Abstract
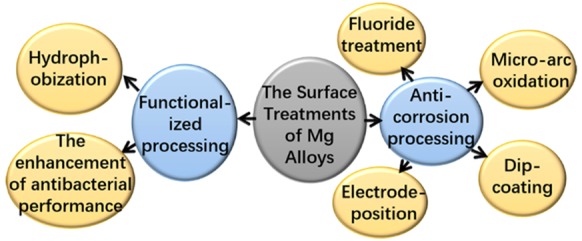
Mg alloys have attracted extensive attention in the biomedical fields owing to their great biocompatibility, suitable mechanical properties, and biodegradability, etc. However, the fast degradation rate restricts the application of Mg alloys. Thus, the surface treatment of Mg alloys is considered as one of the most effective ways to enhance the corrosion resistance of Mg alloys. Nevertheless, simple processing to improve the corrosion resistance can no longer meet the growing biofunctional clinical requirements. Therefore, functionalized processing has become one of the key development directions for surface treatment in the future, such as functionalized Mg alloys with antibacterial property and hydrophobicity. There are few papers that review the functionalized processing of surface treatment. This review summarized and compared the major advances of the surface treatment (anticorrosion processing and functionalized processing) of Mg alloys. Then, some potential research suggestions are proposed, which may provide a reference for the development of Mg alloys.
1. Introduction
Nowadays, Mg alloys have attracted extensive attention especially in the biomedical fields due to their desirable biocompatibility, mechanical properties, and biodegradability.1,2 As a biomedical metal, Mg alloys have a density and elastic modulus similar to natural bone, which can effectively decrease the stress shielding effect. Moreover, secondary surgery can normally be avoided because of the biodegradability of Mg alloys used as implants such as a bone screw. However, Mg has active chemical properties and poor corrosion resistance in an aqueous environment, especially with a chloride ion. The over fast corrosion rate resulted in the serious degradation of mechanical properties of Mg alloys, while the bone heals, which may cause fractures again. At the same time, accumulated hydrogen which was the byproduct of Mg degradation may form an air cavity around implants, and this was disadvantageous for the healing of bone and tissue. Therefore, improving the corrosion resistance of Mg alloys is the primary goal.
Surface treatment, an effective way to enhance anticorrosion properties of Mg alloys, is to form a protective layer on Mg alloys, which can hinder the direct contact between Mg alloys and the physiological environment and thus control the over fast degradation of Mg alloys.3 Four typical methods of anticorrosion processing in surface treatments are fluoride treatment, microarc oxidation (MAO), dip-coating, and electrodeposition. It is a consensus that improving the corrosion resistance of Mg alloys is the fundamental purpose for surface treatment. However, with the application of Mg alloy in practice, new requirements for surface treatment are consistently being put forward, like making the surface of Mg alloys functionalized. Hydrophobization is one of the functionalized processes in surface treatments.4 In addition, the coating with antibacterial property is given a lot of attention, which is meaningful for the healing of patients.5 The functionalized processing could be one of the key development directions of surface treatment in the future. However, there were few papers to review the functionalized processing of surface treatment. In this paper, the latest studies about the surface treatments (anticorrosion processing and functionalized processing) of Mg alloys were reviewed, and the development direction was briefly predicted.
2. Anticorrosion Processing
2.1. Fluoride Treatment
Fluoride treatment is a type of simple and effective chemical treatment, which can form a fluorine-containing coating on the surface of Mg alloys by reacting Mg alloys with hydrofluoric acid solution. The fluoride content in the coating was impacted by HF concentrations, reaction time, temperature, etc. Fluoride treatment can improve the corrosion resistance of Mg alloys, reduce their hemolysis rate, and improve their antiplatelet adhesion. However, it should be noted that the Mg alloy with fluorine-containing coating has some toxicity to the cells. The release of a high concentration of F– in the coating was not conducive to the growth of cells.6
Furthermore, Li et al.7 found that the Mg alloy with fluorine-containing coating slowly degraded layer by layer. However, the result was different from the study of Barajas et al.,8 where SEM images of the cross-sectional of fluoride-treated AZ31after 28 days of immersion displayed that the corrosion of MgF2 coating was not uniform, and fluoride coating peeled off. Wang et al.9 also found that the cracks and localized corrosion were observed on the surface of Mg alloys treated with alkali-fluoride treatment after 20 days of immersion. The difference of three studies may be due to the presence of the second phase in Mg alloys, which results in the defect (irregularly distributed pores) of the fluoride coating and the local stress concentration on the surface of Mg alloys.
Besides enhancing the corrosion resistance of Mg alloys, Lin et al.10 reported that fluoride treatment can maintain the surface features of Mg alloy microstructure. The Mg alloy which was processed by ultrasonic etching in advance displayed a bowl-like nest structure, and this structure remained after fluoride treatment. Hence, fluorine treatment is a good choice for surface treatment of Mg alloys with special morphology, like the papillary-like pits or bowl-like nest structure.
2.2. Microarc Oxidation
Microarc oxidation is ordinarily used to form bioceramic coatings such as MgO coating. The Mg alloy matrix is oxidized under high voltage in the electrolyte, and then a protective ceramic layer is formed in situ on the surface of Mg alloys. The protective layer fabricated by MAO has a high bonding strength with the substrate. Due to the complex reaction process, the MAO coating result is affected by various factors, such as voltage, duty cycle, MAO time, current density, and so on. MAO coating can effectively improve the corrosion resistance of Mg alloys, but the coating contains a large number of pores and cracks.11 The further improvement of corrosion resistance is limited by the pores in the MAO coating. The solution penetrates into the Mg alloy matrix through the pores of the MAO coating, which will decrease the protective effect of the coating. Therefore, researchers generally post-treat the as-deposited MAO coating through other methods to solve this problem. The hydrothermal treatment6 and sol–gel method12 are the common post-treatment methods. In addition to sealing the pores, there is a viewpoint that the semisealing pores formed by the post-treatment of MAO coating pores can be utilized as a medicine loader or to enhance the bonding strength of multiple coatings.
Microarc oxidation (MAO) treatment can enhance the corrosion resistance. However, for biomedical application, the biocompatibility of MAO coating is far from enough, which should be further improved. Some bioactive material such as calcium phosphates can be introduced into the MAO coating. The surface microstructure is also a considerable method to enhance the bioactivity. Meanwhile, as a ceramic coating, the surface of the MAO coating is normally magnetic and electrically insulated. Magnetic or electric simulation is proved to be effective for the osteogenesis.13 Therefore, the electric conductor MAO coating could be a profound field.
2.3. Dip-Coating
Dip-coating is one of the most frequently used sol–gel methods, which mainly includes several procedures of dipping, extraction, cross-linking or curing, and drying. It is simple and easy to operate, but there are many factors for the coating, like solution concentration and withdrawal speed. Table 1 listed common parameters in the process of dip-coating and the thickness of the coating.14−24 These factors have a significant impact on the thickness, pore size, uniformity, and corrosion resistance of the coating.
Table 1. Parameters for Coating Prepared by Dip-Coating and the Thickness of the Coating.
| ref | substrate | coating material | solution concentration | solution temperature | dipping time (s) | dipping number | withdrawal speed | drying, curing, or cross-linking | coating thickness (μm) |
|---|---|---|---|---|---|---|---|---|---|
| (14) | AZ31B, AZ91D | hybrid silica sol | 120 g/L (silica) | 30 cm/min | curing for 30 min at 180 °C | ||||
| (15) | AZ31 | poly(lactide coglycolide) (PLGA) | 2% w/v | 80 mm/min | <1.61 | ||||
| (16) | Mg-32 wt % Ca | polycaprolactone (PCL) | 2.5 wt % | 30 | drying at room temperature (RT) | 70–80 | |||
| (17) | pure Mg | PCL | 5% w/v | 5 | 1 time | 10 mm/60 s | drying at RT for 24 h | 2.88 ± 0.91 | |
| 10 times | 4.33 ± 0.40 | ||||||||
| 50 times | 13.31 ± 0.36 | ||||||||
| (18) | Mg-4Zn-0.2Ca alloy | PCL | 1%, 2.5% and 5% (w/v) | 30 °C | 10 | 3 mm/s | drying in cold air for 5 min | ||
| (19) | Mg3Gd | chitosan | 0.01 mg/mL | drying for 30 min at 60 °C | 2.07 ± 0.50 | ||||
| (20) | AZ31 | poly(l-lactic acid) (PLA) | 8 wt % | RT | 1 mm/s | drying at RT for at least 1 week | |||
| (21) | Mg rods | polyurethane (PU) | 20 wt % | RT | 30 | 1000 μm/s | first, hang-drying at RT for 2 h; then, drying at 50 °C for 48 h | 9.5 ± 0.54 | |
| (22) | AZ91 | gelatin (GEL) | 10% (w/v) | 50 °C | gelation for 1 h by chemical cross-linking | about 40 | |||
| (23) | AZ60 | PLA | 10% (w/v) | 3 times | 220 μm/s | drying at RT | |||
| (24) | AZ91D | PLA | 4% (w/v) | 30 | 20 mm/min | drying at RT |
Dip-coating is widely use to prepare organic coating, which is considered to have superior biological properties.19 To date, the most commonly prepared organic coatings are PCL, PLGA, PLA, and chitosan, etc. Despite the advantages of outstanding organic coating, their disadvantages limit their further application. Organic coatings have poor mechanical strength and thermal instability and easily fall off from the substrate.17 Therefore, it is urgent to solve the problem of poor adhesion of organic coatings prepared by dip-coating on the substrate. Moreover, there is much room for further improvement of the coating quality by optimizing the experimental parameters and freeze-drying.
Besides organic coating, dip-coating can also be used to prepare inorganic coating such as silica sol–gel, Al2O3, ZrO2, and TiO2 coating.12,14 The inorganic coating can effectively enhance the corrosion resistance of Mg alloys, and it possessed strong adhesive strength. However, due to the sintering or drying process at high temperature, many pores and microcracks formed on the surface of Mg alloys during the preparation of the inorganic coating by dip-coating. Although the pretreatment or UV-assisted treatment can slightly reduce the microcracks, the defect of inorganic coating was still a problem which needs further study.25 Even so, dip-coating was widely applied because it is simple, fast, and preferably secure.
2.4. Electrodeposition
The electrodeposition is an electrochemical treatment method and is divided into direct electrodeposition and pulse electrodeposition.26 During the electrodeposition, Mg alloys are used as the cathode of the electrolytic cell, and then the elements in electrolytes will deposit on Mg alloys through oxidation and reduction. As a result, a protective coating is formed on the surface of Mg alloys. Electrodeposition is frequently used to prepare Ca–P coating.27 Hydroxyapatite (HA) is the most common Ca–P coating because it is the main component of bone tissue. Based on HA, Dehghanian et al.28 found that the silicon-substituted hydroxyapatite behaved much better. The coating prepared by electrolyte with the addition of 0.005 mol/L of SiO32– increased corrosion resistance by 7 times and had better cell proliferation performance. The electrodeposited coatings are compact and thin, but they are easily doped with an unexpected element and then form new phases. It is also a good way to prepare the new deposited coating on Mg alloys by changing the composition of the electrolyte.
In addition to the electrolyte, electrodeposition is influenced by many factors, such as the voltage, time, and duty cycle. For instance, the surface roughness of the coating decreased with the increase of voltage, but the coating was more difficult to form; meanwhile, the increase of time can increase the thickness and hydrophobicity of the coating and reduced the corrosion current density.29 The thickness and surface roughness of the coating reduced with a decrease of duty cycle, while the hydrophobicity increased.26 Besides, pH values of electrolyte, temperature, and the pretreatment of the Mg alloy also had an influence on the formation of electrodeposited coating.30,31
Except for the above four methods, there are many other anticorrosion processing methods, such as electrospinning and magnetron sputtering. However, the effect of a single anticorrosion processing method to promote the properties of the Mg alloy is limited. Therefore, combining two or more anticorrosion processing methods together to form the composite coating on the surface of Mg alloys has become a trend.32
3. Functionalized Processing
3.1. Enhancement of Antibacterial Performance
In general, the occurrence of fractures is always accompanied with inflammation or infection. Therefore, it is desirable to provide Mg alloys with antibacterial property. To our best knowledge, bacteria can be killed by the high pH environment caused by degradation of Mg and will not adhere to areas where the corrosion of Mg alloys is active.33,34 However, the antibacterial ability of Mg alloys is dramatically restricted in vivo due to the buffering effect of human fluid and the control in the corrosion of Mg.
To reduce the risk of inflammation and infection, Mg alloy loading with antibacterial drug is a common choice as implants.5 However, the use of antibiotics can induce drug resistance. Hence, a combination of coating with the elements possessing antibacterial ability was studied. Cu and Ag are common additive elements in the coating owing to the inhibitory effect on the proteins in bacterial biofilm.35,36 Moreover, Zou et al.37 prepared a Zn-loaded montmorillonite coating to improve the antibacterial properties of AZ31. Besides the coating with antibacterial ions, Pei et al.38 prepared a carboxymethyl chitosan coating loaded with heparin to make the surface of AZ31B functionalized, which exhibited excellent antibacterial and anticoagulant properties. Wang et al.21 utilized the functionalized PU to improve the antibacterial performance of Mg alloys. Overall, the coating containing ions with antibacterial behavior (Cu2+, Ag+, etc.) had great antibacterial performance. However, the development of the antibacterial materials such as the functionalized chitosan and PU is still necessary because those can avoid the potential toxic side effect of heavy metal ions and provide more options for the applications of Mg alloys under different environments.
3.2. Hydrophobization
The introduction of hydrophobic property can reduce the contact area between Mg alloys and water. The general way to introduce the hydrophobic property is to form a hydrophobic surface on Mg alloys. Table 2 listed hydrophobization and the properties of substrate after hydrophobization in some literature.39−48 It can be found that chemical modification is a common way to prepare the hydrophobic coating. SA and FAS are the common materials in hydrophobic coating. Except for organics, inorganic coating and the organic–inorganic composite coating were studied to improve the corrosion resistance of Mg alloys.
Table 2. Hydrophobization and the Properties of the Substrate after Hydrophobizationa.
| contact angle (deg) |
|||||||
|---|---|---|---|---|---|---|---|
| substrate | treatment | hydrophobic coating | before | after | Ecorr (V) | Icorr (A/cm2) | ref |
| Mg-5Sn-1Zn | MAO (400 V, 5 min) + chemical modification | stearic acid (SA) | 37.1 | 122.5 | –1.53 | 0.07 × 10–6 | (39) |
| Mg-4Li-1Ca | MAO + electrodeposition | zinc stearate | 54.4 ± 0.8 | 153.5 ± 0.5 | –1.65 | 7.68 × 10–8 | (40) |
| AZ61 | electrochemical machining + chemical modification | fluoroalkylsilane (FAS) | 30.9 | 165.2 | –1.4221 | 9.68 × 10–8 | (41) |
| AZ31B | etching + electroplating + chemical modification | SA | 68.5 | 153 | –0.39377 | (42) | |
| Al–Mg | electrochemical etching + low surface energy modification | FAS | 3 ± 0.8 | 160.4 ± 2.2 | –0.52 | (43) | |
| AZ31 | MAO + corrosion inhibitor + hydrophobic wax film technologies | hydrophobic wax | 68.71 ± 0.6 | 106.89 ± 1.1 | –1.412 | 5.764 × 10–9 | (44) |
| AZ31 | anodized treatment + chemical modification | SA | 13.3 | 150.6 | (45) | ||
| sodium laurate | 153.7 | ||||||
| myristic acid | 152 | ||||||
| PFDTMS | 145.5 | ||||||
| Mg-9Al-1Zn | linear laser ablation + annealing treatment | 33 ± 1 | 158.8 ± 2° | –1.556(±0.005) | (6.7 ± 1.2) × 10–6 | (46) | |
| Mg–Li | electroplating + SA modification | super hydrophilic Ni–Cu–SiC | 156.0 | –1.209 | 1.43 × 10–3 | (47) | |
| AZ31 | laser ablation + chemical etching + chemical modification | SA | >150 | (48) | |||
Corrosion potential (Ecorr), corrosion current density (Icorr), and 1H,1H,2H,2H-perfluorodecyltrimethoxysilane (PFDTMS); corrosion solution was NaCl.
As is well-known, different materials had different static contact angles, and the level of static contact angle directly reflects the degree of hydrophobicity of the material. Cui et al.40 formed a superhydrophobic zinc stearate coating on the MAO-coated Mg-4Li-1Ca alloy by electrodeposition. The results showed that the contact angle of zinc stearate coating reached 153.5°, which was super hydrophobic. Xu et al.41 prepared a fluoroalkyl-silane coating on the AZ61 alloy. The contact angle of coating increased from 30.9° to 165.2°. Meanwhile, Icorr of the Mg alloy reduced by 3 orders of magnitude, while Ecorr only increased from −1.5847 to −1.4221 V. Interestingly, the superhydrophilic coatings exhibited great anticorrosion properties as well as superhydrophobic coatings.47 Besides materials, the microstructure of Mg alloys can also impact its hydrophobicity.48 Therefore, both the microstructure and coating materials should be taken into consideration in order to control the hydrophobicity of Mg alloys.
Functionalized processing is developing rapidly with great potential in the future. Except for the properties mentioned above, the coatings with other properties have also been studied. For instance, Wang et al.49 prepared the coating with great blood biocompatibility and the property of endothelialization. Wang et al.50 manufactured the coating with good mineralization ability on the Mg–Zn–Zr–Sr alloy. Therefore, it is no doubt that the employment of functionalized processing promotes the applications of Mg alloys in different fields.
4. Conclusion
Surface treatment is an effective method to improve the performance (appropriate degradation rate) of Mg alloys. It is desired to form a protective layer on the surface of Mg alloys so that the corrosion resistance of Mg alloys can be improved. To date, surface treatments not only improve the corrosion resistance of the Mg alloy but also can make Mg alloys functionalized. According to this, surface treatments of Mg alloys can be divided into two parts: anticorrosion processing and functionalized processing. The anticorrosion processing mainly includes fluoride treatment, MAO, dip-coating, and electrodeposition, and those methods can effectively improve the corrosion resistance of Mg alloys. New properties (such as antibacterial and hydrophobic properties) will be achieved for Mg alloys through the functionalized processing.
Acknowledgments
This work was supported by the project from the National Natural Science Foundation of China (No. 81500897), China Scholarship Council (No. 201408210385), and Foundation of the Education Department of Liaoning Province in China (No. QN2019035).
Biographies

Weiwei Wu received her bachelor’s degree in biomedical engineering (2013–2017) from China Medical University. Now she is pursuing her master’s degree in biophysics at China Medical University, and her research interest is biodegradable Mg alloys.

Ziyuan Wang received a double bachelor’s degree from Queen’s University Belfast and China Medical University. As the top student she graduated with the first class degree in pharmaceutical sciences. She is studying for a masters of research degree in the Department of Chemistry of Imperial College London. She is doing research projects on metal organic frameworks in a nanoscale as a drug delivery system.

Sitian Zang received a master’s degree in Biophysics from China Medical University. Her research during her master’s degree focused on zirconia biomaterials. At present, she is studying for a doctor’s degree at China Medical University.

Xiaoming Yu, Ph.D, associate professor, received his Doctors degree in material physics and chemistry from the Northeastern University. He has been working on biodegradable and biocompatible medical materials and devices including Mg alloy and Ta.

Huazhe Yang received his B.S. (2003) from Shenyang University of Technology and Ph.D. (2008) from Northeastern University (Shenyang, China). He worked as a visiting scholar in Peking University (2009–2010) and Harvard Medical School (2015–2016). He did postdoctoral research at the Institute of Metal Research, Chinese Academy of Sciences (2011–2014). Currently, he is a Professor in China Medical University. His research focuses on the applications of various biomaterials (zirconia, Mg alloys, GelMA hydrogel, etc.) as a drug delivery system and implant materials.

Shijie Chang received his B.S. (2004) from Northeastern University and M.S. (2007) and Ph.D. (2013) from China Medical University. Currently, he is a vice professor in the Department of Biomedical Engineering, China Medical University. His research focuses on Materials Science, Artificial Intelligence, Machine Learning, and Data Analysis.
The authors declare no competing financial interest.
References
- Luo Q.; Zhai C.; Gu Q.; Zhu W.; Li Q. Experimental study and thermodynamic evaluation of Mg-La-Zn system. J. Alloys Compd. 2020, 814, 152297. 10.1016/j.jallcom.2019.152297. [DOI] [Google Scholar]
- Chakraborty Banerjee P.; Al-Saadi S.; Choudhary L.; Harandi S. E.; Singh R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. 10.3390/ma12010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry-Rendon M.; Allain J.; Robledo S.; Echeverria F.; Harmsen M. Coatings for biodegradable magnesium-based supports for therapy of vascular disease: A general view. Mater. Sci. Eng., C 2019, 102, 150–163. 10.1016/j.msec.2019.04.032. [DOI] [PubMed] [Google Scholar]
- Kuang J.; Ba Z.; Li Z.; Jia Y.; Wang Z. Fabrication of a superhydrophobic Mg-Mn layered double hydroxides coating on pure magnesium and its corrosion resistance. Surf. Coat. Technol. 2019, 361, 75–82. 10.1016/j.surfcoat.2019.01.009. [DOI] [Google Scholar]
- Bakhsheshi-Rad H.; Akbari M.; Ismail A.; Aziz M.; Hadisi Z.; Pagan E.; Daroonparvar M.; Chen X. Coating biodegradable magnesium alloys with electrospun poly-L-lactic acid-åkermanite-doxycycline nanofibers for enhanced biocompatibility, antibacterial activity, and corrosion resistance. Surf. Coat. Technol. 2019, 377, 124898. 10.1016/j.surfcoat.2019.124898. [DOI] [Google Scholar]
- Peng F.; Wang D.; Tian Y.; Cao H.; Qiao Y.; Liu X. Sealing the Pores of PEO Coating with Mg-Al Layered Double Hydroxide: Enhanced Corrosion Resistance, Cytocompatibility and Drug Delivery Ability. Sci. Rep. 2017, 7, 8167. 10.1038/s41598-017-08238-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Zhu P.; Chen S.; Zhang B.; Yang K. In vitro study on degradation of AZ31B magnesium alloy with fluoride conversion coating. Mater. Technol. (London, U. K.) 2017, 32, 409–414. 10.1080/10667857.2016.1262311. [DOI] [Google Scholar]
- Barajas J.; Joya J.; Durán K.; Hernández-Barrios C.; Coy A.; Viejo F. Relationship between microstructure and formation-biodegradation mechanism of fluoride conversion coatings synthesised on the AZ31 magnesium alloy. Surf. Coat. Technol. 2019, 374, 424–436. 10.1016/j.surfcoat.2019.06.010. [DOI] [Google Scholar]
- Wang P.; Liu J.; Shen S.; Li Q.; Luo X.; Xiong P.; Gao S.; Yan J.; Cheng Y.; Xi T. In Vitro and in Vivo Studies on Two-Step Alkali-Fluoride-Treated Mg–Zn–Y–Nd Alloy for Vascular Stent Application: Enhancement in Corrosion Resistance and Biocompatibility. ACS Biomater. Sci. Eng. 2019, 5, 3279–3292. 10.1021/acsbiomaterials.9b00140. [DOI] [PubMed] [Google Scholar]
- Lin D.; Hung F.; Yeh M.; Lee H.; Lui T. Development of a novel micro-textured surface using duplex surface modification for biomedical Mg alloy applications. Mater. Lett. 2017, 206, 9–12. 10.1016/j.matlet.2017.06.047. [DOI] [Google Scholar]
- Sankara Narayanan T.S.N.; Park I. S.; Lee M. H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. 10.1016/j.pmatsci.2013.08.002. [DOI] [Google Scholar]
- Xia W.; Li N.; Deng B.; Zheng R.; Chen Y. Corrosion behavior of a sol-gel ZrO2 pore-sealing film prepared on a microarc oxidized aluminum alloy. Ceram. Int. 2019, 45, 11062–11067. 10.1016/j.ceramint.2019.02.192. [DOI] [Google Scholar]
- Fonseca J. H.; Bagne L.; Meneghetti D. H.; Santos G. M. T.; Esquisatto M. A. M.; Andrade T. A. M.; Amaral M. E. C.; Felonato M.; Caetano G. F.; Santamaria M.; Mendonca F. A. S. Electrical stimulation: Complementary therapy to improve the performance of grafts in bone defects?. J. Biomed. Mater. Res., Part B 2019, 107, 924–932. 10.1002/jbm.b.34187. [DOI] [PubMed] [Google Scholar]
- Castro Y.; Durán A. Control of degradation rate of Mg alloys using silica sol–gel coatings for biodegradable implant materials. J. Sol-Gel Sci. Technol. 2019, 90, 198–208. 10.1007/s10971-018-4824-6. [DOI] [Google Scholar]
- Agarwal S.; Morshed M.; Labour M.; Hoey D.; Duffy B.; Curtin J.; Jaiswal S. Enhanced corrosion protection and biocompatibility of a PLGA- silane coating on AZ31 Mg alloy for orthopaedic applications. RSC Adv. 2016, 6, 113871–113883. 10.1039/C6RA24382G. [DOI] [Google Scholar]
- Bakhsheshi-Rad H.; Hamzah E.; Ismail A.; Daroonparvar M.; Yajid M.; Medraj M. Preparation and characterization of NiCrAlY/nano-YSZ/PCL composite coatings obtained by combination of atmospheric plasma spraying and dip coating on Mg-Ca alloy. J. Alloys Compd. 2016, 658, 440–452. 10.1016/j.jallcom.2015.10.196. [DOI] [Google Scholar]
- Park M.; Lee J.; Park C.; Lee S.; Seok H.; Choy Y. Polycaprolactone coating with varying thicknesses for controlled corrosion of magnesium. J. Coat. Technol. Res. 2013, 10, 695–706. 10.1007/s11998-013-9474-6. [DOI] [Google Scholar]
- Prabhu D.; Gopalakrishnan P.; Ravi K. Coatings on implants: Study on similarities and differences between the PCL coatings for Mg based lab coupons and final components. Mater. Des. 2017, 135, 397–410. 10.1016/j.matdes.2017.09.043. [DOI] [Google Scholar]
- Guo Y.; Yu Y.; Han L.; Ma S.; Zhao J.; Chen H.; Yang Z.; Zhang F.; Xia Y.; Zhou Y. Biocompatibility and osteogenic activity of guided bone regeneration membrane based on chitosan-coated magnesium alloy. Mater. Sci. Eng., C 2019, 100, 226–235. 10.1016/j.msec.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Mousa H.; Abdal-hay A.; Bartnikowski M.; Mohamed I.; Yasin A.; Ivanovski S.; Park C.; Kim C. A Multifunctional Zinc Oxide/Poly(Lactic Acid) Nanocomposite Layer Coated on Magnesium Alloys for Controlled Degradation and Antibacterial Function. ACS Biomater. Sci. Eng. 2018, 4, 2169–2180. 10.1021/acsbiomaterials.8b00277. [DOI] [PubMed] [Google Scholar]
- Wang C.; Yi Z.; Sheng Y.; Tian L.; Qin L.; Ngai T.; Lin W. Development of a novel biodegradable and anti-bacterial polyurethane coating for biomedical magnesium rods. Mater. Sci. Eng., C 2019, 99, 344–356. 10.1016/j.msec.2019.01.119. [DOI] [PubMed] [Google Scholar]
- Tiyyagura H.; Fuchs-Godec R.; Gorgieva S.; Arthanari S.; Mohan M.; Kokol V. Biomimetic gelatine coating for less-corrosive and surface bioactive Mg–9Al–1Zn alloys. J. Mater. Res. 2018, 33, 1449–1462. 10.1557/jmr.2018.65. [DOI] [Google Scholar]
- Li B.; Niu J.; Liu H.; Li G. Fabrication and corrosion property of novel 3-aminopropyltriethoxy-modified calcium phosphate/poly(lactic acid) composite coating on AZ60 Mg alloy. Appl. Phys. A: Mater. Sci. Process. 2018, 124, 825. 10.1007/s00339-018-2240-y. [DOI] [Google Scholar]
- Jin J.; Zhou S.; Duan H. Preparation and properties of heat treated FHA@PLA composition coating on micro-oxidized AZ91D magnesium alloy. Surf. Coat. Technol. 2018, 349, 50–60. 10.1016/j.surfcoat.2018.05.043. [DOI] [Google Scholar]
- Li Y.; Yang H.; He Y.; Zhang Q.; Shi Y.; Chen Y. Properties of UV-irradiated TiO2, ZrO2, and TiO2-ZrO2 films as pore-sealing layers on micro-arc-oxidized aluminum alloys. J. Sol-Gel Sci. Technol. 2019, 10.1007/s10971-019-05164-3. [DOI] [Google Scholar]
- Zhang Y.; Lin T. Influence of duty cycle on properties of the superhydrophobic coating on an anodized magnesium alloy fabricated by pulse electrodeposition. Colloids Surf., A 2019, 568, 43–50. 10.1016/j.colsurfa.2019.01.078. [DOI] [Google Scholar]
- Huang W.; Xu B.; Yang W.; Zhang K.; Chen Y.; Yin X.; Liu Y.; Ni Z.; Pei F. Corrosion behavior and biocompatibility of hydroxyapatite/magnesium phosphate/zinc phosphate composite coating deposited on AZ31 alloy. Surf. Coat. Technol. 2017, 326, 270–280. 10.1016/j.surfcoat.2017.07.066. [DOI] [Google Scholar]
- Dehghanian C.; Aboudzadeh N.; Shokrgozar M. Characterization of silicon- substituted nano hydroxyapatite coating on magnesium alloy for biomaterial application. Mater. Chem. Phys. 2018, 203, 27–33. 10.1016/j.matchemphys.2017.08.020. [DOI] [Google Scholar]
- Syu J.; Uan J.; Lin M.; Lin Z. Optically transparent Li–Al–CO3 layered double hydroxide thin films on an AZ31 Mg alloy formed by electrochemical deposition and their corrosion resistance in a dilute chloride environment. Corros. Sci. 2013, 68, 238–248. 10.1016/j.corsci.2012.11.023. [DOI] [Google Scholar]
- Han J.; Blawert C.; Tang S.; Yang J.; Hu J.; Zheludkevich M. Effect of Surface Pre-Treatments on the Formation and Degradation Behaviour of a Calcium Phosphate Coating on Pure Magnesium. Coatings 2019, 9, 259. 10.3390/coatings9040259. [DOI] [Google Scholar]
- Aboudzadeh N.; Dehghanian C.; Shokrgozar M. Effect of electrodeposition parameters and substrate on morphology of Si-HA coating. Surf. Coat. Technol. 2019, 375, 341–351. 10.1016/j.surfcoat.2019.07.016. [DOI] [Google Scholar]
- Bakhsheshi-Rad H.; Ismail A.; Aziz M.; Hadisi Z.; Omidi M.; Chen X. Antibacterial activity and corrosion resistance of Ta2O5 thin film and electrospun PCL/MgO-Ag nanofiber coatings on biodegradable Mg alloy implants. Ceram. Int. 2019, 45, 11883–11892. 10.1016/j.ceramint.2019.03.071. [DOI] [Google Scholar]
- Yu X.; Ibrahim M.; Liu Z.; Yang H.; Tan L.; Yang K. Biofunctional Mg coating on PEEK for improving bioactivity. Bioactive Materials 2018, 3, 139–143. 10.1016/j.bioactmat.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuola M.; Minan A.; Grillo C.A.; Cortizo M.C.; Fernandez Lorenzo de Mele M.A. Corrosion protection of AZ31 alloy and constrained bacterial adhesion mediated by a polymeric coating obtained from a phytocompound. Colloids Surf., B 2018, 172, 187–196. 10.1016/j.colsurfb.2018.08.025. [DOI] [PubMed] [Google Scholar]
- Chen J.; Zhang Y.; Ibrahim M.; Etim I.; Tan L.; Yang K. In vitro degradation and antibacterial property of a copper-containing micro-arc oxidation coating on Mg-2Zn-1Gd-0.5Zr alloy. Colloids Surf., B 2019, 179, 77–86. 10.1016/j.colsurfb.2019.03.023. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Shi L.; Ji X.; Li J.; Han Z.; Li S.; Zeng R.; Zhang F.; Wang Z. Corrosion resistance and antibacterial properties of polysiloxane modified layer-by-layer assembled self-healing coating on magnesium Alloy. J. Colloid Interface Sci. 2018, 526, 43–50. 10.1016/j.jcis.2018.04.071. [DOI] [PubMed] [Google Scholar]
- Zou Y.; Wang J.; Cui L.; Zeng R.; Wang Q.; Han Q.; Qiu J.; Chen X.; Chen D.; Guan S.; Zheng Y. Corrosion resistance and antibacterial activity of zinc-loaded montmorillonite coatings on biodegradable magnesium alloy AZ31. Acta Biomater. 2019, 98, 196–214. 10.1016/j.actbio.2019.05.069. [DOI] [PubMed] [Google Scholar]
- Pei Y.; Zhang G.; Zhang C.; Wang J.; Hang R.; Yao X.; Zhang X. Corrosion resistance, anticoagulant and antibacterial properties of surface-functionalized magnesium alloys. Mater. Lett. 2019, 234, 323–326. 10.1016/j.matlet.2018.09.140. [DOI] [Google Scholar]
- Luo D.; Liu Y.; Yin X.; Wang H.; Han Z.; Ren L. Corrosion inhibition of hydrophobic coatings fabricated by micro-arc oxidation on an extruded Mg-5Sn-1Zn alloy substrate. J. Alloys Compd. 2018, 731, 731–738. 10.1016/j.jallcom.2017.10.017. [DOI] [Google Scholar]
- Cui L.; Liu H.; Tang S.; Hu J.; Lu X.; Blawert C.; Lin T.; et al. Corrosion resistance of a superhydrophobic micro-arc oxidation coating on Mg-4Li-1Ca alloy. J. Mater. Sci. Technol. (Shenyang, China) 2017, 33, 1263–1271. 10.1016/j.jmst.2017.10.010. [DOI] [Google Scholar]
- Xu W.; Song J.; Sun J.; Lu Y.; Yu Z. Rapid Fabrication of Large-Area, Corrosion-Resistant Superhydrophobic Mg Alloy Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. 10.1021/am2010527. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Gu Z.; Xin Y.; Yuan N.; Ding J. Facile formation of super-hydrophobic nickel coating on magnesium alloy with improved corrosion resistance. Colloids Surf., A 2018, 538, 500–505. 10.1016/j.colsurfa.2017.11.055. [DOI] [Google Scholar]
- Ma N.; Chen Y.; Zhao S.; Li J.; Shan B.; Sun J. Preparation of super-hydrophobic surface on Al–Mg alloy substrate by electrochemical etching. Surf. Eng. 2019, 35, 394–402. 10.1080/02670844.2017.1421883. [DOI] [Google Scholar]
- Li Z.; Yu Q.; Zhang C.; Liu Y.; Liang J.; Wang D.; Zhou F. Synergistic effect of hydrophobic film and porous MAO membrane containing alkynol inhibitor for enhanced corrosion resistance of magnesium alloy. Surf. Coat. Technol. 2019, 357, 515–525. 10.1016/j.surfcoat.2018.10.054. [DOI] [Google Scholar]
- Wu L.; Wu J.; Zhang Z.; Zhang C.; Zhang Y.; Tang A.; Li L.; Zhang G.; Zheng Z.; Atrens A.; Pan F. Corrosion resistance of fatty acid and fluoroalkylsilane-modified hydrophobic Mg-Al LDH films on anodized magnesium alloy. Appl. Surf. Sci. 2019, 487, 569–580. 10.1016/j.apsusc.2019.05.121. [DOI] [Google Scholar]
- Wei D.; Wang J.; Wang H.; Liu Y.; Li S.; Li D. Anti-corrosion behaviour of superwetting structured surfaces on Mg-9Al-1Zn magnesium alloy. Appl. Surf. Sci. 2019, 483, 1017–1026. 10.1016/j.apsusc.2019.03.286. [DOI] [Google Scholar]
- Ji P.; Long R.; Hou L.; Wu R.; Zhang J.; Zhang M. Study on hydrophobicity and wettability transition of Ni-Cu-SiC coating on Mg-Li alloy. Surf. Coat. Technol. 2018, 350, 428–435. 10.1016/j.surfcoat.2018.07.038. [DOI] [Google Scholar]
- Li D.; Wang H.; Luo D.; Liu Y.; Han Z.; Ren L. Corrosion resistance controllable of biomimetic superhydrophobic microstructured magnesium alloy by controlled adhesion. Surf. Coat. Technol. 2018, 347, 173–180. 10.1016/j.surfcoat.2018.04.078. [DOI] [Google Scholar]
- Wang P.; Xiong P.; Liu J.; Gao S.; Xi T.; Cheng Y. A silk-based coating containing GREDVY peptide and heparin on Mg–Zn–Y–Nd alloy: improved corrosion resistance, hemocompatibility and endothelialization. J. Mater. Chem. B 2018, 6, 966–978. 10.1039/C7TB02784B. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li X.; Chen M.; Zhao Y.; You C.; Li Y.; Chen G. In Vitro and in Vivo Degradation Behavior and Biocompatibility Evaluation of Microarc Oxidation-Fluoridated Hydroxyapatite-Coated Mg–Zn–Zr–Sr Alloy for Bone Application. ACS Biomater. Sci. Eng. 2019, 5, 2858–2876. 10.1021/acsbiomaterials.9b00564. [DOI] [PubMed] [Google Scholar]


