studies demonstrated the effect of resveratrol in treating NAFLD in rodent models.
Mechanically, resveratrol activates AMPK74, 75, 76 and/or SIRT1 signaling to suppress lipogenesis in liver71, 72, 73 or induces white adipose browning via activating AMPK77. Resveratrol inhibits the NLRP3 inflammasome activation to attenuate hepatic metainflammation78; and modulates autophagy and NFκB1 activity in diet-induced NAFLD murine models79,80. Resveratrol also inhibits liver X receptor alpha (LXR)-dependent hepatic lipogenesis through antioxidant activity81. Although no direct report showed how nuclear factor erythroid 2-related factor 2 (NRF2) activation mediates the hepatoprotective role of resveratrol in treating NAFLD, resveratrol was demonstrated to activate NRF2 and thus protect cells from oxidative stress both in vivo and in vitro, dependent on the presence of NRF282,83.
Notably, resveratrol is a poorly-absorbed polyphenol and is metabolized rapidly, so it could be mainly detected in the small intestine after gavage for 1 h in rats127. Consistent with its predominant distribution in the intestines when dosed by gavage, resveratrol was found to target the duodenal AMPK–SIRT1 axis and vagal gut–brain neuronal axis to reverse the metabolic syndrome in obese and diabetic rats84. In addition, resveratrol is partly metabolized by gut microbiota to produce resveratrol derivatives that could have similar biological effects with resveratrol, and resveratrol changes the composition of gut microbiota to yield beneficial metabolic outcomes85,86. Resveratrol improves the HFD-induced gut microbiota dysbiosis by increasing the Bacteroidetes-to-Firmicutes ratios, significantly inhibiting the growth of Enterococcus faecalis, and increasing the growth of Lactobacillus and Bifidobacterium87. Another study further demonstrates that resveratrol could be metabolized by the human gut microbiota to yield dihydroresveratrol, 3,4′-dihydroxy-trans-stilbene and 3,4-dihydroxybibenzyl lunularin; and two strains, Slackia equolifaciens and Adlercreutzia equolifaciens, were identified as dihydroresveratrol producers88. Resveratrol also maintains junctions between intestinal cells, contributes to gut barrier integrity and could prevent “leaky gut”86. Thus, resveratrol activates NRF2 signaling and both hepatic and intestinal SIRT1 and AMPK signaling, modulates cell death and regulates gut microbiota and intestine integrity that could affect NAFLD. However, the translation of its effect from mouse models to humans remains uncertain.
2.3.2.2. Curcumin
Curcumin, a natural polyphenol, is the major active compound of turmeric and has been historically used in TCM or Ayurvedic medicine. Preclinical murine models demonstrate that curcumin attenuates NAFLD128 and clinical trials demonstrate curcumin as a promising, but not proven, treatment for NAFLD129. Curcumin is currently in phase II/III clinical trials (Table 1). However, the clinical benefit for curcumin in treating NAFLD is still uncertain. In a trial of 80 patients in Iran, curcumin significantly improved fatty liver (78.9% by curcumin vs. 27.5% by placebo) and other NAFLD parameters including aminotransferases130. Extensive preclinical studies support the hepatoprotective role of curcumin in treating NAFLD and NASH. Curcumin attenuates NFKB1 activation and improves methionine-choline-deficient (MCD) diet-induced NASH in mice89, HFD-induced NASH in rabbits90, and prevents fatty liver in fructose-fed rats91. Curcumin also retards the NASH progression in a NASH-hepatocarcinoma cancer mouse model131. Mechanically, curcumin improves NASH by inhibiting apoptosis and protecting mitochondria in HFD-induced NAFLD in rats92, and limits the fibrogenic evolution of MCD diet-induced steatohepatitis in vivo, as well as directly reduces tissue inhibitor of metallopeptidase 1 (TIMP1) secretion and oxidative stress in HSC in vitro93. Curcumin inhibits expression of receptors for advanced glycation end-products in HCS in vitro by inducing PPARG and inhibiting oxidative stress94. In addition, other studies found that curcumin eliminated the effect of advanced glycation end-products by interrupting leptin signaling95, and attenuated the effects of insulin on stimulating HSC activation via affecting insulin signaling and oxidative stress96. Curcumin activated the NRF2, FXR and LXR signaling pathways to regulate metabolism and attenuate NAFLD in mice97, and attenuated HFD-induced hepatic steatosis by activating AMPK and increasing PPARA signaling99. Curcumin also decreased oleic acid-induced lipid accumulation via AMPK phosphorylation in vitro in hepatocarcinoma cells98. By using Nlrp3-deficient mice, curcumin was confirmed to inhibit NLRP3 inflammasome activation and suppress interleukin 1B (IL1B) secretion and inflammation in HFD-fed mice depending on the presence of NLRP3100.
Curcumin repressed NLRP3 inflammasome activation via the TLR4/MYD88/NFKB1 and P2X purinoceptor 7 (P2X7) signaling in macrophages101. It protects against LPS-induced septic shock via suppressing NLRP3 inflammasome activation132. Curcumin also alleviated HFD-induced metabolic endotoxemia and intestinal inflammation and attenuated hepatic steatosis in rats via modulation of gut microbiota102. Curcumin remains at a high concentration in the gastrointestinal tract after oral intake, and is known to affect the abundance of Prevotellaceae, Bacteroidaceae, and Rikenellaceae103. Thus, curcumin decreases NAFLD via activating AMPK, NRF2 and nuclear receptors to modulate lipid metabolism and oxidative stress, while it also inhibits NLRP3 inflammasome activation and gut microbiota to modulate inflammation and decrease hepatic steatosis, but its effect in clinical NAFLD patients still needs further validation.
2.3.2.3. Berberine
Berberine is a natural alkaloid present in Coptis Chinensis and several other Chinese herbal medicines. Although most commonly used for treating diarrhea in China, extensive experimental studies demonstrated efficacy of berberine as a hypolipidemic and NAFLD protectant both in various rodent NAFLD/NASH animal models and NAFLD/NASH patients133,134. In one trial among 184 NAFLD patients from three medical centers, berberine plus lifestyle intervention resulted in a significant NAFLD improvement compared with lifestyle intervention alone24. In another trial on 80 patients, berberine treatment markedly decreased serum levels of lipid species and ceramides135. Due to a limitation in the number and quality of clinical trials, further efficacy validation of berberine on NAFLD patients is warranted136. A phase 4 clinical trial (ClinicalTrials.gov Identifier: NCT03198572) of berberine was launched for treating NASH (Table 1). Many mechanisms were suggested to explain how berberine might alleviate NAFLD. Berberine improves obesity by activating thermogenesis in both brown adipose tissue and white adipose tissue104. Berberine was found to improve NAFLD/NASH by suppressing ER stress, NLRP3 inflammasome activation and liver inflammation in genetically-obese db/db mice, MCD diet-fed mice or in Apoe–/– mice26,105,106,137,138. Berberine inhibited NLRP3 inflammasome activation in vitro and in vivo in two unrelated murine models, MCD-induced NASH and acetaminophen-induced acute liver injury, and modulating P2X7 signaling to antagonize NLRP3 inflammasome activation in LPS-treated macrophages in vitro106. Berberine also attenuated palmitate-induced NLRP3 inflammasome activation via triggering autophagy in macrophages in vitro and attenuated HFD-induced insulin resistance in vivo105. Berberine is known to activate NRF2 signaling and PPARG, and suppress oxidative stress-related liver injury in rats107, while NRF2 activation by berberine is also found in macrophage108. Berberine ameliorates fatty acid-induced oxidative stress in both HFD-fed mice and fatty acid-treated human hepatoma cells109. However, there is still no direct evidence to demonstrate a direct role for berberine in NRF2 activation in vitro with NRF2-driven luciferase activity. In addition, berberine activation of central, peripheral and adipose AMPK may be of value in treating diabetes and fatty liver104,110,111.
Interestingly, berberine, known to have extremely low bioavailability (<1%), was mainly distributed in intestines after gavage and was reported to modulate hepatic lipid metabolism by directly modulating gut microbiota and microbial bile acid metabolism through intestinal FXR signaling23,112. Berberine increased short-chain fatty acid-producing bacteria including Allobaculum, Bacteriodes, Blautia, Butyricoccus, and Phascolarctobacterium in HFD-fed rats113,114.
Berberine could be metabolized by the gut microbiota into a form that is more rapidly absorbed in the intestine115 and thus nitroreductases, encoded by intestinal bacteria, could promote intestinal absorption of berberine116. Berberine could induce the production of gut microbiota-derived bioactive metabolites such as butyrate and thus improve energy metabolism117. Thus, berberine targets multiple molecular hepatoprotective mechanisms related with organs including liver, macrophage, gut, microbiota and adipose. Its translational effect in treating NASH is in a phase 4 clinical trials.
2.3.2.4. Silymarin
Silymarin, also known as milk thistle extract, has mixed components that show potent hepatoprotective effects in various types of liver diseases139. As multiple drug mixtures, the effective components include taxifolin, isosilybin A, silybin A, silybin B, silibinin, or their mixed forms (Fig. 2). Preclinically, silymarin or its hepatoprotective component(s) were demonstrated to alleviate NASH in MCD diet-fed C57BL/6J mice140 and in db/db mice141, and in HFD-induced NAFLD in hamsters120 and mice142. Oral administration of silymarin improved a juvenile model of NASH143. A clinical trial with 64 NASH patients registered in Iran demonstrated that silymarin significantly decreased serum ALT and AST144. Similarly, several other clinical studies indicate that silymarin could improve NAFLD139,145. However, other clinical studies also indicate that the effect of silymarin in treating NAFLD or NASH is limited. One trial with 99 NASH patients demonstrated that silymarin only reduced liver fibrosis and reduced NAFLD activity scores less than 30%27. A systematic meta-analysis of 10,904 publications suggested that silymarin only minimally reduces ALT and AST without clinical relevance146. Thus, the data on silymarin clinical outcomes are still controversial. To further establish the clinical effects of silymarin in treating NAFLD, a phase 4 clinical study (ClinicalTrials.gov Identifier: NCT02973295) was launched for evaluating the effect of silymarin in treating NAFLD (Table 1) and the results outcome is expected to be obtained within 1–3 years.
The controversial data of silymarin clinical outcomes may result from patient variance and different dosing regimens. However, it is still generally believed that all the three main silymarin-contained flavonoids, silybin, silydianin and silychristin, have poor-bioavailability, which might be the cause of its compromised hepatoprotective effect in clinical patients. Indeed, some studies have tried to improve the bioavailability of silymarin by using several methods. For example, phytosomes (silybin–phosphatidylcholine complexed lipid-compatible molecules) were tested to enhance bioavailability33. A clinical study revealed that the efficacy of a silibinin/vitamin E/phospholipid complex improved insulin resistance and NAFLD147. The beneficial effect of this complex in NAFLD patients was further confirmed in another multicenter trial148.
A mechanism was suggested that silibinin restored NAD+ levels to induce the SIRT1/AMPK pathway in HFD-fed mice and palmitate-treated HepG2 cells118. Silymarin or silibinin also activated FXR signaling or AMPK phosphorylation to modulate lipid metabolism in HFD-fed rodents119,120. Silybin alleviated NLRP3 inflammasome assembly via the NAD+/SIRT2 pathway in a rodent NAFLD model121. Silymarin is also a known antioxidant122, which could increase NRF2 translocation in MCD diet-fed mouse livers149. Although several other polyphenols such as quercetin and naringenin were demonstrated to modulate the gut microbiota150, to the best of our knowledge, there are no studies investigating how silymarin affects the gut microbiota. As one type of several mixed polyphenols, silymarin has a potential role in altering intestinal microbiota that may in turn mediate its effects on metabolic diseases. Thus, silymarin affects multiple targets, including FXR, NRF2, SIRT1, SIRT2, AMPK and NLRP3 inflammasome to restore NAFLD or NASH. Clinical results indicate a promising, but still not conclusive, role for silymarin in treating NAFLD or NASH.
2.3.2.5. Silymarin-contained mixtures
Silymarin is also used as an important ingredient for complex mixtures. For example, one nutraceutical mixture contains ingredients of natural origin, including fish oil, 70% docosahexaenoic acid, phosphatidylcholine, silymarin, choline bitartrate, curcumin, d-α-tocopherol and choline151. This mixture was registered in ClinicalTrials.gov (NCT02369536) for treating NAFLD (Table 1), but no results have been posted. Another example is siliphos, a silibinin-phosphatidylcholine complex. The main purpose of forming silybin-phosphatidylcholine complex is to facilitate the transmembrane ability of silybin and improve the absorption of this poorly absorbed flavonoid to enhance the hepatoprotective effect33. Siliphos prevented mitochondrial dysfunction, oxidative stress, inflammation and liver fibrosis in a rodent model of NASH152.
2.3.2.6. THM beyond the registration
Other THM, although clinical trials not currently registered in ClinicalTrials.gov for treating NAFLD and NASH, showed potential for treating NAFLD in preclinical studies. With some single compounds as examples, a recent study demonstrated that withaferin A, the main bioactive component isolated from the ayurvedic medicine Withania Somnifera, has a potent hepatoprotective effect in both MCD diet-induced NASH and HFD-induced NASH153, while other studies revealed that glycyrrhizin or its active metabolite glycyrrhetinic acid were both shown to have a potent hepatoprotective effect in MCD diet-induced NASH and in HFD-induced NAFLD models154, 155, 156. In addition, baicalein attenuated MCD diet-induced NASH in rats via enhancing NRF2 signaling157 and protected AML-12 cells from lipotoxicity by suppressing ER stress and Thioredoxin interacting protein/NLRP3 inflammasome activation158. The multiple component TCM is exemplified by Picrorhiza kurroa, which is used in Indian Ayurvedic medicine for the treatment of digestive problems, was recently demonstrated to potently lower HFD-induced hepatic lipid accumulation159. Shenlin Baizhu powder, a widely used classical TCM formulation, suppressed p38 mitogen-activated protein kinase signaling in NASH160,161. TJ-9 (termed as “Xiao Chai Hu Tang” in China and “Sho-saiko-to” in Japan), which is in clinical studies for treating hepatitis C, also showed effects in experimental NAFLD and NASH models162,163. In addition, tanshinone IIA demonstrated protective effects in improving NAFLD in preclinical models164. Similarly, Bofutsushosan (kampo medicine)165, Jiang Tang Xiao Ke Granule166, 167, 168, Chaihu-Shugan-San Decotion169,170, Chinese bayberry juice171, wolfberry172, 173, 174, 175, shirazi's thyme176, ginger177,178, and ginseng179, 180, 181, 182 or ginseng-derived active component ginsenoside Rb2 and Rg1183,184, have shown potential for improving obesity-related metabolic syndrome and NAFLD that deserves further study. Furthermore, a meta-analysis of 8 RCTs with a total of 800 patients suggested that danshen, a TCM under clinical trial for treating coronary heart disease, and from which the TCM Tanshinone IIA is isolated, may have positive effects on NAFLD that deserve future multicenter large-sample RCTs185.
3. How to develop THM for treating NAFLD?
3.1. Reverse-pharmacology-guided approach
In contrast to the “bench-to-bedside” process used for modern drug development, most THM already in the clinic, lack preclinical evidence and large-scale randomized clinical trials to further verify their safety and efficacy. The advantages of a reverse pharmacology approach for clinically-used THM features a “beside (clinical efficacy)-to-bench (preclinical phenotype and mechanism)”186,187. In the case of THM for treating NAFLD, most THM, although claimed to be hepatoprotective in treating liver diseases in traditional practice, the mechanisms have not been comprehensively defined. Due to a general lack modern research evidence, most market-available hepatoprotective THM are prescribed or used over-the-counter under general directions for treating acute or chronic liver diseases without any clearly-defined types of liver diseases. Although liver diseases share some common pathological mechanisms, different types of liver diseases vary in their pathological processes and thus the druggable therapy targets differ. To determine the molecular mechanisms and which types of liver disease are suitable for being treated by each THM, more studies are urgently needed using preclinical NAFLD models to guide the clinical use of THM, a process of reverse pharmacology188. In addition, the reverse pharmacokinetics analysis of THM also benefits the clinical use, target/mechanism study and THM-derived drug discovery as reviewed previously187.
3.2. Experiment design for testing the role of THM in treating NALFD
NAFLD is one type of common chronic liver disease. To better support the hepatoprotective effects and explore the mechanisms of THM (which is already used in clinics), preclinical studies based on the reverse-pharmacology-guided approach are needed. The experiment designs for testing the role of THM in treating NAFLD is discussed in detail below.
3.2.1. NAFLD model choice
In the clinic, while the large majority of NAFLD patients are obese, many NAFLD patients are lean4,5. To better mimic pathological characters of the predominant obese NAFLD patients, most preclinical NAFLD animal models have obesity and obesity-associated metabolic syndrome such as insulin resistance and glucose intolerance. There are also NAFLD models that do not show increased body weight and/or insulin resistance. For example, NAFLD mice maintained on a MCD diet have decreased body weight, and are free of insulin resistance. The most frequently-used NAFLD models and their respective characteristics (which aspect could be emphasized by each model) were reviewed previously189.
3.2.2. Drug dosing design
THM choice, drug dose, drug dosing route, and drug dosing regimen are the major concerns for THM drug delivery design. A THM for anti-NAFLD effects could be mainly from known hepatoprotective herbs, repurposing from traditional herbs with unknown hepatoprotective effects or herb-derived active components or metabolites. Drug doses should be designed based on publications related to the investigated THM. Short-term pilot studies for testing the effective dose or dose-dependency of THM in treating NAFLD are preferred for starting the full investigation of THM in treating NAFLD, especially by using the MCD diet-induced NASH model. A two-week MCD diet feeding could be long enough to cause significant serum ALT and AST increases for testing the efficacy of the pilot dosing method190.
NAFLD animal models are usually long-term, and thus daily intravenous injection is difficult to carry out in rodent models. Oral intake by gavage, dietary supplementation as well as intraperitoneal injection are the most-frequently-used dosing methods for THM in mouse models. Dose regimen choice could be divided into two major types based on whether the THM is tested for examining the preventive effect or therapeutic effect. For evaluation of a preventive effect, mice are treated with a THM coincident with the induced onset of the disease or prior to the induced onset of the disease until termination of the experiments. To examine the therapeutic effect of a THM, the disease is induced and then the THM is administrated. For example with the MCD diet-induced NASH model, THM is usually dosed from the first day of MCD diet feeding to test the preventive effect, while for therapeutic effect, THM needs to be administered after NASH is established by MCD diet feeding for 6 weeks154. Body weight-independent effects of THM in treating NAFLD is also of concern, mainly for determining whether the hepatoprotective effect of a THM is the result or cause of body weight change. For the HFD-induced obese NAFLD model, the drug is usually dosed for a relatively-short time at the early stages of onset of disease symptoms prior to body weight change, and any early anti-NAFLD markers such as ALT, AST, hepatic triglyceride or total cholesterol are measured44. It is notable that the MCD diet-induced NASH model is a lean NASH model. This model could be relatively feasible for examining whether a THM-reduced MCD diet-induced NASH is due to body weight change153,154.
3.2.3. New technology and omics-based high throughput screening for target/mechanism exploration
Evolution of new technologies have yielded multiple types of omics analysis (metagenomics, transcriptomics, metabolomics, and proteomics) that facilitates the high throughput screening for target/molecular mechanism exploration. First, 16S ribosomal RNA analysis enables metagenomics analysis of gut microbiota. Exploration of the microbiome in both mouse and human NAFLD/NASH not only largely clarifies the roles of intestinal microbiota in metabolic diseases, but also provides pharmacological implications for herbs in treating NAFLD150. Detailed microbiota changes have been clarified for many herbs such as resveratrol, berberine and curcumin, as described in Section 2.3.2 of this review. Second, RNA sequencing (RNA-Seq) using next-generation sequencing helps analysis of the cellular transcriptome, while single-cell RNA-Seq further deconstructs the transcriptomes of complex tissues at the single-cell level and thus could tell how different types of individual liver cells (hepatocytes, Kupfer cells, HSCs, monocytes) and even zonal differences in liver hepatocytes, contribute to NAFLD/NASH progression or treatment. Most recently, by using single-cell secretome gene analysis in combination with quantitative proteomics and liver RNA-Seq analysis, a marked presence of macrophages that were characterized with high levels of triggering receptor expressed on myeloid cells 2 was uncovered as a hallmark of mouse and human NASH191. Third, metabolomic analysis of serum metabolomes of NAFLD patients and NASH mice identified two major subtypes of NAFLD and found markers that differentiate steatosis from NASH in each subtype192. In addition, targeted lipidomic analysis is also a powerful way to determine how THM modulates lipid metabolism during treating NAFLD. For example, with Eclipta prostrata as chemical probes in combination with lipidomic, it demonstrated the therapeutic potential of this THM in treating NAFLD and revealed four lipid species as potential biomarkers for NAFLD prognosis193.
3.2.4. Hypothesis-based molecular mechanism exploration
Hypothesis-based target exploration is aimed to validate whether a known pharmacotherapy target is targeted by THM. The pathogenesis and pharmacotherapy targets of NAFLD were reviewed previously35 and summarized in Fig. 1. The NAFLD pathogenesis and molecular targets combined with the molecular or chemical information of THM can yield a hypothesis and a potential anti-NAFLD target, and then whether the hypothesis fits in vitro or in genetically-modified mice in vivo can be examined. Hypothesis-based target exploration belongs to the reverse-pharmacology-guided approach. The hypothesis-based target exploration methodology was extensively used to investigate many herbs and the frequently hypothesized mechanisms are listed in Table 2 and Fig. 3.
Figure 3.
Role and mechanisms of frequently-studied traditional herbs in treating NAFLD. Four frequently-studied THM (silymarin, curcumin, berberine, and resveratrol) are registered in ClinicalTrials.gov for clinically testing their safety and efficacy in treating NAFLD. Berberine and silymarin were subjected to a phase 4 clinical trial. These four representative traditional herbs show hepatoprotective effects in improving NAFLD/NASH in preclinical rodent mouse models via various molecular pathways mainly including cell death modulation (via inducing autophagy or inhibiting hepatocyte apoptosis), lipid metabolism modulation (via activating FXR, PPARA, PPARG, AMPK, SIRT1 or antagonizing LXR), anti-inflammation (via inhibiting TLR/MYD88 or NLRP3 inflammasome pathway), anti-oxidative stress (via activating NRF2), modulating liver-gut axis (via changing microbiota composition, repairing leaky gut to reduce the release of LPS or harmful bacteria, modulating intestinal FXR signaling or SIRT1 signaling, or producing microbiota products such as active bile acids or herb drug metabolites).
4. Challenges and hints
Clinical trials need to be launched in order to determine whether a THM is effective for treating NAFLD194. In general, many factors such as drug dose variance, inter-individual patient variability and differences between rodents and patients make clinical trial design challenging. Compounds used as diet supplements also need to be subject to clinical trials35. In addition, most THM has a very low bioavailability, which possibly compromises their therapeutic effects. Notably, although some herbs, such as berberine and resveratrol, have low bioavailability (less than 1%), leading to lack of hepatic accumulation and concentrations far below the required effective dose, they still show hepatoprotective effects84,112. This interesting phenotype drives studies to explore whether and how THM work without being systematically absorbed. It's known that some THM directly work at least partially through gut microbiota or intestinal-liver signaling84,112,195. On the other hand, THM that have multiple components may show pharmacological effect via the combined actions of several components through multiple targets/mechanisms make THM study even more complicated196,197, which is also an important point for THM study and clinical use, but not emphasized in detail in this review.
5. Summary
THM are valuable sources for developing novel anti-NASH drugs. Beyond the related and diverse philosophy systems to emphasize the holistic health balance, THM itself at least provides valuable sources of anti-NASH drugs, lead compounds or adjuvant complements for novel drug discovery. Market-available THM are usually directed or prescribed for treating virus-induced hepatitis or generally-defined acute and chronic liver diseases. NAFLD is one chronic liver disease that includes many pathological aspects, including steatosis, lipotoxicity, oxidative stress, ER stress, and insulin resistance. Increasingly, herbal products have been studied via well-designed controlled trials that provide evidence supporting beneficial effects in liver disease. Due to the variance of complicated clinical trial conditions for drug dosing methods and patient choice, most results are still inconclusive. Thus, large-scale multicenter RCTs for THM are urgently needed, with two herbs (silymarin and berberine) already in phase 4 clinical trials. On one hand, the research for THM is increasing rapidly, and THM has global popularity and recognition beyond Asian countries. On the other hand, with increasing use of THM, THM-induced toxicity has gathered increasing attention that attracts both consumer attention and research attention. Hopefully with more and more extensive and well-designed reverse-pharmacology-guided research for THM, people will be able to use these ancient herbs in a more safe and rational way based on solid evidence.
Acknowledgments
Diane Cooper, MS, NIH Library, participated in writing the manuscript. This work was supported by the intramural research program of the National Cancer Institute, National Institutes of Health.
Author contributions
Tingting Yan conceived the manuscript and figures; Tingting Yan and Nana Yan wrote the manuscript; Ping Wang made the figures; Haiping Hao, Yangliu Xia and Guangji Wang reviewed and edited the manuscript; Frank J. Gonzalez supervised and edited the manuscript.
Conflicts of interest
The authors declare no conflict of interest for publishing this manuscript.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.11.017.
Contributor Information
Tingting Yan, Email: Tingting.yan@nih.gov.
Frank J. Gonzalez, Email: gonzalef@mail.nih.gov.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wree A., Broderick L., Canbay A., Hoffman H.M., Feldstein A.E. From NAFLD to NASH to cirrhosis—new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 2.Michelotti G.A., Machado M.V., Diehl A.M. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J.Z., Dai Y.N., Wang Y.M., Zhou Q.Y., Yu C.H., Li Y.M. Prevalence of nonalcoholic fatty liver disease and economy. Dig Dis Sci. 2015;60:3194–3202. doi: 10.1007/s10620-015-3728-3. [DOI] [PubMed] [Google Scholar]
- 4.Fan J.G., Kim S.U., Wong V.W. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 6.Oseini A.M., Sanyal A.J. Therapies in non-alcoholic steatohepatitis (NASH) Liver Int. 2017;37 Suppl 1:97–103. doi: 10.1111/liv.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassidy S., Syed B.A. Nonalcoholic steatohepatitis (NASH) drugs market. Nat Rev Drug Discov. 2016;15:745–746. doi: 10.1038/nrd.2016.188. [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., van Natta M.L., Abdelmalek M.F. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Connolly J.J., Ooka K., Lim J.K. Future Pharmacotherapy for non-alcoholic steatohepatitis (NASH): review of phase 2 and 3 trials. J Clin Transl Hepatol. 2018;6:264–275. doi: 10.14218/JCTH.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumida Y., Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53:362–376. doi: 10.1007/s00535-017-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Lassailly G., Caiazzo R., Buob D., Pigeyre M., Verkindt H., Labreuche J. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149:379–388. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Pengpid S., Peltzer K. Utilization of traditional and complementary medicine in Indonesia: results of a national survey in 2014–15. Complement Ther Clin Pract. 2018;33:156–163. doi: 10.1016/j.ctcp.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Peltzer K., Pengpid S. Utilization and practice of traditional/complementary/alternative medicine (T/CAM) in southeast Asian nations (ASEAN) member states. Stud Ethno-Med. 2015;9:209–218. [Google Scholar]
- 16.Seeff L.B., Curto T.M., Szabo G., Everson G.T., Bonkovsky H.L., Dienstag J.L. Herbal product use by persons enrolled in the hepatitis C antiviral long-term treatment against cirrhosis (HALT-C) trial. Hepatology. 2008;47:605–612. doi: 10.1002/hep.22044. [DOI] [PubMed] [Google Scholar]
- 17.Seeff L.B., Bonkovsky H.L., Navarro V.J., Wang G. Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology. 2015;148:517–532. doi: 10.1053/j.gastro.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Shi K.Q., Fan Y.C., Liu W.Y., Li L.F., Chen Y.P., Zheng M.H. Traditional Chinese medicines benefit to nonalcoholic fatty liver disease: a systematic review and meta-analysis. Mol Biol Rep. 2012;39:9715–9722. doi: 10.1007/s11033-012-1836-0. [DOI] [PubMed] [Google Scholar]
- 19.Ratziu V., Goodman Z., Sanyal A. Current efforts and trends in the treatment of NASH. J Hepatol. 2015;62:S65–S75. doi: 10.1016/j.jhep.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 20.Pan S.Y., Litscher G., Gao S.H., Zhou S.F., Yu Z.L., Chen H.Q. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid Based Complement Altern Med. 2014;2014:525340. doi: 10.1155/2014/525340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu J. China plans to modernize traditional medicine. Nature. 2007;446:590–591. doi: 10.1038/446590a. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y., Pan Q., Cai W., Shen F., Chen G.Y., Xu L.M. Modulation of gut microbiota by berberine improves steatohepatitis in high-fat diet-fed BALB/c Mice. Arch Iran Med. 2016;19:197–203. [PubMed] [Google Scholar]
- 23.Yan H.M., Xia M.F., Wang Y., Chang X.X., Yao X.Z., Rao S.X. Efficacy of berberine in patients with non-alcoholic fatty liver disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134172. e0134172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan X.L., Wang J., Tang X.Y., Li Y.X., Xia P., Gao X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J Transl Med. 2015;13:24–34. doi: 10.1186/s12967-015-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z., Li B., Meng X., Yao S., Jin L., Yang J. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci Rep. 2016;6:20848. doi: 10.1038/srep20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wah Kheong C., Nik Mustapha N.R., Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:1940–1949 e8. doi: 10.1016/j.cgh.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Wicklow B., Wittmeier K., t'Jong G.W., McGavock J., Robert M., Duhamel T. Proposed trial: safety and efficacy of resveratrol for the treatment of non-alcoholic fatty liver disease (NAFLD) and associated insulin resistance in adolescents who are overweight or obese adolescents— rationale and protocol. Biochem Cell Biol. 2015;93:522–530. doi: 10.1139/bcb-2014-0136. [DOI] [PubMed] [Google Scholar]
- 28.Poulsen M.K., Nellemann B., Bibby B.M., Stodkilde-Jorgensen H., Pedersen S.B., Gronbaek H. No effect of resveratrol on VLDL–TG kinetics and insulin sensitivity in obese men with nonalcoholic fatty liver disease. Diabetes Obes Metab. 2018;20:2504–2509. doi: 10.1111/dom.13409. [DOI] [PubMed] [Google Scholar]
- 29.Poulsen M.K., Nellemann B., Stodkilde-Jorgensen H., Pedersen S.B., Gronbaek H., Nielsen S. Impaired insulin suppression of VLDL–triglyceride kinetics in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2016;101:1637–1646. doi: 10.1210/jc.2015-3476. [DOI] [PubMed] [Google Scholar]
- 30.Faghihzadeh F., Adibi P., Rafiei R., Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837–843. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P.W., Chen F.X., Li D., Ling W.H., Guo H.H. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine (Baltim) 2015;94:e758. doi: 10.1097/MD.0000000000000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kidd P., Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos) Altern Med Rev. 2005;10:193–203. [PubMed] [Google Scholar]
- 33.Wong M.C.S., Huang J.L.W., George J., Huang J., Leung C., Eslam M. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16:57–73. doi: 10.1038/s41575-018-0055-0. [DOI] [PubMed] [Google Scholar]
- 34.Lazaridis N., Tsochatzis E. Current and future treatment options in non-alcoholic steatohepatitis (NASH) Expert Rev Gastroenterol Hepatol. 2017;11:357–369. doi: 10.1080/17474124.2017.1293523. [DOI] [PubMed] [Google Scholar]
- 35.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez F.J., Jiang C.T., Xie C., Patterson A.D. Intestinal farnesoid X receptor signaling modulates metabolic disease. Dig Dis. 2017;35:178–184. doi: 10.1159/000450908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharifnia T., Antoun J., Verriere T.G., Suarez G., Wattacheril J., Wilson K.T. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. 2015;309:G270–G278. doi: 10.1152/ajpgi.00304.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray K. NAFLD. Leaky guts: intestinal permeability and NASH. Nat Rev Gastroenterol Hepatol. 2015;12:123. doi: 10.1038/nrgastro.2015.15. [DOI] [PubMed] [Google Scholar]
- 39.Leung C., Rivera L., Furness J.B., Angus P.W. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 40.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Wan X., Xu C., Yu C., Li Y. Role of NLRP3 inflammasome in the progression of NAFLD to NASH. Chin J Gastroenterol Hepatol. 2016;2016:6489012. doi: 10.1155/2016/6489012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazankov K., Jorgensen S.M.D., Thomsen K.L., Moller H.J., Vilstrup H., George J. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 43.Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Xie C., Yagai T., Luo Y., Liang X., Chen T., Wang Q. Activation of intestinal hypoxia-inducible factor 2alpha during obesity contributes to hepatic steatosis. Nat Med. 2017;23:1298–1308. doi: 10.1038/nm.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang C., Xie C., Li F., Zhang L., Nichols R.G., Krausz K.W. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Investig. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G., Xie C., Lu S., Nichols R.G., Tian Y., Li L. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metabol. 2017;26:672–685. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wree A., Eguchi A., McGeough M.D., Pena C.A., Johnson C.D., Canbay A. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frasinariu O.E., Ceccarelli S., Alisi A., Moraru E., Nobili V. Gut–liver axis and fibrosis in nonalcoholic fatty liver disease: an input for novel therapies. Dig Liver Dis. 2013;45:543–551. doi: 10.1016/j.dld.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Paolella G., Mandato C., Pierri L., Poeta M., Di Stasi M., Vajro P. Gut–liver axis and probiotics: their role in non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:15518–15531. doi: 10.3748/wjg.v20.i42.15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripathi A., Debelius J., Brenner D.A., Karin M., Loomba R., Schnabl B. The gut–liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang C., Xie C., Lv Y., Li J., Krausz K.W., Shi J. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iacono A., Raso G.M., Canani R.B., Calignano A., Meli R. Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem. 2011;22:699–711. doi: 10.1016/j.jnutbio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Federico A., D'Aiuto E., Borriello F., Barra G., Gravina A.G., Romano M. Fat: a matter of disturbance for the immune system. World J Gastroenterol. 2010;16:4762–4772. doi: 10.3748/wjg.v16.i38.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrese M., Cabrera D., Kalergis A.M., Feldstein A.E. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294–1303. doi: 10.1007/s10620-016-4049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tosello-Trampont A.C., Landes S.G., Nguyen V., Novobrantseva T.I., Hahn Y.S. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duarte N., Coelho I.C., Patarrao R.S., Almeida J.I., Penha-Goncalves C., Macedo M.P. How inflammation impinges on NAFLD: a role for Kupffer cells. BioMed Res Int. 2015;2015:1–11. doi: 10.1155/2015/984578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wree A., McGeough M.D., Pena C.A., Schlattjan M., Li H.Y., Inzaugarat M.E. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med. 2014;92:1069–1082. doi: 10.1007/s00109-014-1170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mridha A.R., Wree A., Robertson A.A.B., Yeh M.M., Johnson C.D., van Rooyen D.M. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037–1046. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pilegaard H., Saltin B., Neufer P.D. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xia B., Cai G.H., Yang H., Wang S.P., Mitchell G.A., Wu J.W. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1007110. e1007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Granneman J.G., Burnazi M., Zhu Z., Schwamb L.A. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285:E1230–E1236. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- 63.Kuang J., Zhang Y., Liu Q., Shen J., Pu S., Cheng S. Fat-specific sirt6 ablation sensitizes mice to high-fat diet-induced obesity and insulin resistance by inhibiting lipolysis. Diabetes. 2017;66:1159–1171. doi: 10.2337/db16-1225. [DOI] [PubMed] [Google Scholar]
- 64.Taher J., Farr S., Adeli K. Central nervous system regulation of hepatic lipid and lipoprotein metabolism. Curr Opin Lipidol. 2017;28:32–38. doi: 10.1097/MOL.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 65.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 66.Pimentel G.D., Micheletti T.O., Pace F., Rosa J.C., Santos R.V.T., Lira F.S. Gut-central nervous system axis is a target for nutritional therapies. Nutr J. 2012;11:22–30. doi: 10.1186/1475-2891-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gastaldelli A., Marchesini G. Time for glucagon like peptide-1 receptor agonists treatment for patients with NAFLD?. J Hepatol. 2016;64:262–264. doi: 10.1016/j.jhep.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 68.Thon M., Hosoi T., Ozawa K. Possible integrative actions of leptin and insulin signaling in the hypothalamus targeting energy homeostasis. Front Endocrinol (Lausanne) 2016;7:138–144. doi: 10.3389/fendo.2016.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez-Sanchez N., Seoane-Collazo P., Contreras C., Varela L., Villarroya J., Rial-Pensado E. Hypothalamic AMPK-ER stress–JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metabol. 2017;26:212–229. doi: 10.1016/j.cmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao Y., Deng Y., Yuan F., Xia T., Liu H., Li Z. ATF4/ATG5 signaling in hypothalamic proopiomelanocortin neurons regulates fat mass via affecting energy expenditure. Diabetes. 2017;66:1146–1158. doi: 10.2337/db16-1546. [DOI] [PubMed] [Google Scholar]
- 71.Wang G.L., Fu Y.C., Xu W.C., Feng Y.Q., Fang S.R., Zhou X.H. Resveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling pathway. Biochem Biophys Res Commun. 2009;380:644–649. doi: 10.1016/j.bbrc.2009.01.163. [DOI] [PubMed] [Google Scholar]
- 72.Andrade J.M., Paraiso A.F., de Oliveira M.V., Martins A.M., Neto J.F., Guimaraes A.L. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915–919. doi: 10.1016/j.nut.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 73.Tian Y., Ma J., Wang W., Zhang L., Xu J., Wang K. Resveratrol supplement inhibited the NF-kappaB inflammation pathway through activating AMPKalpha-SIRT1 pathway in mice with fatty liver. Mol Cell Biochem. 2016;422:75–84. doi: 10.1007/s11010-016-2807-x. [DOI] [PubMed] [Google Scholar]
- 74.Shang J., Chen L.L., Xiao F.X., Sun H., Ding H.C., Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y., Chen M.L., Zhou Y., Yi L., Gao Y.X., Ran L. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol Nutr Food Res. 2015;59:1443–1457. doi: 10.1002/mnfr.201500016. [DOI] [PubMed] [Google Scholar]
- 76.Alberdi G., Rodriguez V.M., Macarulla M.T., Miranda J., Churruca I., Portillo M.P. Hepatic lipid metabolic pathways modified by resveratrol in rats fed an obesogenic diet. Nutrition. 2013;29:562–567. doi: 10.1016/j.nut.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Wang S., Liang X., Yang Q., Fu X., Rogers C.J., Zhu M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha 1. Int J Obes. 2015;39:967–976. doi: 10.1038/ijo.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang S.J., Lim Y. Resveratrol ameliorates hepatic metaflammation and inhibits NLRP3 inflammasome activation. Metabolism. 2014;63:693–701. doi: 10.1016/j.metabol.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 79.Li L., Hai J., Li Z., Zhang Y., Peng H., Li K. Resveratrol modulates autophagy and NF-kappaB activity in a murine model for treating non-alcoholic fatty liver disease. Food Chem Toxicol. 2014;63:166–173. doi: 10.1016/j.fct.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 80.Ji G., Wang Y., Deng Y., Li X., Jiang Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health Dis. 2015;14:134–142. doi: 10.1186/s12944-015-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin S.H., Yang J.H., Shin B.Y., Seo K., Shin S.M., Cho I.J. Resveratrol inhibits LXR alpha-dependent hepatic lipogenesis through novel antioxidant sestrin2 gene induction. Toxicol Appl Pharmacol. 2013;271:95–105. doi: 10.1016/j.taap.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 82.Chen C.Y., Jang J.H., Li M.H., Surh Y.J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 83.Ungvari Z., Bagi Z., Feher A., Recchia F.A., Sonntag W.E., Pearson K. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cote C.D., Rasmussen B.A., Duca F.A., Zadeh-Tahmasebi M., Baur J.A., Daljeet M. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat Med. 2015;21 doi: 10.1038/nm.3821. 498-U284. [DOI] [PubMed] [Google Scholar]
- 85.Chaplin A., Carpene C., Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 2018;10:1651–1679. doi: 10.3390/nu10111651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bird J.K., Raederstorff D., Weber P., Steinert R.E. Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota. Adv Nutr. 2017;8:839–849. doi: 10.3945/an.117.016568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qiao Y., Sun J., Xia S., Tang X., Shi Y., Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5:1241–1249. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- 88.Bode L.M., Bunzel D., Huch M., Cho G.S., Ruhland D., Bunzel M. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr. 2013;97:295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- 89.Leclercq I.A., Farrell G.C., Sempoux C., dela Pena A., Horsmans Y. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol. 2004;41:926–934. doi: 10.1016/j.jhep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 90.Ramirez-Tortosa M.C., Ramirez-Tortosa C.L., Mesa M.D., Granados S., Gil A., Quiles J.L. Curcumin ameliorates rabbits's steatohepatitis via respiratory chain, oxidative stress, and TNF-alpha. Free Radic Biol Med. 2009;47:924–931. doi: 10.1016/j.freeradbiomed.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 91.Li J.M., Li Y.C., Kong L.D., Hu Q.H. Curcumin inhibits hepatic protein-tyrosine phosphatase 1B and prevents hypertriglyceridemia and hepatic steatosis in fructose-fed rats. Hepatology. 2010;51:1555–1566. doi: 10.1002/hep.23524. [DOI] [PubMed] [Google Scholar]
- 92.Wang L., Lv Y., Yao H., Yin L., Shang J. Curcumin prevents the non-alcoholic fatty hepatitis via mitochondria protection and apoptosis reduction. Int J Clin Exp Pathol. 2015;8:11503–11509. [PMC free article] [PubMed] [Google Scholar]
- 93.Vizzutti F., Provenzano A., Galastri S., Milani S., Delogu W., Novo E. Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab Investig. 2010;90:104–115. doi: 10.1038/labinvest.2009.112. [DOI] [PubMed] [Google Scholar]
- 94.Lin J., Tang Y., Kang Q., Feng Y., Chen A. Curcumin inhibits gene expression of receptor for advanced glycation end-products (RAGE) in hepatic stellate cells in vitro by elevating PPARgamma activity and attenuating oxidative stress. Br J Pharmacol. 2012;166:2212–2227. doi: 10.1111/j.1476-5381.2012.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang Y., Chen A. Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling. Lab Investig. 2014;94:503–516. doi: 10.1038/labinvest.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin J., Zheng S., Chen A. Curcumin attenuates the effects of insulin on stimulating hepatic stellate cell activation by interrupting insulin signaling and attenuating oxidative stress. Lab Investig. 2009;89:1397–1409. doi: 10.1038/labinvest.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan C., Zhang Y., Zhang X., Aa J., Wang G., Xie Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed Pharmacother. 2018;105:274–281. doi: 10.1016/j.biopha.2018.05.135. [DOI] [PubMed] [Google Scholar]
- 98.Kang O.H., Kim S.B., Seo Y.S., Joung D.K., Mun S.H., Choi J.G. Curcumin decreases oleic acid-induced lipid accumulation via AMPK phosphorylation in hepatocarcinoma cells. Eur Rev Med Pharmacol Sci. 2013;17:2578–2586. [PubMed] [Google Scholar]
- 99.Um M.Y., Hwang K.H., Ahn J., Ha T.Y. Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase. Basic Clin Pharmacol Toxicol. 2013;113:152–157. doi: 10.1111/bcpt.12076. [DOI] [PubMed] [Google Scholar]
- 100.Yin H.P., Guo Q., Li X., Tang T.T., Li C.L., Wang H.X. Curcumin suppresses IL-1 beta secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J Immunol. 2018;200:2835–2846. doi: 10.4049/jimmunol.1701495. [DOI] [PubMed] [Google Scholar]
- 101.Kong F., Ye B., Cao J., Cai X., Lin L., Huang S. Curcumin represses NLRP3 inflammasome activation via TLR4/MyD88/NF-kappaB and P2X7R signaling in PMA-induced macrophages. Front Pharmacol. 2016;7:369. doi: 10.3389/fphar.2016.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng W., Wang H., Zhang P., Gao C., Tao J., Ge Z. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim Biophys Acta Gen Subj. 2017;1861:1801–1812. doi: 10.1016/j.bbagen.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 103.Shen L., Liu L., Ji H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr Res. 2017;61:1361780. doi: 10.1080/16546628.2017.1361780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Z., Zhang H., Li B., Meng X., Wang J. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun. 2014;5:5493–5507. doi: 10.1038/ncomms6493. [DOI] [PubMed] [Google Scholar]
- 105.Zhou H., Feng L., Xu F., Sun Y., Ma Y., Zhang X. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: a new mechanism linking berberine to insulin resistance improvement. Biomed Pharmacother. 2017;89:864–874. doi: 10.1016/j.biopha.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 106.Vivoli E., Cappon A., Milani S., Piombanti B., Provenzano A., Novo E. NLRP3 inflammasome as a target of berberine in experimental murine liver injury: interference with P2X(7) signalling. Clin Sci. 2016;130:1793–1806. doi: 10.1042/CS20160400. [DOI] [PubMed] [Google Scholar]
- 107.Mahmoud A.M., Hozayen W.G., Ramadan S.M. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARgamma, and suppressing oxidative stress and apoptosis in rats. Biomed Pharmacother. 2017;94:280–291. doi: 10.1016/j.biopha.2017.07.101. [DOI] [PubMed] [Google Scholar]
- 108.Dinesh P., Rasool M. Berberine, an isoquinoline alkaloid suppresses TXNIP mediated NLRP3 inflammasome activation in MSU crystal stimulated RAW 264.7 macrophages through the upregulation of Nrf2 transcription factor and alleviates MSU crystal induced inflammation in rats. Int Immunopharmacol. 2017;44:26–37. doi: 10.1016/j.intimp.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 109.Sun Y., Yuan X., Zhang F., Han Y., Chang X., Xu X. Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells. Sci Rep. 2017;7:11340. doi: 10.1038/s41598-017-11860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee Y.S., Kim W.S., Kim K.H., Yoon M.J., Cho H.J., Shen Y. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 111.Kim W.S., Lee Y.S., Cha S.H., Jeong H.W., Choe S.S., Lee M.R. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296:E812–E819. doi: 10.1152/ajpendo.90710.2008. [DOI] [PubMed] [Google Scholar]
- 112.Sun R., Yang N., Kong B., Cao B., Feng D., Yu X. Orally administered berberine modulates hepatic lipid metabolism by altering microbial bile acid metabolism and the intestinal FXR signaling pathway. Mol Pharmacol. 2017;91:110–122. doi: 10.1124/mol.116.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang X., Zhao Y., Xu J., Xue Z., Zhang M., Pang X. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X., Zhao Y., Zhang M., Pang X., Xu J., Kang C. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042529. e42529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng R., Shou J.W., Zhao Z.X., He C.Y., Ma C., Huang M. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci Rep. 2015;5:12155. doi: 10.1038/srep12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y., Tong Q., Shou J.W., Zhao Z.X., Li X.Y., Zhang X.F. Gut microbiota-mediated personalized treatment of hyperlipidemia using berberine. Theranostics. 2017;7:2443–2451. doi: 10.7150/thno.18290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y., Shou J.W., Li X.Y., Zhao Z.X., Fu J., He C.Y. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism. 2017;70:72–84. doi: 10.1016/j.metabol.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 118.Salomone F., Barbagallo I., Godos J., Lembo V., Currenti W., Cina D. Silibinin restores NAD+ levels and induces the SIRT1/AMPK pathway in non-alcoholic fatty liver. Nutrients. 2017;9 doi: 10.3390/nu9101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gu M., Zhao P., Huang J., Zhao Y., Wang Y., Li Y. Silymarin ameliorates metabolic dysfunction associated with diet-induced obesity via activation of farnesyl X receptor. Front Pharmacol. 2016;7:345. doi: 10.3389/fphar.2016.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cui C.X., Deng J.N., Yan L., Liu Y.Y., Fan J.Y., Mu H.N. Silibinin capsules improves high fat diet-induced nonalcoholic fatty liver disease in hamsters through modifying hepatic de novo lipogenesis and fatty acid oxidation. J Ethnopharmacol. 2017;208:24–35. doi: 10.1016/j.jep.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 121.Zhang B.L., Xu D., She L.L., Wang Z.X., Yang N., Sun R.B. Silybin inhibits NLRP3 inflammasome assembly through the NAD+/SIRT2 pathway in mice with nonalcoholic fatty liver disease. FASEB J. 2018;32:757–767. doi: 10.1096/fj.201700602R. [DOI] [PubMed] [Google Scholar]
- 122.Surai P.F. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants. 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Charytoniuk T., Drygalski K., Konstantynowicz-Nowicka K., Berk K., Chabowski A. Alternative treatment methods attenuate the development of NAFLD: a review of resveratrol molecular mechanisms and clinical trials. Nutrition. 2017;34:108–117. doi: 10.1016/j.nut.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 124.Poulsen M.M., Vestergaard P.F., Clasen B.F., Radko Y., Christensen L.P., Stodkilde-Jorgensen H. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoshino J., Conte C., Fontana L., Mittendorfer B., Imai S., Schechtman K.B. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metabol. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elgebaly A., Radwan I.A., AboElnas M.M., Ibrahim H.H., Eltoomy M.F., Atta A.A. Resveratrol supplementation in patients with non-alcoholic fatty liver disease: systematic review and meta-analysis. J Gastrointest Liver Dis. 2017;26:59–67. doi: 10.15403/jgld.2014.1121.261.ely. [DOI] [PubMed] [Google Scholar]
- 127.Liang L., Liu X., Wang Q., Cheng S., Zhang S., Zhang M. Pharmacokinetics, tissue distribution and excretion study of resveratrol and its prodrug 3,5,4′-tri-O-acetylresveratrol in rats. Phytomedicine. 2013;20:558–563. doi: 10.1016/j.phymed.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 128.Farzaei M.H., Zobeiri M., Parvizi F., El-Senduny F.F., Marmouzi I., Coy-Barrera E. Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients. 2018;10:855–882. doi: 10.3390/nu10070855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.White C.M., Lee J.Y. The impact of turmeric or its curcumin extract on nonalcoholic fatty liver disease: a systematic review of clinical trials. Pharm Pract (Granada) 2019;17:1350. doi: 10.18549/PharmPract.2019.1.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rahmani S., Asgary S., Askari G., Keshvari M., Hatamipour M., Feizi A. Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytother Res. 2016;30:1540–1548. doi: 10.1002/ptr.5659. [DOI] [PubMed] [Google Scholar]
- 131.Afrin R., Arumugam S., Rahman A., Wahed M.I., Karuppagounder V., Harima M. Curcumin ameliorates liver damage and progression of NASH in NASH-HCC mouse model possibly by modulating HMGB1-NF-kappaB translocation. Int Immunopharmacol. 2017;44:174–182. doi: 10.1016/j.intimp.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 132.Gong Z., Zhou J., Li H., Gao Y., Xu C., Zhao S. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Mol Nutr Food Res. 2015;59:2132–2142. doi: 10.1002/mnfr.201500316. [DOI] [PubMed] [Google Scholar]
- 133.Kong W.J., Wei J., Abidi P., Lin M.H., Inaba S., Li C. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y., Zhang L., Song H., Ji G. Update on berberine in nonalcoholic fatty liver disease. Evid Based Complement Altern Med. 2013;2013:308134. doi: 10.1155/2013/308134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chang X., Wang Z., Zhang J., Yan H., Bian H., Xia M. Lipid profiling of the therapeutic effects of berberine in patients with nonalcoholic fatty liver disease. J Transl Med. 2016;14:266–276. doi: 10.1186/s12967-016-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wei X., Wang C., Hao S., Song H., Yang L. The therapeutic effect of berberine in the treatment of nonalcoholic fatty liver disease: a meta-analysis. Evid Based Complement Altern Med. 2016;2016:3593951. doi: 10.1155/2016/3593951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang J., Ma X.J., Li L., Wang L., Chen Y.G., Liu J. Berberine ameliorates non-alcoholic steatohepatitis in ApoE–/– mice. Exp Ther Med. 2017;14:4134–4140. doi: 10.3892/etm.2017.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo T., Woo S.L., Guo X., Li H., Zheng J., Botchlett R. Berberine ameliorates hepatic steatosis and suppresses liver and adipose tissue inflammation in mice with diet-induced obesity. Sci Rep. 2016;6:22612. doi: 10.1038/srep22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cacciapuoti F., Scognamiglio A., Palumbo R., Forte R., Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol. 2013;5:109–113. doi: 10.4254/wjh.v5.i3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ou Q., Weng Y., Wang S., Zhao Y., Zhang F., Zhou J. Silybin alleviates hepatic steatosis and fibrosis in NASH mice by inhibiting oxidative stress and involvement with the NF-κB pathway. Dig Dis Sci. 2018;63:3398–3408. doi: 10.1007/s10620-018-5268-0. [DOI] [PubMed] [Google Scholar]
- 141.Salamone F., Galvano F., Cappello F., Mangiameli A., Barbagallo I., Li Volti G. Silibinin modulates lipid homeostasis and inhibits nuclear factor kappa B activation in experimental nonalcoholic steatohepatitis. Transl Res. 2012;159:477–486. doi: 10.1016/j.trsl.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 142.Ni X.J., Wang H.Y. Silymarin attenuated hepatic steatosis through regulation of lipid metabolism and oxidative stress in a mouse model of nonalcoholic fatty liver disease (NAFLD) Am J Transl Res. 2016;8:1073–1081. [PMC free article] [PubMed] [Google Scholar]
- 143.Marin V., Gazzin S., Gambaro S.E., Dal Ben M., Calligaris S., Anese M. Effects of oral administration of silymarin in a juvenile murine model of non-alcoholic steatohepatitis. Nutrients. 2017;9:1006–1025. doi: 10.3390/nu9091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Solhi H., Ghahremani R., Kazemifar A.M., Hoseini Yazdi Z. Silymarin in treatment of non-alcoholic steatohepatitis: a randomized clinical trial. Caspian J Intern Med. 2014;5:9–12. [PMC free article] [PubMed] [Google Scholar]
- 145.Aller R., Izaola O., Gomez S., Tafur C., Gonzalez G., Berroa E. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease: a randomized clinical pilot study. Eur Rev Med Pharmacol Sci. 2015;19:3118–3124. [PubMed] [Google Scholar]
- 146.de Avelar C.R., Pereira E.M., de Farias Costa P.R., de Jesus R.P., de Oliveira L.P.M. Effect of silymarin on biochemical indicators in patients with liver disease: systematic review with meta-analysis. World J Gastroenterol. 2017;23:5004–5017. doi: 10.3748/wjg.v23.i27.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Federico A., Trappoliere M., Tuccillo C., de Sio I., Di Leva A., Del Vecchio Blanco C. A new silybin-vitamin E-phospholipid complex improves insulin resistance and liver damage in patients with non-alcoholic fatty liver disease: preliminary observations. Gut. 2006;55:901–902. doi: 10.1136/gut.2006.091967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Andreone P., Brisc M.C., Chiaramonte M., Federico A., Floreani A., Freni M.A. Silybin conjugated with phosphatidylcholine and vitamin E improves liver damage in patients with NAFLD: the results of a randomized multicentre double-blind vs. placebo trial. J Hepatol. 2011;54:S330–S331. [Google Scholar]
- 149.Kim M., Yang S.G., Kim J.M., Lee J.W., Kim Y.S., Lee J.I. Silymarin suppresses hepatic stellate cell activation in a dietary rat model of non-alcoholic steatohepatitis: analysis of isolated hepatic stellate cells. Int J Mol Med. 2012;30:473–479. doi: 10.3892/ijmm.2012.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen L., Ji H.F. Intestinal microbiota and metabolic diseases: pharmacological implications. Trends Pharmacol Sci. 2016;37:169–171. doi: 10.1016/j.tips.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 151.Abenavoli L., Izzo A.A., Milic N., Cicala C., Santini A., Capasso R. Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res. 2018;32:2202–2213. doi: 10.1002/ptr.6171. [DOI] [PubMed] [Google Scholar]
- 152.Serviddio G., Bellanti F., Giudetti A.M., Gnoni G.V., Petrella A., Tamborra R. A silybin-phospholipid complex prevents mitochondrial dysfunction in a rodent model of nonalcoholic steatohepatitis. J Pharmacol Exp Ther. 2010;332:922–932. doi: 10.1124/jpet.109.161612. [DOI] [PubMed] [Google Scholar]
- 153.Patel D.P., Yan T., Kim D., Dias H.B., Krausz K.W., Kimura S. Withaferin A improves non-alcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371:360–374. doi: 10.1124/jpet.119.256792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yan T., Wang H., Cao L., Wang Q., Takahashi S., Yagai T. Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab Dispos. 2018;46:1310–1319. doi: 10.1124/dmd.118.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun X., Duan X., Wang C., Liu Z., Sun P., Huo X. Protective effects of glycyrrhizic acid against non-alcoholic fatty liver disease in mice. Eur J Pharmacol. 2017;806:75–82. doi: 10.1016/j.ejphar.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 156.Wu X., Zhang L., Gurley E., Studer E., Shang J., Wang T. Prevention of free fatty acid-induced hepatic lipotoxicity by 18beta-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology. 2008;47:1905–1915. doi: 10.1002/hep.22239. [DOI] [PubMed] [Google Scholar]
- 157.Xin H.G., Zhang B.B., Wu Z.Q., Hang X.F., Xu W.S., Ni W. Treatment with baicalein attenuates methionine-choline deficient diet-induced non-alcoholic steatohepatitis in rats. Eur J Pharmacol. 2014;738:310–318. doi: 10.1016/j.ejphar.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 158.Zhang J., Zhang H., Deng X., Zhang Y., Xu K. Baicalin protects AML-12 cells from lipotoxicity via the suppression of ER stress and TXNIP/NLRP3 inflammasome activation. Chem Biol Interact. 2017;278:189–196. doi: 10.1016/j.cbi.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 159.Shetty S.N., Mengi S., Vaidya R., Vaidya A.D. A study of standardized extracts of Picrorhiza kurroa Royle ex Benth in experimental nonalcoholic fatty liver disease. J Ayurveda Integr Med. 2010;1:203–210. doi: 10.4103/0975-9476.72622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang Q.H., Xu Y.J., Liu Y.Z., Liang Y.J., Feng G.F., Zhang Y.P. Effects of chaihu-shugan-san and shen-ling-bai-zhu-san on p38 MAPK pathway in kupffer cells of nonalcoholic steatohepatitis. Evid Based Complement Altern Med. 2014;2014:671013. doi: 10.1155/2014/671013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang Q., Xu Y., Feng G., Hu C., Zhang Y., Cheng S. p38 MAPK signal pathway involved in anti-inflammatory effect of Chaihu-Shugan-San and Shen-ling-bai-zhu-San on hepatocyte in non-alcoholic steatohepatitis rats. Afr J Tradit, Complementary Altern Med. 2014;11:213–221. doi: 10.4314/ajtcam.v11i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takahashi Y., Soejima Y., Kumagai A., Watanabe M., Uozaki H., Fukusato T. Japanese herbal medicines shosaikoto, inchinkoto, and juzentaihoto inhibit high-fat diet-induced nonalcoholic steatohepatitis in db/db mice. Pathol Int. 2014;64:490–498. doi: 10.1111/pin.12199. [DOI] [PubMed] [Google Scholar]
- 163.Takahashi Y., Soejima Y., Kumagai A., Watanabe M., Uozaki H., Fukusato T. Inhibitory effects of Japanese herbal medicines sho-saiko-to and juzen-taiho-to on nonalcoholic steatohepatitis in mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087279. e87279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li X.X., Lu X.Y., Zhang S.J., Chiu A.P., Lo L.H., Largaespada D.A. Sodium tanshinone IIA sulfonate ameliorates hepatic steatosis by inhibiting lipogenesis and inflammation. Biomed Pharmacother. 2019;111:68–75. doi: 10.1016/j.biopha.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 165.Ono M., Ogasawara M., Hirose A., Mogami S., Ootake N., Aritake K. Bofutsushosan, a Japanese herbal (Kampo) medicine, attenuates progression of nonalcoholic steatohepatitis in mice. J Gastroenterol. 2014;49:1065–1073. doi: 10.1007/s00535-013-0852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu N., Fang X., Zhao D., Mu Q., Zuo J., Ma Y. Anti-diabetic effects of jiang tang xiao ke granule via PI3K/Akt signalling pathway in type 2 diabetes kkay mice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168980. e0168980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mo F.F., An T., Zhang Z.J., Liu Y.F., Liu H.X., Pan Y.Y. Jiang tang xiao ke granule play an anti-diabetic role in diabetic mice pancreatic tissue by regulating the mRNAs and microRNAs associated with PI3K-Akt signaling pathway. Front Pharmacol. 2017;8:795. doi: 10.3389/fphar.2017.00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Y., An H., Pan S.Y., Zhao D.D., Zuo J.C., Li X.K. Jiang tang xiao ke granule, a classic Chinese herbal formula, improves the effect of metformin on lipid and glucose metabolism in diabetic mice. Evid Based Complement Altern Med. 2016;2016 doi: 10.1155/2016/1592731. 1592731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liang Y., Zhang Y., Deng Y., Liang S., He Y., Chen Y. Chaihu-shugan-san decoction modulates intestinal microbe dysbiosis and alleviates chronic metabolic inflammation in NAFLD rats via the NLRP3 inflammasome pathway. Evid Based Complement Altern Med. 2018;2018:9390786. doi: 10.1155/2018/9390786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jiang W.N., Li D., Jiang T., Guo J., Chen Y.F., Wang J. Protective effects of chaihu shugan san on nonalcoholic fatty liver disease in rats with insulin resistance. Chin J Integr Med. 2018;24:125–132. doi: 10.1007/s11655-016-2252-4. [DOI] [PubMed] [Google Scholar]
- 171.Guo H., Zhong R., Liu Y., Jiang X., Tang X., Li Z. Effects of bayberry juice on inflammatory and apoptotic markers in young adults with features of non-alcoholic fatty liver disease. Nutrition. 2014;30:198–203. doi: 10.1016/j.nut.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 172.Xiao J., Wang F., Liong E.C., So K.F., Tipoe G.L. Lycium barbarum polysaccharides improve hepatic injury through NF-κB and NLRP3/6 pathways in a methionine choline deficient diet steatohepatitis mouse model. Int J Biol Macromol. 2018;120:1480–1489. doi: 10.1016/j.ijbiomac.2018.09.151. [DOI] [PubMed] [Google Scholar]
- 173.Li G., Zhou F., Chen Y., Zhang W., Wang N. Kukoamine A attenuates insulin resistance and fatty liver through downregulation of SREBP-1c. Biomed Pharmacother. 2017;89:536–543. doi: 10.1016/j.biopha.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 174.Jia L., Li W., Li J., Li Y., Song H., Luan Y. Lycium barbarum polysaccharide attenuates high-fat diet-induced hepatic steatosis by up-regulating SIRT1 expression and deacetylase activity. Sci Rep. 2016;6:36209. doi: 10.1038/srep36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xiao J., Xing F., Huo J., Fung M.L., Liong E.C., Ching Y.P. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci Rep. 2014;4:5587. doi: 10.1038/srep05587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zamani N., Shams M., Nimrouzi M., Zarshenas M.M., Abolhasani Foroughi A., Fallahzadeh Abarghooei E. The effects of Zataria multiflora Boiss. (Shirazi thyme) on nonalcoholic fatty liver disease and insulin resistance: a randomized double-blind placebo-controlled clinical trial. Complement Ther Med. 2018;41:118–123. doi: 10.1016/j.ctim.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 177.Li J., Wang S., Yao L., Ma P., Chen Z., Han T.L. 6-Gingerol ameliorates age-related hepatic steatosis association with regulating lipogenesis, fatty acid oxidation, oxidative stress and mitochondrial dysfunction. Toxicol Appl Pharmacol. 2019;362:125–135. doi: 10.1016/j.taap.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 178.Lai Y.S., Lee W.C., Lin Y.E., Ho C.T., Lu K.H., Lin S.H. Ginger essential oil ameliorates hepatic injury and lipid accumulation in high fat diet-induced nonalcoholic fatty liver disease. J Agric Food Chem. 2016;64:2062–2071. doi: 10.1021/acs.jafc.5b06159. [DOI] [PubMed] [Google Scholar]
- 179.Hong S.H., Suk K.T., Choi S.H., Lee J.W., Sung H.T., Kim C.H. Anti-oxidant and natural killer cell activity of Korean red ginseng (Panax ginseng) and urushiol (Rhus vernicifera Stokes) on non-alcoholic fatty liver disease of rat. Food Chem Toxicol. 2013;55:586–591. doi: 10.1016/j.fct.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 180.Lee S.B., Cho H.I., Jin Y.W., Lee E.K., Ahn J.Y., Lee S.M. Wild ginseng cambial meristematic cells ameliorate hepatic steatosis and mitochondrial dysfunction in high-fat diet-fed mice. J Pharm Pharmacol. 2016;68:119–127. doi: 10.1111/jphp.12487. [DOI] [PubMed] [Google Scholar]
- 181.Hong M., Lee Y.H., Kim S., Suk K.T., Bang C.S., Yoon J.H. Anti-inflammatory and antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease. J Ginseng Res. 2016;40:203–210. doi: 10.1016/j.jgr.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jeong H., Kim J.W., Yang M.S., Park C., Kim J.H., Lim C.W. Beneficial effects of Korean red ginseng in the progression of non-alcoholic steatohepatitis via FABP4 modulation. Am J Chin Med. 2018;46:1581–1607. doi: 10.1142/S0192415X18500817. [DOI] [PubMed] [Google Scholar]
- 183.Huang Q., Wang T., Yang L., Wang H.Y. Ginsenoside Rb2 alleviates hepatic lipid accumulation by restoring autophagy via induction of Sirt1 and activation of AMPK. Int J Mol Sci. 2017;18:1063–1077. doi: 10.3390/ijms18051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xu Y., Yang C., Zhang S., Li J., Xiao Q., Huang W. Ginsenoside Rg1 protects against non-alcoholic fatty liver disease by ameliorating lipid peroxidation, endoplasmic reticulum stress, and inflammasome activation. Biol Pharm Bull. 2018;41:1638–1644. doi: 10.1248/bpb.b18-00132. [DOI] [PubMed] [Google Scholar]
- 185.Peng H., He Y., Zheng G., Zhang W., Yao Z., Xie W. Meta-analysis of traditional herbal medicine in the treatment of nonalcoholic fatty liver disease. Cell Mol Biol (Noisy-Le-Grand) 2016;62:88–95. [PubMed] [Google Scholar]
- 186.Sanyal A.J., Neuschwander-Tetri B.A., Tonascia J. End points must be clinically meaningful for drug development in nonalcoholic fatty liver disease. Gastroenterology. 2016;150:11–13. doi: 10.1053/j.gastro.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 187.Hao H.P., Zheng X., Wang G.J. Insights into drug discovery from natural medicines using reverse pharmacokinetics. Trends Pharmacol Sci. 2014;35:168–177. doi: 10.1016/j.tips.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 188.Saeidnia S., Gohari A.R., Manayi A. Reverse pharmacognosy and reverse pharmacology; two closely related approaches for drug discovery development. Curr Pharmaceut Biotechnol. 2016;17:1016–1022. doi: 10.2174/1389201017666160709200208. [DOI] [PubMed] [Google Scholar]
- 189.Hebbard L., George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 190.Stankovic M.N., Mladenovic D.R., Duricic I., Sobajic S.S., Timic J., Jorgacevic B. Time-dependent changes and association between liver free fatty acids, serum lipid profile and histological features in mice model of nonalcoholic fatty liver disease. Arch Med Res. 2014;45:116–124. doi: 10.1016/j.arcmed.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 191.Xiong X., Kuang H., Ansari S., Liu T., Gong J., Wang S. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol Cell. 2019;75:644–660. doi: 10.1016/j.molcel.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Alonso C., Fernandez-Ramos D., Varela-Rey M., Martinez-Arranz I., Navasa N., van Liempd S.M. Metabolomic identification of subtypes of nonalcoholic steatohepatitis. Gastroenterology. 2017;152:1449–1461. doi: 10.1053/j.gastro.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hussein N.S., Helmy A.S., Sherif N.M., Ghanem H.Z., Ibrahim N.A., El Gendy A.N.G. Lipidomic analysis reveals the efficiency of eclipta prostrata on diet-induced nonalcoholic fatty liver disease in rats. J Pharm Biomed Anal. 2019;165:224–232. doi: 10.1016/j.jpba.2018.11.060. [DOI] [PubMed] [Google Scholar]
- 194.Sanyal A.J., Brunt E.M., Kleiner D.E., Kowdley K.V., Chalasani N., Lavine J.E. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xu J., Chen H.B., Li S.L. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med Res Rev. 2017;37:1140–1185. doi: 10.1002/med.21431. [DOI] [PubMed] [Google Scholar]
- 196.Wang Y., Fan X., Qu H., Gao X., Cheng Y. Strategies and techniques for multi-component drug design from medicinal herbs and traditional Chinese medicine. Curr Top Med Chem. 2012;12:1356–1362. doi: 10.2174/156802612801319034. [DOI] [PubMed] [Google Scholar]
- 197.Chen M., Yang F., Yang X., Lai X., Gao Y. Systematic understanding of mechanisms of a Chinese herbal formula in treatment of metabolic syndrome by an integrated pharmacology approach. Int J Mol Sci. 2016;17:2114–2127. doi: 10.3390/ijms17122114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.