Abstract
Nitric oxide (NO) is a ubiquitous, endogenously produced, water-soluble signaling molecule playing critical roles in physiological processes. Nitric oxide plays pleiotropic roles in cancer and, depending on its local concentration, may lead to either tumor progression or tumor suppression. Addition of NO group to a cysteine residue within a protein, termed as S-nitrosylation, plays diverse regulatory roles and affects processes such as metabolism, apoptosis, protein phosphorylation, and regulation of transcription factors. The process of S-nitrosylation has been associated with development of different cancers, including breast cancer. The present review discusses different mechanisms through which NO acts, with special emphasis on breast cancers, and provides detailed insights into reactive nitrogen species, posttranslational modifications of proteins mediated by NO, dual nature of NO in cancers, and the implications of S-nitrosylation in cancers. Our review will generate interest in exploring molecular regulation by NO in different cancers and will have significant therapeutic implications in the management and treatment of breast cancer.
Keywords: Breast cancer, nitric oxide, nitric oxide synthase, S-nitrosylation, reactive nitrogen species, redox switch, nitrosative stress
Introduction
Breast cancer, although termed as one disease, is one of the most diverse cancers displaying heterogeneous expression of progesterone receptor (PR), estrogen receptor (ER), and human epidermal growth factor receptor 2 (HER2) in patients with different breast cancers, which also give rise to intertumor and intratumor heterogeneity and often lead to different clinical presentations in terms of histology, prognosis, and responsiveness to treatment.1 One of the main reasons behind the broad heterogeneity is variation in transcriptional programs which could help in providing a distinct molecular profile for each tumor.2 Early onset of breast cancer with an aggressive phenotype has been observed in young women, making breast cancer awareness and screening among younger women a priority.3 Research performed in the last few decades has highlighted the role of genomic alteration in driving breast cancers and has drawn special attention to role of genomic drivers, impact of DNA repair defects, and resistant clones in the disease.4 One of the reasons behind DNA damage and impaired DNA repair is oxidative stress. Increasing evidence suggests that a situation of oxidative stress arises when an imbalance in the rate of generation and disposal of reactive oxygen species (ROS) or reactive nitrogen species (RNS) occurs. The generated reactive species are capable of playing a dual role and can cause oxidative damage as well as act as molecular signals and activate stress responses, beneficial for the organism.5 Reactive nitrogen species play a crucial role in physiological regulation in cells by displaying pleiotropic effects on cellular targets. An elevated level of RNS induces nitrosative stress and has been implicated in cell injury and death.6
The development of cancers including breast cancer is affected by various intracellular and extracellular factors, including reactive oxygen and nitrogen species (RONS). The cellular accumulation of RNS is implicated to play a role in cancer initiation and progression by causing alterations in gene expression profile, deregulating signal transduction pathways, and inducing abnormal protein modifications.7 As cancer cells are adapted to grow in low oxygen concentration or hypoxia, they undergo metabolic reprogramming to meet their elevated demands of nutrients and energy for proliferation and survival. Metabolic adaptations of an increased rate of anaerobic glycolysis, decreased oxidative phosphorylation generation, and overall mitochondrial dysfunction govern cancer growth.8 As reported in different cancers, hypoxic condition results in an increased production of RONS.9 Cancer cells with chronic inflammatory conditions and elevated concentration of reactive species face oxidative (imbalance between generation of ROS and antioxidants) and nitrosative stress (imbalance between production and elimination of RNS), leading to DNA damage and impaired DNA repair.10 Elevated levels of RONS have high reactivity and, hence under normal physiologic conditions, require proper redox balance between RONS generation and elimination by the internal antioxidant system.11 The clean-up of elevated intracellular RONS concentration controls the reversible modifications of regulatory proteins caused by oxidative/nitrosative stress and acts as redox switches controlling activities of intracellular downstream effectors of different cell signaling pathways via ROS/RNS signaling.12 Reactive nitrogen species have been implicated in disturbed cellular homeostasis leading to deleterious consequences by deregulation of signaling pathways causing protein modifications.13
Nitric Oxide, Nitric Oxide Synthase, and RNS
Nitric oxide (NO) is a highly diffusible and reactive diatomic free radical. Present in gaseous state at room temperature, NO has pleiotropic functions and plays a critical role in multiple biological processes, such as neurotransmission, vasodilatation, and macrophage-mediated immunity. In addition, NO can also act a messenger molecule and play a role in promoting as well as inhibiting cancer.14
Nitric oxide synthase (NOS; EC 1.14.13.39) enzyme helps in the synthesis of NO from l-arginine in the presence of oxygen and is ubiquitously expressed in malignant cancers.15 Nitric oxide synthase also requires nicotinamide adenine dinucleotide phosphate (NADPH), flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), and (6R-)5,6,7,8-tetrahydrobiopterin (BH4) as cofactors. All isoforms of the NOS enzyme, neuronal “n”NOS (NOS1), inducible “i”NOS (NOS2), and endothelial “e”NOS (NOS3), present in mammalian cells are encoded by three distinct genes, are homodimers16 and share strong homology (Figure 1).17
Figure 1.
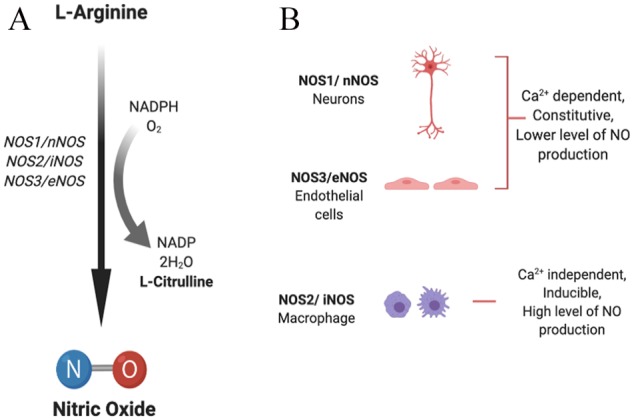
(A) Synthesis of nitric oxide. (B) Nitric oxide synthase (NOS) isoforms: neuronal (NOS1/nNOS), inducible (NOS2/iNOS), and endothelial (NOS3/eNOS) isoforms catalyze the process of NO generation in the presence of cofactors via oxidation of l-arginine to l-citrulline. NADPH indicates nicotinamide adenine dinucleotide phosphate; NO, nitric oxide.
NOS1 and NOS3 are dependent on calcium levels for activity and hence produce lower but transient concentrations of NO. They are continuously expressed in neurons and endothelial cells, whereas NOS2 is a calcium-independent, inducible isoform and, once induced, results in continuous production of higher concentrations of NO.13 The expression of nNOS and eNOS can also be activated or inhibited via different protein kinase–mediated phosphorylations, while expression of iNOS can be regulated transcriptionally by multiple factors, including cytokines (interleukin [IL]-1β, interferon [IFN]-γ, tumor necrosis factor [TNF]-α), bacterial endotoxin (lipopolysaccharide [LPS]), and oxidative stress.14
Nitric oxide is the primary, common progenitor for all RNSs, and different RNSs are formed by NO-dependent reactions. Peroxynitrite (ONOOH−) is formed by a fast reaction between NO and O−2, which forms secondary RNS by further reaction. Nitric oxide reacts with its intracellular environment to form other reactive metabolites including peroxynitrite, nitrite, nitrate, or S-nitroso-thiols that induce genotoxic effects leading to DNA damage. Peroxynitrite species are capable of causing single-strand DNA breaks by attacking the sugar-phosphate backbone of DNA (Figure 2).14
Figure 2.
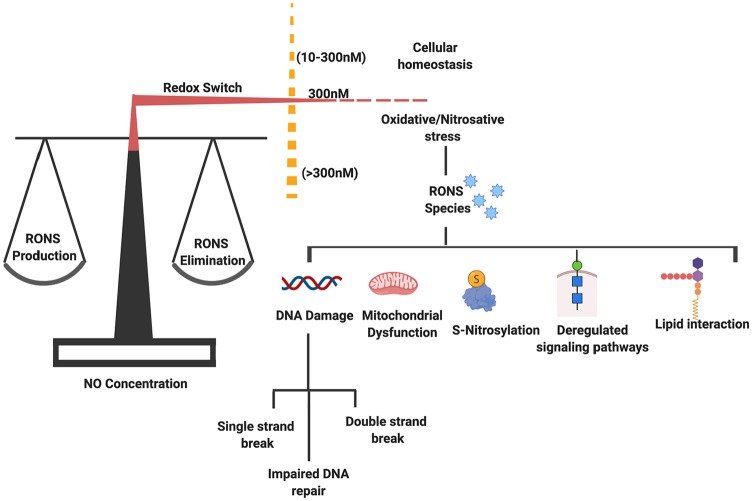
A redox switch mechanism helps in maintaining a fine balance between ROS/RNS generation and elimination. However, a lower concentration of NO helps in maintaining cellular homeostasis. An imbalance in their level results in generation of oxidative and nitrosative stress. The reactive species (RONS) play a role in development of pathological condition via acting on different pathways and causing varied effects in the form of DNA damage, mitochondrial dysfunction, deregulated S-nitrosylation, and lipid interaction. NO indicates nitric oxide; RNS, reactive nitrogen species; RONS, reactive oxygen and nitrogen species; ROS, reactive oxygen species.
The products formed after oxidation reactions (oxidation of glutathione [GSH]), nitration (nitrotyrosine formation), or nitrosation (S-nitrosoglutathione [GSNO] formation) are of high biological significance and help in generation of more NO donors and deregulate cell signaling.18 Posttranslational modifications induced after RNS exposure often lead to differential interactions with other cellular targets and induce varied effects depending on their local concentration.6 RONSs cause modifications such as tyrosine nitration, S-nitrosylation, S-sulfenylation, S-glutathionylation, and cysteine oxidation in residues of regulatory protein that play critical role in carcinogenesis. Furthermore, they also cause alterations in the activities of transcription factors and intracellular effectors of different signaling pathways.7
Posttranslational Modifications Mediated by NO
Reactive nitrogen species are produced under normal physiological conditions in cells and are monitored and properly controlled by different mechanisms to maintain redox homeostasis, ie, proper regulation of oxidative and nitrosative stress. To maintain normal cellular physiology, redox mechanism plays a lead role in the regulation of signal transduction pathways involved in proper functioning of cellular targets that are prone to malfunction associated with human disease. As mentioned previously, NO mediates some of the major types of protein posttranslational modifications (PTMs) including S-nitrosylation, S-glutathionylation, and tyrosine nitration.19 Mechanistically, NO regulates protein function by modification of cysteine thiol residues and transition metal centers, by S-nitrosylation of single critical cysteine residue present inside an acid-base or hydrophobic structural motif, or by inducing specific signals after PTM that can be used for self-defense against microbes and cancer cells.20
S-nitrosylation is one of the most important types of PTM in which an NO group is covalently attached to the thiol side chain of a cysteine residue. This type of PTM serves as a critical mechanism behind redox-based physiological regulation and plays an important role in posttranslational regulation of a wide variety of protein influenced by NO. It is now evident that proteins from almost all functional classes are substrates for S-nitrosylation, and the entire process of S-nitrosylation and de-nitrosylation is highly specific and is tightly regulated by virtue of structural motifs, allosteric regulators, and molecular interactions between target protein and NOS.21 S-nitrosothiols (SNOs), resulting from S-nitrosylation, may also cause alteration in cellular function of a variety of proteins.21,22 S-nitrosoglutathione, a low-molecular-weight SNO formed after S-nitrosylation of antioxidant GSH, is the major type of SNO in the cell and serves as an NO reservoir in cells.23,24 GSNO also facilitates transnitrosylation reactions and transfers its NO group to a new cysteine thiol group.21 As S-nitrosylation is a highly regulated, reversible mechanism, generated SNO can be broken down either enzymatically by thioredoxins in a very specific manner25 or non-enzymatically by antioxidants such as ascorbate or GSH. Indirectly, S-nitrosoglutathione reductase (GSNOR) also controls SNO levels by decomposing GSNO.26
The other important type of PTM is S-glutathionylation. It is the reversible process in which GSH is covalently added to cysteine residues in target proteins and results in alteration in molecular charge, mass, and structure-associated function and might prevent protein degradation via proteolysis or sulfhydryl overoxidation. Presence of excess S-glutathionylated proteins in serum may also serve as useful oxidative or nitrosative stress biomarkers in diseased individuals.27 This process has emerged as a candidate mechanism in maintaining intracellular redox state by having a control over the generation of RONS associated with a functional responses and stress signaling.19 Although the process of tripeptide GSH addition is promoted by oxidative and nitrosative stress, it also occurs in the cell under unstressed conditions. As proper folding of proteins in endoplasmic reticulum is dependent on balanced redox environment, a redox stress condition in the ER can affect rates of S-glutathionylation.28 Glutathione acts as a biological redox buffer and a balanced GSH (reduced)/GSSG (oxidized) ratio is maintained via GSH peroxidase and GSH reductase enzymes in controlled oxidation/reduction reactions. A decrease in GSH mediated by RONS may cause cell death via apoptosis or necrosis.29
Protein tyrosine nitration is another type of PTM and is caused by covalent modification by NO-derived oxidants such as peroxynitrite (ONOO−) and nitrogen dioxide (•NO2) and involves the formation of intermediate Tyr• radical from tyrosine.30 Peroxynitrite is a potent, short-lived, oxidizing, and nitrating agent; by its secondary radicals, it can directly or indirectly promote protein and lipid modifications.31 Nitration of protein tyrosine residues occurs when a nitro group (-NO2) substitutes hydrogen at the third position in the phenolic ring, leading to the formation of 3-nitrotyrosine (3-NT) as product.32 The formation of 3-NT in proteins indicates an oxidative PTM that favors pro-oxidant processes. Excess ROS along with NO and derivatives forms peroxynitrite as a nitrating species, which by tyrosine nitration modifies key properties of protein and can cause profound structural and functional effects33 and, overall, may serve as a marker of nitroxidative stress in diseased condition.34
Dual Role of NO in Cancer
Nitric oxide depending on its concentration and locations plays dichotomous role in cancer development. Its protumor as well as antitumor effect makes it a very interesting molecule in the tumor microenvironment. At lower concentrations, NO supports carcinogenesis, whereas at higher concentration, it becomes cytotoxic to cancer cells and induces apoptosis by forming peroxynitrite.35 As reviewed in detail by Choudhary et al, NO plays tumoricidal or tumor-inhibiting role, depending on its concentration and location. Nitric oxide has cytostatic or cytotoxic effect on the growth of cancer cells by shifting cellular metabolism, inhibiting DNA synthesis, activating caspases, and upregulating expression of multiple apoptosis-associated proteins or it plays a tumor-promoting role by different mechanisms, including (a) genotoxic effect—creating toxic and mutagenic species, directly inducing modification in DNA in the form of strand breaks and nucleic acid oxidation and deamination, impaired DNA repair; (b) antiapoptotic effects—by inhibiting caspase activity by S-nitrosylation of the cysteine residue, causing loss of p53 repressor activity GC to AT mutations, increasing B-cell lymphoma 2 (Bcl)-2 expression, activating cyclo-oxygenase (COX), blocking cytochrome C release, and suppressing ceramide generation; (c) angiogeneic effect—via dilating eNOS-mediated arteriolar vessels, increasing hyperpermeability of vascular endothelium, vascular endothelial growth factor (VEGF) release, increase in permeability of tumor vasculature, and production of proangiogenic factors; (d) metastatic effect—via upregulated expression of matrix metalloproteinase 2 (MMP2), MMP-9, and VEGF; (e) dampened immune response against tumor—by suppressed leukocyte proliferation and infiltration.17
As NOSs are ubiquitously expressed in different malignant cancers, NO derived from cancer cell promotes cancer progression, whereas NO derived from host stromal cell acts differentially and inhibits growth of NO-sensitive cancers but promotes growth of NO-resistant cancers15. There is increasing interest in studying the role of NO and NOSs in cancer growth and progression, and studies conducted in different cancers, including brain,36,37 gastric,38 colorectal,39 lung,40 prostate,41 bladder,42 head and neck,43 pancreatic,44 and breast cancer,45-50 emphasize its critical role in these various cancers.
Nitric Oxide and S-Nitrosylation Implications in Breast Cancer
Breast cancer is highly heterogeneous, displaying great heterogeneity in different areas of the same tumor in ER, PR, and HER2 expression.1 It was found that in invasive ductal carcinoma (a breast cancer subtype), NO biosynthesis was significantly higher in grade III tumors with respect to grade II, and it was further reported that NOS expression correlates with tumor grade in breast cancer.51 After investigations in benign, malignant, and normal breast tissue samples, an inverse correlation was found between metastatic potential of tumors and presence of iNOS protein in tumor cell, and it was suggested that tumor cell–mediated endogenous iNOS expression might play an inhibitory effect on the metastatic potential in breast cancer52 or tumors with high NOS activity indicated a lower proliferation rate and grade.53 However, interestingly, it was shown by Vakkala et al54 that iNOS-positive breast tumor and stromal cells showed increased vascularization and higher apoptotic indices, suggesting iNOS-mediated angiogenesis and apoptosis-promoting role in breast carcinoma (Figure 3).
Figure 3.
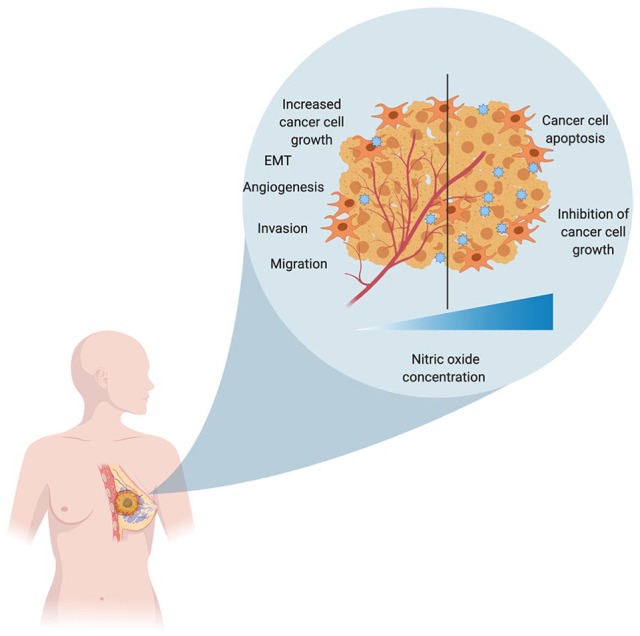
NO plays a role in both tumor progression as well as tumor regression depending on the concentration of NO in the tumor microenvironment. A lower concentration of NO promotes tumor progression by increasing their proliferation, migration, invasion, and chemoresistance, whereas a higher concentration of NO has cytotoxic effects and causes inhibition of their growth and apoptosis. EMT indicates epithelial-to-mesenchymal transition; NO, nitric oxide.
Studies on the role of eNOS in breast cancer concluded that eNOS is expressed in breast tumors and its expression positively correlates with ER expression and negatively with tumor grade and lymph node status.55 Expression of NOS has been observed in advanced breast cancers56,57 and breast cancer cell lines.58 Previous findings have suggested that tumor-derived eNOS promotes the growth and metastasis of tumors by stimulating migration, invasiveness, and angiogenesis in tumor cells.59 NO-associated nitro-tyrosine levels were also found to be correlated with reduced disease-free and overall survival in breast cancer.49 In benign breast cancer tissues, eNOS synthase expression was predominantly found localized in apocrine metaplastic cells and entire endothelia, whereas no eNOS activity was found in infiltrating duct carcinoma cells of poorly differentiated type.60 In breast cancer, the tumor microenvironment45 and its metabolism61 also play a significant role in chemoresistance and metastasis in an NOS2-dependent manner.
Steroid hormones, estrogen and progesterone, both control NOS activity; estrogen increases the eNOS activity, whereas progesterone activates iNOS activity. An increased apoptosis rate in response to progesterone is associated with high levels of NO produced by iNOS, whereas low levels of NO produced by eNOS could be behind the proliferative effect of estrogen. All these findings implicate correlation between NOS expression and hormones in breast cancer development.62 Furthermore, a gene signature analysis performed on estrogen receptor–negative (ER−) breast cancer associated with poor disease outcome revealed that Ets-1, a transcriptional mediator of oncogenic NO signaling, promotes the aggressive phenotype in ER− breast cancer through Ras/MEK/ERK signaling pathway.63
c-Src acts as an upstream regulator of the estrogen-stimulated phosphatidylinositol 3-kinase (PI3K)/Akt/eNOS signaling pathway. Estrogen results in rapid activation of eNOS by PI3K/Akt-dependent Src kinase. Estrogen causes a complex formation between ER, c-Src, and P85 (PI3K regulatory subunit) and results in the activation of PI3K and Akt.64 As already discussed above, NO promotes cancer by activating several oncogenic signaling pathways, such as PI3K/Akt and ERK-1/2 pathways. Protein phosphatase 2A (PP2A), a tumor suppressor, negatively regulates the same pathways that are activated by NO in cancers. Activating PP2A in ER− breast cancer would be a novel mechanism to antagonize NO signaling–mediated effects that promote breast cancer.65
In summary, NOS2-derived NO can be considered as a driver of breast cancer progression by targeting multiple cell signaling pathways, including hypoxia inducible factor-1 alpha stabilization, COX2 activity, phosphoinositide 3-kinase/protein kinase B, mitogen-activated protein kinase (MAPK), epidermal growth factor receptor (EGFR), and Ras pathways.66 Increased expression of NOS2 in breast cancer is associated with a basal-like gene expression pattern and is a predictor of poor survival in patients with ER− breast cancer.67 The coexpression of NOS2 and COX2 is reported to promote tumor growth and reduce survival in patients with ER− breast cancer by their cross-talk.68 A better understanding of steroid hormones and molecular mechanism of their interactions with NO will help in development of novel ways and strategies for effective breast cancer treatment.
The role of Ets family transcription factors has been well studied in different cancers.69,70 Ets-1, a proto-oncogene and member of the same family, is known to promote invasive phenotype by supporting angiogenesis and extracellular matrix remodeling and is associated with poor prognosis in breast cancer.71 One of the mechanisms to promote invasiveness is by binding to MMP-9 gene, which harbors a binding site for Ets-1.72 It also acts as a downstream effector of HER2 and also increases MMP-1 expression.73 A high expression of HER2 is associated with aggressive metastasis in breast cancer cells via MMP-1 and MMP-9 expression.74
Nitric oxide–mediated S-nitrosylation regulates a wide variety of protein functions. The specificity with which this process targets critical cysteine residues and signals protein-protein interactions is controlled by different acid-base and hydrophobic motifs.75 It has been recently identified that more than 3000 proteins impacted by NO signaling are largely managed by S-nitrosylation, and a basal level of S-nitrosylation helps in maintaining tissue homeostasis.76 An increased level of intracellular NO leads to elevated S-nitrosylation in breast cancer and has emerged as an important mechanism promoting breast carcinomas. S-nitrosylation of Ras leads to activation of Ets-1 caused by MAPK-dependent phosphorylation and results in an aggressive breast cancer phenotype.77 S-nitrosylation of H-Ras also restricts Raf-1 activation and further signals propagation via ERK-1/2.78 It has also been reported that NO signaling leads to tyrosine phosphorylation of EGFR. Further analysis revealed that S-nitrosylation of EGFR and Src results in activation of oncogenic signaling in human basal-like breast cancer.79 NO also results in modification of human ER structure by S-nitrosylation, which impairs its DNA-binding activity in turn, leading to obstruction of estrogen-dependent gene transcription.80
Specifically, S-nitrosylation also plays a critical role in breast cancer angiogenesis and metastasis by targeting different pathways. As recently reviewed in detail by Ehrenfeld et al,81 S-nitrosylation of target proteins promotes tumor cell epithelial-to-mesenchymal transition (EMT) and helps in their migration and invasion by promoting adhesion to the endothelium and intravasation and extravasation. In basal-like human breast cancers, Src and EGFR S-nitrosylation activates a network of oncogenic signaling and leads to increased EMT and stem cell–like phenotype and contributes to chemoresistance.79 NO-mediated S-nitrosylation of c-Src kinase at Cys (498) residue stimulates its kinase activity and helps in cancer cell invasion and metastasis.82 In ER− breast cancers, S-nitrosylation activates different target molecules, such as EGFR, Src, Ras, and CD63 which further initiate associated oncogenic signaling pathways including Ras/ERK, PI3K/Akt, nuclear factor κB, β-catenin, and AP-1.63,83 Ras is reported to be abnormally activated in different types of breast cancers showing overexpression of EGF/ErbB-2 receptors; its activation was further found to be related to MAPK activity.84 S-nitrosothiol formation by S-nitrosylation and its homeostasis is often impaired in many cancers. In HER2+ breast cancers, alteration in SNO homeostasis gives a survival advantage to the tumors and reduces their trastuzumab sensitivity.85
S-nitrosoglutathione reductase, an oxidoreductase, helps in denitrosylation and is capable of reducing NO completely86; a mediated targeting could be effective in breast cancer treatment. Flavone also restrains NO production and lessens protein S-nitrosylation in breast cancer cells by inhibiting NOS activity in a dose-dependent manner and, therefore, could be of anticancer use.87
Nitric Oxide–Mediated Strategies for Cancer Treatment
Accumulating evidence implicates defective levels of NO with different diseases. Lower concentration (picomolar to nanomolar range) of NO is present in normal physiological conditions in a cell, but a sudden increase in the concentration (micromolar range) results in development of pathological conditions. The concentration of not only NO but also other reactive species, generated after reacting with NO, strongly contributes to pathological conditions.88 Peroxynitrite, generated from NO and superoxide anion in diffusion-controlled reaction, causes a wide range of cellular alterations by interacting with DNA, lipids, and proteins either directly via oxidative reactions or indirectly via radical-mediated mechanisms. Peroxynitrite generation triggers cellular responses causing cell necrosis or apoptosis which have been implicated in disease conditions such as heart failure, diabetes, infectious diseases, neurodegenerative diseases as well as cancer.89-91
Designing novel therapeutics to modulate NO bioavailability by finding ways to increase NO synthase activity, strengthening nitrate-nitrite-NO pathway, designing novel-class drugs for NO-donating function, and limiting NO metabolism and also finding ways to regulate downstream targets such as phosphodiesterases and soluble guanylyl cyclases will be of value.92 As either an excess or an absence of NO may lead to different pathologies, different strategies of NO regulation show great potential. In conditions of decreased NO production caused by arginine deficiency (reduced arginine production from citrulline), a supplementation of citrulline might be curative.93 Apart from citrulline, targeting other limiting factors such as tetrahydrobiopterin (BH4) could also be of benefit. Other than arginine or citrulline supplementation, additional approaches such as providing NO donors in the form of inhaled NO and other nitrite sources, NOS3 regulating agents, or targeting endogenous NOS inhibitors such as asymmetric dimethylarginine could have potential therapeutic benefits.94,95 Use of NO-donating nonsteroidal antiinflammatory drugs (NSAIDs), such as NO-aspirin and NO-ibuprofen, have shown to decrease prostate cancer cell growth and induce apoptosis in a dose-dependent manner.96 A wide variety of other NO donors have been shown to be effective in treating prostate and bladder cancers.97 Finding similar use of NO donors in breast cancer may be of high benefit (Figure 4).
Figure 4.
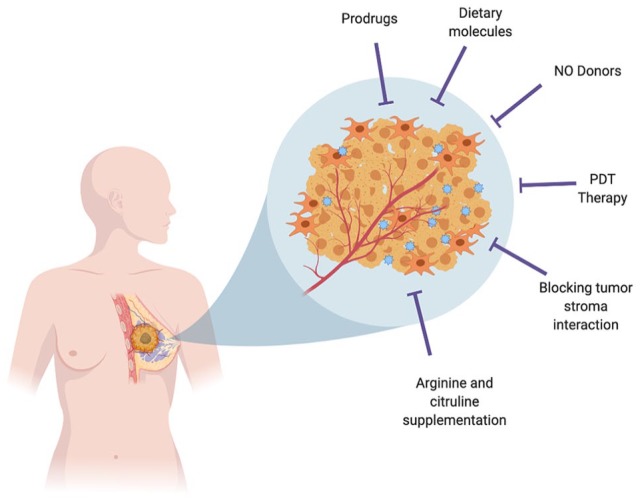
Strategies to increase NO bioavailability in the tumor microenvironment: Use of prodrugs, NO donors, dietary molecules, photodynamic therapy, arginine and citrulline supplementation, and blocking tumor stroma interaction could be effective in treating cancers. NO indicates nitric oxide; PDT, photodynamic therapy.
Other therapeutic ways are to deliver NO specifically to solid tumors by using polymer NO donors,98 or nanoparticle-based systems could be useful.99 Photodynamic therapy (PDT) is a different therapeutic approach used in treatment of certain cancers and involves the use of photosensitizer (PS) and irradiation with light of specific wavelength. PDT treatment causes iNOS/NO induction in the tumor as well as its microenvironment and could play significant role in NO-mediated cytotoxicity and act as a chemosensitizing agent.100 The use of NO prodrug such as JS-K which is a NO-releasing diazeniumdiolate(s) has also shown to be effective in different cancers.101 Use of potential dietary chemopreventive agents such as magnolol,102 cardamom,103 and curcumin104 has shown to be effective against NO-induced gene modulation and tumor progression. As reviewed in detail by Vahora et al,35 use of dietary agents would be of benefit in NO-mediated halting of cancer progression in different cancers, including breast cancer.
Progress made in the last few years in the area of NO and the mechanism behind its action has exponentially increased the overall understanding of NO signaling. Research performed to identify novel strategies via identifying promising new drug candidates, different dietary constituents, or other mechanisms will help in NO-mediated targeting of tumor cells.
Conclusions
Previous research has helped us to gain an insight into the dual role of NO. Biphasic response to NO in cancers is dependent on its levels and may inhibit or promote cancer growth and survival. Expression of several genes involved in tumor biology is regulated by NO and largely by NO-mediated PTM of proteins. Of these, S-nitrosylation has been depicted as a process involved in every phase of cancer progression. It affects a wide variety of proteins important in maintaining cellular functions. Affected proteins lead to cellular dysfunctions contributing to cancer onset, growth, progression, invasion, and metastasis. The exact role of NO in different cancers is determined by the primary organ affected, stage of cancer, and types of cells constituting the tumor microenvironment. Further research into regulation of critical proteins in cancer by RNS may be helpful in developing targeted therapies for cancer and, in particular, breast cancer.
Author Contributions
DM and VP contributed equally in the preparation of manuscript. All authors contributed to discussions and writing of the manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by discretionary funds from the School of Graduate Studies, Rutgers University.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Debabrata Banerjee  https://orcid.org/0000-0003-0658-9023
https://orcid.org/0000-0003-0658-9023
References
- 1. Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12:381-394. [DOI] [PubMed] [Google Scholar]
- 2. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747-752. [DOI] [PubMed] [Google Scholar]
- 3. Narod SA. Breast cancer in young women. Nat Rev Clin Oncol. 2012;9:460-470. [DOI] [PubMed] [Google Scholar]
- 4. Arnedos M, Vicier C, Loi S, et al. Precision medicine for metastatic breast cancer—limitations and solutions. Nat Rev Clin Oncol. 2015;12:693-704. [DOI] [PubMed] [Google Scholar]
- 5. Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016: 1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez MC, Andriantsitohaina R. Reactive nitrogen species: molecular mechanisms and potential significance in health and disease. Antioxid Redox Signal. 2009;11:669-702. [DOI] [PubMed] [Google Scholar]
- 7. Moldogazieva NT, Lutsenko SV, Terentiev AA. Reactive oxygen and nitrogen species-induced protein modifications: implication in carcinogenesis and anticancer therapy. Cancer Res. 2018;78:6040-6047. [DOI] [PubMed] [Google Scholar]
- 8. DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith KA, Waypa GB, Schumacker PT. Redox signaling during hypoxia in mammalian cells. Redox Biol. 2017;13:228-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149-156. [DOI] [PubMed] [Google Scholar]
- 11. He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532-553. [DOI] [PubMed] [Google Scholar]
- 12. Moldogazieva NT, Mokhosoev IM, Feldman NB, Lutsenko SV. ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic Res. 2018;52:507-543. [DOI] [PubMed] [Google Scholar]
- 13. Adams L, Franco MC, Estevez AG. Reactive nitrogen species in cellular signaling. Exp Biol Med (Maywood). 2015;240:711-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12:311-320. [DOI] [PubMed] [Google Scholar]
- 15. Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521-534. [DOI] [PubMed] [Google Scholar]
- 16. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choudhari SK, Chaudhary M, Bagde S, Gadbail AR, Joshi V. Nitric oxide and cancer: a review. World J Surg Oncol. 2013;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel RP, McAndrew J, Sellak H, et al. Biological aspects of reactive nitrogen species. Biochim Biophys Acta. 1999;1411:385-400. [DOI] [PubMed] [Google Scholar]
- 19. Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928-4944. [DOI] [PubMed] [Google Scholar]
- 20. Stamler JS, Lamas S, Fang FC. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106:675-683. [DOI] [PubMed] [Google Scholar]
- 21. Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150-166. [DOI] [PubMed] [Google Scholar]
- 22. Astier J, Rasul S, Koen E, et al. S-nitrosylation: an emerging post-translational protein modification in plants. Plant Sci. 2011;181:527-533. [DOI] [PubMed] [Google Scholar]
- 23. Durner J, Gow AJ, Stamler JS, Glazebrook J. Ancient origins of nitric oxide signaling in biological systems. Proc Natl Acad Sci U S A. 1999;96:14206-14207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Begara-Morales JC, Sanchez-Calvo B, Chaki M, et al. Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs). Front Plant Sci. 2016;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001; 410:490-494. [DOI] [PubMed] [Google Scholar]
- 27. Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD. Causes and consequences of cysteine S-glutathionylation. J Biol Chem. 2013;288:26497-26504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv. 2007;7:313-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiong Y, Uys JD, Tew KD, Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15:233-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sawa T, Akaike T, Maeda H. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J Biol Chem. 2000;275:32467-32474. [DOI] [PubMed] [Google Scholar]
- 32. Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018;14:618-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Radi R. Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res. 2013;46:550-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ischiropoulos H. Protein tyrosine nitration—an update. Arch Biochem Biophys. 2009;484:117-121. [DOI] [PubMed] [Google Scholar]
- 35. Vahora H, Khan MA, Alalami U, Hussain A. The potential role of nitric oxide in halting cancer progression through chemoprevention. J Cancer Prev. 2016;21: 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bakshi A, Nag TC, Wadhwa S, Mahapatra AK, Sarkar C. The expression of nitric oxide synthases in human brain tumours and peritumoral areas. J Neurol Sci. 1998;155:196-203. [DOI] [PubMed] [Google Scholar]
- 37. Tran AN, Boyd NH, Walker K, Hjelmeland AB. NOS expression and NO function in glioma and implications for patient therapies. Antioxid Redox Signal. 2017;26:986-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feng CW, Wang LD, Jiao LH, Liu B, Zheng S, Xie XJ. Expression of p53, inducible nitric oxide synthase and vascular endothelial growth factor in gastric precancerous and cancerous lesions: correlation with clinical features. BMC Cancer. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ambs S, Bennett WP, Merriam WG, et al. Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. J Natl Cancer Inst. 1999;91:86-88. [DOI] [PubMed] [Google Scholar]
- 40. Ambs S, Bennett WP, Merriam WG, et al. Vascular endothelial growth factor and nitric oxide synthase expression in human lung cancer and the relation to p53. Br J Cancer. 1998;78:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee KM, Kang D, Park SK, et al. Nitric oxide synthase gene polymorphisms and prostate cancer risk. Carcinogenesis. 2009;30:621-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin Z, Chen S, Ye C, Zhu S. Nitric oxide synthase expression in human bladder cancer and its relation to angiogenesis. Urol Res. 2003;31:232-235. [DOI] [PubMed] [Google Scholar]
- 43. Gallo O, Masini E, Morbidelli L, et al. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J Natl Cancer Inst. 1998;90:587-596. [DOI] [PubMed] [Google Scholar]
- 44. Fujita M, Somasundaram V, Basudhar D, et al. Role of nitric oxide in pancreatic cancer cells exhibiting the invasive phenotype. Redox Biol. 2019;22:101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heinecke JL, Ridnour LA, Cheng RY, et al. Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc Natl Acad Sci U S A. 2014;111:6323-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang L, Zeng M, Fu BM. Inhibition of endothelial nitric oxide synthase decreases breast cancer cell MDA-MB-231 adhesion to intact microvessels under physiological flows. Am J Physiol Heart Circ Physiol. 2016;310:H1735-H1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zintzaras E, Grammatikou M, Kitsios GD, et al. Polymorphisms of the endothelial nitric oxide synthase gene in breast cancer: a genetic association study and meta-analysis. J Hum Genet. 2010;55:743-748. [DOI] [PubMed] [Google Scholar]
- 48. Pervin S, Singh R, Chaudhuri G. Nitric oxide-induced cytostasis and cell cycle arrest of a human breast cancer cell line (MDA-MB-231): potential role of cyclin D1. Proc Natl Acad Sci U S A. 2001;98:3583-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakamura Y, Yasuoka H, Tsujimoto M, et al. Nitric oxide in breast cancer: induction of vascular endothelial growth factor-C and correlation with metastasis and poor prognosis. Clin Cancer Res. 2006;12:1201-1207. [DOI] [PubMed] [Google Scholar]
- 50. Ridnour LA, Barasch KM, Windhausen AN, et al. Nitric oxide synthase and breast cancer: role of TIMP-1 in NO-mediated Akt activation. PLoS ONE. 2012;7:e44081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72: 41-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tschugguel W, Schneeberger C, Unfried G, et al. Expression of inducible nitric oxide synthase in human breast cancer depends on tumor grade. Breast Cancer Res Treat. 1999;56:145-151. [DOI] [PubMed] [Google Scholar]
- 53. Reveneau S, Arnould L, Jolimoy G, et al. Nitric oxide synthase in human breast cancer is associated with tumor grade, proliferation rate, and expression of progesterone receptors. Lab Invest. 1999;79:1215-1225. [PubMed] [Google Scholar]
- 54. Vakkala M, Kahlos K, Lakari E, Paakko P, Kinnula V, Soini Y. Inducible nitric oxide synthase expression, apoptosis, and angiogenesis in in situ and invasive breast carcinomas. Clin Cancer Res. 2000;6:2408-2416. [PubMed] [Google Scholar]
- 55. Martin JH, Begum S, Alalami O, Harrison A, Scott KW. Endothelial nitric oxide synthase: correlation with histologic grade, lymph node status and estrogen receptor expression in human breast cancer. Tumour Biol. 2000;21:90-97. [DOI] [PubMed] [Google Scholar]
- 56. Loibl S, von Minckwitz G, Weber S, et al. Expression of endothelial and inducible nitric oxide synthase in benign and malignant lesions of the breast and measurement of nitric oxide using electron paramagnetic resonance spectroscopy. Cancer. 2002;95:1191-1198. [DOI] [PubMed] [Google Scholar]
- 57. Alagol H, Erdem E, Sancak B, Turkmen G, Camlibel M, Bugdayci G. Nitric oxide biosynthesis and malondialdehyde levels in advanced breast cancer. Aust N Z J Surg. 1999;69:647-650. [DOI] [PubMed] [Google Scholar]
- 58. Zeillinger R, Tantscher E, Schneeberger C, et al. Simultaneous expression of nitric oxide synthase and estrogen receptor in human breast cancer cell lines. Breast Cancer Res Treat. 1996;40:205-207. [DOI] [PubMed] [Google Scholar]
- 59. Jadeski LC, Hum KO, Chakraborty C, Lala PK. Nitric oxide promotes murine mammary tumour growth and metastasis by stimulating tumour cell migration, invasiveness and angiogenesis. Int J Cancer. 2000;86:30-39. [DOI] [PubMed] [Google Scholar]
- 60. Tschugguel W, Knogler W, Czerwenka K, et al. Presence of endothelial calcium-dependent nitric oxide synthase in breast apocrine metaplasia. Br J Cancer. 1996;74:1423-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Salimian Rizi B, Achreja A, Nagrath D. Nitric oxide: the forgotten child of tumor metabolism. Trends Cancer. 2017;3:659-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pance A. Nitric oxide and hormones in breast cancer: allies or enemies? Future Oncol. 2006;2:275-288. [DOI] [PubMed] [Google Scholar]
- 63. Switzer CH, Cheng RY, Ridnour LA, Glynn SA, Ambs S, Wink DA. Ets-1 is a transcriptional mediator of oncogenic nitric oxide signaling in estrogen receptor-negative breast cancer. Breast Cancer Res. 2012;14:R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Haynes MP, Li L, Sinha D, et al. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem. 2003;278:2118-2123. [DOI] [PubMed] [Google Scholar]
- 65. Switzer CH, Glynn SA, Ridnour LA, et al. Nitric oxide and protein phosphatase 2A provide novel therapeutic opportunities in ER-negative breast cancer. Trends Pharmacol Sci. 2011;32:644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Basudhar D, Somasundaram V, de Oliveira GA, et al. Nitric oxide synthase-2-derived nitric oxide drives multiple pathways of breast cancer progression. Antioxid Redox Signal. 2017;26:1044-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Glynn SA, Boersma BJ, Dorsey TH, et al. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest. 2010;120:3843-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Basudhar D, Glynn SA, Greer M, et al. Coexpression of NOS2 and COX2 accelerates tumor growth and reduces survival in estrogen receptor-negative breast cancer. Proc Natl Acad Sci U S A. 2017;114:13030-13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Turner DP, Findlay VJ, Moussa O, Watson DK. Defining ETS transcription regulatory networks and their contribution to breast cancer progression. J Cell Biochem. 2007;102:549-559. [DOI] [PubMed] [Google Scholar]
- 71. Lincoln DW, II, Bove K. The transcription factor Ets-1 in breast cancer. Front Biosci. 2005;10:506-511. [DOI] [PubMed] [Google Scholar]
- 72. Nazir SU, Kumar R, Singh A, et al. Breast cancer invasion and progression by MMP-9 through Ets-1 transcription factor. Gene. 2019;711:143952. [DOI] [PubMed] [Google Scholar]
- 73. Park YH, Jung HH, Ahn JS, Im YH. Ets-1 upregulates HER2-induced MMP-1 expression in breast cancer cells. Biochem Biophys Res Commun. 2008;377: 389-394. [DOI] [PubMed] [Google Scholar]
- 74. Kim S, Han J, Shin I, Kil WH, Lee JE, Nam SJ. A functional comparison between the HER2(high)/HER3 and the HER2(low)/HER3 dimers on heregulin-β1-induced MMP-1 and MMP-9 expression in breast cancer cells. Exp Mol Med. 2012;44:473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hess DT, Matsumoto A, Nudelman R, Stamler JS. S-nitrosylation: spectrum and specificity. Nat Cell Biol. 2001;3:E46-E49. [DOI] [PubMed] [Google Scholar]
- 76. Furuta S. Basal s-nitrosylation is the guardian of tissue homeostasis. Trends Cancer. 2017;3:744-748. [DOI] [PubMed] [Google Scholar]
- 77. Marshall HE, Foster MW. S-nitrosylation of Ras in breast cancer. Breast Cancer Res. 2012;14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Raines KW, Cao GL, Lee EK, Rosen GM, Shapiro P. Neuronal nitric oxide synthase-induced S-nitrosylation of H-Ras inhibits calcium ionophore-mediated extracellular-signal-regulated kinase activity. Biochem J. 2006;397:329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Switzer CH, Glynn SA, Cheng RY, et al. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol Cancer Res. 2012;10:1203-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Garban HJ, Marquez-Garban DC, Pietras RJ, Ignarro LJ. Rapid nitric oxide-mediated S-nitrosylation of estrogen receptor: regulation of estrogen-dependent gene transcription. Proc Natl Acad Sci U S A. 2005;102:2632-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ehrenfeld P, Cordova F, Duran WN, Sanchez FA. S-nitrosylation and its role in breast cancer angiogenesis and metastasis. Nitric Oxide. 2019;87:52-59. [DOI] [PubMed] [Google Scholar]
- 82. Rahman MA, Senga T, Ito S, Hyodo T, Hasegawa H, Hamaguchi M. S-nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide-mediated cell invasion. J Biol Chem. 2010;285:3806-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Switzer CH, Ridnour LA, Cheng R, et al. S-nitrosation mediates multiple pathways that lead to tumor progression in estrogen receptor-negative breast cancer. For Immunopathol Dis Therap. 2012;3:117-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. von Lintig FC, Dreilinger AD, Varki NM, Wallace AM, Casteel DE, Boss GR. Ras activation in human breast cancer. Breast Cancer Res Treat. 2000;62:51-62. [DOI] [PubMed] [Google Scholar]
- 85. Canas A, Lopez-Sanchez LM, Penarando J, et al. Altered S-nitrosothiol homeostasis provides a survival advantage to breast cancer cells in HER2 tumors and reduces their sensitivity to trastuzumab. Biochim Biophys Acta. 2016;1862:601-610. [DOI] [PubMed] [Google Scholar]
- 86. Rizza S, Filomeni G. Role, targets and regulation of (de)nitrosylation in malignancy. Front Oncol. 2018;8:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhu W, Yang B, Fu H, et al. Flavone inhibits nitric oxide synthase (NOS) activity, nitric oxide production and protein S-nitrosylation in breast cancer cells. Biochem Biophys Res Commun. 2015;458:590-595. [DOI] [PubMed] [Google Scholar]
- 88. Paolo S. Nitric oxide in human health and disease. http://www.els.net/WileyCDA/ElsArticle/refId-a0003390.html. Updated 2013.
- 89. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Knott AB, Bossy-Wetzel E. Nitric oxide in health and disease of the nervous system. Antioxid Redox Signal. 2009;11:541-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Burgner D, Rockett K, Kwiatkowski D. Nitric oxide and infectious diseases. Arch Dis Child. 1999;81:185-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14:623-641. [DOI] [PubMed] [Google Scholar]
- 93. Hoang HH, Padgham SV, Meininger CJ. L-arginine, tetrahydrobiopterin, nitric oxide and diabetes. Curr Opin Clin Nutr Metab Care. 2013;16:76-82. [DOI] [PubMed] [Google Scholar]
- 94. Luiking YC, Engelen MP, Deutz NE. Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care. 2010;13:97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. El-Hattab AW, Emrick LT, Craigen WJ, Scaglia F. Citrulline and arginine utility in treating nitric oxide deficiency in mitochondrial disorders. Mol Genet Metab. 2012;107:247-252. [DOI] [PubMed] [Google Scholar]
- 96. Royle JS, Ross JA, Ansell I, Bollina P, Tulloch DN, Habib FK. Nitric oxide donating nonsteroidal anti-inflammatory drugs induce apoptosis in human prostate cancer cell systems and human prostatic stroma via caspase-3. J Urol. 2004;172:338-344. [DOI] [PubMed] [Google Scholar]
- 97. Seabra AB, Duran N. Nitric oxide donors for prostate and bladder cancers: current state and challenges. Eur J Pharmacol. 2018;826:158-168. [DOI] [PubMed] [Google Scholar]
- 98. Studenovsky M, Sivak L, Sedlacek O, et al. Polymer nitric oxide donors potentiate the treatment of experimental solid tumours by increasing drug accumulation in the tumour tissue. J Control Release. 2018;269:214-224. [DOI] [PubMed] [Google Scholar]
- 99. Quinn JF, Whittaker MR, Davis TP. Delivering nitric oxide with nanoparticles. J Control Release. 2015;205:190-205. [DOI] [PubMed] [Google Scholar]
- 100. Rapozzi V, Della Pietra E, Bonavida B. Dual roles of nitric oxide in the regulation of tumor cell response and resistance to photodynamic therapy. Redox Biol. 2015;6:311-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liu Z, Li G, Gou Y, et al. JS-K, a nitric oxide prodrug, induces DNA damage and apoptosis in HBV-positive hepatocellular carcinoma HepG2.2.15 cell. Biomed Pharmacother. 2017;92:989-997. [DOI] [PubMed] [Google Scholar]
- 102. Kim KM, Kim NS, Kim J, et al. Magnolol suppresses vascular endothelial growth factor-induced angiogenesis by inhibiting Ras-dependent mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. Nutr Cancer. 2013;65:1245-1253. [DOI] [PubMed] [Google Scholar]
- 103. Sengupta A, Ghosh S, Bhattacharjee S. Dietary cardamom inhibits the formation of azoxymethane-induced aberrant crypt foci in mice and reduces COX-2 and iNOS expression in the colon. Asian Pac J Cancer Prev. 2005;6:118-122. [PubMed] [Google Scholar]
- 104. Nasr M, Selima E, Hamed O, Kazem A. Targeting different angiogenic pathways with combination of curcumin, leflunomide and perindopril inhibits diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharmacol. 2014; 723:267-275. [DOI] [PubMed] [Google Scholar]