Abstract

DNA molecular compaction/decompaction is of great significance for the exploration of basic life processes, the research of biomedical and genetic engineering, and so forth. However, the detailed mechanism of DNA compaction/decompaction caused by surfactants remains an open and challenging problem that has not been fully solved so far. In this paper, a sort of novel solid substrate, nanoPAA-ZnCl2-AuLs, with good stability and high sensitivity, was prepared by a self-assembly method. Based on this substrate, the surface-enhanced Raman scattering (SERS) technology was employed to investigate characteristics of interactions between DNA molecules and surfactants at a single molecular level. SERS spectra of calf thymus DNA (ctDNA), cetyl trimethyl ammonium bromide (CTAB), and sodium dodecyl sulfate (SDS) with a concentration as low as 10–9 M, and SERS spectra of ctDNA–CTAB and ctDNA–CTAB–SDS composites were collected, respectively. The interactions between ctDNA and surfactants were analyzed by changes in SERS spectra, for example, disappearances and appearances of SERS bands and relative changes of peak intensity, in which CTAB resulted in the compaction of the DNA molecule while SDS induced the decompaction of the ctDNA–CTAB complex. Moreover, UV–visible spectrophotometry was employed to demonstrate the compaction/decompaction of ctDNA molecules caused by surfactants. The local binding modes of ctDNA molecules and surfactant molecules were expounded. This work will be helpful for understanding biological processes such as DNA compaction and recombination within nucleus or/and cells and for the development of gene therapy technologies.
1. Introduction
Exploring the conformation changes of DNA molecules in detail and revealing their mechanisms accurately, such as the compaction/decompaction of DNA molecules induced by the interaction of various surfactants, are both of great significance for expanding fields of basic research in molecular biology and developing practical technologies of biomedical engineering.
The fact that DNA molecules can be used as carriers of drugs1 or nonviral genes has received much attention.2,3 During the delivery process, DNA molecules are compacted into small particles under the action of surfactants, by which they are protected from the degradation of nucleases, and then they penetrate through the membrane and move inside the cell under external forces. Subsequently, the DNA complexes are decompacted to release the drugs/nonviral genes. With this method, the amount of effective drugs/non-viral genes absorbed by a cell will be substantially increased. This technique has been utilized for gene transfer, gene transfection, gene therapy, and so on,4−6 and this procedure has been employed as a typical model for DNA extraction7 and purification.8
In order to investigate the interaction between DNA molecules and surfactants, scientists have proposed many methods,9−13 including zeta potential measurement, dynamic light scattering measurement, agarose gel electrophoresis, conductivity measurement, tension measurement, circular dichroism, isothermal titration calorimetry, fluorescence imaging, and so forth. By evaluating the overall properties (e.g. electrical, optical, and mechanical) of surfactants and DNA molecules, interactions between surfactants and DNA molecules can be deduced. However, because detections are carried out on a large number of molecules with the concentration of ca. 10–5 M, these traditional technologies are only capable of reflecting average effect. Furthermore, it is required to use complex chemical methods to covalently connect optical or electroactive indicators to the specific sites of DNA molecules, which is complicated and difficult to operate.
The local binding pattern of surfactant to DNA molecule at single molecular level was unable to be directly determined until the 1990s. A variety of techniques have been developed to accurately explore the interaction information between DNA molecules and surfactants, that is optical tweezers, atomic force microscopy, magnetic tweezers, and other biological single molecule manipulation techniques.14−17 However, the measurement accuracies are severely affected by environmental conditions, that is temperature, mechanical vibration, airflow, and/or electronic noise.18 So far, it is still an openly challenging problem that needs a lot of efforts to elucidate the mechanism of surfactants-induced DNA molecular compaction and decompaction at a single-molecule level.
Raman scattering spectroscopy is one of the efficient technologies and has been used to study structural information of single biomolecules.19,20 Each molecule has its own unique vibrational modes (molecular vibration, lattice vibration, rotation, etc.),21 which can be identified by Raman scattering spectra. For example, peak position reflects the composition of the sample, peak shift reflects the tension or stress, peak intensity reveals the total amount of the substance, peak width expresses the quality, and Raman polarization reflects the symmetry and orientation of the crystal.22 By analyzing the Raman spectra, the information of molecular structures and slight variations can be distinctly obtained. However, the intensity of Raman spectra is much weaker than that of the Rayleigh spectra, only 10–3 to 10–6 times. Some molecular information cannot be revealed.
In recent years, surface-enhanced Raman scattering (SERS) technology has been rapidly developed and made good progress,23 the intensity of Raman spectra can be selectively enhanced by 106 times. SERS can investigate the sample structure at a single molecule level and explore the molecular interaction model. It has been used to study the configuration and conformation, and analyze the structure of biological molecules.24,25 As for the principle of SERS intensity enhancement, there are widely accepted explanations of physical and chemical mechanisms. The physical mechanism is that when a beam of laser excites the surface of SERS substrate, the surface plasmon resonance is generated, the local electric field intensity is increased, and the peak intensity of Raman spectra is enhanced in a large frequency range. The region where the local electric field intensity increases through surface plasmon resonance is defined as a hot spot. The main content of the chemical enhancement mechanism is that the chemical bond formed between the molecules absorbed onto the surface of the metal substrate and the metal surface can change the polarizability of the sample molecules, resulting in some selective enhancement of Raman peaks.
For SERS applications, selection of SERS-active substrates is crucial. Generally, SERS substrates can be divided into two categories according to the types of substrate materials. That is, one is metal nanoparticle sol,26,27 and the other is a solid nanostructure.28,29 About the former, the SERS measurement is performed by adding an analyte sample to a metal nanoparticle sol, and the nanoparticles are aggregated to generate an electromagnetic hot spot; thus, the SERS effect will be achieved. Applications of colloidal particles are generally limited to their irregular size and shape and uncontrollable aggregations. In addition, analytes are easily contaminated by nanoparticle sols, which would severely affect the accuracy of SERS detection. As for the latter, solid nanostructures may include nanostructures prepared directly on a solid substrate using nanofabrication methods, or assembly of metal nanoparticles on a solid substrate. SERS technology based on solid substrate has more advantages, for example, convenient operation, high stability, diversified forms, and controllable structure.
Our research group has developed multiple solid complex substrates.28,30 Here, the nanoporous anodic alumina (nanoPAA)-ZnCl2-AuLs substrate is first prepared and experimentally demonstrated that it can be capable of detecting rhodamine 6G (R6G) solution with a concentration as low as 10–11 M [Figure S1]. Compared with the Au nanoparticle film over a glass slide, the SERS intensity of R6G solution on nanoPAA-ZnCl2-AuLs substrates is enhanced by 7.8 times.31 Furthermore, nanoPAA-ZnCl2-AuLs composite as SERS substrate has more advantages such as great stability, uniformity, and reproducibility.
In this paper, based on the self-made nanoPAA-ZnCl2-AuLs substrate, the compaction of DNA under the action of cetyl trimethyl ammonium bromide (CTAB) and the decompaction of DNA–CTAB complex under the action of sodium dodecyl sulfate (SDS) were investigated by SERS. In addition, UV–visible spectrophotometry is also employed to expound the mechanism of action of DNA compaction/decompaction as a reference.
2. Experimental Section
2.1. Materials
Both CTAB (impurities ≤ 0.001% and assay ≥ 99%) and SDS (assay ≥ 98.5%), linear surfactant molecules, were purchased from Sigma-Aldrich Corporation, and their purity are analytical grade. Calf thymus DNA (ctDNA) was also purchased from Sigma-Aldrich Corporation. It can be found that the ratios of the absorption of the ctDNA solutions at 260–280 nm were more than 1.8, indicating proteins were absent. R6G was purchased from Aladdin Industrial Corporation.
During experiments, samples of ctDNA and surfactants were prepared at room temperature. ctDNA solutions were kept at a fixed concentration, that is, 10–7 M. According to different ratio, for example, CCTAB/CDNA = 5, various amounts of surfactants were mixed with DNA molecules in sample cells.
2.2. nanoPAA-ZnCl2-AuLs Solid-Stated Substrates
The preparation process of nanoPAA-ZnCl2-AuLs solid-stated substrates is described as shown in Figure 1A. First, nanoPAA membranes were made with standard two-step anodic oxidation method. Second, several drops of ZnCl2 solution with the concentration of 0.001 M were added onto the template. After that, the samples were stored at a certain temperature of 25 °C for 7 days. Then, ZnCl2 nanofilm was self-organized on the top surface of nanoPAA membrane. Finally, a gold layer with thickness of ca. 25 nm was deposited on the top surface by vacuum thermal evaporator and then sprinkled a layer of gold nanoparticles with 50 nm diameter.
Figure 1.
nanoPAA-ZnCl2-AuLs solid substrate. (A) A diagram sketch of the preparation process; (B) top surface of the nanoPAA membrane; (C) nanoPAA-ZnCl2 composite film, the dotted ellipse marks the nanoPAA; (D) nanoPAA-ZnCl2-AuL composite film.
Figure 1B shows a typical scanning electron microscopy (SEM) image of nanoPAA membrane. Figure 1C displays the substrate of nanoPAA-ZnCl2 composite film, in which the nanoPAA membrane marked with dotted ellipse can be clearly observed. Figure 1D shows a SEM image of nanoPAA-ZnCl2 coated by 25 nm Au layer. During experiments, the nanoPAA-ZnCl2-AuLs substrate was fixed on a clean glass slides first, and then a certain amount of sample solution was transferred onto the solid substrate by a transfer liquid gun.
R6G, one of the most commonly used probe molecules, was employed to determine SERS enhancement factors (EFs) and reproducibility of the substrate because of its high adsorption energy yielding Raman enhancement.
About the SERS EFs, the spectra of 10–9 M and 100 mM R6G were collected on the nanoPAA-ZnCl2-AuLs substrate and the quartz slide (Figure S2), respectively. After a simple calculation, the EF of the SERS-active substrate was 9.18 × 107 at a Raman shift of 1313 cm–1.
For reproducibility of nanoPAA-ZnCl2-AuLs substrates, the SERS spectra of 10–9 M R6G were measured at 9 differently located spots randomly under the same conditions (Figure S3). Experimental data revealed that all the deviations are less than 20%, indicating the substrate has good reproducibility and is suitable for SERS applications.
2.3. Characterization
The SERS spectra were measured by an confocal Raman microscopy system (Alpha 500R, WITec, Germany) with a 532 nm laser sources (here, only used for the Raman spectrum of DNA fiber), 633 nm (beam size about ca. 1 μm, objective with 20×), and a EMCCD (UHTS 300). SERS spectra were collected with the Raman shifts ranging from 0 to 2750 cm–1 with a 600 g mm–1 grating. The spectra were processed by WITec Project Four software.
The SERS spectra of ctDNA, CTAB, SDS, DNA–CTAB complex solution, and DNA–CTAB–SDS complex solution were separately detected on different pieces cut from an identical substrate. After a 10 μL droplet of solution was dripped onto the surface of the substrate and was dried at room temperature, molecules were completely adsorbed for SERS measurements.
In addition, UV–vis spectrophotometry (DS-11, DeNovix, USA) was used to collect the spectra of UV absorption of the sample solution. The spectra were recorded in a range of wavelength from 220 to 350 nm.
For the DNA molecule compaction, a series of CTAB were added into the DNA solutions with different concentration ratios of CTAB to DNA of 0, 0.1, 1, 3, 5, 8, 100, and 1000. About 3 μL of DNA–CTAB complex solution was taken for UV absorption spectrum after cultivated for 24 h. As for the DNA molecule decompaction, SDS surfactants were added into the DNA–CTAB complex with different concentration ratios of SDS to CTAB of 0, 0.02, 0.2, 1, and 20. About 3 μL of DNA–CTAB–SDS solution was taken for UV absorption spectrum after cultivated for 24 h.
3. Results and Discussion
Based on the self-made nanoPAA-ZnCl2-AuLs substrates, both the compression of ctDNA molecules caused by surfactant CTAB and the decompression of ctDNA molecules caused by surfactant SDS were investigated by SERS technology under room temperature. All of the following measurements were repeated more than 3 times.
3.1. SERS Spectra
3.1.1. ctDNA Molecule
Figure 2 displays Raman spectra of ctDNA molecules. Figure 2A shows typical Raman spectrum of ctDNA fiber and SERS spectrum of ctDNA solution with concentration of 10–8 M; Figure 2B shows SERS spectrum of ctDNA molecule with a low concentration of 10–9 M, the illustration in the upper left corner is the chemical molecular structure of ctDNA molecule.
Figure 2.
(A) Raman spectrum of ctDNA fiber and SERS spectrum of ctDNA solution (10–8 M); and (B) SERS spectrum of ctDNA solution (10–9 M), the inset is the molecular structure of ctDNA.
From Figure 2A, it can be observed clearly that the main characteristic Raman peaks of ctDNA are at 673, 733, 784, 807, 980, 1013, 1092, 1106, 1156, 1183, 1251, 1304, 1336, 1381, 1422, 1470, 1485, 1510, and 1570 cm–1. These characteristic peaks reveal structural information of DNA molecule illustrated as follows.32−34 The bands at 673 cm–1 is assigned to thymine (T), and the 733 cm–1 is assigned to the symmetric stretching vibration of adenine (A). The 784 cm–1 is related to the symmetric stretching vibration of phosphodiester O–P–O. The 807 cm–1 is designated as the main chain vibration mode of the phosphate. The 980 cm–1 is assigned to deoxyribose. The 1013 cm–1 is the contribution of C–O stretching vibration of deoxyribose. The 1092 cm–1 is attributed to the symmetric stretching vibration of O=P=O in PO2–. The 1106 cm–1 is attributed to the stretching vibration of O–P–O in the phosphate. The band at 1156 cm–1 is attributed to the phosphate skeleton vibration mode, the band at 1183 cm–1 is assigned to C–N stretching outside the base, and the band at 1251 cm–1 is attributed to adenine (A) and cytosine (C). The bands at 1304, 1336, and 1510 cm–1 are all attributed to the vibration of adenine (A). The 1381 cm–1 is assigned to thymine (T), adenine (A), and guanine (G), while the bands at 1422, 1470, 1485, and 1570 cm–1 are attributed to adenine (A) and guanine (G). It can be noted that the SERS intensity of 10–8 M DNA solution at 1323, 1381, 1470, 1485, and 1570 cm–1 are significantly increased compared with the rest of the bands. These bands are attributed to the characteristic peaks of bases and deoxyribose. These characteristic Raman peaks are usually undetectable when the concentration of DNA solution is extremely low, reaching the level of a single molecule. Under the effect of hotspots, the SERS intensity of DNA molecule is considerably increased.
In Figure 2B, the concentration of ctDNA solution was decreased to 10–9 M. the SERS intensity at 1510 cm–1 is significantly increased compared with the rest of the bands in 10–9 M ctDNA solution. It has been proved that spectral quality and reproducibility may be severely influenced by the large changes of the conformation of DNA molecules and/or the bulk density of DNA molecules on the substrate in previous studies.35 The vibration mode of the adsorbed molecule has a large polarization component perpendicular to the SERS substrate,36 which results in efficient enhancement of the Raman peaks. The strong intensity enhancement of peak at 1510 cm–1 can be assigned to the orientation of the adenine (A) ring plane of the ctDNA molecule that maybe perpendicular to the Au surfaces.37 Therefore, it can be deduced that DNA molecules are absorbed on the substrate surface via the chemisorption of adenine (A).
3.1.2. Surfactant CTAB
CTAB is a typical cationic surfactant with good coordination with anionic, nonionic, and amphoteric surfactants. It is an amphiphilic molecular system with a hydrophobic chain at one end and a hydrophilic polar head at the other end. Generally, the hydrophilic polar head is defined as the CTAB head, and the hydrophobic chain is defined as the CTAB tail. Some research groups38 have studied the conformational changes of DNA molecules caused by CTAB with various techniques. However, the detailed mechanism and the sites acting on DNA molecules are still not fully understood.
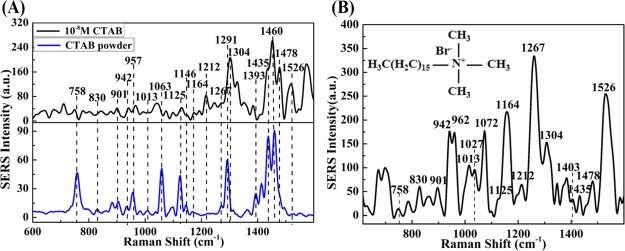
Figure 3 shows Raman spectra of CTAB surfactant. Figure 3A shows a typical Raman spectrum of the CTAB powder and SERS spectrum of CTAB solution with a concentration of 10–8 M; Figure 3B shows SERS spectrum of CTAB molecule solution with a lower concentration of 10–9 M, the illustration in the upper left corner is the chemical molecular structure of CTAB.
Figure 3.
(A) Raman spectrum of CTAB powder and SERS spectrum of CTAB solution (10–8 M); and (B) SERS spectrum of CTAB solution (10–9 M), the inset is the molecular structure of CTAB.
From Figure 3A, it can be seen clearly that the characteristic Raman peaks of CTAB mainly appear at 758, 830, 901, 942, 957, 1013, 1063, 1125, 1146, 1164, 1212, 1267, 1291, 1393, 1435, 1460 cm–1, and so forth. These characteristic peaks can reflect structural information of CTAB molecular illustrated as follows.39−42 The 758 cm–1 is attributed to methyl rocking vibration from the (CH3)3N+ group. The bands at 830 cm–1 corresponds to the CH3 deformation. The 901, 942, and 957 cm–1 bands probably correspond to methyl rocking vibration from the (CH3)3N+ group and C–N+ stretching modes. The bands at 1013, 1063, and 1125 cm–1 are attributed to the C–C stretching vibration. The 1146 cm–1 band is assignable to C–C antisymmetric stretch. The band at 1164 cm–1 has its origin in the CH2 rocking mode, and the 1212 cm–1 is attributed to the CH2 wag vibration. The strongest line at 1267 cm–1 is assigned to the δ(C–H) vibrations of the −CH2–N+(CH3)3 group. The 1291 cm–1 is assigned to the CH2 twisting, while the bands at 1393 cm–1 is assigned to the wag vibration of CH2 and the deformation vibration of CH3. The band at 1435 cm–1 is attributed to CH2 bending vibration, and the 1460 cm–1 is assigned to CH2 scissors. It can be noted that there are new bands at 1478 and 1526 cm–1, and the SERS intensity at of bands 1212, 1304, 1435, and 1460 cm–1 are significantly increased compared with the rest of the bands in 10–8 M CTAB solution. Except for the case of 1526 cm–1, which can be assigned to the bending of methyl group in (CH3)3N+ group, the rest are characteristic peaks of CH2 in the structure.
In Figure 3B, the SERS bands of 10–9 M CTAB solution are the same as most bands shown in Figure 3A, while there is a new Raman peak at 1027 cm –1 related to C–C band. In addition, the SERS intensity of at 942, 962, 1164, 1267, and 1526 cm–1 are significantly increased compared with the rest of the bands in 10–9 M CTAB solution. The sharply enhancement at 942, 962, 1267, and 1526 cm–1 can be attributed to the methyl groups of the head group adsorbed well on the gold surface. The reason of the strongest increase at 1267 cm–1 band is that the orientation of the C–H vibrations of the −CH2–N+(CH3)3 group is consistent with the polarization direction of the incident light.42 The reason of the band blue-shifted to 962 from 957 cm–1 is the chemical adsorption of the methyl groups from the (CH3)3N+ group to the substrate. The enhancements of CH2 at 1164 cm–1 and C–C at 1027 cm–1 are also assigned to the alkane chain being close to the substrate surface. The main reason of these bands of blue-shifted to 1072, 1304, and 1403 cm–1, is the chemical adsorption of CH2 to the substrate. So, during experiments, both the head methyl groups and the hydrocarbon chain of CTAB are near the hot spot of substrate surface.39
3.1.3. Surfactant SDS
SDS is another typical sensitive anionic surfactant. It is an amphiphilic molecular system with hydrophilicity at one end and hydrophobic chain at the other end. The hydrophilic polarity head is defined as the SDS head, while the hydrophobic chain is defined as the SDS tail. SDS can bind to CTAB and destroy the combination of CTAB and DNA to study the decompression of DNA molecules.
Figure 4 is the Raman spectra of SDS. Figure 4A shows a typical Raman spectrum of SDS powder and SERS spectrum of SDS solution with a concentration of 10–8 M; Figure 4B shows SERS spectrum of SDS solution with a low concentration of 10–9 M, the illustration in the upper left corner is the chemical molecular structure of SDS.
Figure 4.
(A) Raman spectrum of SDS powder and SERS spectrum of SDS solution (10–8 M); and (B) SERS spectrum of SDS solution (10–9 M), the inset is the molecular structure of SDS.
From Figure 4A, it can be seen clearly that the characteristic Raman peaks of SDS mainly are at 687, 830, 895, 995, 1060, 1081, 1125, 1217, 1300, 1321, 1365, 1437, 1454, 1478, 1537 cm–1, and so forth. These characteristic peaks reveal abundant structural information of SDS molecular illustrated as follows.43−46 The bands 687 cm–1 belonged to an SO3 vibration. The 830 cm–1 belonged to CO–SO3 stretching vibration. The 895 cm–1 is attributed to CH3 rocking, and the 995 cm–1 is attributed to the stretching vibration of S–OC. The 1060 cm–1 is assigned to the full-reflex vibration of the C–C skeleton and may be mixed with SO3 vibration, and the 1081 cm–1 is attributed to twist mode of the C–C skeleton and may be mixed with SO3 vibration. The band at 1125 cm–1 is attributed to the full-reflex vibration of the C–C skeleton, and the 1217 cm–1 is a characteristic of the S=O bond. The 1300 and 1321 cm–1 are assigned to the CH2 twist. The SERS feature at 1365 cm–1 can be attributed to the CH2 wag and the band at 1437, 1454, and 1478 cm–1 are attributed to the CH2 bending modes. The 1537 cm–1 is attributed to CH2 rocking vibration. It can be noted that there is a new Raman peak at 1578 cm–1, and the SERS intensity of 10–8 M SDS solution at 1321, 1365, and 1478 cm–1 are greatly increased compared with the rest bands, these bands are characteristic peaks of CH2.
In Figure 4B, the SERS bands of 10–9 M SDS are the same as the most bands shown in Figure 4A, except for the appearance of a new band at 736 cm–1 and the intensity increase of the peaks at 1217, 1370, 1478, and 1578 cm–1 compared with the rest of the bands. The significantly enhanced 1217 cm–1 is one characteristic peak of the head group, and the main reason of great enhancement of 1217 cm–1 is that the vibration of S=O bond has a large polarization component perpendicular to the SERS substrate. The band blue-shift to 1370 from 1365 cm–1 can be assigned to the CH2 of the chain interacted with the gold surface. The characteristic peaks of CH2 at 736, 1370, 1478, and 1578 cm–1 are also enhanced, and the main reason is that the alkane chain is close to the surface. From these stretching modes and vibrational modes of the OSO3 moiety and CH2, it can be inferred that the SDS is absorbed on the gold surface, and both the polar head and the nonpolar hydrocarbon chain are close to the gold surface with the CH2.
3.1.4. Composite of ctDNA–CTAB
Figure 5 is the information of the composite of ctDNA–CTAB. Figure 5A shows the SERS spectrum of a ctDNA–CTAB composite, in which the black and blue characteristic numbers represent the wavenumbers of DNA and CTAB, respectively. The concentration of DNA molecules is 1 × 10–9 M. Figure 5B is a schematic diagram of the ctDNA molecule in the compaction state induced by surfactant CTAB.
Figure 5.
Composite of ctDNA–CTAB. (A) SERS spectrum, black and blue characters represent the wave number of DNA and CTAB, respectively; (B) schematic diagram of a ctDNA molecule under compaction state induced by CTAB.
From Figure 5A, some characteristic peaks of DNA and CTAB can be clearly observed. For example, for the DNA molecules, bands are at 653, 807, 1092, 1106, and 1422 cm–1. The band at 653 cm–1 is attributable to deoxyribose, the band at 807 cm–1 is designated as symmetrical vibration mode of O–P–O phosphate backbone, the band at 1092 cm–1 is attributed to the symmetric stretching vibration of O=P=O in PO2–, the 1106 cm–1 is attributed to the stretching vibration of O–P–O in the phosphate, and the 1422 cm–1 band is attributed to the bending vibration of the CH2 group of deoxyribose. While, for the CTAB, some characteristic peaks are also observed at 758, 962, 1027, 1146, 1164, 1212, 1304, 1446, and 1478 cm–1. The bands at 758 and 962 cm–1 belong to methyl rocking from the (CH3)3N+ group, the 1027 cm–1 is assigned to C–C stretching vibration, the 1146 cm–1 is assignable to C–C anti-symmetric stretch, the band at 1164 cm–1 belongs to the CH2 rocking, the 1212 cm–1 can be attributed to CH2 wag, the 1304 cm–1 is related to the CH2 twisting, the 1446 cm–1 is attributed to CH2 bending vibration, and the 1478 cm–1 is related to the CH2 scissors.
Comparing the characteristic peak of ctDNA molecules shown in Figure 5A with that shown in Figure 2B, it can be found that: (1) the number of characteristic peaks of DNA molecules are less in Figure 5A. Both the phosphate group at 807, 1092, and 1106 cm–1 and the deoxyribose at 1422 cm–1 are observed, while the characteristic peak of the base disappears, which indicates that the DNA molecule has been compressed from the free state and some components like the base was hidden and undetectable. (2) When DNA molecules are free (Figure 2B), the peak intensity ratio of the phosphate group to the deoxyribose is I1092/I1422 = 6.86, while it is I1092/I1422 = 0.42 as DNA molecules are in compaction state of DNA–CTAB (Figure 5A). The ratio of DNA phosphoric acid to deoxyribose reduces by about 16 times in the compressed state, indicating that the action of the phosphate group with the substrate is weakened because the phosphate group is further away from the hot spot in the substrate. It can be inferred that the ctDNA molecule are tightly packed by CTAB.
Furthermore, comparing the characteristic peaks of CTAB shown in Figure 5A with that shown in Figure 3B, it can be observed that (1) the characteristic peaks of CTAB, both the head group and the hydrophobic tail, are all appeared. The peak intensity of hydrocarbon chains at 1212, 1446, and 1478 cm–1 are enhanced compared with the rest bands, indicating the number of hydrocarbon chains increase sharply at the hot spot of the substrate. The band blue-shift to 1446 from 1435 cm–1 suggests that CH2 in hydrocarbon chain exposed to the hot spot interact with the surface of the substrate by chemical adsorption. (2) The peak intensity ratio of the head group to the hydrocarbon chain is I962/I1027 = 2.22 in the pure CTAB, while it is I962/I1027 = 0.69 in the DNA–CTAB complex. The ratio reduces about 3 times in the DNA–CTAB complex, which reveals that the action of the head with the substrate is sharply reduced. According to the essences of peak intensity of SERS spectrum, it can be concluded that the number of the head group interacting with the substrate decreases greatly because the head group leaves the substrate surface to bind to the phosphoric acid by the electrostatic effect. This point is also corroborated by the disappearance of the 1267 cm–1 of the Raman characteristic peaks of CTAB head.
The process of surfactant CTAB interacting with DNA molecules to compress DNA can be described as following: at first, the head of CTAB combine with the phosphate group of the DNA, and then, the hydrophobic interaction among the CTAB hydrocarbon chains bring about the bending of DNA molecules to achieve a tightly compaction conformation. CTAB wrap around the compressed DNA molecules, hydrocarbon chains is exposed to the outside.
3.1.5. Composite of DNA–CTAB–SDS
Figure 6 shows the composite of ctDNA–CTAB–SDS. Figure 6A is the SERS spectra of ctDNA–CTAB–SDS composite, in which the black, blue, and red character numbers represent the wavenumber of DNA, CTAB, and SDS, respectively. The concentration of DNA molecules is 1 × 10–9 M. Figure 6B is a schematic diagram of composite ctDNA–CTAB–SDS in the decompaction state that DNA is decompressed from the ctDNA–CTAB compaction to a free state induced by the action of surfactant SDS.
Figure 6.
Composite of ctDNA–CTAB–SDS: (A) SERS spectra of composite ctDNA–CTAB–SDS, black, blue, and red characters represent the DNA, CTAB, and SDS, respectively; (B) schematic diagram of ctDNA–CTAB–SDS in decompaction state.
From Figure 6A, some characteristic peaks of DNA, CTAB, and SDS can be clearly observed. (1) Bands at 697, 733, 784, 980, 1092, 1251, 1510, and 1570 cm–1 are all related to the ctDNA molecules. The band at 697 and 980 cm–1 are attributable to deoxyribose. The band at 733 cm–1 is attributed to the symmetric stretching vibration of adenine (A). The 784 cm–1 is related to the symmetric stretching of phosphodiester O–P–O. The band at 1092 cm–1 is attributed to the symmetric stretching vibration of O=P=O in PO2–. The 1251 cm–1 is because of adenine (A) and cytosine (C), and the 1510 cm–1 is assigned to adenine (A). The 1570 cm–1 is related to adenine (A) and guanine (G). (2) There are Raman characteristic features of CTAB, for example, 758, 942, 1027, 1072, 1125, 1403, 1435, and 1526 cm–1. As mentioned above, the band at 758 and 1526 cm–1 are assigned to CH3 rocking from N+(CH3)3 group. The 942 cm–1 is derived from the methyl rocking of the (CH3)3N+ group and C–N+ stretching mode. The band at 1027, 1072, and 1125 cm–1 are assigned to the C–C stretching vibration. The 1403 cm–1 is attributed to CH2 wag and CH3 deformation. The 1435 cm–1 is attributed to CH2 bending vibration. (3) The characteristic peaks of SDS at 895, 1125, 1217, 1370, 1478, and 1578 cm–1 can also be found. It is known that the 895 cm–1 is assigned to CH3 rocking vibration, the band at 1125 cm–1 belongs to C–C stretching vibration, the 1217 cm–1 band is a characteristic of the S=O bond, the 1370 cm–1 is attributed to the CH2 wag, and the 1478 cm–1 is attributed to the CH2 bending. The 1578 cm–1 is assigned to CH2 rocking vibration.
Analyzing the SERS characteristic spectra can provide us an insight into the effects of surfactant CTAB–SDS on the compaction and decompaction of DNA molecules at the single molecule level. (1) In Figure 6A, there are characteristic peaks of the deoxyribose at 980 cm–1 and phosphate groups at 784 and 1092 cm–1, and bases of DNA molecule at 733, 1251, 1510, and 1570 cm–1. The main components of the DNA molecule are exposed and can be detected, which indicate that the DNA molecule has been decompressed from the compaction state. (2) The intensity at 1570 cm–1 is enhanced greatly compared with the rest peaks of ctDNA molecule in Figure 6A, which indicates that the adenine (A) ring plane might be perpendicular to the surface of substrate and interact with it. Thus, the DNA molecule is close to the substrate surface by adenine (A). (3) The peak intensity ratio of the base to the phosphate group is I1251/I1092 = 0.87 in Figure 2B, while it is I1251/I1092 = 0.86 after the SDS decompressed the ctDNA–CTAB complex in Figure 6A. The ratio is barely changed, revealing that the configuration of DNA molecule are basically recovered to the original state, but the DNA molecules in the decompaction state can no longer have the same conformation as the original one. (4) Compared with the SERS spectrum of CTAB solution in Figure 3B, characteristic peaks of the head group at 758, 942, and 1526 cm–1 and the hydrocarbon chain at 1027, 1072, 1125, 1403, and 1435 cm–1 still exist in a complex state of DNA–CTAB–SDS shown in Figure 6A. It can be observed that the peak intensity of the hydrophobic chain at 1403 cm–1 is enhanced compared with the rest bands in Figure 6A, which can be attributed to the chemical adsorption of CH2 and CH3 to the SERS substrate. About the characteristic Raman pattern of CTAB, in Figure 3B the peak intensity ratio of the head to the hydrocarbon chain is I1526/I1072 = 2.04, while it is I1526/I1072 = 1.62 after the SDS decompressed the ctDNA–CTAB complex. The ratio is reduced by 1.3 times, indicating the effect of CTAB head group interacting with the substrate is weakened slightly but still in close proximity to the substrate, while the CH2 of the hydrocarbon chain still interacts with the substrate surface. (5) Compared with the SERS spectrum of SDS solution in Figure 4B, characteristic peaks of the head group at 895 and 1217 cm–1 and the hydrocarbon chain at 1370, 1478, and 1578 cm–1 still exist in a complex state of DNA–CTAB–SDS shown in Figure 6A. The peak intensity ratio of the head to the hydrocarbon chain is I1217/I1478 = 0.81 in pure SDS solution, while it is I1217/I1478 = 0.72 after the SDS decompressed the DNA–CTAB complex, which indicates that the action of the head and the tail with the SERS substrate are not significantly changed. (6) Combined with (4) and (5), it can be concluded that the negative head of SDS neutralize the positive head of CTAB when SDS interact with the complex DNA–CTAB composite, and the interaction not only disbands the DNA from the DNA–CTAB compaction state to a decompaction state, but also lead to the head group of CTAB no longer directly acting on the substrate. From Figure 6A, it should also be noted that the peak intensity at 1125 cm–1 and the C–C bond in both CTAB and SDS, is significantly enhanced, which can be assigned to the part of C–C chain being perpendicular to the solid surface of the SERS substrate. After SDS combined with CTAB by electrostatic and hydrophobic, their head group and hydrocarbon chain interact with the SERS substrate, and the part of C–C chains in CTAB and SDS are vertical to the substrate.
The decompaction process of DNA after adding surfactant SDS can be described as follows: at first, the compacted conformation of DNA–CTAB complex is destroyed by SDS combining with CTAB through electrostatic and hydrophobic bonding; then, the DNA is released from the DNA–CTAB complex and extended by the electrostatic repulsion of the phosphate group. The DNA molecule is decompressed but cannot have the original conformation.
3.2. UV–Vis Spectra
To characterize
the internal construction of DNA–CTAB compaction and DNA–CTAB–SDS
decompaction, the ultraviolet–visible spectroscopy (UV–vis)
technology was employed. UV–vis, an ultraviolet–visible
absorption spectrum, belongs to the electronic spectrum, and is generated
by the transition of valence electrons.47 The ultraviolet visible spectrum and the degree of absorption, produced
by the absorption of ultraviolet and visible light from molecules
or ions of a sample, can be determined and inferred for the composition
and structure of the sample. Generally, the technique measures the
absorption of light across the ultraviolet and visible light wavelengths
through a liquid sample. With a constant light path length and known
absorption coefficient, the concentration of a compound in question
can be determined from the light absorbed by the sample according
to the Beer–Lambert’s law:  , where a is the absorbance
of the solution, ε is the molar absorptivity, l is the thickness, and c is the concentration.
, where a is the absorbance
of the solution, ε is the molar absorptivity, l is the thickness, and c is the concentration.
3.2.1. UV–Vis Spectra of DNA–CTAB Compaction
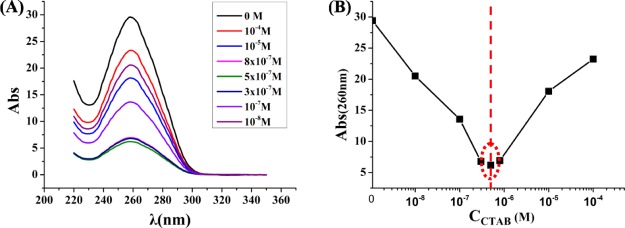
There is strong absorption of DNA in a range of wavelength from 240 to 290 nm. The maximum absorption peak at 260 nm is caused by its conjugated double bond in the nucleotide bases. To determine the relationship between the amount of cationic surfactant CTAB and DNA molecules in the compacted/decompacted state, the absorption spectra of DNA molecules interacted with CTAB at various concentration ratios are systematically investigated. As shown in Figure 7, the DNA concentration is 10–7 M; while, the CTAB concentrations are 0, 10–8, 10–7, 3 × 10–7, 5 × 10–7, 8 × 10–7, 10–5, and 10–4 M, respectively.
Figure 7.
(A) Absorption spectra of DNA molecules in compacted state under the action of cationic CTAB at different concentrations; (B) absorption peak values of DNA–CTAB complex around 260 nm at various CCTAB; the connecting line is just to guide the eyes.
Figure 7A shows the absorption spectra of DNA–CTAB complex at different concentrations. Figure 7B displays the absorbance of DNA–CTAB at 260 nm changing with different concentrations of CTAB (CCTAB). It can be observed clearly from Figure 7B, the absorbance of DNA decreases first and then increases with increasing CTAB concentration, and the absorption nadir is the CCTAB = 5 × 10–7 M. That is, as the CCTAB increases, the absorption value decreases when CCTAB < 5 × 10–7 M, which indicates that the DNA molecules are gradually compressed, both the number of DNA directly exposed and the total area of DNA exposed gradually reduce; when the CCTAB = 5 × 10–7 M, DNA molecule is under the most compressed state; when the CCTAB > 5 × 10–7 M, the absorbance has an apparently increase, which reveals the DNA–CTAB complexes are decompressed as an excessive amount of CTAB interacting with them; when the CTAB concentration is about 10–4 M, the absorbance is similar to the value of the DNA solution without CTAB. At this time, all DNA molecules are under completely decompaction state.
3.2.2. UV–Vis Spectra of DNA–CTAB with SDS
DNA molecules can be decompressed when anionic surfactant SDS is added into the DNA–CTAB complex. As shown in Figure 8, the SDS concentrations are respectively 0, 10–8, 10–7, 5 × 10–7, and 10–5 M. For comparison, the absorption spectra without SDS is also provided.
Figure 8.
(A) Absorption spectra of DNA–CTAB complexes in decompaction state with added anionic surfactant SDS at different concentrations and (B) absorption peak values of DNA–CTAB complex solutions at various CSDS around 260 nm, the connecting line is just to guide the eyes.
Figure 8A displays the absorption spectra of the ctDNA–CTAB complex when surfactant SDS is added to the solution. Figure 8B shows the tendency of the absorbance at 260 nm with the concentration of SDS (CSDS). It can be seen from Figure 8B that the absorbance at 260 nm first rises and then stabilizes with CSDS increasing, and the threshold value of CSDS = 10–7 M. That is, when CSDS < 10–7 M, the absorption peak gradually increases with the increasing of CSDS, indicating the concentration of SDS is insufficient enough to break down the DNA–CTAB complex, that is, the amount of anionic SDS is not large enough to neutralize the cationic CTAB. The absorbance gradually increases with the increase of CSDS, indicating that DNA–CTAB complex is gradually decompressed. When CSDS > 10–7 M, the absorbance of solution is near to that of the pure DNA solution, which indicates the DNA is fully released from the DNA–CTAB compressed state. Furthermore, as the SDS concentration continues to increase, there is no significant change in the absorption value at 260 nm, indicating that the addition of excessive SDS has little effect on the absorption of DNA in the decompaction state.
From Figures 7 and 8, it can be deduced that both the excessive cationic CTAB and the anionic SDS could lead to the decompaction of the DNA–CTAB complex.
3.3. Mechanism of Interaction
Figure 9 is a schematic diagram of ctDNA molecule compaction and decompaction induced by surfactants of cationic CTAB and anionic SDS.
Figure 9.
Schematic diagram of DNA molecule compaction under surfactant CTAB and decompaction interacted with surfactants of SDS or CTAB.
The compaction process of ctDNA molecules interacted with surfactant CTAB can be described as following: positively charged CTA+ ions (i.e. head of CTAB) approach and bind to the negatively charged binding site of DNA (phosphate group) by electrostatic action. The hydrophobic action of the CTAB hydrophobic chains results in the double helix DNA bend, shrink, and even compaction. The decompaction of DNA–CTAB is induced by two ways, one is the interaction with surfactant SDS and the other is the effect of excess surfactant CTAB. As for the former, because of the strong electrostatic ion pairing and hydrophobic effect between the anionic SDS and cationic CTAB, CTAB is detached from the DNA–CTAB compressed complex to interact with SDS, causing the DNA molecules in the decompaction state. With regard to the latter, the hydrophobic tail of the excessive free CTAB acts on the DNA–CTAB complex to form micelles by the similar compatible principle when the concentration of CTAB is further increased, which results in the separation of CTAB from the DNA–CTAB compaction state and the release of DNA molecule.
4. Conclusions
To understand the mechanism of DNA molecular compaction/decompaction is very meaningful for life sciences and biomedicine. In this paper, a sort of solid nanofilm of nanoPAA-ZnCl2-AuLs was prepared by the standard two-step anodization method combined with self-assembly method, which was with high sensitivity and reproducibility. The EF of the SERS-active substrate was evaluated to be 9.18 × 107 at a Raman shift of 1313 cm–1. Then, based on this novel solid substrate, SERS technique was used to study the interaction between ctDNA molecules and surfactants (e.g., CTAB and SDS) which resulted in the DNA molecule compaction and decompaction. The SERS spectra of ctDNA, CTAB, and SDS were all obtained, even though the sample concentration was as low as 10–9 M. The SERS spectra of the compacted ctDNA–CTAB complex and the decompacted DNA–CTAB–SDS with DNA concentration of 10–9 M were also obtained. For the compaction, it is found that the ratio of DNA phosphoric acid to deoxyribose reduces by about 16 times in the DNA–CTAB complex, and the characteristic peak of the base disappears. In addition, the peak intensity ratio of the head group to the hydrocarbon chain reduces about 3 times in the DNA–CTAB complex, indicating that the DNA is compacted and the hydrophobic chain of CTAB is exposed at the outermost layer. For the decompaction, the characteristic peak components of the DNA molecule in a state of decompression is the same as the natural state, the SDS peak intensity ratio of the head to the hydrocarbon chain is not significantly changed, and the CTAB peak intensity ratio of the head to the hydrocarbon chain is reduced by 1.3 times, which indicates that the DNA is decompressed but no longer has the original conformation, and the CTAB head is not directly electrostatically adsorbed on the substrate but acts on the substrate after electrostatic adsorption with the SDS. The mechanism of DNA compression and decompression caused by surfactants is that positively charged CTA+ ions bind to negatively charged phosphate groups through electrostatic interaction and compress DNA through hydrophobic interaction of hydrophobic chains. The combination of strong electrostatic ion pairing and hydrophobic effect between SDS and CTAB results in the release of DNA from the compacted state, which is extended because of the mutual repulsion of phosphate groups in the DNA skeleton. In addition, UV–visible spectrophotometry was also employed to detect the compaction and decompaction under different concentrations of the surfactant. When the concentration ratio of CTAB to DNA is 5, the DNA was completely compressed, while SDS (not less than 0.2 times of CTAB) or excess CTAB could bring the DNA–CTAB complex to a decompressed state. The combination mode of DNA with surfactant and the mechanism of DNA compaction and decompaction are very practically helpful for the future research of DNA as drug or gene carriers for disease treatment.
Acknowledgments
This Project was supported by the National Natural Science Foundation of China (grant nos. 61775181, 61378083), the International Cooperation Foundation of the National Science and Technology Major Project of the Ministry of Science and Technology of China (grant no. 2011DFA12220), the Major Research Plan of the National Natural Science Foundation of China (grant no. 91123030), the Natural Science Foundation of Shaanxi Province of China (grant nos. 14JS106, 16JS102, 2010JS113), and the Natural Science Basic Research Program of Shaanxi Province-Major Basic Research Project (2016ZDJC-15, S2018-ZC-TD-0061).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03294.
Sensitivity, EFs, and reproducibility of a nanoPAA-ZnCl2-AuLs substrate (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Tan X.; Li B. B.; Lu X.; Jia F.; Santori C.; Menon P.; Li H.; Zhang B.; Zhao J. J.; Zhang K. Light-Triggered, Self-Immolative Nucleic Acid-Drug Nanostructures. J. Am. Chem. Soc. 2015, 137, 6112–6115. 10.1021/jacs.5b00795. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Palazzolo S.; Bayda S.; Corona G.; Toffoli G.; Rizzolio F. DNA Nanotechnology for Cancer Therapy. Theranostics 2016, 6, 710–725. 10.7150/thno.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer M. A.; Simanek E. E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- Vijayanathan V.; Agostinelli E.; Thomas T.; Thomas T. J. Innovative approaches to the use of polyamines for DNA nanoparticle preparation for gene therapy. Amino Acids 2014, 46, 499–509. 10.1007/s00726-013-1549-2. [DOI] [PubMed] [Google Scholar]
- Safinya C. R.; Deek J.; Beck R.; Jones J. B.; Leal C.; Ewert K. K.; Li Y. Liquid crystal assemblies in biologically inspired systems. Liq. Cryst. 2013, 40, 1748–1758. 10.1080/02678292.2013.846422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Feng L.; Hao J.; Dong S. Compaction and decompaction of DNA dominated by the competition between counterions and DNA associating with cationic aggregates. Colloids Surf., B 2015, 134, 105–112. 10.1016/j.colsurfb.2015.06.038. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif A.; Osman G. Comparison of three genomic DNA extraction methods to obtain high DNA quality from maize. Plant Methods 2017, 13, 2–9. 10.1186/s13007-016-0152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajkowski A.; Cieslak J.; Beaucage S. L. Solid-Phase Purification of Synthetic DNA Sequences. J. Org. Chem. 2016, 81, 6165–6175. 10.1021/acs.joc.6b01020. [DOI] [PubMed] [Google Scholar]
- Chaudhuri T.; Pan A.; Das S.; Moulik S. P. Ratiometric Interactions of Anionic Surfactants with Calf Thymus DNA Bound Cationic Surfactants: Study II. J. Surfactants Deterg. 2018, 21, 127–137. 10.1002/jsde.12016. [DOI] [Google Scholar]
- Ren W.; Liu H.; Yang W.; Fan Y.; Yang L.; Wang Y.; Liu C.; Li Z. A cytometric bead assay for sensitive DNA detection based on enzyme-free signal amplification of hybridization chain reaction. Biosens. Bioelectron. 2013, 49, 380–386. 10.1016/j.bios.2013.05.055. [DOI] [PubMed] [Google Scholar]
- López-López M.; López-Cornejo P.; Martín V. I.; Ostos F. J.; Checa-Rodríguez C.; Prados-Carvajal R.; Lebrón J. A.; Huertas P.; Moyá M. L. Importance of hydrophobic interactions in the single-chained cationic surfactant-DNA complexation. J. Colloid Interface Sci. 2018, 521, 197–205. 10.1016/j.jcis.2018.03.048. [DOI] [PubMed] [Google Scholar]
- Li X.; Sun D.; Chen Y.; Wang K.; He Q.; Wang G. Studying compaction-decompaction of DNA molecules induced by surfactants. Biochem. Biophys. Res. Commun. 2018, 495, 2559–2565. 10.1016/j.bbrc.2017.12.151. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Wang H.; He C.; Qiao F.; Wang S.; Wang Y. DNA Condensation Induced by a Star-Shaped Hexameric Cationic Surfactant. ACS Appl. Mater. Interfaces 2017, 9, 23333–23341. 10.1021/acsami.7b04317. [DOI] [PubMed] [Google Scholar]
- Bustamante C.; Bryant Z.; Smith S. B. Ten years of tension: single-molecule DNA mechanics. Nature 2003, 421, 423–427. 10.1038/nature01405. [DOI] [PubMed] [Google Scholar]
- Ritort F.; Mihardja S.; Smith S. B.; Bustamante C. Condensation transition in DNA-polyaminoamide dendrimer fibers studied using optical tweezers. Phys. Rev. Lett. 2006, 96, 118301. 10.1103/physrevlett.96.118301. [DOI] [PubMed] [Google Scholar]
- Husale S.; Grange W.; Karle M.; Bürgi S.; Hegner M. Interaction of cationic surfactants with DNA: a single-molecule study. Nucleic Acids Res. 2008, 36, 1443–1449. 10.1093/nar/gkm1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.; Xu L.; Dong S.; Hao J. Thermo-reversible capture and release of DNA by zwitterionic surfactants. Soft Matter 2016, 12, 7495–7504. 10.1039/c6sm00704j. [DOI] [PubMed] [Google Scholar]
- Neuman K. C.; Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler H. J.; Ashton L.; Bird B.; Cinque G.; Curtis K.; Dorney J.; Esmonde-White K.; Fullwood N. J.; Gardner B.; Martin-Hirsch P. L.; Walsh M. J.; McAinsh M. R.; Stone N.; Martin F. L. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. 10.1038/nprot.2016.036. [DOI] [PubMed] [Google Scholar]
- Lawson L. S.; Rodriguez J. D. Raman Barcode for Counterfeit Drug Product Detection. Anal. Chem. 2016, 88, 4706–4713. 10.1021/acs.analchem.5b04636. [DOI] [PubMed] [Google Scholar]
- Rygula A.; Majzner K.; Marzec K. M.; Kaczor A.; Pilarczyk M.; Baranska M. Raman spectroscopy of proteins: a review. J. Raman Spectrosc. 2013, 44, 1061–1076. 10.1002/jrs.4335. [DOI] [Google Scholar]
- Raman C. V.; Krishnan K. S. A new type of secondary radiation. Nature 1928, 121, 501. 10.1038/121501c0. [DOI] [Google Scholar]
- Laing S.; Jamieson L. E.; Faulds K.; Graham D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. Chem. 2017, 1, 0060. 10.1038/s41570-017-0060. [DOI] [Google Scholar]
- Nie S.; Emory S. R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. 10.1126/science.275.5303.1102. [DOI] [PubMed] [Google Scholar]
- Muntean C. M.; Leopold N.; Tripon C.; Coste A.; Halmagyi A. Surface-enhanced Raman spectroscopy of genomic DNA from in vitro grown tomato (Lycopersicon esculentum Mill.) cultivars before and after plant cryopreservation. Spectrochim. Acta, Part A 2015, 144, 107–114. 10.1016/j.saa.2015.02.085. [DOI] [PubMed] [Google Scholar]
- Reguera J.; Langer J.; Jiménez de Aberasturi D.; Liz-Marzán L. M. Anisotropic metal nanoparticles for surface enhanced Raman scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. 10.1039/c7cs00158d. [DOI] [PubMed] [Google Scholar]
- Fazio B.; D’Andrea C.; Foti A.; Messina E.; Irrera A.; Donato M. G.; Villari V.; Micali N.; Maragò O. M.; Gucciardi P. G. SERS detection of Biomolecules at Physiological pH via aggregation of Gold Nanorods mediated by Optical Forces and Plasmonic Heating. Sci. Rep. 2016, 6, 26952. 10.1038/srep26952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui C.; Wang K.; Wang S.; Ren J.; Bai X.; Bai J. SERS activity with tenfold detection limit optimization on a type of nanoporous AAO-based complex multilayer substrate. Nanoscale 2016, 8, 5920–5927. 10.1039/c5nr06771e. [DOI] [PubMed] [Google Scholar]
- Chiang C.-Y.; Liu T.-Y.; Su Y.-A.; Wu C.-H.; Cheng Y.-W.; Cheng H.-W.; Jeng R.-J. Au Nanoparticles Immobilized on Honeycomb-Like Polymeric Films for Surface-Enhanced Raman Scattering (SERS) Detection. Polymers 2017, 9, 93. 10.3390/polym9030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Wang K.; Zhou Y.; Wang S.; Zhang C.; Wang G.; Bai J. Synthesis and photoluminescence enhancement of nano-PAA-ZnCl2 with controllable dimension and morphology. Appl. Surf. Sci. 2016, 390, 122–130. 10.1016/j.apsusc.2016.08.036. [DOI] [Google Scholar]
- Sui C.; Wang K.; Zhou Y.; Hao B.; Bai X.; Bai J.. A Novel Multilayer Composite Nanostructure for Single-Molecule SERS Detection. Submitted. [Google Scholar]
- Garcia-Rico E.; Alvarez-Puebla R. A.; Guerrini L. Direct surface-enhanced Raman scattering (SERS) spectroscopy of nucleic acids: from fundamental studies to real-life applications. Chem. Soc. Rev. 2018, 47, 4909–4923. 10.1039/c7cs00809k. [DOI] [PubMed] [Google Scholar]
- Prescott B.; Steinmetz W.; Jr T. G. Characterization of DNA structures by laser Raman spectroscopy. Biopolymers 1984, 23, 235–256. 10.1002/bip.360230206. [DOI] [PubMed] [Google Scholar]
- Goodwin D. C.; Brahms J. Form of DNA and the nature of interactions with proteins in chromatin. Nucleic Acids Res. 1978, 5, 835–850. 10.1093/nar/5.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhoumi A.; Zhang D.; Tam F.; Halas N. J. Surface-enhanced Raman spectroscopy of DNA. J. Am. Chem. Soc. 2008, 130, 5523–5529. 10.1021/ja800023j. [DOI] [PubMed] [Google Scholar]
- Zhang R.-Y.; Pang D.-W.; Zhang Z.-L.; Yan J.-W.; Yao J.-L.; Tian Z.-Q.; Mao B.-W.; Sun S.-G. Investigation of ordered ds-DNA monolayers on gold electrodes. J. Phys. Chem. B 2002, 106, 11233–11239. 10.1021/jp025817o. [DOI] [Google Scholar]
- Grajcar L.; Baron M.-H. A SERS probe of adenyl residues available for intermolecular interactions. Part I—adenyl ‘fingerprint’. J. Raman Spectrosc. 2001, 32, 912–918. 10.1002/jrs.760. [DOI] [Google Scholar]
- Hianik T.; Wang X.; Tashlitsky V.; Oretskaya T.; Ponikova S.; Antalík M.; Ellis J. S.; Thompson M. Interaction of cationic surfactants with DNA detected by spectroscopic and acoustic wave techniques. Analyst 2010, 135, 980–986. 10.1039/c0an00070a. [DOI] [PubMed] [Google Scholar]
- Dendramis A. L.; Schwinn E. W.; Sperline R. P. A surface-enhanced Raman scattering study of CTAB adsorption on copper. Surf. Sci. 1983, 134, 675–688. 10.1016/0039-6028(83)90065-1. [DOI] [Google Scholar]
- Foucault R.; Birke R. L.; Lombardi J. R. SERS of Surfactants in Monolayer and Multibilayer Forms on an Electrified Ag Surface. Langmuir 2003, 19, 8818–8827. 10.1021/la034631t. [DOI] [Google Scholar]
- Duan H.; Jia L.; Chen M.; Ge S.; Guo X. Surface-enhanced Raman scattering based on colloidal Ag nanoparticles for quantitative detection of cetyltrimethyl ammonium bromide. Mater. Express 2016, 6, 77–82. 10.1166/mex.2016.1278. [DOI] [Google Scholar]
- Huang X.; El-Sayed I. H.; Qian W.; El-Sayed M. A. Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface Raman spectra: a potential cancer diagnostic marker. Nano Lett. 2007, 7, 1591–1597. 10.1021/nl070472c. [DOI] [PubMed] [Google Scholar]
- Picquart M. Vibrational model behavior of SDS aqueous solutions studied by Raman scattering. J. Phys. Chem. 1986, 90, 243–250. 10.1021/j100274a008. [DOI] [Google Scholar]
- Paschoal A. R.; Ayala A. P.; Pinto R. C. F.; Paschoal C. W. A.; Tanaka A. A.; Boaventura Filho J. S.; José N. M. About the SDS inclusion in PDMS/TEOS ORMOSIL: a vibrational spectroscopy and confocal Raman scattering study. J. Raman Spectrosc. 2011, 42, 1601–1605. 10.1002/jrs.2908. [DOI] [Google Scholar]
- Valmalette J. C.; Tan Z.; Abe H.; Ohara S. Raman scattering of linear chains of strongly coupled Ag nanoparticles on SWCNTs. Sci. Rep. 2014, 4, 5238. 10.1038/srep05238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamil R. F. V.; Corio P.; Agostinho S. M. L.; Rubim J. C. Effect of sodium dodecylsulfate on copper corrosion in sulfuric acid media in the absence and presence of benzotriazole. J. Electroanal. Chem. 1999, 472, 112–119. 10.1016/s0022-0728(99)00267-3. [DOI] [Google Scholar]
- Li P.; Hur J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. 10.1080/10643389.2017.1309186. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.