Abstract
Bioinspired superhydrophobic surfaces have attracted great interest due to their special functions and wide applications. However, it is still a big challenge to construct a durable superhydrophobic coating for large-scale applications due to its easy destruction by the mechanochemical attack. In this mini-review, we present the state-of-the-art developments in the rational design of mechanochemical durable and self-healing superhydrophobic surfaces. First, the mechanically durable superhydrophobic surfaces are constructed to endure mechanical damage by adjusting the surface morphology and increasing the binding force between the substrates and the modified materials. Second, chemical damages also have been taken into consideration to develop chemically robust superhydrophobic surfaces, such as chemical etching, ultraviolet (UV)-light irradiation, and bioerosion, etc. Third, endowing superhydrophobic coatings with self-healing function can effectively improve the durability and prolong the lifespan of the coatings by releasing low-surface-energy agents or regenerating topographic structures. Finally, the challenges and future perspectives in developing super durable bioinspired superhydrophobic surfaces by structure design and chemistry control are discussed. The innovative points provided in this mini-review will provide deep fundamental insight for prolonging the lifetime of the superhydrophobic surfaces and enable their practical applications in the near future.
Introduction
By mimicking the natural organisms, such as lotus leaves and gecko feet, bioinspired surfaces with special wettability have been successfully constructed by rational design, which exhibit a high water contact angle exceeding 150° and a sliding angle lower than 10°. As we know, superhydrophobic surfaces should have two features: (i) appropriate hierarchical micro-/nanostructures and (ii) low surface energy.1 Due to the unique superhydrophobicity and oleophobicity, the superwetting surfaces have been widely used in self-cleaning, anti-icing, water collection, and oil–water separation, etc.2
Recently, superhydrophobic surfaces have been fabricated not only by “top-down” or “bottom-up” methods on bulk materials but also by multiple steps via creating rough surfaces first and then modified with low-surface-energy materials.3 The fabrication techniques include the solvothermal/hydrothermal method, chemical vapor deposition, dip/spray-coating technique, electrospinning, etc. However, the development of artificial superhydrophobic surfaces for real-world applications is severely limited by the poor mechanical and chemical stability. The superhydrophobic surfaces usually lose their special wettability due to the destruction of topographic structures and depletion of low-surface-energy agents when subjecting to scratch, washing, and abrasion, or working in an acid/alkali/salt environment, high temperature, and under UV-light irradiation. For example, a facile one-step approach was adopted to fabricate superhydrophobic coatings via the sol–gel processing of long-chain fluoroalkylsilane.4 However, the coating was easily damaged by a pencil scratch due to the low adhesion between fluoroalkylsilane and glass. Therefore, it is essential to construct mechanochemical robust superhydrophobic coatings by increasing the adhesion with the substrates.
As an alternative to the design of mechanochemical durable surfaces, superhydrophobic surfaces with intelligent self-healing functions have been developed to overcome durability challenges and prolong the lifespan of the superhydrophobic coatings.5 Inspired by living organisms, releasing low-surface-energy agents and reforming topographic structures are two main techniques to fabricate self-healing superhydrophobic surfaces. The former method can be easily realized by storing the low-surface-energy components inside the rough structures and then releasing them onto the damaged surface during the self-healing process, while the latter method can be carried out by regenerating the hierarchical micro-/nanostructures again by some catalysts or triggers, which is comparatively difficult and not normally used.6 However, how to accelerate the migration speed of low-surface-energy components onto the damaged surface and regenerate topographic structures via a facile method is still a critical challenge.
In this mini-review, we present the state-of-the-art developments in the rational design of mechanochemical durable and self-healing superhydrophobic surfaces (Figure 1), including dip/spray coating, electrospinning, wet chemical method, a layer-by-layer assembly technique, 3D printing, and phase separation technique, etc. First, the physical and chemical methods, such as a combination of micro- and nanostructure design, cross-linking technique, forming covalent bonds, and introducing elastic and soft materials, are introduced to synthesize mechanically durable superhydrophobic surfaces. Second, chemical robust superhydrophobic coatings have been developed to endure chemical etching, such as UV-light irradiation, bioerosion, and high-temperature attack, etc. Third, two main strategies to endow superhydrophobic coatings with self-healing function by releasing low-surface-energy agents and regenerating topographic structures are summarized, which can effectively enhance the durability and prolong the lifetime of the superhydrophobic coatings. Finally, the challenges and future perspectives in developing super durable bioinspired superhydrophobic surfaces for practical applications are discussed. This mini-review will provide fundamental insight into understanding the working mechanism of fabricating mechanochemical robust superhydrophobic surfaces, thus inspiring researchers to explore advanced methods for improving the durability and prolonging the lifetime of superhydrophobic surfaces at a low cost.
Figure 1.
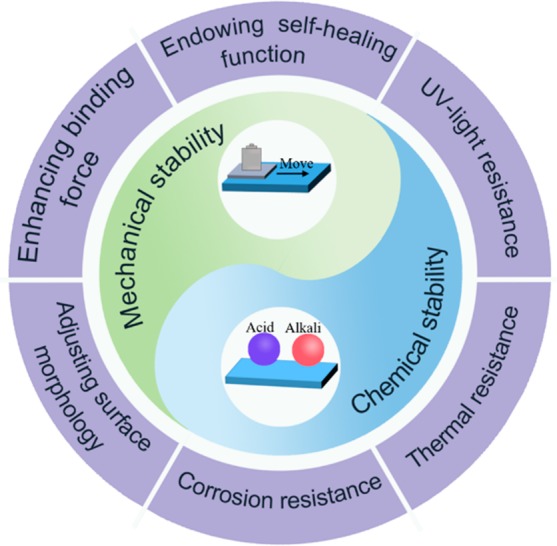
Rational design of mechanochemical durable and self-healing superhydrophobic surfaces by different strategies.
Mechanically Durable Superhydrophobic Surfaces
With the rapid development of nanotechnology and materials science, superhydrophobic surfaces have been widely used in our daily life. When compared to low-surface-energy factors, surface roughness is a more vital element to construct mechanically durable superhydrophobic surfaces, which can be destroyed by mechanical attack. Therefore, keeping the hierarchical structure intact and improving the adhesion with the substrates are two effective ways to endure mechanical damage. It can be realized by adjusting the surface morphology and increasing the binding force between modified materials and substrates via cross-linking, forming covalent bonds, and introducing elastic/soft materials.
Adjusting the Surface Morphology
Encapsulating nanocomposite coatings with a polymer film is an important way to enhance the durability of superhydrophobic surfaces. The incorporated nanoparticles provide rough structures, while the hydrophobic polymers act as nanoparticle binding and provide low tension surface.7 This process can be easily realized by a simple layer-by-layer assembly technique, dip-/spray-coating, and a wet chemical method. When the nanostructures are fixed stably on the substrate, the combination of superhydrophobicity with the smart surface texture brings a mechanical robust coating (Figure 2a).
Figure 2.
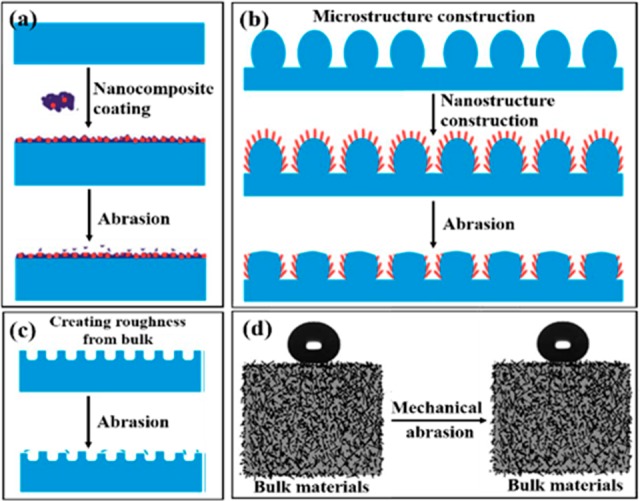
(a) Mechanical durability of nanocomposite coating surface, (b) optimized rough micro-/nanostructure surface, (c) creating micro-/nanostructures on a bulk surface, and (d) filling hydrophobic particles into bulk materials. (a–c) Reproduced with permission from ref (7). (d) Reproduced with permission from ref (10).
Since microstructures can stand more pressure than nanostructures, many researchers have fabricated hierarchical micro-/nanostructures by using the nanostructures to enhance the roughness and/or lower the surface energy and microroughness as a cushion and shelter to improve the mechanical stability and protect fragile nanoscaled topographies. As displayed in Figure 2b, optimizing the hierarchical micro-/nanostructures can effectively enhance their mechanical stability. Our group has fabricated raspberry-like hierarchical structures consisting of micro-/nanoscaled particles by an aerosol-assisted chemical vapor deposition.8 It displayed superior robustness and retained a similar structure even after tape-peeling and sandpaper abrasion. Benefitting from this design, it can be applied on various substrates to resist chemical damage in any harsh environment.
Besides, creating micro-/nanostructures on inherent bulk materials and filling hydrophobic micro-/nanoparticles into bulk materials are also effective means to endure the mechanical attack. Roughness could be directly grown on the surface of bulk materials by chemical etching, electrochemical anodization, template method, and 3D printing method. The roughness comes from the substrate matrix as a whole, which avoids the interfacial problems, leading to good adhesion with the substrate and excellent mechanical durability when compared to the nanocoatings deposited on the substrate (Figure 2c). For example, a biomimetic superhydrophobic surface with eggbeater structure was innovatively developed by a 3D printing technique.9 This method can introduce hierarchical roughness and low-surface-energy agents simultaneously, which is promising for large-scale production with low cost and high speed. Similarly, the modified bulk materials exhibit superior durability of superhydrophobicity and long lifetime because the encapsulated particles inside are difficult to be removed by mechanical abrasion (Figure 2d), such as electrospinning nanofibers, graphene-based aerogels, etc.10,11
Increasing the Binding Force between the Modified Materials and the Substrate
Compared to adjusting the surface morphology, increasing the binding force between the coating layer and substrate is a more facile and promising strategy to improve the mechanical durability of superhydrophobic coatings for commercialization, which can be divided into three routes: forming covalent bonds between the coating layer and substrate, cross-linking with the coating layer, and introducing elastic/soft materials into the coating layer.
Forming Covalent Bonds between the Coating Layer and the Substrate
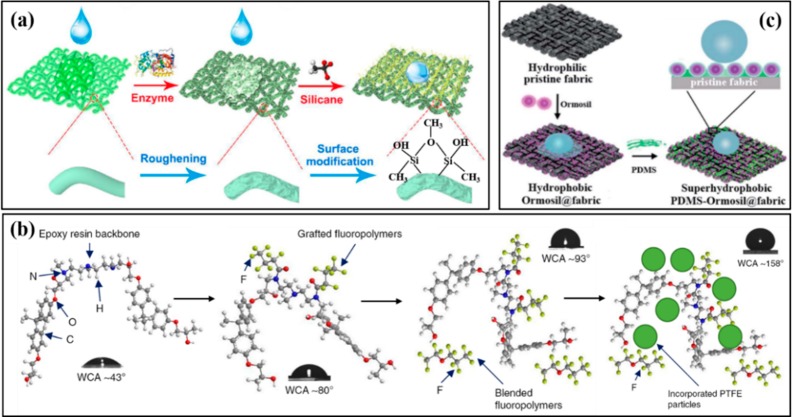
Compared to van der Waals forces, forming covalent bonds between the coating layer and substrate is much stronger to endure the mechanical and chemical attack. However, it requires both the substrate (usually fabric, glass, stone, etc.) and the modified micro-/nanoparticles (usually inorganic such as TiO2, SiO2, etc.) to have chemical reactive groups. Cellulose-based fabric, such as cotton, silk fabric, etc., with hydroxyl groups can react with silanol, epoxide, or azide groups to form covalent bonds first and then are decorated by hydrophobic low-surface-energy agents, resulting in superhydrophobicity, while for glass, metal, and other synthetic fabrics, they inherently have less reactive groups on their surfaces and must be pretreated by chemical and plasma etching treatment, etc., or be predecorated by mussel-inspired dopamine to form amino and hydroxyl groups on its surface. For example, our group has prepared superhydrophobic cellulose-based fabrics (silk, cotton, and wool fabric) by an environmentally friendly enzyme-etching approach, followed by modification with methyltrichlorosilane via a chemical bond (Figure 3a).12 It was demonstrated that the superhydrophobic cellulose-based fabrics exhibited excellent mechanical durability against the long cycling abrasion and laundering tests. In short, the covalent bonds can effectively enhance the adhesion with the substrate, making the superhydrophobic surfaces endure a more severe mechanochemical attack than that of the superhydrophobic coating on the substrate only with physical contact.
Figure 3.
Schematic illustration for fabricating mechanochemical durable superhydrophobic (a) enzyme-etching fabric with a covalent bond, (b) cross-linked fluoropolymers/epoxy/PTEE superhydrophobic coating, and (c) PDMS-modified cotton fabric, respectively. (a) Reproduced with permission from ref (12). (b) Reproduced with permission from ref (1). (c) Reproduced with permission from ref (17).
Cross-Linking with the Coating Layer
In addition to forming covalent bonds, cross-linking with the coating layer has been considered as another promising technique to improve the durability of superhydrophobic coatings.13 This method can be used for both polymer coating and polymeric nanocomposite coating, which can be realized by the addition of cross-linking agents and catalysts or induced by UV-light irradiation and heating treatment, etc. After cross-linking, the polymer can form a stable network, and the micro-/nanoparticles are encapsulated by the network with a strong bond, improving the adhesion between the network and the substrate simultaneously. For example, Liu et al. successfully constructed superhydrophobic coatings on glasses with a three-dimensional continuous network and low-surface-energy hierarchical structure by a facile UV-induced cross-linking vinyltriethoxysilane with silica particles.14 Making full use of the 3D continuous superhydrophobic network, the robust coating exhibited outstanding stability to both mechanical damage (sandpaper abrasion) and chemical damage (strong acid/alkali/salt solutions). Besides, Tiwari’s group synthesized a robust superhydrophobic all-organic nanocomposite coating by cross-linking fluoropolymers with epoxy backbone, followed by incoporating polytetrafluoroethylene (PTEE) nanoparticles, which not only possessed mechanical and chemical stability but also exhibited excellent resistance to high-speed liquid impact due to the high adhesion with the substrate (Figure 3b).1 The cross-linking technique can not only increase the binding force between the micro-/nanoparticles and polymer by embedding them inside the network but also enhance the adhesion between the network and the substrate, exhibiting promising prospects in practical applications.
Introducing Elastic/Soft Materials into the Coating Layer
Compared to brittle materials, the nanocomposite coating by incorporating the micro/nanoparticles into elastic and soft materials can endure mechanical damage, and the adhesion with the substrate can even be improved via dispersing energy with deformation.15 The nanocomposite coating with elastic and soft materials is like a spring. When loaded force is released, the superhydrophobic rough surfaces can recover their morphology as the pristine state. At the same time, the elastic and soft materials are compatible with various substrates, such as fabric, metal, and stone. Benefitting from these advantages, large quantities of elastic and soft materials have been developed to construct mechanical robust superhydrophobic surfaces by a dip-/spray-coating technique, such as PDMS, resin, and polyurethane, etc.16 Among them, PDMS is the most widely used fluorine-free elastic polymer binder because of its wonderful mechanochemical stability and high adhesion with the substrate, which was widely used to synthesize superhydrophobic coatings by phase separation. For example, our group synthesized superhydrophobic cotton fabrics by first decorating a silica aerogel film, followed by PDMS top-coating via a simple dip-coating technique (Figure 3c).17 Due to the high binding strength between the superhydrophobic coating and cotton fabric, it can withstand 100 cycles of abrasion and 5 cycles of accelerated machine wash, without any morphology changes. Besides, it was very stable in strongly acidic and alkaline solutions. Recently, an unstable system for creating hierarchical micro-/nanostructures via phase separation is also raised for constructing superhydrophobicity. Kang et al. successfully fabricated a superhydrophobic coating driven by Marangoni instability and photopolymerization of PDMS.18 Under UV irradiation, the uneven evaporation and cluster formation of the polymer surfaces in ethanol formed micro- and nanoscale roughness. PDMS acted as a binder and provided low-surface-energy agents simultaneously. This approach provides a rapid and efficient route to the generation of superhydrophobic surfaces, which may be extended to a wide variety of polymers. It is also promising for commercialization of superhydrophobic surfaces with an eco-friendly approach without using any toxic organic solvent solutions.
Chemically Robust Superhydrophobic Surfaces
In addition to the physical resistance against mechanical damage, chemical attack should also be taken into consideration in the real-world applications, which destroys the rough structures and exhausts the low-surface-energy agents, resulting in loss of superhydrophobicity. The UV-light irradiation resistance, corrosion resistance, and thermal resistance will be discussed in the following sections.
UV-Light Irradiation Resistance
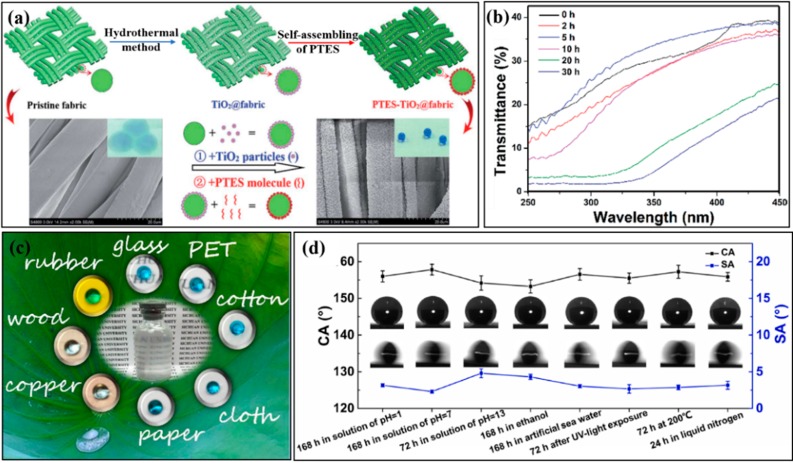
The superhydrophobic surfaces outdoors are prone to be destroyed by UV-light irradiation, especially for outdoor-wearing textiles, tents, canopy covers, etc. The UV-light irradiation not only accelerates the aging process of the substrate but also destroys the structure of the superhydrophobic components. Therefore, employing UV-light absorption and inert inorganic micro-/nanoparticles (TiO2, ZnO, SiO2, etc.) has been adopted to resist UV-light damage. Titanium dioxide has been widely used to fabricate superhydrophobic surfaces with UV-light irradiation resistance due to its excellent UV-light absorption property, chemical stability, and high bond energy between hydrophobic substrates and agents. For example, our group decorated cotton fabric with flower-like TiO2 nanoparticles by a facile hydrothermal method first, followed by fluoroalkylsilane modification (Figure 4a).19 The TiO2@fabric not only exhibited excellent resistance to abrasion but also displayed effectiveness in shielding UV radiation due to high ultraviolet absorbance and the scattering characteristics of flower-like TiO2 nanostructures (Figure 4b).
Figure 4.
(a) Schematic illustration of the procedure to construct superhydrophobic TiO2 particle-decorated cotton fabric. (b) UV transmission measurements for various deposition durations of TiO2 nanoparticles coated onto cotton fabric samples. (c) A fluorine-free superhydrophobic coating with high flexibility on different substrates. (d) Water contact angle (CA) and sliding angle (SA) on the superhydrophobic surface after the treatment at different conditions. (a,b) Reproduced with permission from ref (19). (c,d) Reproduced with permission from ref (22).
Apart from inorganic micro-/nanoparticles, some polymers (like polypyrrole) and carbon materials (like graphene, CNT) also have the ability to resist UV-light irradiation. For example, robust superhydrophobic poly(ethylene terephthalate) fabrics were successfully synthesized by coating with CNT and PDMS by an immersing technique.20 The as-obtained fabrics possess remarkable mechanical durability to washing and abrasion and chemical stability against different pH solutions and UV light. However, the color of the bulk materials will become black and may not be suitable for fabrics.
Corrosion Resistance
For corrosion resistance, acid, alkaline, and salt environments have a great influence on the durability and lifetime of the superhydrophobic surfaces. When exposed directly to the corrosive solution, the superhydrophobic molecules can easily be destroyed, and the superhydrophobic coating would detach from the substrate due to the hydrolysis of chemical interfacial bonding. Up to now, stabilizing the superhydrophobic coating and keeping a stable air layer between the rough structures and solution are the two main approaches to resist various kinds of chemical damages. For example, our group coated fabrics with polydopamine first and then modified them with the stearic acid to synthesize superhydrophobic surfaces, exhibiting chemical stability in alkali and salt solutions due to the stability and strong binding force of the superhydrophobic coatings.21 Besides, a fluorine-free superhydrophobic coating was prepared by functionalized silica nanoparticles and polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene, which has been applied on different substrates by a facile dip/spray-coating technique, such as glass, metal, and polymeric matrix (Figure 4c).22 The as-created surfaces displayed strong resistance not only to chemical corrosions (immersed in acid, alkaline, and salt solution for a long time) but also to high/low temperature and UV irradiation, without losing superhydrophobic property (Figure 4d).
What’s more, the bioerosion (like fungus and bacteria) is also noteworthy. It will destroy the intrinsic structure of the materials, resulting in metal rusting and degradation of cellulose-based fabrics. Therefore, constructing superhydrophobic coatings and incorporating transition metals or noble metals are effective and efficient for bioerosion resistance. Li’s group fabricated superhydrophobic cotton-derived carbon fibers modified with nanocopper by a facile and scalable method.23 It exhibited outstanding antibacterial activity against Escherichia coli and Staphylococcus aureus with antibacterial ratios of >92% due to the superhydrophobicity and strong oxidizing ability of the oxygen reactive radicals by introduction of Cu. Similarly, a superhydrophobic fabric with durable photo-, bio-, and magneto-activities was also developed by in situ synthesis of TiO2@Fe3O4@Ag nanocomposites on its surface.24 These facile methods are effective and efficient for fabricating superhydrophobic surface with bioerosion property.
Thermal Resistance
In addition to UV-light irradiation and corrosion damage, thermal resistance has been widely investigated to prolong the lifespan of the superhydrophobic surfaces because the structure and chemical interfacial bonds can be easily destroyed at high temperatures. Recently, modifying the surface coating with inorganic materials, Cl–-containing materials, graphene, CNT, and other novel materials has been adopted to construct fire-resistant superhydrophobic surfaces. For example, our group has synthesized 3D superhydrophobic aluminum hydroxide nanoparticle-coated cellulose composite aerogels.25 Due to the existence of the inorganic nanoparticles in the bulk materials, the cellulose composite aerogels exhibited remarkable mechanical stability, thermal stability, and fire retardancy.
Except for the aforementioned harsh environmental conditions, some other extreme conditions, such as low temperature, low/high pressure, high humidity, etc., should also be paid much attention to construct chemically robust superhydrophobic surfaces.
Intelligent Self-Healing Superhydrophobic Surfaces
Inspired by the naturally superhydrophobic insect wings and plant leaves, always keeping special wettability throughout their lifetime, endowing artificial superhydrophobic surfaces with intelligent self-healing function has been proposed to maintain mechanochemical durability. They are meaningful and promising in practical applications to stand much more severe destructions, such as knife-cut, scratch, etc. The self-healing process can be realized by two means: releasing low-surface-energy agents and regenerating topographic structures.
Releasing Low-Surface-Energy Agents
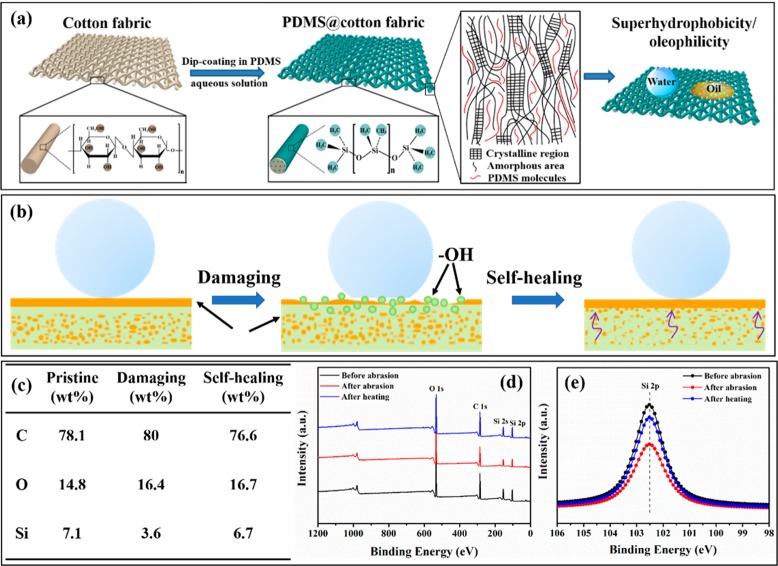
When the hydrophobic leaves are damaged, they can regenerate the hydrophobic epicuticular wax layer and maintain superhydrophobicity throughout its lifetime. By mimicking this phenomenon, releasing low-surface-energy agents inside is the most commonly used technique to construct artificial robust and self-healing superhydrophobic surfaces. Generally speaking, large quantities of low-surface-energy agents (usually fluoroalkylsilane, PDMS, etc.) are injected into the inner space of the rough matrix by the dip-/spray-coating method. Once the superhydrophobic coating is decomposed or degraded by mechanochemical attack (abrasion, washing, plasma damage, etc.), the superhydrophobic molecules will migrate to the damaged surface when triggered by mechanical force, high temperature, and UV-light, which exhibits higher self-healing speed than longtime placement at room temperature. Recently, we fabricated a mechanochemically robust superhydrophobic cotton fabric with intelligent self-healing nature by a “PDMS-in-water” emulsion approach via phase separation (Figure 5a).26 After dip-coating and drying, PDMS was uniformly dispersed in cotton fiber and grafted on the bulk surface of cotton fabric via a strong interfacial binding force, enhancing inherent hierarchical microstructures and decreasing the surface energy simultaneously. Therefore, the superhydrophobic coatings not only displayed superior chemical stability but also could repair the superhydrophobicity throughout the whole lifetime though damaged by machine washing or abrasion, due to the self-diffusion process of PDMS molecules from the inner part to the outer surface of the cotton fibers to minimize surface free energy (Figure 5b–e). This environmentally friendly and cost-effective method can be employed on various materials in the nanoscience field for environment and energy applications. Besides, Zhang et al. first fabricated totally waterborne, mechanically robust, and self-healing superhydrophobic coatings by a combination of polyurethane adhesive and hexadecyl polysiloxane-modified SiO2.27 Except for the high mechanical stability and chemical stability against corrosive liquids and UV irradiation, the coatings also showed fast and stable self-healing capability owing to migration of the healing agent (hexadecyl polysiloxane) to the damaged surface. However, when the low-surface-energy components are exhausted, the superhydrophobicity cannot be recovered any more. From this view, self-healing superhydrophobic surfaces can be achieved by modifying the rough surfaces with the superhydrophobic agents again via the dip-/spraying-coating method.
Figure 5.
(a) Schematic illustration of the procedure to construct superhydrophobic PDMS@cotton fabric and (b) working mechanism of self-healing process by releasing low-surface-energy agents. (c) Line scan results of content changes of C, O, and Si elements and (d) wide and (e) narrow XPS spectra of PDMS@cotton fabric before abrasion, after abrasion, and after heating treatment. Reproduced with permission from ref (26).
Regenerating Topographic Structures
Traditionally, hierarchical micro-/nanoparticles have low adhesion with the substrate, and they can easily peel off from the substrate after mechanical or chemical destruction. Therefore, regenerating topographic structures is another important strategy for constructing robust self-healing superhydrophobic surfaces. Chen’s group synthesized a self-healing superhydrophobic surface containing polystyrene, fluorinated SiO2 nanoparticles, low-surface-energy agents, and TiO2 nanoparticles.28 When the micro-/nanostructures were destroyed by mechanical abrasion, polystyrene was decomposed to regenerate the roughness by the photocatalysis of TiO2 nanoparticles under UV-light irradiation to maintain superhydrophobicity. Recently, shape-memory polymers have appeared to fabricate the mechanochemical robust and self-healing superhydrophobic surfaces.29 Though it was damaged by mechanical force and the surface became flat, it would recover its original roughness due to the memory effect. However, there are little works on regenerating rough structures. Therefore, it is still a big challenge to be addressed, and more efforts should be put in this area.
Conclusions and Outlook
In summary, the state-of-the-art fabrication methods on mechanochemical durable and self-healing superhydrophobic surfaces are presented in this mini-review. The strategies of adjusting surface morphology and increasing the adhesion with the substrate are firstly introduced to synthesize mechanically durable superhydrophobic surfaces. Then, chemically robust superhydrophobic surfaces have been developed to endure chemical etching, such as UV-light irradiation, bioerosion, and high temperature, etc. Endowing superhydrophobic coatings with self-healing function by releasing low-surface-energy agents and reforming topographic structures can also enhance the durability and prolong the lifetime of the superhydrophobic coatings. These strategies all contribute to constructing mechanochemical robust superhydrophobic surfaces and enable them in practical applications in the following decades.
Though great progress has been achieved in constructing durable and self-healing superhydrophobic surfaces, there still exist some critical challenges. First, the superhydrophobic coatings usually contain fluorine-containing compounds and are often synthesized by the wet chemical method using organic solvent, which is toxic and harmful to human beings. It is essential to construct artificial robust and self-healing superhydrophobicity via an environmentally friendly technique. Second, though the adhesion with the substrate has been improved by cross-linking or forming covalent bonds, it can only stand mild mechanical damage, such as abrasion, washing, etc. In order to adapt the real-world usage, it is meaningful to construct durable superhydrophobic surfaces against severe mechanical damages (deep scratch, knife-cut, etc.) by forming much stronger interfacial chemical bonds. Third, most of the self-healing processes can be realized by longtime placement or triggered by mechanical force, heating, and UV-light irradiation, etc. Thus, how to accelerate the speed of the migration/mobility of low-surface-energy molecules onto the damaged surface is still a big challenge. Fourth, the traditional methods for fabricating mechanochemical durable and self-healing superhydrophobic materials are limited to large-scale production and cannot meet the industrial requirements. The novel techniques should be developed for mass production by creating roughness and providing low-surface-energy simultaneously, such as 3D/4D printing, an unstable system for constructing superhydrophobicity via phase separation, etc. Finally, various testing methods have been conducted to evaluate the mechanical and chemical stability of the superhydrophobicity, making it difficult to compare the efficiency of different fabrication techniques. Therefore, the testing methods should be unified according to the actual applications, such as abrasion standard, washing standard, etc. We believe that with more efforts put in this field the artificial mechanochemical durable superhydrophobic surfaces can be successfully constructed with multifunctions within a short time. It can also be applied in various environmental and energy applications, such as textile finishing, oil–water separation, anti-icing, catalysis, and lithium-ion batteries, etc.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (Grant No. 2016YFB0303101), the National Nature Science Foundation of China (Grant Nos. 21501127 and 21875040), and Nantong Science and Technology Project (No. JC2018105). This work was also supported by the Open Project Program of Key Laboratory of Eco-textiles, Ministry of Education, Jiangnan University (No. KLET1803), the Large Instruments Open Foundation of Nantong University (No. KBJN1927), and Science and Technology Development Fund, Macau SAR (file nos. FDCT-0057/2019/A1 and 0092/2019/A2).
Biographies

Mingzheng Ge is a full professor at the School of Textile & Clothing in Nantong University in 2019. He received his Ph.D. degree from the College of Textile and Clothing Engineering in Soochow University in 2018. During 2016-2017, he was a visiting scholar in Nanyang Technological University (NTU, Singapore). His research interests include intelligent surfaces/interfaces with special wettability, advanced materials for photocatalysis, and wearable energy storage electronics.

Yuekun Lai is a Fujian province “Minjiang Scholar” chair professor at the College of Chemical Engineering at Fuzhou University, China. He received his Ph.D. degree from the Department of Chemistry, Xiamen University. During 2009–2011, he worked as a research fellow at the School of Materials Science and Engineering, Nanyang Technological University, Singapore. In July 2011, he moved to Muenster University with Prof. Harald Fuchs and Prof. Lifeng Chi. During 2013–2018, he was a Professor at the School of Textile and Clothing Engineering in Soochow University. He was selected as the 2018, 2019 Highly Cited Researchers (Clarivate) and the recipient of the 2016 Journal of Materials Chemistry A (RSC) Emerging Investigators and 2019 Advanced Materials Interfaces (Wiley) Hall of Fame awards. His current research topics are bioinspired intelligent materials with special wettability, multifunctional fabrics, and TiO2 nanostructures for energy and environmental applications.

Yuxin Tang is an Assistant Professor in the Institute of Applied Physics and Materials Engineering (IAPME) at the University of Macau. He obtained his B.S. and M.S. degrees at the Nanjing University of Aeronautics and Astronautics in 2006 and 2009, respectively, and graduated from Nanyang Technological University (NTU, Singapore) with a Ph.D. in materials science (2013). After his postdoctoral fellow training at NTU, he joined IAPME as Assistant Professor in 2018. His research interest is rational design of advanced functional materials for energy conversion and storage and environmental remediation.
Author Contributions
The manuscript was written through the contributions of all authors.
The authors declare no competing financial interest.
References
- Peng C.; Chen Z.; Tiwari M. K. All-Organic Superhydrophobic Coatings with Mechanochemical Robustness and Liquid Impalement Resistance. Nat. Mater. 2018, 17, 355–360. 10.1038/s41563-018-0044-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Tang Y.; Deng J.; Li W.; Xia H.; Zhu Z.; Lv Z.; Wei J.; Li W.; Persson C.; Malyi O. I.; Antonietti M.; Chen X. Correlating the Peukert’s Constant with Phase Composition of Electrode Materials in Fast Lithiation Processes. ACS Materials Lett. 2019, 1, 519–525. 10.1021/acsmaterialslett.9b00320. [DOI] [Google Scholar]
- Chen K.; Wu Y.; Zhou S.; Wu L. Recent Development of Durable and Self-Healing Surfaces with Special Wettability. Macromol. Rapid Commun. 2016, 37, 463–485. 10.1002/marc.201500591. [DOI] [PubMed] [Google Scholar]
- Liu S.; Liu X.; Latthe S.; Gao L.; An S.; Yoon S.; Liu B.; Xing R. Self-Cleaning Transparent Superhydrophobic Coatings through Simple Sol-Gel Processing of Fluoroalkylsilane. Appl. Surf. Sci. 2015, 351, 897–903. 10.1016/j.apsusc.2015.06.016. [DOI] [Google Scholar]
- Dalawai S.; Aly Saad Aly M.; Latthe S.; Xing R.; Sutar R.; Nagappan S.; Ha C.; Kumar Sadasivuni K.; Liu S. Recent Advances in Durability of Superhydrophobic Self-Cleaning Technology: A Critical Review. Prog. Org. Coat. 2020, 138, 105381. 10.1016/j.porgcoat.2019.105381. [DOI] [Google Scholar]
- Kobina Sam E.; Kobina Sam D.; Lv X.; Liu B.; Xiao X.; Gong S.; Yu W.; Chen J.; Liu J. Recent Development in the Fabrication of Self-Healing Superhydrophobic Surfaces. Chem. Eng. J. 2019, 373, 531–546. 10.1016/j.cej.2019.05.077. [DOI] [Google Scholar]
- Xue C. H.; Ma J. Z. Long-Lived Superhydrophobic Surfaces. J. Mater. Chem. A 2013, 1, 4146–4161. 10.1039/c2ta01073a. [DOI] [Google Scholar]
- Li S.; Page K.; Sathasivam S.; Heale F.; He G.; Lu Y.; Lai Y.; Chen G.; Carmalt C. J.; Parkin I. P. Efficiently Texturing Hierarchical Superhydrophobic Fluoride-Free Translucent Films by AACVD with Excellent Durability and Self-cleaning Ability. J. Mater. Chem. A 2018, 6, 17633–17641. 10.1039/C8TA05402A. [DOI] [Google Scholar]
- Yang Y.; Li X.; Zheng X.; Chen Z.; Zhou Q.; Chen Y. 3D-Printed Biomimetic Super-Hydrophobic Structure for Microdroplet Manipulation and Oil/Water Separation. Adv. Mater. 2018, 30, 1704912. 10.1002/adma.201704912. [DOI] [PubMed] [Google Scholar]
- Jing X.; Guo Z. Biomimetic Super Durable and Stable Surfaces with Superhydrophobicity. J. Mater. Chem. A 2018, 6, 16731–16768. 10.1039/C8TA04994G. [DOI] [Google Scholar]
- Wan C.; Lu Y.; Jiao Y.; Jin C.; Sun Q.; Li J. Ultralight and Hydrophobic Nanofibrillated Cellulose Aerogels from Coconut Shell with Ultrastrong Adsorption Properties. J. Appl. Polym. Sci. 2015, 132, 42037. 10.1002/app.42037. [DOI] [Google Scholar]
- Cheng Y.; Zhu T.; Li S.; Huang J.; Mao J.; Yang H.; Gao S.; Chen Z.; Lai Y. A Novel Strategy for Fabricating Robust Superhydrophobic Fabrics by Environmentally-Friendly Enzyme Etching. Chem. Eng. J. 2019, 355, 290–298. 10.1016/j.cej.2018.08.113. [DOI] [Google Scholar]
- Su X.; Li H.; Lai X.; Chen Z.; Zeng X. 3D Porous Superhydrophobic CNT/EVA Composites for Recoverable Shape Reconfiguration and Underwater Vibration Detection. Adv. Funct. Mater. 2019, 29, 1900554. 10.1002/adfm.201900554. [DOI] [Google Scholar]
- Liu M.; Luo Y.; Jia D. Facile, Solvent-Free Fabrication of A Robust 3-Dimensional Continuous Superhydrophobic Coating with Wettability Control and Abrasion Healing. Chem. Eng. J. 2019, 368, 18–28. 10.1016/j.cej.2019.02.162. [DOI] [Google Scholar]
- Tian T.; Sharma C. S.; Ahuja N.; Varga M.; Selvakumar R.; Lee Y. T.; Chiu Y. C.; Shih C. J. An Elastic Interfacial Transistor Enabled by Superhydrophobicity. Small 2018, 14, 1804006. 10.1002/smll.201804006. [DOI] [PubMed] [Google Scholar]
- Jung K. K.; Jung Y.; Choi C. J.; Ko J. S. Highly Reliable Superhydrophobic Surface with Carbon Nanotubes Immobilized on a PDMS/Adhesive Multilayer. ACS Omega 2018, 3, 12956–12966. 10.1021/acsomega.7b01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C.; Ge M.; Huang J.; Li S.; Deng S.; Zhang S.; Chen Z.; Zhang K.; Al-Deyab S. S.; Lai Y. Robust Fluorine-Free Superhydrophobic PDMS-Ormosil@Fabrics for Highly Effective Self-Cleaning and Efficient Oil-Water separation. J. Mater. Chem. A 2016, 4, 12179–12187. 10.1039/C6TA04420D. [DOI] [Google Scholar]
- Kang S.; Hwang S.; Jin S.; Choi C.; Kim J.; Park B.; Lee D.; Lee C. A Rapid One-Step Fabrication of Patternable Superhydrophobic Surfaces Driven by Marangoni Instability. Langmuir 2014, 30, 2828–2834. 10.1021/la500266f. [DOI] [PubMed] [Google Scholar]
- Huang J. Y.; Li S. H.; Ge M. Z.; Wang L. N.; Xing T. L.; Chen G. Q.; Liu X. F.; Al-Deyab S. S.; Zhang K. Q.; Chen T.; Lai Y. K. Robust Superhydrophobic TiO2@Fabrics for UV Shielding, Self-cleaning and Oil-Water Separation. J. Mater. Chem. A 2015, 3, 2825–2832. 10.1039/C4TA05332J. [DOI] [Google Scholar]
- Guo X.; Xue C.; Li M.; Li X.; Ma J. Fabrication of Robust, Superhydrophobic, Electrically Conductive and UV-Blocking Fabrics via Layer-by-Layer Assembly of Carbon Nanotubes. RSC Adv. 2017, 7, 25560–25565. 10.1039/C7RA02111A. [DOI] [Google Scholar]
- Dong X.; Gao S.; Huang J.; Li S.; Zhu T.; Cheng Y.; Zhao Y.; Chen Z.; Lai Y. A Self-Roughened and Biodegradable Superhydrophobic Coating with UV Shielding, Solar-Induced Self-Healing and Versatile Oil-Water Separation Ability. J. Mater. Chem. A 2019, 7, 2122–2128. 10.1039/C8TA10869B. [DOI] [Google Scholar]
- Wang F.; Pi J.; Song F.; Feng R.; Xu C.; Wang X.; Wang Y. A Superhydrophobic Coating to Create Multi-Functional Materials with Mechanical/Chemical/Physical Robustness. Chem. Eng. J. 2020, 381, 122539. 10.1016/j.cej.2019.122539. [DOI] [Google Scholar]
- Jiao Y.; Wan C.; Zhang W.; Bao W.; Li J. Carbon Fibers Encapsulated with Nano-Copper:A Core-Shell Structured Composite for Antibacterial and Electromagnetic Interference Shielding Applications. Nanomaterials 2019, 9, 460. 10.3390/nano9030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harifi T.; Montazer M. Photo-, Bio-, and Magneto-Active Colored Polyester Fabric with Hydrophobic/Hydrophilic and Enhanced Mechanical Properties through Synthesis of TiO2/Fe3O4/Ag Nanocomposite. Ind. Eng. Chem. Res. 2014, 53, 1119–1129. 10.1021/ie403052m. [DOI] [Google Scholar]
- He C.; Huang J.; Li S.; Meng K.; Zhang L.; Chen Z.; Lai Y. Mechanically Resistant and Sustainable Cellulose-Based Composite Aerogels with Excellent Flame Retardant, Sound-Absorption, and Superantiwetting Ability for Advanced Engineering Materials. ACS Sustainable Chem. Eng. 2018, 6, 927–936. 10.1021/acssuschemeng.7b03281. [DOI] [Google Scholar]
- Ge M.; Cao C.; Liang F.; Liu R.; Zhang Y.; Zhang W.; Zhu T.; Yi B.; Tang Y.; Lai Y. A “PDMS-in-water” Emulsion Enables Mechanochemically Robust Superhydrophobic Surfaces with Self-Healing Nature. Nanoscale Horiz. 2020, 5, 65-73. 10.1039/C9NH00519F. [DOI] [Google Scholar]
- Li Y.; Li B.; Zhao X.; Tian N.; Zhang J. Totally Waterborne, Nonfluorinated, Mechanically Robust, and Self-Healing Superhydrophobic Coatings for Actual Anti-Icing. ACS Appl. Mater. Interfaces 2018, 10, 39391–39399. 10.1021/acsami.8b15061. [DOI] [PubMed] [Google Scholar]
- Chen K.; Zhou S.; Wu L. Facile Fabrication of Self-Repairing Superhydrophobic Coatings. Chem. Commun. 2014, 50, 11891–11894. 10.1039/C3CC49251F. [DOI] [PubMed] [Google Scholar]
- Lv T.; Cheng Z.; Zhang E.; Kang H.; Liu Y.; Jiang L. Self-Restoration of Superhydrophobicity on Shape Memory Polymer Arrays with Both Crushed Microstructure and Damaged Surface Chemistry. Small 2017, 13, 1503402. 10.1002/smll.201503402. [DOI] [PubMed] [Google Scholar]