Abstract
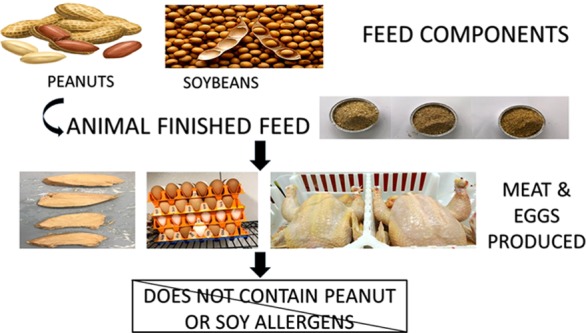
Previous studies have demonstrated that allergenic feed proteins from peanuts in the diets of layer hens are not detected in the eggs produced. Hence, in this study, we aimed to determine if soy and/or peanut proteins in poultry feed rations of broiler chickens or layer hens would be transferred or detectable in the meat or eggs produced. To meet this objective, 99 layer hens and 300 broiler chickens were equally divided into treatment groups and fed one of three experimental diets: control soybean meal and corn diet, whole unblanched high-oleic peanut and corn diet (HO PN), or a control diet spiked supplemented with oleic acid (OA) oil. At termination, broiler chickens were processed, and chicken breast samples of the left pectoralis muscle were collected, and eggs were collected from layers. Total protein extracts from pooled egg samples and chicken breast samples were subjected to enzyme-linked immunosorbent assay (ELISA) methods and immunoblotting analysis with rabbit antipeanut agglutinin antibodies and rabbit antisoy antibodies for the detection of peanut and soy proteins. Peanut and soy proteins were undetected in all pooled egg samples and individual chicken breast meat samples using immunoblotting techniques with rabbit antipeanut agglutinin and rabbit antisoy antibodies. Moreover, quantitative ELISA allergen detection methods determined all pooled egg samples and individual meat samples as “not containing” peanut or soy allergens. Therefore, this study helps to evaluate the risk associated with the potential transfer of allergenic proteins from animal feed to the products produced for human consumption.
Introduction
Studies have estimated that more than 26 million American adults1 and approximately 8% of children2,3 within the United States suffer from food allergies.4 In the United States, approximately 200 000 Americans annually require urgent medical care due to food allergies, correlating to emergency medical care every 3 min due to food hypersensitivity responses.5 Without proven treatment and/or prevention strategies, consumers with hypersensitivity responses to foods must refrain from all potentially offending foods to minimize the risk of systemic anaphylaxis. Therefore, consumers must rely upon the accuracy of food ingredient labeling and the avoidance of hidden ingredients that appear in packaged food, due to cross-contact with food allergens during the manufacturing process. Thus, strict adherence within food manufacturing facilities to food allergen sanitation methods defined in an effective Allergen Control program6−8 is an extremely important public health concern to the food-allergic consumer.
However, studies conducted by Fæste et al. (2014)9 suggested that dietary avoidance of food allergens with stringent food labeling might not be adequate to identify all potential allergenic proteins in foods. This study suggests that allergic responses to food may be possible when consuming meat products produced from animals fed diets containing allergens.9 In this study, zebrafish were fed a parasitic nematode found within fish and marine animals, Anisakis simplex, which cause food-allergic symptoms in humans when consumed.10,11 Fæste et al. (2014) demonstrated that the flesh of the zebrafish contained A. simplex nematode proteins after 14 days of feeding fishmeal spiked with A. simplex, and upon consumption, it elicited an allergenic response in a sensitized consumer.9 Moreover, Armentia et al. (2006) reported the detection of A. simplex allergenic peptides in chicken meat produced from poultry fed fishmeal containing A. simplex and A. simplex Ig-E responses in sensitized consumers of these chicken meat products.12
Fishmeal is normally prepared using processed and cooked fish remnants directly on factory ships.13,14 However, studies have shown allergenic peptides isolated from A. simplex to be highly protease and thermally stable.15 Consequently, we aimed to detect peanut and/or soy peptides in the meat or eggs produced from broiler chickens or layer hens fed diets containing soy (soybean meal) or high-oleic peanuts. Soybean meal, which contains soy allergens, is commonly used as a conventional poultry ration in poultry meat and egg production and therefore might transfer to the meat and/or eggs produced. Previous studies within our lab have demonstrated the effective use of high-oleic peanuts as an alternative poultry feed ingredient to effectively enrich the meat or eggs produced.16 Subsequently, in this study, we aimed to determine if allergenic peptides found in poultry feed are detectable in the eggs or meat produced from broiler chickens and laying hens fed diets containing high-oleic peanuts.
Results and Discussion
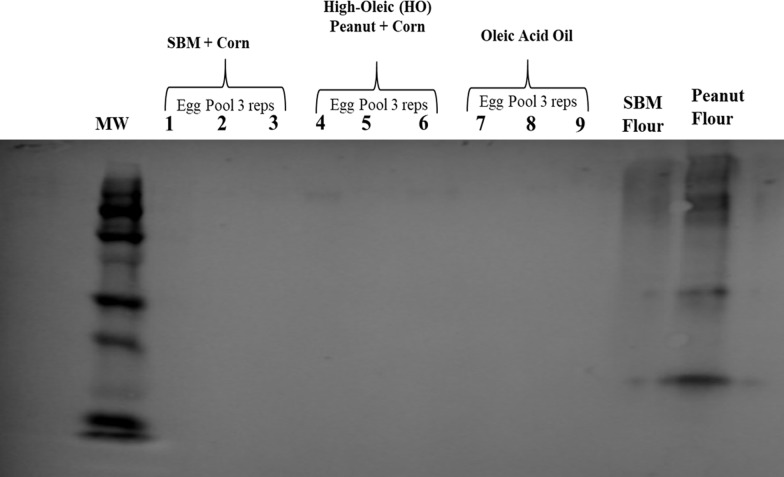
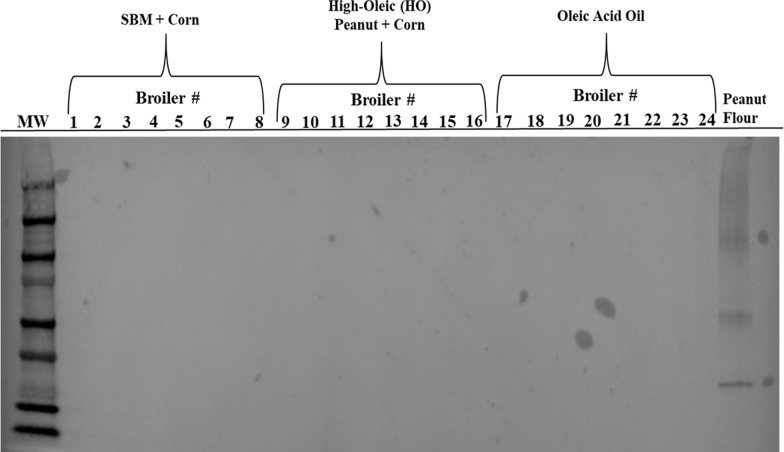
For the detection of peanut or soy peptides in the eggs or meat produced from poultry fed diets containing soybean meal or high-oleic peanuts, total protein extracts from pooled egg and individual chicken breast samples were analyzed by immunoblotting methods. Total protein extracts from pooled egg samples were not detected using rabbit antipeanut agglutinin antibodies, while reactive with total proteins extracted from peanut flour (Figure 1), thus implying that peanut proteins cannot be detected in the eggs produced from egg-producing hens fed a high-oleic peanut-containing diet for 8 weeks. Moreover, total protein extracts from breast samples from broilers fed a high-oleic peanut diet for 6 weeks were also nonreactive with rabbit antipeanut agglutinin antibodies, while total proteins extracted from the positive control, peanut flour were detected (Figure 2). Hence, allergenic peanut proteins from the broiler diet were not detected in the meat produced.
Figure 1.
Immunoreactivity of protein extracts from eggs produced from layer hens fed a high-oleic peanut diet. Total proteins were extracted from pooled egg samples (three replicates per treatment, with 10 eggs pooled per replicate) and electrophoretically ran on a 10% polyacrylamide gel with 75 μg of total protein per lane. Resolved proteins were transferred to a nitrocellulose membrane and immunoblotted with rabbit IgG antipeanut agglutinin antibodies (1:1000), and detection was determined with horseradish peroxidase (HRP) activity. Positive control = peanut flour extracted proteins (75 μg). Negative control = soybean flour extracted proteins (75 μg).
Figure 2.
Immunoreactivity of protein extracts from chicken breast samples collected from broiler chickens fed a high-oleic peanut diet. Total protein extracts from chicken breast samples (eight samples per treatment) were ran using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 75 μg of total protein per lane. Resolved proteins were transferred to a nitrocellulose membrane and immunoblotted with rabbit IgG antipeanut agglutinin antibodies (1:1000); detection was determined using chromogenic peroxidase substrate with HRP. Positive control = peanut flour extracted proteins (75 μg).
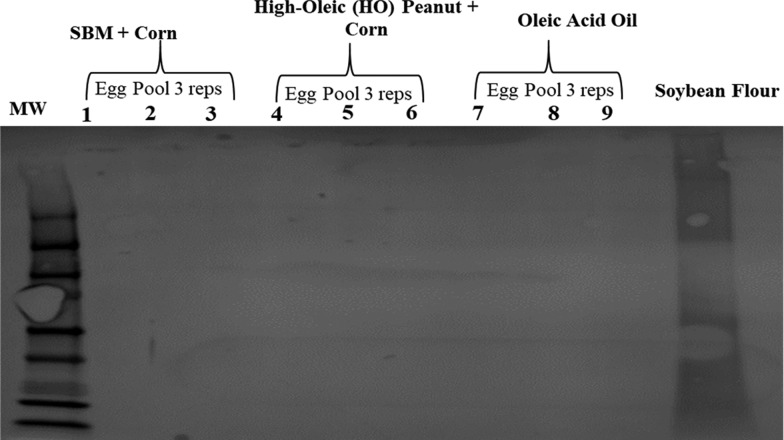
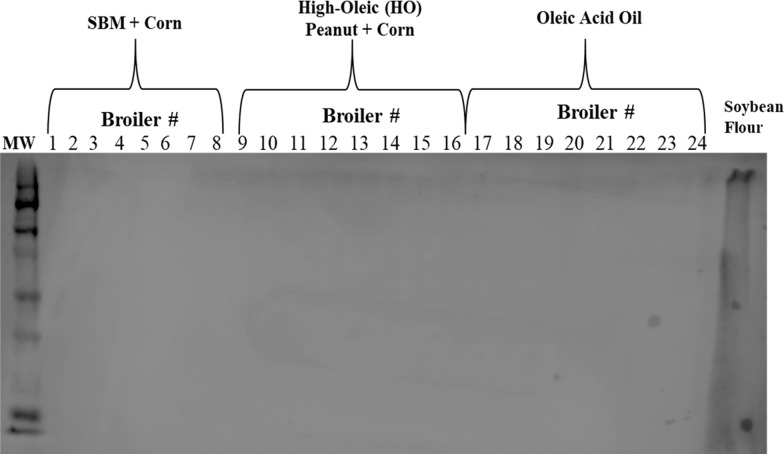
Subsequently, total protein extracts were immunoblotted with rabbit antisoy primary antibodies to determine the presence of soy peptides in the eggs or meat produced from layer hens and broilers, respectively, fed diets containing soybean meal. All egg (Figure 3) and chicken breast total protein samples (Figure 4) were nonreactive with rabbit antisoy primary antibodies, while reactive with soybean flour total protein extracts, thus suggesting that soy proteins cannot be detected in the eggs and/or meat produced by egg-producing layers or broiler chickens fed soy diets. In parallel, other studies have also demonstrated that peptide allergens such as peanuts are not detected in the eggs produced from layer hens fed experimental peanut diets for 10 weeks.16
Figure 3.
Immunoreactivity of protein extracts from pooled egg samples produced from layer hens fed a high-oleic peanut-containing diet for 8 weeks. Protein extracts from pooled egg samples (three replicates per treatment, with 10 eggs pooled per replicate) were run by SDS-PAGE on a 10% polyacrylamide gel with 75 μg of total protein per lane. Immunoreactivity of resolved proteins was determined by immunoblotting with nitrocellulose membrane and rabbit IgG antisoy antibodies (1:1000) and detection using chromogenic peroxidase substrate with HRP activity. Soybean flour extracted proteins (75 μg) = positive control.
Figure 4.
Immunoreactivity of protein extracts from chicken breast samples produced from broilers fed a high-oleic peanut-containing diet for 6 weeks. Protein extracts from chicken breast samples (eight samples per treatment) were run on a 10% SDS-PAGE l with 75 μg of total protein per lane. Resolved proteins were immunoblotted using nitrocellulose membrane and rabbit IgG antisoy antibodies (1:1000) and detection with chromogenic peroxidase substrate and HRP activity. Soybean flour extracts (75 μg) = positive control.
Notwithstanding, previous studies (Armentia et al., 2006) reported hypersensitivity responses in A. simplex-sensitized patients by allergens found in meat consumed from chickens fed fishmeal diets containing A. simplex allergenic peptides.12 While Armentia et al. (2006) focused on the transfer of A. simplex larval peptides from poultry diets to the meat produced, we aimed to investigate the transfer of immunoreactive proteins found in feedstock rations of poultry to the meat or eggs produced. Moreover, we aimed to address the safety concerns of food-allergic consumers regarding the potential transfer of allergenic peptides from animal feed to the food produced for human consumption using immunoblotting techniques with antigen-specific antibodies for detection.
While the United States Food and Drug Administration (U.S. FDA) has not established allergen reference doses for precautionary food labeling within manufacturing facilities, the Allergen Bureau, a nonprofit international industry-based organization, started by food industry within Australia and New Zealand issued the voluntary incidental trace allergen labeling (VITAL) initiative.17 The VITAL initiative provided food manufacturers’ with (1) defined allergen reference doses, (2) manufacturing allergen sanitation methods and risk management strategies, and (3) consistent precautionary labeling of food allergens of packaged food products.18,19 In March 2006 (U.S. FDA, 2006), The Allergen Threshold Working Group at the Center for Food Safety and Applied Nutrition posted a report regarding food allergen thresholds and that “The Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA), requiring the label of a food product that “is” or “contains” an ingredient of the eight major known food allergens, declare the presence of that allergen within the product labeling”.20 The report posted summarized the scientific knowledge of food allergen research in a dose–response relationship and highlighted the strengths and weaknesses of each study. However, no conclusive allergen reference doses were identified in this summary report, and any decisions for establishing thresholds for food allergens would require additional factors not covered to date (July 16, 2018), and therefore The Allergen Threshold Working Group has yet to make a decision on the establishment of food allergen thresholds until present.20
In the VITAL initiative, food products containing less than 2 ppm of peanut proteins or less than 10 ppm of soy proteins do not require declaration of allergens in food labeling; however, food products containing 2–20 ppm of peanut proteins or 10–100 ppm of soy proteins must be labeled declaring “trace” amounts or “may be present” of these allergens within the food labeling.17 Therefore, in this study, we aimed to define the number of allergens detected in pooled egg samples and individual meat samples based upon the VITAL reference doses,17 using enzyme-linked immunosorbent assay (ELISA) methods and to also validate the immunoblotting results of this study.
This study determined that pooled egg and individual meat samples had less than an average of 2 ppm (mg/kg) of peanut proteins and per the VITAL reference doses defined as not containing peanut allergens, which validates the immunoblotting results of this study (Table 4). Interestingly, pooled egg samples from layers fed the control diet contained an average of 0.1 ppm of peanut proteins, in contrast to 0 ppm of peanut protein in eggs produced from layer hens fed the high-oleic peanut and oleic acid (OA) diet, suggesting that pooled egg samples from hens fed the control diet may have contained minute amounts of peanut proteins due to laboratory cross-contamination during assay methods or handling (Table 4). Pooled egg and individual meat samples were determined by ELISA to have less than an average of 4 ppm of soy proteins and per the VITAL reference doses defined as not containing soy allergens, which also validate the immunoblotting results of this study (Table 4). In summary, this study demonstrates that allergenic proteins in the feed of broiler chickens and layer hens are not detected in the meat and/or eggs produced, which parallels previous high-oleic peanut-feeding studies with egg-producing layer hens.16 Therefore, this study helps to evaluate the associated health risk to the food-allergic consumer of meat or eggs produced from poultry fed diets containing allergenic food proteins.
Table 4. Composition of Three Experimental Grower (15–42 days) Dietsa for Meat-Type Chickens.
| % |
|||
|---|---|---|---|
| ingredient | controlb | HO PNc | OAd |
| corn, yellow | 56.75 | 56.9 | 60.48 |
| wheat, middlings | 0.98 | 2.40 | 4.20 |
| soybean meal | 26.20 | 18.4 | 25.30 |
| high-oleic peanut | 0.0 | 12.0 | 0.0 |
| oleic acid oil | 0.00 | 0.00 | 6.0 |
| poultry meal | 7.50 | 7.50 | 7.50 |
| poultry fat | 6.09 | 0.00 | 0.00 |
| salt | 0.3 | 0.2 | 0.3 |
| limestone | 0.8 | 0.8 | 0.8 |
| sodium bicarbonate | 0.0 | 0.2 | 0.0 |
| di-calcium phosphate | 0.79 | 0.87 | 0.78 |
| dl-methionine | 0.21 | 0.24 | 0.22 |
| l-lysine | 0.005 | 0.15 | 0.02 |
| l-threonine | 0.0 | 0.0 | 0.0 |
| choline chloride | 0.10 | 0.10 | 0.10 |
| vitamin premixe | 0.05 | 0.05 | 0.05 |
| mineral premixf | 0.20 | 0.20 | 0.20 |
| selenium premixg | 0.05 | 0.05 | 0.05 |
Isocaloric (3190 kcal/kg of energy), isonitrogenous (21% protein).
Conventional corn + soybean.
Unblanched (peanut skin intact) raw high-oleic peanuts + corn.
Control diet spiked with 6.0% oleic fatty acid oil.
Vitamin premix supplied the following per kg of diet: 13 200 IU vitamin A, 4000 IU vitamin D3, 33 IU vitamin E, 0.02 mg vitamin B12, 0.13 mg biotin, 2 mg menadione (K3), 2 mg thiamine, 6.6 mg riboflavin, 11 mg d-pantothenic acid, 4 mg vitamin B6, 55 mg niacin, and 1.1 mg folic acid.
Mineral premix supplied the following per kg of diet: manganese, 120 mg; zinc, 120 mg; iron, 80 mg; copper, 10 mg; iodine, 2.5 mg; and cobalt.
1 mg selenium premix provided 0.2 mg Se (as Na2SeO3) per kg of diet.
Conclusions
In summary, these results imply that peanut proteins found in peanut poultry diets were not detected in the eggs or meat produced from layers or broilers and therefore would not be expected to elicit an allergic response in peanut-sensitized individuals. Other work has revealed that a substantial portion of incoming dietary proteins in the diets of monogastric animals (piglets and humans) are digested during metabolism within the gut mucosa, with the aid of digestive enzymes secreted from chief cells, pancreas, and intestinal cells,21,22 and thus do not remain intact as polypeptide chains upon transport from the intestine to the use within the tissues for reproduction, growth, development, repair, or maintenance.22 Regardless, it is common knowledge that many allergenic food proteins are stable to digestive proteases and acid denaturation and have an increased likelihood of reaching the intestinal mucosa unchanged where absorption might occur, and these allergenic proteins could potentially be incorporated intact within the tissues or found within the products of production animals fed diets with allergenic proteins. Nonetheless, this study indicates that allergenic proteins found in the diets of production animals were not detected in the products produced using immunoblotting or ELISA detection methods. As a consequence, feeding high-oleic peanuts as a poultry feed ingredient to egg-producing hens and meat-type chickens does not appear to pose a public health issue for the peanut-sensitized consumer (Table 1).
Table 1. Detectiona of Peanut and/or Soy Proteins in Meat or Eggs Produced by Broilers or Layer Hens Fedb One of Three Experimental Diets Including a High-Oleic Peanut Diet.
| pooled
egg samplesc | ||
|---|---|---|
| protein allergen | peanut contentd | soy contentd |
| treatment group | ppm (mg/kg) | |
| controle | 0.11 ± 0.08 | 3.34 ± 1.23 |
| HO PNf | 0.00 ± 0.00 | 3.14 ± 0.15 |
| OAg | 0.00 ± 0.00 | 3.02 ± 0.25 |
| meat
samplesh | ||
|---|---|---|
| treatment group | ppm (mg/kg) | |
| controle | 1.66 ± 0.46 | 0.050 ± 0.01 |
| HO PNf | 1.35 ± 0.55 | 0.083 ± 0.03 |
| OAg | 1.28 ± 0.26 | 0.136 ± 0.03 |
| VITALi threshold | ≥2 ppm | ≥10 ppm |
By enzyme-linked immunosorbent assay (ELISA) (RIDASCREEN FAST peanut and RIDASCREEN FAST soya allergen)(R-Biopharm, Darmstadt, Germany).
For 6 weeks.
Total protein extracts, three replicates per treatment, 10 pooled eggs per replicate.
Average ± standard error.
Conventional soybean meal + corn diet for broilers and layers.
Unblanched (peanut skin intact) raw high-oleic peanuts + corn diet.
Control diet spiked with oleic fatty acid oil.
Total protein extracts chicken breast samples, eight replicates per treatment.
Voluntary incidental trace allergen labeling.
Materials and Methods
Layer Hen-Experimental Design, Animal Husbandry, and Dietary Treatments
With 57 week-old Leghorn hens, we compared three isonitrogenous (18% protein), isocaloric (3080 kcal/kg metabolizable energy) dietary treatments (Table 2) prepared 2 weeks prior to the onset of the study and maintained in feed storage bins in NCSU Feed Mill in a cool dry location. The control diet layer diet was a conventional mash soybean meal + corn diet (control) composed predominately of yellow corn, corn gluten, soybean meal, wheat bran, and vegetable oil, with NCSU vitamin, mineral, and selenium premix (Table 2). Treatment 2 was a high-oleic peanut + corn diet prepared using aflatoxin-free whole nonroasted unblanched shelled high-oleic peanuts. Prior to inclusion within the diet, whole raw high-oleic peanuts were crushed using a Roller Mill. The high-oleic peanut + corn diet (HO PN) was composed predominately of yellow corn, corn gluten, raw whole high-oleic peanuts, and wheat bran, supplemented with amino acids l-lysine, dl-methionine, l-tryptophan, and l-threonine along with NCSU vitamin, mineral, and selenium premix. The oleic fatty oil diet (OA) was prepared using the control diet supplemented with 2.64% (% by weight) food-grade oleic fatty acid oil (Millipore Sigma, Burlington, MA). Experimental diets were analyzed and determined to be free of aflatoxin and microbiological contaminants by the North Carolina Department of Agriculture and Consumer Services, Food and Drug Protection Division Laboratory (Raleigh, NC).
Table 2. Composition of Three Experimental Dietsa for Egg-Producing Hens.
| % (by
weight) |
|||
|---|---|---|---|
| ingredient | soybean meal + corn (control)b | high-oleic peanut + corn (HO PN)c | oleic acid oil (OA)d |
| corn, yellow | 46.38 | 39.00 | 52.28 |
| corn gluten meal | 5.00 | 10.35 | 5.00 |
| soybean meal | 21.40 | 0.00 | 20.35 |
| high-oleic peanut | 0.00 | 20.00 | 0.00 |
| oleic acid oil | 0.00 | 0.00 | 2.64 |
| vegetable fat | 7.80 | 0.00 | 0.00 |
| wheat bran | 6.00 | 16.80 | 6.0 |
| calcium carbonate | 10.90 | 10.80 | 11.30 |
| di-calcium phosphorus | 1.60 | 1.41 | 1.51 |
| salt, plain | 0.25 | 0.25 | 0.25 |
| dl-methionine | 0.10 | 0.08 | 0.10 |
| l-lysine | 0.00 | 0.53 | 0.00 |
| l-tryptophan | 0.00 | 0.03 | 0.00 |
| l-threonine | 0.00 | 0.13 | 0.00 |
| choline chloride | 0.17 | 0.22 | 0.17 |
| MYC-Out 65 | 0.05 | 0.05 | 0.05 |
| NCSU vitamin premixe | 0.10 | 0.10 | 0.10 |
| NCSU trace mineral mixf | 0.20 | 0.20 | 0.20 |
| NCSU selenium mixg | 0.05 | 0.05 | 0.05 |
Isocaloric (3080 kcal/kg), isonitrogenous (18% protein).
Conventional corn + soybean.
Unblanched (peanut skin intact) raw high-oleic peanuts (HO PN) + corn.
Control diet spiked with 2.64% oleic fatty acid oil.
Vitamin premix supplied the following per kg of diet: 13 200 IU vitamin A, 4000 IU vitamin D3, 33 IU vitamin E, 0.02 mg vitamin B12, 0.13 mg biotin, 2 mg menadione (K3), 2 mg thiamine, 6.6 mg riboflavin, 11 mg d-pantothenic acid, 4 mg vitamin B6, 55 mg niacin, and 1.1 mg folic acid.
Mineral premix supplied the following per kg of diet: manganese, 120 mg; zinc, 120 mg; iron, 80 mg; copper, 10 mg; iodine, 2.5 mg; and cobalt.
1 mg selenium premix provided 0.2 mg Se (as Na2SeO3) per kg of diet.
Ninety-nine 57 week-old Brown Leghorn layer hens (NCSU-University-maintained poultry flock) were randomly assigned to 33 animals per treatment group with three replicates/treatment of 11 animals per replicate. Animals were housed individually in conventional layer batter cages in one room. Animals were provided experimental feed and water ad libitum and provided with 14 h of light daily for 8 weeks. The Institutional Animal Care and Use Committee at North Carolina State University (Raleigh, NC) approved all experimental animal protocols (approved protocol 17-001-A) prior to the onset of this study. Egg samples were collected and pooled per replicate at termination (week 8) of the study and subsequently used for protein extraction. Egg samples were pooled in a sterile Whirl-pak bag (Thermo Fisher Scientific, Rockford, IL) and homogeneously mixed using a Stomacher Lab Blender.
Broiler Meat-Type Chicken-Experimental Design, Animal Husbandry, and Dietary Treatments
On the day of hatch, male broiler chicks (Ross 708) were randomly placed into 30 wire battery cages with 10 birds per cage (N = 100). Broiler chicks were randomly assigned and fed ad libitum one of three isocaloric, isonitrogenous dietary treatments with a starter diet (3120 kcal/kg, 23% protein) from days 0 to 14 and a grower diet (3190 kcal/kg, 21% protein) from days 15 to 42 (Table 3, starter diet; Table 4, grower diet). Antioxidant, santoquin (Novus International, Saint Charles, MO), was added (1%) to the diets to prevent rancidity. Broilers were fed a conventional soybean meal and corn control diet, or a high-oleic peanut and corn diet (HO PN) or a control diet spiked with oleic fatty acid oil (OA) for 6 weeks.
Table 3. Composition of Three Experimental Starter (0–14 days) Dietsa for Meat-Type Chickens.
| % |
|||
|---|---|---|---|
| ingredient | controlb | HO PNc | OAd |
| corn, yellow | 52.5 | 53.86 | 55.72 |
| wheat, middlings | 0.13 | 0.00 | 3.10 |
| soybean meal | 32.5 | 26.2 | 31.80 |
| high-oleic peanut | 0.0 | 10.2 | 0.0 |
| oleic acid oil | 0.00 | 0.00 | 5.50 |
| poultry meal | 6.0 | 6.0 | 6.0 |
| poultry fat | 5.50 | 0.00 | 0.00 |
| salt | 0.3 | 0.2 | 0.3 |
| limestone | 0.9 | 0.9 | 0.9 |
| sodium bicarbonate | 0.1 | 0.3 | 0.1 |
| di-calcium phosphate | 0.97 | 1.05 | 0.96 |
| dl-methionine | 0.37 | 0.39 | 0.37 |
| l-lysine | 0.22 | 0.35 | 0.23 |
| l-threonine | 0.11 | 0.15 | 0.12 |
| choline chloride | 0.10 | 0.10 | 0.10 |
| vitamin premixe | 0.05 | 0.05 | 0.05 |
| mineral premixf | 0.20 | 0.20 | 0.20 |
| selenium premixg | 0.05 | 0.05 | 0.05 |
Isocaloric (3080 kcal/kg), isonitrogenous (18% protein).
Conventional corn + soybean.
Unblanched (peanut skin intact) raw high-oleic peanuts + corn.
Control diet spiked with 5.5% oleic fatty acid oil.
Vitamin premix supplied the following per kg of diet: 13 200 IU vitamin A, 4000 IU vitamin D3, 33 IU vitamin E, 0.02 mg vitamin B12, 0.13 mg biotin, 2 mg menadione (K3), 2 mg thiamine, 6.6 mg riboflavin, 11 mg d-pantothenic acid, 4 mg vitamin B6, 55 mg niacin, and 1.1 mg folic acid.
Mineral premix supplied the following per kg of diet: manganese, 120 mg; zinc, 120 mg; iron, 80 mg; copper, 10 mg; iodine, 2.5 mg; and cobalt.
1 mg selenium premix provided 0.2 mg Se (as Na2SeO3) per kg of diet.
The HO PN diet was prepared using peanut crumbles prepared from aflatoxin-free whole nonroasted unblanched high-oleic peanuts. The oleic-fatty-acid-oil-supplemented (6%) diet (OA) was prepared by supplementing the control diet with 6% food-grade oleic fatty acid oil (Millipore Sigma, Burlington, MA). Experimental diets were analyzed and determined to be free of aflatoxin and microbiological contaminants by the North Carolina Department of Agriculture and Consumer Services, Food and Drug Protection Division Laboratory (Raleigh, NC).
At termination, one bird from each of 10 pens for each treatment group was selected for processing (N = 30). Broilers with an individual body weight within one-half standard deviation from the experimental mean body weight were selected for processing. Birds were removed from feed only 10 h prior to termination and processing. The left pectoralis major sections were removed from each bird and chilled in an ice bath for 4 h and subsequently sealed and stored at −80 °C until protein extraction.
Protein Extraction, SDS-PAGE, and Immunoblotting Analysis
A 1 mg section of the left pectoralis major breast muscle from eight broiler chickens and a 1 mL sample of pooled shell egg samples were utilized for protein extraction. Total protein extraction, SDS-PAGE analysis, immunoblotting, and detection techniques were followed using methods described by Toomer et al. (2019) with a rabbit IgG antipeanut agglutinin primary antibody 1:1000 dilution (Lifespan Biosciences, Seattle, WA) or rabbit antisoy primary antibody 1:1000 dilution (Sigma-Aldrich, St. Louis, MO) and donkey antirabbit IgG-HRP (Santa Cruz Biotechnology, Dallas, TX).16
Enzyme-Linked Immunosorbent Assay (ELISA) Food Allergen Detection
ELISA assays were performed for the detection of peanut and soy allergens using total protein extracts from chicken breast and pooled egg samples in triplicate with the RIDASCREEN FAST Peanut and RIDASCREEN FAST Soya allergen detection kits per the manufacturer’s instructions (R-Biopharm, Darmstadt, Germany).
Acknowledgments
This work was supported by funds from the North Carolina Peanut Growers Association (Funding Source 572099-87361) and the Market Quality & Handling Research Unit-Agricultural Research Service-United States Department of Agriculture (CRIS Project Number 6070-43440-011-00D). The authors would gratefully like to acknowledge the following: students and staff of the Prestage Department of Poultry Science-North Carolina State University, staff of North Carolina State University Feed Mill, Birdsong Peanuts for donation of high-oleic peanuts, and the students and staff of the Market Quality & Handling Research Unit-ARS for their contributions to this study. Photographs of chicken breast, eggs, whole chickens, and feed samples are courtesy of Ondulla Toomer; the image of whole soybeans is within the public domain (amazon advertisement), and the image of peanuts is within the public domain (peanut clipart image collection).
The authors declare no competing financial interest.
References
- Gupta R. S.; Warren C. M.; Smith B. M.; Jiang J.; Blumenstock J. A.; Davis M. M.; Schleimer R. P.; Nadeau K. C. Prevalence and Severity of Food Allergies among US Adults. JAMA Network Open 2019, 2, e185630 10.1001/jamanetworkopen.2018.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Muhsen S.; Clarke A. E.; Kagan R. S. Peanut allergy: an overview. Can. Med. Assoc. J. 2003, 168, 1279–1285. [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S.; Warren C. M.; Smith B. M.; Blumenstock J. A.; Jiang J.; Davis M. M.; Nadeau K. C. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 2018, 142, e20181235 10.1542/peds.2018-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARP (Food Allergy Research and Resource Program). Allergenic Foods and their Allergens. https://farrp.unl.edu/informallbig8 (accessed July 31, 2019).
- Clark S.; Espinola J.; Rudders S. A.; Banerji A.; Camargo C. A. Frequency of US emergency department visits for food-related acute allergic reactions. J. Allergy Clin. Immunol. 2011, 127, 682–683. 10.1016/j.jaci.2010.10.040. [DOI] [PubMed] [Google Scholar]
- Taylor S. L.; Hefle S. L. Food allergen labeling in the USA and Europe. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 186–190. 10.1097/01.all.0000225158.75521.ad. [DOI] [PubMed] [Google Scholar]
- Jackson L. S.; Al-Taher F. M.; Moorman M.; DeVries J. W.; Tippett R.; Swanson K. M.; Fu T. J.; Salter R.; Dunaif G.; Estes S.; Albillos S.; Gendel S. M. Cleaning and other control and validation strategies to prevent allergen cross-contact in food-processing operations. J. Food Prot. 2008, 71, 445–458. 10.4315/0362-028X-71.2.445. [DOI] [PubMed] [Google Scholar]
- Röder M.; Baltruweit I.; Gruyters H.; Ibach A.; Mücke I.; Matissek R.; Vieths S.; Holzhauser T. Allergen sanitation in the food industry: a systematic industrial scale approach to reduce hazelnut cross-contamination of cookies. J. Food Prot. 2010, 73, 1671–1679. 10.4315/0362-028X-73.9.1671. [DOI] [PubMed] [Google Scholar]
- Fæste C. K.; Jonscher K. R.; Dooper M. M.; Egge-Jacobsen W.; Moen A.; Daschner A.; Egaas E.; Christians U. Characterization of potential novel allergens in the fish parasite Anisakis simplex. EuPa Open Proteomics 2014, 4, 140–155. 10.1016/j.euprot.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo M. D. D.; Audícana M.; Diez J. M.; Munoz D.; Ansotegui I. J.; Fernández E.; García M.; Etxenagusia M.; Moneo I.; Fernández de Corres L. Anisakis simplex, a relevant etiologic factor in acute urticaria. Allergy 1997, 52, 576–579. 10.1111/j.1398-9995.1997.tb02603.x. [DOI] [PubMed] [Google Scholar]
- Aibinu I. E.; Smooker P. M.; Lopata A. L. Anisakis Nematodes in Fish and Shellfish- from infection to allergies. Int. J. Parasitol. 2019, 9, 384–393. 10.1016/j.ijppaw.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentia A.; Martin-Gil F. J.; Pascual C.; Martín-Esteban M.; Callejo A.; Martínez C. Anisakis simplex allergy after eating chicken meat. J. Invest. Allergol. Clin. Immunol. 2006, 16, 258–263. [PubMed] [Google Scholar]
- Miles R. D.; Jacob J. P.. Fishmeal in Poultry Diets: Understanding the Production of this Valuable Feed Ingredient; Animal Science Department, Florida Cooperative ExtensionService, Institute of food and Agricultural Sciences, University of Florida, 2003. http://edis.ifas.ufl.edu (accessed July 31, 2019). [Google Scholar]
- Moneo I.; Caballero M. L.; Gonzaez-Muñoz M.; Rodriguez-Mahillo A. I.; Rodriguez-Perez R.; Silva A. Isolation of a heat-resistant allergen from the fish parasite Anisakis simplex. Parasitol. Res. 2005, 96, 285–289. 10.1007/s00436-005-1362-2. [DOI] [PubMed] [Google Scholar]
- Caballero M. L.; Moneo I. Several allergens from Anisakis simplex are highly resistant to heat and pepsin treatments. Parasitol. Res. 2004, 93, 248–251. 10.1007/s00436-004-1099-3. [DOI] [PubMed] [Google Scholar]
- Toomer O. T.; Hulse-Kemp A. M.; Dean L. L.; Boykin D. L.; Malheiros R.; Anderson K. E. Feeding high-oleic peanuts to layer hens enhances egg yolk color and oleic fatty acid content in shell eggs. Poult. Sci. 2019, 98, 1732–1748. 10.3382/ps/pey531. [DOI] [PubMed] [Google Scholar]
- Allergen Bureau. Seventh Workshop on Food Allergen Methodologies. http://allergenbureau.net/wp-content/uploads/2014/03/VITAL-a-threshold-approach-to-allergen-labelling.pdf (accessed July 29, 2013).
- Allen K. J.; Remington B. C.; Baumert J. L.; Crevel R. W.; Houben G. F.; Brooke-Taylor S.; Kruizinga A. G.; Taylor S. L. Allergen reference doses for precautionary labeling (VITAL 2.0): clinical implications. J. Allergy Clin. Immunol. 2014, 133, 156–164. 10.1016/j.jaci.2013.06.042. [DOI] [PubMed] [Google Scholar]
- Taylor S. B.; Christensen G.; Grinter K.; Sherlock R.; Warren L. The Allergen Bureau VITAL Program. J. AOAC Int. 2018, 101, 77–82. 10.5740/jaoacint.17-0392. [DOI] [PubMed] [Google Scholar]
- U.S. FDA. The Allergen Threshold Working Group. Approaches to Establish Thresholds for Major Food Allergens and for Gluten in Food. https://www.fda.gov/food/food-labeling-nutrition/approaches-establish-thresholds-major-food-allergens-and-gluten-food (accessed Nov 25, 2006).
- Stoll B.; J. Henry J.; Reeds P. J.; Yu H.; Jahoor F.; Burrin D. G. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J. Nutr. 1998, 128, 606–614. 10.1093/jn/128.3.606. [DOI] [PubMed] [Google Scholar]
- Tomé D.; Bos C. Dietary protein and nitrogen utilization. J. Nutr. 2000, 130, 1868S–1873S. 10.1093/jn/130.7.1868S. [DOI] [PubMed] [Google Scholar]