Short abstract
Increased reactive oxygen species production and oxidative stress have been implicated in the pathogenesis of numerous neurodegenerative conditions including among others Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, Friedrich’s ataxia, multiple sclerosis, and stroke. The endogenous antioxidant response pathway protects cells from oxidative stress by increasing the expression of cytoprotective enzymes and is regulated by the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2). In addition to regulating the expression of antioxidant genes, NRF2 has also been shown to exert anti-inflammatory effects and modulate both mitochondrial function and biogenesis. This is because mitochondrial dysfunction and neuroinflammation are features of many neurodegenerative diseases as well NRF2 has emerged as a promising therapeutic target. Here, we review evidence for a beneficial role of NRF2 in neurodegenerative conditions and the potential of specific NRF2 activators as therapeutic agents.
Keywords: dementia and neurological disorders, neurodegenerative diseases, bioenergetics
Introduction
Reactive oxygen species (ROS) serve as critical intracellular signaling molecules but in excess can cause oxidative stress and damage organelles and macromolecules, eventually leading to cell death (Zuo et al., 2015). Increased ROS production and oxidative stress have been implicated in the pathogenesis of numerous neurodegenerative conditions, including, among others, Alzheimer’s disease (AD; Behl, 2005), Parkinson’s disease (PD; Trist et al., 2019), Huntington’s disease (HD; Browne and Beal, 2006), Friedrich’s ataxia (Lupoli et al., 2018), multiple sclerosis (MS; Di Filippo et al., 2010), and stroke (Rodrigo et al., 2013). Aging is the primary risk factor for most neurodegenerative diseases, and oxidative stress is known to increase with aging. Although increased oxidative stress is the cumulative result of many factors including changes in tissue antioxidant status, increased mitochondrial dysfunction, and altered metal homeostasis, the majority of the excessive ROS are produced by the mitochondria (Buendia et al., 2016). The endogenous antioxidant response pathway protects cells from oxidative stress by increasing the expression of cytoprotective enzymes that can scavenge free radicals and reduce the risk of cellular damage caused by ROS (Itoh et al., 1997; Motohashi and Yamamoto, 2004; Buendia et al., 2016). The transcription factor nuclear factor erythroid 2-related factor 2 (NRF2, also called NFE2L2) regulates this pathway by binding to antioxidant response elements (AREs) in the promoters of antioxidant genes (Itoh et al., 1997; Motohashi and Yamamoto, 2004). These AREs are cis-acting enhancer sequences. Past studies identified a necessary ARE core sequence of RTGACnnnGC, but this sequence alone is insufficient to mediate induction and requires additional flanking nucleotides that can vary between different AREs (Wasserman and Fahl, 1997).
NRF2 is a member of the cap-n-collar family of basic leucine zipper proteins. NRF2 is normally bound in the cytosol to Kelch-Like ECH-Associated Protein 1 (KEAP1) that targets it for degradation by the proteasome. However, in the presence of electrophiles or oxidative stress, the nucleophilic cysteine sulfhydryl groups on KEAP1 are modified, resulting in an allosteric conformational change that diminishes KEAP-dependent degradation of NRF2 and allows the transcription factor to accumulate in the nucleus (Kobayashi et al., 2006). While this is the classical mode of NRF2 activation, other mechanisms, including phosphorylation, can also result in its dissociation from KEAP1 and increased nuclear localization (Huang et al., 2002; Chen et al., 2019; Xiao et al., 2019). Within the nucleus, NRF2 forms a heterodimer with small musculoaponeurotic Maf proteins and binds to ARE consensus sequence in the promoter of target genes (Esteras et al., 2016).
NRF2 is ubiquitously expressed (Moi et al., 1994) and in the brain is an important defense against toxic insults in both glial cells as well as neurons (Lee et al., 2003; Jakel et al., 2007; Chen et al., 2009; Vargas and Johnson, 2009). In addition to upregulating numerous antioxidant enzymes, NRF2 can also increase expression of anti-inflammatory mediators, Phase I and II drug-metabolizing enzymes as well as mitochondrial pathways (Nguyen et al., 2009; Sandberg et al., 2014; Dinkova-Kostova and Abramov, 2015; Buendia et al., 2016; Hayashi et al., 2017; Sivandzade et al., 2019).
Antioxidant Role of NRF2
Within the central nervous system, upregulation of NRF2 target genes, such as heme oxygenase-1 (HMOX1), glutathione S-transferase (GST), superoxide dismutase (SOD), catalase (CAT), NAD(P)H dehydrogenase (quinone) 1 (NQO1), and others, can make neurons more resistant to oxidative insults (Chen et al., 2000; Satoh et al., 2006; Giordano et al., 2007; Tanito et al., 2007; Lim et al., 2008).
Anti-Inflammatory Role of NRF2
Because of the cross talk that exists between antioxidant and anti-inflammatory pathways, many of the anti-inflammatory and mitochondrial actions of NRF2 have been considered secondary to its antioxidant effects. For example, the classical proinflammatory transcription factor NFkB is activated by oxidative stress that can be blocked by the NRF2-dependent induction of antioxidant target genes, and thus, the transcription of proinflammatory cytokines is decreased (Lee et al., 2009a; Bellezza et al., 2012). However, NRF2 has been shown to directly regulate the expression of anti-inflammatory mediators such as interleukin (IL)-17D, CD36, macrophage receptor with collagenous structure, and G protein-coupled receptor kinase (Thimmulappa et al., 2002; Ishii et al., 2004; Saddawi-Konefka et al., 2016). Moreover, NRF2 has recently been implicated in reducing the expression of the proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-6, IL-8, and IL-1β in microglia, macrophages, monocytes, and astrocytes (Kobayashi et al., 2016; Quinti et al., 2017).
Mitochondrial Role of NRF2
A complex interplay also exists between mitochondrial function and antioxidant response. By inducing free-radical scavenging enzymes, NRF2 protects mitochondria from oxidative damage. NRF2 can also directly regulate mitochondrial biogenesis as well as function and by improving mitochondrial function diminishes overproduction of intracellular ROS. NRF2 activation affects the expression of many mitochondrial enzymes important for proper bioenergetic function including malic enzyme 1, isocitrate dehydrogenase 1, glucose-6-phosphate dehydrogenase, and 6-phosphogluconate-dehydrogenase (Morgan et al., 2013; Ku and Park, 2017). A role has also been described for NRF2 in maintaining mitochondrial integrity and regulating mitochondrial biogenesis. It has been reported that NRF2 activation protects mitochondria by opening the mitochondrial permeability transition pore (Lee et al., 2000; Greco and Fiskum, 2010; Greco et al., 2011) and that declining NRF2 signaling is associated with increased damage to mitochondrial DNA (mtDNA; Li et al., 2018). In addition, NRF2 activation modulates the expression of ATP synthase subunit α and NDUFA4, two components of the electron transport chain (Abdullah et al., 2012; Agyeman et al., 2012), and mice in which NRF2 has been deleted have lower mtDNA globally than wild-type (WT) mice (Zhang et al., 2013). In addition, NRF2 activation affects the expression of several regulators of mitochondrial biogenesis including Sirt1 (sirtuin 1), PPARγ (peroxisome proliferator-activated receptor γ), and Pgc1α (PPARγ coactivator 1α), considered to be the master regulator of biogenesis (Cho et al., 2010; Bellezza et al., 2012; Lai et al., 2014; Ping et al., 2015; Huang et al., 2017a; Song et al., 2018).
Role of NRF2 in Aging
NRF2 expression and activity are diminished in both aged mice and humans (Suh et al., 2004; Collins et al., 2009; Duan et al., 2009; Demirovic and Rattan, 2011; Cheng et al., 2012; Rahman et al., 2013). Because oxidative stress, inflammation, and mitochondrial dysfunction are all features of the aging brain and because aging is the primary risk factor for most neurodegenerative diseases, NRF2 has emerged as an attractive target for clinical intervention. In fact, the NRF2-activating compound dimethyl fumarate (DMF) is an existing FDA-approved therapy for use in MS. This review will primarily focus on the therapeutic potential of NRF2 activation in other neurodegenerative conditions.
NRF2 and MS
MS is a chronic inflammatory neurodegenerative disorder that currently affects more than 2.3 million people worldwide (Browne et al., 2014). Demyelination, astrocytosis, axonal degeneration, and sclerotic plaques are all features of the autoimmune response that occurs in the disease (Compston and Coles, 2008). Elevated levels of proinflammatory cytokines are seen in the cerebrospinal fluid (CSF) of MS patients (Khaibullin et al., 2017) as have increased levels of chemokines involved in B cell migration (Kalinowska-Lyszczarz et al., 2011). Large-scale gene expression studies using either peripheral blood monocytes or brain tissue from MS patients have also found altered expression of several genes involved in the activation of T and B cells (Ramanathan et al., 2001).
Oxidative stress and mitochondrial dysfunction are also prominent features of the disease. The activation of microglia and macrophages is a major contributor to the increased ROS seen in MS (Genestra, 2007). These elevated ROS levels along with the enhanced inflammatory response negatively affect mitochondrial function. Mitochondrial injury, oxidative stress, and altered metabolism are thought to be connected to the formation of plaques and subsequent neurodegeneration in both white and gray matter lesions (Fischer et al., 2012).
Several mouse models of MS effectively recapitulate the inflammatory response, mitochondrial dysfunction, and oxidative stress seen in the disease. In the experimental autoimmune encephalomyelitis (EAE) model, mitochondrial dysfunction appears early in the disease progression with damage to the mitochondria being evident even before the inflammatory processes of the disease develop (Qi et al., 2006; Sadeghian et al., 2016). EAE mice also show high levels of oxidative damage in the spinal cord along with elevated levels of oxidative stress-induced enzymes (Wang et al., 2017a) and reduced frequency of TH1, TH17, and B cell MHCII expression (Schulze-Topphoff et al., 2016). Similar observations have been made in the lipopolysaccharide (LPS)-induced model of MS. Elevated TNFα, IL-6, and IL-10 is seen in the blood of LPS-treated animals as are increased markers of oxidative stress (Yang et al., 2018). Widespread mitochondrial dysfunction is also observed throughout the brains of LPS-treated animals (Noh et al., 2014).
Multiple reports suggest a role for NRF2 in MS pathogenesis. Loss of NRF2 has been shown to result in more rapid onset and a more severe clinical course following EAE treatment that was accompanied by increased glial activation and exacerbated spinal cord damage and axonal degeneration as well as increased levels of proinflammatory cytokines (Johnson et al., 2010; Larabee et al., 2016; Table 1). Conversely, a number of NRF2-activating compounds have shown beneficial effects in MS model systems. NRF2 activation by resveratrol, lycopene, quercetin, and ferulic acid has been shown to reduce LPS-induced neurotoxicity, improve synaptic and mitochondrial function, and reduce inflammatory markers as well as gliosis (Chen et al., 2017; Khan et al., 2018; Rehman et al., 2019; Wang et al., 2019). In the EAE model, DMF treatment increased NRF2 activation in neurons and glial cells and improved disease score ratings, an effect that was lost with NRF2 deletion (Linker et al., 2011).
NRF2 activation as a clinical target is also well established in MS. DMF, also known as Tecfidera or BG-12, was licensed as an oral therapy for relapsing remitting MS in 2013. DMF treatment has been evaluated in Phase I and Phase II trials and has been shown to reduce relapse rates and decrease the number and progression of lesions, even though no differences were seen on the expanded disability status scale (Kappos et al., 2008; Fox et al., 2012; Gold et al., 2012; Table 2). DMF has also been shown to be comparable or superior to several other MS treatments. DMF-treated patients had lower annualized relapse rates and 12-week disability progression when compared with patients treated with glatiramer acetate (Chan et al., 2017). Patients who switched from first-generation platform disease-modifying therapies to DMF also saw reductions in annualized relapse rates and lesions, and switching to DMF treatment was found to be comparable with fingolimod and superior to teriflunomide (Fernandez et al., 2017; Prosperini et al., 2018; Ontaneda et al., 2019; Table 2).
Table 2.
Summary of Clinical Interventions With NRF2-Activating Compounds.
| Disease | Intervention | Outcome |
|---|---|---|
| MS | DMF | - Reduced relapse rates and decreased the number and progression of lesions (Kappos et al., 2008; Fox et al., 2012; Gold et al., 2012)- Increased no evidence of disease status (Hammer et al., 2018; Prosperini et al., 2018) |
| Omega 3 fatty acids | - Decreased inflammatory markers and increased antioxidant capacity (Gallai et al., 1995) | |
| Vitamin D | - Improved cognitive function in Vitamin D-deficient patients (Darwish et al., 2017) | |
| Vitamin D + omega 3 fatty acids | - Decreased inflammatory markers as well as increased antioxidant capacity and improvement on expanded disability status scale (Kouchaki et al., 2018) | |
| Lipoic acid | - Decreased inflammatory markers as well as increased antioxidant capacity improvement on expanded disability status scale (Khalili et al., 2014) | |
| AD | Vitamin E | - Improved cognitive function and activities of daily life (Sano et al., 1997; Dysken et al., 2014) |
| Omega 3 fatty acid + alpha lipoic acid | - Slowed decline in mini-mental state exam and in activities of daily life (Shinto et al., 2014) | |
| PD | N-acetylcysteine | - Increased antioxidant activity and improved scores on Unified Parkinson’s Disease Rating Scale (Coles et al., 2018) |
| Vitamin D + omega 3 fatty acids | - Increased total antioxidant capacity and improved scores on Unified Parkinson’s Disease Rating Scale (Taghizadeh et al., 2017) | |
| Friedrich’s Ataxia | Omaveloxolone | - Improved scores on modified Friedrich’s ataxia rating scale (Reata Pharmaceuticals, 2019) |
| Stroke | Vitamin E | - Improvement on Matthew Scale and Barthel index as well as decreased plasma lipid peroxidation (Daga et al., 1997) |
| Soybean isoflavones | - Increased brachial flow-mediated dilation, enhanced antioxidant markers, and decreased circulating proinflammatory cytokines as well as markers of oxidative damage (Li and Zhang, 2017) |
Note. DMF = dimethyl fumarate; MS = multiple sclerosis; AD = Alzheimer’s disease; PD = Parkinson’s disease.
DMF treatment has been shown to increase the expression of the NRF2 target gene NQO1 in the blood of MS patients (Gopal et al., 2017; Hammer et al., 2018), and those patients who showed a significant increase in NQO1 expression after 4 to 6 weeks of treatment were more likely to achieve no evidence of disease activity status 1 year later (Hammer et al., 2018; Table 2).
The exact mechanism by which DMF exerts its beneficial effects has not been fully described, although multiple mechanisms including shifting toward anti-inflammatory immune balance, inhibition of T cell activation, induction of B and T cell apoptosis, inhibition of proinflammatory cytokines, and reduction of memory T cells have all been suggested as contributing factors (Treumer et al., 2003; Longbrake et al., 2016; Schulze-Topphoff et al., 2016; Smith et al., 2017; Montes Diaz et al., 2018a). There have been many clinical and basic science studies of DMF in MS. An excellent and thorough review of the history and mechanism of DMF as an MS therapy was recently published by Montes Diaz et al. (2018b).
Treatment with other NRF2-activating compounds has likewise demonstrated beneficial results in MS patients. It has been reported that Vitamin D, omega 3 fatty acids, and lipoic acid all result in decreased inflammatory markers, increased antioxidant capacity, and improvements on the expanded disability status scale (Gallai et al., 1995; Khalili et al., 2014; Kouchaki et al., 2018; Table 2), although NRF2 activation was not investigated as a mechanism in any of these studies. Vitamin D supplementation has also been associated with improved cognitive function in MS patients, especially those that were vitamin D deficient (Darwish et al., 2017; Table 2).
NRF2 and AD
AD is the most common form of dementia. It currently affects an estimated 5.7 million people, and this number is predicted to reach 14 million by 2050 (Alzheimer's Association, 2017). The two pathological hallmarks of AD are intracellular neurofibrillary tangles comprised of the protein tau and extracellular β-amyloid (Aβ) plaques. The relationship between these hallmarks and the inflammation, oxidative stress, and mitochondrial dysfunction that occurs in AD is complex. The Aβ plaques activate nearby microglia which can result in increased release of proinflammatory cytokines and ROS which can result in mitochondrial dysfunction (Galasko and Montine, 2010). However, oxidative stress and neuroinflammation are also believed to facilitate the pathological protein aggregation seen in AD (Di Bona et al., 2010).
Increased oxidative stress is considered to be an early event in AD brains (Lovell and Markesbery, 2007), and diminished antioxidant capacity along with increased markers of oxidative stress are evident in both the blood and brains of AD patients (Gubandru et al., 2013; Schrag et al., 2013; Zabel et al., 2018). Reduced neuronal mitochondrial function and number have also been reported in AD patients (Hirai et al., 2001), and studies suggest that alterations in mitochondrial bioenergetics in the brain precede and may even induce cognitive decline in AD (Yao et al., 2009).
It has been reported that NRF2 nuclear expression is decreased in AD and a recent meta-analysis of microarray datasets identified 31 downregulated ARE genes in AD patients (Ramsey et al., 2007; Kanninen et al., 2008; Wang et al., 2017b). Similarly, in transgenic mouse models of AD, loss of NRF2 has been shown to increase levels of Aβ and phosphorylated tau (Branca et al., 2017; Rojo et al., 2017); increase glial activation, markers of oxidative stress, and neurodegeneration; and exacerbate cognitive decline (Rojo et al., 2017; Rojo et al., 2018; Table 1).
Table 1.
Summary of Studies Using NRF2KO in Neurodegenerative Disease Models.
| Disease | Result of loss of NRF2 |
|---|---|
| MS | - More severe clinical course, increased glial activation and exacerbated spinal cord damage, increased proinflammatory cytokines, and axonal degeneration in response to EAE (Johnson et al., 2010; Larabee et al., 2016) |
| AD | - Enhanced Aβ and tau pathology, increased glial activation, markers of oxidative stress and neurodegeneration, and exacerbated cognitive decline in transgenic mouse models (Branca et al., 2017; Rojo et al., 2017, 2018) |
| PD | - Increased sensitivity to MPTP with enhanced dopaminergic cell loss and microglial activation (Chen et al., 2009; Rojo et al., 2010)- Increased vulnerability to 6-OH dopamine-induced cell loss (Jakel et al., 2007)- Increased inflammation, protein misfolding, and neuronal death in transgenic model of αSyn accumulation (Lastres-Becker et al., 2016) |
| HD | - Increased vulnerability to 3-NP and malonic acid-induced lesions in the striatum (Shih et al., 2005) |
| Stroke | - Increased infarct size, enhanced inflammatory response, and neurobehavioral deficits (Shih et al., 2005; Li et al., 2013) |
Note. NRF2 = nuclear factor erythroid 2-related factor 2; MS = multiple sclerosis; EAE = experimental autoimmune encephalomyelitis; AD = Alzheimer’s disease; PD = Parkinson’s disease; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; HD = Huntington’s disease; 3-NP = 3-nitropropionic acid.
Conversely, the activation of NRF2 has been shown to be beneficial in models of AD. A recent study found that NRF2 activation by sulforaphane reduces amyloid secretion and normalizes cytokine in AD astrocytes derived from human-induced pluripotent stem cells (Oksanen et al., 2019). In in vivo studies, the NRF2-activating compound tert-Butylhydroquinone (tBHQ) was found to protect against cell death in isolated neurons exposed to Aβ (Kanninen et al., 2008), and oral curcumin, another NRF2 activator, has been shown to prevent synaptic degradation and improve spatial learning in the 5xFAD mouse model of Aβ accumulation (Zheng et al., 2017). DMF has likewise been shown to protect against Aβ-induced cell death in vitro (Campolo et al., 2018) and also to improve spatial memory in a rat model of AD (Majkutewicz et al., 2016).
While therapies specifically targeting NRF2 have yet to be investigated clinically, there is some evidence that treatment with antioxidant compounds could be beneficial. Trials with vitamin E have shown slight improvements in cognitive function and activities of daily life at high doses (Sano et al., 1997; Dysken et al., 2014), and treatment with a combination of omega 3 fatty acids and alpha lipoic acid slowed decline in mini-mental state exam as well as activities of daily life (Shinto et al., 2014; Table 2). Although these compounds are known to activate NRF2, none of these studies investigated that specifically.
NRF2 in PD
PD is a progressive and incurable movement disorder that is accompanied by varying degrees of cognitive dysfunction and dementia and is the second most common neurodegenerative disease (Goris et al., 2007; Tufekci et al., 2011). PD is characterized by loss of dopaminergic neurons in the substantia nigra and the accumulation of intracellular protein aggregates known as Lewy bodies, enriched with α-synuclein (αSyn; Bhat et al., 2018). Oxidative stress, mitochondrial dysfunction, and neuroinflammation have all been implicated in the development and progression of the disease (Navarro and Boveris, 2009; Di Filippo et al., 2010).
Imaging studies have observed mitochondrial dysfunction in the dopamine neurons in the substantia nigra early in PD progression (Hattingen et al., 2009), and reduced expression and activity of enzymes in the electron transport chain has been observed in the brains of PD patients throughout the course of the disease (Schapira et al., 1989, 1990; Trimmer et al., 2000). In late-stage PD patients, increased mutations in mtDNA are seen which lead to dysfunction in Complex I and increased oxidative stress (Schapira, 2008; Moon and Paek, 2015). Increased markers of oxidative damage along with decreased antioxidant enzyme activity have also been observed in the blood of PD patients as well (Wei et al., 2018). Inflammation is also prevalent in PD patients. Proinflammatory cytokines such as TNFα and IL-6 are known to be elevated in both the brain and CSF of patients with PD (Dzamko et al., 2015; Hirsch et al., 2012). In fact, it has even been hypothesized that PD pathogenesis may result from an inflammatory infection of the gut and that inflammation then spreads systemically (Weller et al., 2005).
There are multiple lines of evidence suggesting that NRF2 may be a viable therapeutic target for PD. NRF2 expression and activity has been shown to be altered in nigral dopaminergic neurons in PD patients (Schipper et al., 1998; Ramsey et al., 2007) and a functional haplotype in the human NRF2 promoter that increases transcriptional activity of the gene is associated with decreased risk and delayed onset of PD (von Otter et al., 2010). Pharmacological alteration of NRF2 expression levels can phenocopy this protective haplotype in mice (Huang et al., 2017b; Meng et al., 2017).
In addition, in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD, decreased NRF2 activity has been reported, and it was observed that loss of NRF2 exacerbated the phenotype (Chen et al., 2009; Table 1). Dopaminergic neuronal loss and microglial activation were both also more severe in mice in which NRF2 has been deleted (Rojo et al., 2010; Table 1). These mice show a similar increase in vulnerability to 6-OH dopamine, and this damage can be prevented by transplantation of astrocytes overexpressing NRF2 (Jakel et al., 2007; Table 1). Likewise, in a model of αSyn accumulation, loss of NRF2 exacerbated inflammation, protein misfolding, and neuronal death (Lastres-Becker et al., 2016; Table 1).
Activation of NRF2 has been associated with neuroprotection in various PD model systems. In MPTP-treated mice, NRF2 activation, via siRNA mediated knockdown of KEAP1, was associated with a reduction in oxidative stress and neuroinflammation (Williamson et al., 2012). NRF2 activation by sulforaphane also induced the expression of antioxidant enzymes and protected against dopaminergic neuronal loss in MPTP-treated mice (Jazwa et al., 2011) as did a NRF2-activating synthetic triterpenoid that also reduced ROS levels and upregulated genes involved in mitochondrial biogenesis (Yang et al., 2009). In addition, in neuroblastoma-overexpressing αSyn, treatment with tBHQ reduced ROS levels and improved mitochondrial respiratory rates (Fu et al., 2018).
Although NRF2 has not yet been targeted in clinical interventions in PD, epidemiological evidence suggests that high levels of consumption of the NRF2-activating vitamins E and C are associated with decreased risk of PD (Zhang et al., 2002; Seidl and Potashkin, 2011). In addition, 4 weeks of treatment with N-acetyl cysteine, which also activates NRF2, improved scores on the Unified Parkinson’s Disease Rating Scale and increased peripheral markers for antioxidant activity (Coles et al., 2018) as did 12 weeks of intervention with omega 3 fatty acids and vitamin E (Taghizadeh et al., 2017; Table 2). However, NRF2 activation was not specifically implicated as a mechanism of action in either of these studies.
NRF2 and HD
HD is an autosomal dominant neurodegenerative disorder caused by a polyglutamine expansion in the huntingtin protein (Jimenez-Sanchez et al., 2017). Its primary symptoms are motor impairment, cognitive decline, and psychiatric problems which worsen as the disease progresses. Degeneration of both the neostriatal and cerebral cortex are believed to contribute to the cognitive impairment and motor symptoms (Vonsattel et al., 1985; Halliday et al., 1998). As the huntingtin protein aggregates, it becomes toxic to neurons and promotes the generation of ROS (Goswami et al., 2006). Oxidative stress is particularly prominent in the neostriatum of HD brains (Sorolla et al., 2008) and is thought to be an important driver of degeneration. Markers of increased oxidative stress are also evident peripherally. Plasma lipid peroxidation is increased, and glutathione levels are decreased in HD patients. This imbalance can be observed prior to symptom onset and continues to be proportional to the severity of the disease as it progresses (Chen et al., 2007; Klepac et al., 2007). Increased oxidative damage to proteins as well as mtDNA has also been observed in the brains of HD patients (Chen et al., 2007; Duran et al., 2010; Johri and Beal, 2012). Postmortem analysis also revealed that the levels of mitochondrial enzymes involved in oxidative phosphorylation are also depressed in the brains of HD patients, especially the basal ganglia (Chen et al., 2007; Duran et al., 2010). In addition to reports of increased oxidative stress (Browne et al., 1997; Browne and Beal, 2006; del Hoyo et al., 2006; Chen et al., 2007; Klepac et al., 2007; Chang et al., 2012) and mitochondrial dysfunction (Browne, 2008; Damiano et al., 2010; Kim et al., 2010), chronic inflammation is also evident in HD (Pavese et al., 2006; Tai et al., 2007; Bjorkqvist et al., 2008; Politis et al., 2011). Inflammation appears to be an early event in the pathogenesis of HD, and PET imaging has revealed increased microglial activation that is already evident in the striatum and cortex of presymptomatic HD carriers (Tai et al., 2007; Politis et al., 2011). Plasma concentrations of inflammatory cytokines are likewise elevated prior to the onset of disease symptoms, and increased immune activity persists in the CSF of HD patients (Bjorkqvist et al., 2008).
The same mitochondrial dysfunction, oxidative stress, and neuroinflammation are also seen in the brains of animal models of HD, and using these models, studies have demonstrated an important role for NRF2. In the 3-nitropropionic acid (3-NP)-induced model of HD, NRF2 activation is reduced and mice in which NRF2 has been knocked out have been reported to be significantly more vulnerable to both 3-NP and malonic acid-induced lesions in striatum (Shih et al., 2005a; Table 1). Conversely, intrastriatal injections of NRF2 overexpressing astrocytes has been found to be protective against 3-NP and malonic acid-induced damage (Calkins et al., 2005).
Pharmacological activation of NRF2 likewise shows beneficial results in mouse models of HD. DMF treatment protects cortical and striatal neurons, slows weight loss, helps maintain motor function, and increases lifespan in WT but not mice lacking NRF2 (Ellrichmann et al., 2011; Jin et al., 2013). Oral administration of NRF2-activating synthetic triterpenoids similarly attenuated motor deficits, increased longevity, and reduced oxidative stress in a transgenic HD mouse model (Stack et al., 2010), and a NRF2-activating compound isolated from the plant Panax ginseng Meyer decreased ROS and restored antioxidant enzyme levels in the striatum and ameliorated behavioral impairments in the 3-NP mouse model (Gao et al., 2015). In addition, sulforaphane has beneficial effects in both transgenic and 3-NP-induced models of HD. Sulforaphane promoted huntingtin protein degradation and reduced cytotoxicity in both central and peripheral tissues of transgenic animals (Liu et al., 2014) and was also able to suppress 3-NP-induced proinflammatory cytokine production in the striatum and improve behavioral impairments (Jang and Cho, 2016).
In humans, disrupted NRF2 signaling has also been observed. The NRF2 target antioxidant enzymes glutathione peroxidase and SOD1 are reduced in the leukocytes of HD patients compared with controls (Chen et al., 2007). In addition, it has been reported that in striatal neurons, abnormal huntingtin protein disturbs NRF2 signaling, promoting mitochondrial dysfunction and increased oxidative stress (Kim et al., 2010). Although NRF2-activating compounds have not been tested in clinical trials in primary monocytes from HD patients, NRF2 induction by the KEAP1 modifying small molecule C151 repressed IL-1, IL-6, IL-8, and TNFα production (Quinti et al., 2017).
NRF2 and Friedrich’s Ataxia
Friedrich’s ataxia is an inherited degenerative neuromuscular disorder for which there are no approved therapies (Aranca et al., 2016). It is the most common hereditary ataxia affecting about 1 in 50,000 people worldwide (Polek et al., 2013; Vankan, 2013). The disease is an autosomal recessive condition primarily affecting the dorsal root ganglia, cerebellar dentate nuclei, and the heart. It is caused by a GAA repeat expansion mutation in the frataxin gene. This leads to transcriptional silencing which results in a progressive decrease of frataxin protein expression. Frataxin helps assemble iron sulfur clusters necessary for proper mitochondrial function (Li et al., 2008). In Friedrich’s ataxia patients, the decreased frataxin expression causes mitochondrial iron overload, impaired ATP production, and increased oxidative stress (Li et al., 2008; Santos et al., 2010; Aranca et al., 2016). The reduced ATP production is believed to account for the progressive muscle weakness, fatigue, and decreased coordination seen in patients with the disease. The increase in oxidative stress is also thought to be pathogenic, leading to chronic depletion of antioxidants and causing neurodegeneration (Nickel et al., 2014). Cerebellar granule neurons are particularly susceptible to these changes exhibiting significant increases in ROS and lipid peroxidation along with reduced glutathione. Studies using these cerebellar granule neurons have shown that reduced frataxin production leads to increased ROS which induces mitochondrial impairments (Abeti et al., 2015, 2016).
NRF2 signaling dysfunction has been widely reported in animal models of Friedrich’s ataxia. A conditional frataxin knockout mouse line showed decreased NRF2 expression and increased KEAP1 expression (Anzovino et al., 2017) and in the mouse motor neuron cell line NSC-34, frataxin shRNA likewise reduced NRF2 expression and activity (Paupe et al., 2009). In contrast, inducing NRF2 with sulforaphane or the NRF2-activating compound EPI-7443 in neural stem cells isolated from a Friedrich’s ataxia mouse model was shown to reestablish proper differentiation which was previously impaired in those cells (La Rosa et al., 2019). In cultured motor neurons, sulforaphane also increased frataxin levels as well as neurite number and extension (Petrillo et al., 2017). Similar reductions in NRF2 expression have been seen in fibroblasts isolated from patients (Paupe et al., 2009; Petrillo et al., 2017), and sulforaphane treatment likewise increased frataxin levels and enhanced neurite outgrowth in these cells (Petrillo et al., 2017).
The NRF2-activating compound omaveloxolone has been shown to be beneficial in the KIKO and the YG8R mouse models of Friedrich’s ataxia as well as in fibroblasts isolated from human patients. In these models, omaveloxolone restored Complex I activity, increased glutathione levels, decreased ROS, and restored the mitochondrial membrane potential preventing cell death (Abeti et al., 2018). Based on these promising preclinical studies, omaveloxolone is currently undergoing clinical testing. A recent press release announced the results of their Phase II trial that found that 48 weeks of treatment with the compound was generally well tolerated and resulted in significantly improved scores on the modified Friedrich’s ataxia rating scale (Reata Pharmaceuticals, 2019; Table 2).
NRF2 and Stroke
Stroke is the second leading cause of death worldwide and the leading cause of acquired adult disability (Lozano et al., 2012; Murray et al., 2012). Ischemic stroke is characterized by decreased blood flow to parts of the brain resulting in injury to brain tissue and impaired neurologic functioning (Jauch et al., 2013; Ding et al., 2017). A cascade of biochemical events occur following an ischemic stroke that promote tissue damage including inflammatory activation and mitochondrial dysfunction leading to an overproduction of ROS. Eventual restoration of blood flow (reperfusion) exacerbates inflammatory responses and ROS generation leading to further damage from oxidative stress, including degradation of vascular wall proteins and deterioration of blood–brain barrier (BBB) integrity (Stephenson et al., 2000; Kunz et al., 2008; Harari and Liao, 2010; Pradeep et al., 2012).
Animal studies have shown that mice in which NRF2 has been deleted have enhanced infarct size, inflammatory response, and neurobehavioral deficits when compared with NRF2 expressing mice (Shih et al., 2005b; Li et al., 2013; Table 1). Conversely, NRF2 activation has been shown to be beneficial in protecting the brain from injury. tBHQ treatment attenuated neonatal hypoxic-ischemic brain damage in rats (Zhang et al., 2018), and the NRF2-activating compound metformin likewise reduced infarct volume and was able to attenuate cognitive impairments in a mouse model of transient middle cerebral artery occlusion (Kaisar et al., 2017). Trichostatin A-induced NRF2 activation (resulting from decreased KEAP1 expression) similarly increased neuronal cell viability and reduced infarct volume following stroke (Wang et al., 2012).
A number of plant-derived NRF2-activating compounds have also been shown to have beneficial effects in rodent models of stroke. When given prior to injury, the plant-derived flavanol (-)-epicatechin was able to reduce infarct size and the subsequent cognitive impairment, but this effect was lost in mice that do not express NRF2. Posttreatment also improved the same outcome but in a time-sensitive manner (Shah et al., 2010). NRF2 activation by sulforaphane increased the expression of cytoprotective genes in brain tissue and preserved BBB integrity (Zhao et al., 2007), while ginkgolides and bilobalide have been shown to activate NRF2 and decrease both cerebral ROS levels as well as infarct volume ratios in middle cerebral artery occlusion rats (Liu et al., 2019b). Activation of NRF2 by the isoquercetin also attenuated oxidative stress and neuronal loss following ischemia/reperfusion injury in mice by inhibiting NFkB activation (Dai et al., 2018).
Clinical research into NRF2 activation as a stroke therapy is limited, but a few dietary interventions with compounds that can activate NRF2 do suggest a potential benefit. Following ischemic stroke, 6 weeks of vitamin E supplementation was shown to elicit a greater improvement in both the Matthew scale and Barthel index than placebo. This was accompanied by a significant reduction in plasma lipid peroxidation suggesting increased antioxidant activity, although it is unknown the contribution of NRF2 activation specifically to this effect because while vitamin E can activate NRF2, it also possesses free radical scavenging properties in and of itself (Daga et al., 1997; Table 2). However, when patients with ischemic stroke were given soybean isoflavones for 24 weeks, NRF2 activation was implicated in the improvements observed. Supplementation resulted in increased brachial flow-mediated dilation and NRF2 and SOD expression along with decreased circulating levels of C-reactive protein, 8-isoprostane, malondialdehdye, IL-6, and TNFα. The effects on circulating oxidative stress markers were lost when NRF2 was silenced (Li and Zhang, 2017; Table 2).
The potential benefits of NRF2 activation as a means of preventing stroke are somewhat less clear. A number of large-scale prospective studies have been conducted investigating supplementary with vitamins and have yielded conflicting results. In one study of 8,171 female health professionals with a history of cardiovascular disease who received vitamin C, vitamin E, or beta carotene for a mean follow-up time of 9 years, it was determined that those individuals who received vitamin C or vitamin E experienced fewer strokes (Cook et al., 2007), although again, whether this was due to their abilities to activate NRF2 specifically was not determined. Conversely, in another study where more than 20,000 adults with coronary artery disease, occlusive arterial disease, or diabetes were given supplementation with a combination of vitamin E, vitamin C, and beta carotene for 5 years, there was no difference in the incidence of stroke (Heart Protection Study Collaborative Group, 2002). More studies are clearly needed to determine whether directly targeting NRF2 would be beneficial as a preventative agent for ischemic stroke.
NRF Activating Compounds
While NRF2 activation occurs endogenously in response to increased oxidative stress, it can also be induced by exogenous agents. As previously mentioned, many botanically derived and synthetic compounds have been shown to potently activate the NRF2 pathway.
Botanically Derived NRF2 Activators
Sulforaphane is an organic isothiocyanate found in cruciferous plants such as broccoli, brussels sprouts, cabbage, and cauliflower. Sulforaphane activates NRF2 through direct electrophilic modification of the cysteines on KEAP1 allowing for dissociation of NRF2 and nuclear translocation (Takaya et al., 2012). This activation has been reported to increase the expression of antioxidant enzymes in the hippocampus of treated animals (Wang et al., 2014). Sulforaphane can also modulate mitochondrial dynamics through both NRF2-dependent and -independent mechanisms (O'Mealey et al., 2017; de Oliveira et al., 2018). It has also been demonstrated to inhibit TNFα-induced NFkB activation both through directly blocking the interaction of NFkB and its consensus sequence as well as inhibiting IkB-α phosphorylation and degradation (Moon et al., 2009; Checker et al., 2015). These effects of sulforaphane have resulted in neuroprotection in both in vitro and in vivo in models of stroke, traumatic brain injury (TBI), AD, PD, HD, and MS (Zhao et al., 2005, 2006; Han et al., 2007; Kwak et al., 2007; Zhao et al., 2007; Jazwa et al., 2011; Kim et al., 2013; Srivastava et al., 2013; Liu et al., 2014; Jang and Cho, 2016; Yoo et al., 2019).
Lycopene is an aliphatic hydrocarbon carotenoid that can be found in plants such as tomatoes, papayas, and watermelons. Lycopene has been shown to increase antioxidant enzyme activity and decrease inflammatory response in both d-galactose model and transgenic models of AD (Yu et al., 2017; Zhao et al., 2017a). It also attenuated Aβ-induced mitochondrial damage in isolated cortical neurons (Qu et al., 2011; Qu et al., 2016) and reduced proinflammatory cytokines in the brain of Aβ-exposed animals (Liu et al., 2018). Similar effects of lycopene on oxidative and mitochondrial damage have been demonstrated in a pentylenetetrazol-induced model of seizure where it also decreased convulsive activity (Bhardwaj and Kumar, 2016; Kumar et al., 2016). Lycopene also decreased markers of oxidative stress and reduced neuronal cell loss in models of PD and stroke (Prema et al., 2015; Lei et al., 2016) and was able to attenuate BBB disruption and neurological deficits in a model of subarachnoid hemorrhage (Wu et al., 2015).
Curcumin is a polyphenol derived from Curcuma longa rhizomes and has potent antioxidative and anti-inflammatory properties (Agarwal et al., 2011; Kakkar and Kaur, 2011; Dong et al., 2018). Curcumin activates NRF2 both by electrophilic modification of KEAP1 as well as by repression of KEAP1 expression (Ren et al., 2019; Robledinos-Anton et al., 2019). Curcumin treatment has been shown to suppress proinflammatory gene expression through prevention of NFkB activation in microglial cells (Cui et al., 2010; Zhang et al., 2010). In a model of cerebral ischemia and reperfusion, this downregulation of NFkB resulted from NRF2 activation by curcumin and reduced overall brain edema as well as neurological dysfunction (Li et al., 2016). Curcumin was similarly beneficial in models of intracerebral hemorrhage and TBI where again its neuroprotective effects were linked to NRF2 activation (Wang et al., 2015; Kobayashi et al., 2016; de Alcantara et al., 2017; He et al., 2019).
Epigallocatechin gallate (EGCG) is the most abundant catechin found in green tea. It was shown to upregulate NRF2 activity via phosphorylation downstream of p38MAPK and ERk1/2 signaling pathways (Yang et al., 2015), although it can also activate NRF2 through electrophilic disruption of its association with KEAP1 (Mori et al., 2010). In mice, EGCG has been shown to protect against ischemia reperfusion injury in a NRF2-dependent manner (Han et al., 2014) and has also been shown to inhibit NFkB activity, reduce Aβ fibrilization, and improve memory in Aβ exposed animals (Lee et al., 2009b). Neuroprotective effects of EGCG have also been demonstrated in vitro and in vivo in models of PD, MS, and TBI accompanied by increased NRF2 activity and enhanced antioxidant activity and reduced inflammatory responses (Ma et al., 2010; Itoh et al., 2013; Wu, 2016; Semnani et al., 2017; Xu et al., 2018).
Resveratrol is a bioactive polyphenol found in fruits such as grapes and berries. Resveratrol activates NRF2 by phosphorylation via p38MAPK (Shi et al., 2018). Resveratrol treatment has potent antioxidant and anti-inflammatory effects and has been shown to regulate mitochondrial biogenesis (Chiang et al., 2018; Chuang et al., 2019). The NRF2-activating and mitochondrial effects of resveratrol are thought to underlie its protective effects in a rotenone model of PD (Gaballah et al., 2016; Peng et al., 2016). Activation of NRF2 by resveratrol also has been shown to both prevent against ischemic injury and mitigate the oxidative stress induced by ischemic injury in rodents (Narayanan et al., 2015; Gao et al., 2018). In addition, resveratrol treatment can also attenuate TBI-induced cognitive deficits in mice via NRF2 activation (Shi et al., 2018) and can ameliorate cellular and mitochondrial damage in a drosophila model of spinocerebellar ataxia by upregulating the same pathway (Wu et al., 2018).
Alpha lipoic acid is another naturally occurring NRF2-activating compound with neuroprotective effects. While it can be found in low amounts in a number of plants including spinach, broccoli, carrots, and beets, it is more frequently consumed as a dietary supplement. The exact mechanisms by which alpha lipoic acid activates NRF2 is not currently known, although the compound can form lipoyl-cysteinyl mixed disulfides on KEAP1 which would prevent newly synthesized NRF2 from binding to its chaperone (Dinkova-Kostova et al., 2002; Kobayashi et al., 2006). However, alpha lipoic acid has also been shown to activate protein kinase C, one of the kinases that can activate NRF2 that could represent an alternative mechanism of activation (Sen et al., 1999). Activation of NRF2 by alpha lipoic acid has been shown to inhibit cell loss following TBI (Xia et al., 2019) as well as reduce infarct volume, oxidative damage, and edema and promote neurologic recovery following stroke (Lv et al., 2017). In models of PD, alpha lipoic acid has been shown to decrease ROS, upregulate mitochondrial biogenesis, restore ATP content, and preserve dopaminergic neurons (Zaitone et al., 2012; Zhao et al., 2017b), although whether NRF2 activation was required for these effects was not investigated. Alpha lipoic acid also potently reduces inflammation in mouse models of MS (Morini et al., 2004; Chaudhary et al., 2015), yet, again, these studies did not investigate whether this was linked to NRF2 activation.
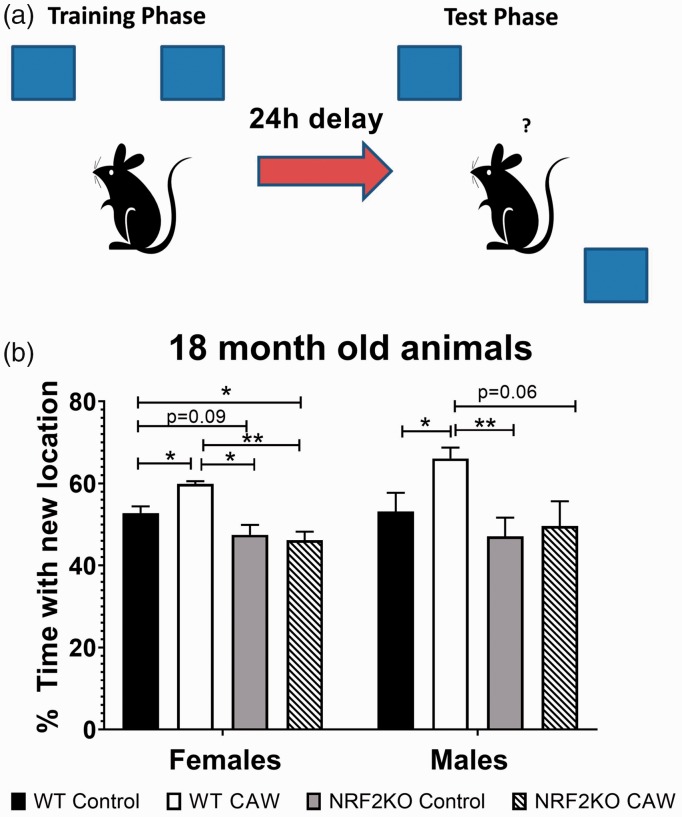
The medicinal plant Centella asiatica (L) Urban also contains many NRF2-activating compounds. The plant contains high levels of four triterpene compounds, Asiatic acid, madecassic acid, asiaticoside, and madecassoside (MS) that have all been shown to activate NRF2 but do not possess strong electrophilic properties (Yang et al., 2016; Jiang et al., 2017; Fan et al., 2018; Liu et al., 2019c; Meng et al., 2019). Centella asiatica also contains many caffeoylquinic acids that we and others have shown can activate NRF2 as well (Boettler et al., 2011; Gray et al., 2014; Liang et al., 2019). Our lab has demonstrated that a water extract of Centella asiatica can activate NRF2 in neuroblastoma cells and isolated primary neurons as well as in the brains of treated animals, and this activation is accompanied by improved mitochondrial function, enhanced synaptic density, and improved cognitive function in mouse models of aging and AD (Gray et al., 2015; Gray et al., 2017a, 2017b; Gray et al., 2018a, 2018b). Other groups have likewise shown antioxidant, anti-inflammatory, and cognitive-enhancing effects of the plant in rodent models of chemically induced neurotoxicity, stroke, seizure, PD, and HD (Gupta et al., 2003; Flora and Gupta, 2007; Shinomol and Muralidhara, 2008a, 2008b; Haleagrahara and Ponnusamy, 2010; Prakash and Kumar, 2013; Tabassum et al., 2013; Doknark et al., 2014). Work is ongoing in our lab to determine the requirement of NRF2 activation for the beneficial effects of Centella asiatica in both normal as well as pathological aging. Our most recent work has indicated that the cognitive benefits of the water extract of Centella asiatica (CAW), at least in healthy aging, do require NRF2. The Object Location Memory task is a test of spatial memory wherein the mouse using identical objects one of which is moved during testing to a location distinct from where it was during training (Figure 1(a)). If the mouse remembers the training location, it should spend a greater amount of time with the object in the new location. NRF2KO mice exhibited cognitive impairments relative to WT animals and while long-term CAW treatment improved the performance in the Object Location Memory test of 18-month-old WT mice but had no effect on age-matched NRF2 knockout mice (Figure 1(b)).
Figure 1.
Object location memory. CAW treatment (2g/L for 13 months) improved performance in 18-month-old WT but not NRF2KO mice. NRF2KO mice were also impaired relative to age-matched WT animals. n = 4–8, *p< .05, **p < .01.
WT = wild-type; NRF2KO = nuclear factor erythroid 2-related factor 2 knockout.
Synthetic Activators of the NRF2/ARE Pathway
DMF is an enoate ester formed by the condensation of fumaric acid and methanol. It is currently an FDA-approved disease-modifying drug for the treatment of relapsing MS under the name Tecfidera® (Deeks, 2016). Because DMF is a thiol-reactive electrophile, it is thought to primarily activate NRF2 via cysteine modification on KEAP1 (Saidu et al., 2019), although it has also been shown to affect NRF2 phosphorylation via PI3K and ERK1/2 pathways in neutrophils (Muller et al., 2016). Perhaps because of its well-documented anti-inflammatory and antioxidant effects, DMF has also been shown to be neuroprotective in many different neurodegenerative conditions. It has also been shown to prevent hippocampal injury following ischemia, reduce edema volume, and protect BBB integrity in mouse models of stroke (Kunze et al., 2015; Yao et al., 2016; Liu et al., 2019a) as well as improve cognitive function in models of AD and subarachnoid hemorrhage (Liu et al., 2015; Majkutewicz et al., 2016; Majkutewicz et al., 2018). In addition, as mentioned previously, DMF protects against both αSyn and Aβ toxicity and can reduce tau hyperphosphorylation (Lastres-Becker et al., 2016; Campolo et al., 2018; Bahn and Jo, 2019).
tBHQ is another known activator of NRF2 commonly used as a food preservative. tBHQ is an electrophile with the ability to disrupt the KEAP1/NRF2 complex and regulate oxidative stress. tBHQ has demonstrated antioxidant and neuroprotective effects. Treatment with tBHQ reduced oxidative stress and prevented neuronal toxicity and Aβ formation in NT2N neurons (Eftekharzadeh et al., 2010) and prevented Aβ-induced cell death in rats (Nouhi et al., 2011). It has also been shown to decrease secondary injury and improve function recovery following TBI and attenuate neurological injury after intracerebral hemorrhage in mice (Sukumari-Ramesh and Alleyne, 2016; Chandran et al., 2018).
Metformin is a highly prescribed antihyperglycemic drug that is often used as a treatment for type II diabetes. However, metformin can also activate NRF2, likely through its induction of AMPK which subsequently phosphorylates NRF2 (Ashabi et al., 2015; Joo et al., 2016), and has been shown to be neuroprotective in many neurodegenerative model systems. Metformin protects against oxidative stress-induced BBB damage, and this was determined to be through NRF2 activation (Prasad et al., 2017). Metformin is also well known for its effects on mitochondrial function and biogenesis (Vial et al., 2019) and also has documented antioxidant and anti-inflammatory effects in rodent models of ischemic stroke, AD, PD, and MS (Ashabi et al., 2015; Katila et al., 2017; Ou et al., 2018; Mudgal et al., 2019).
The tricyclic compound acetylenic tricyclic bis(cyano enone) (TBE-31) is considered to be one of the most potent NRF2 inducers because it contains two electrophilic Michael acceptors (Dinkova-Kostova et al., 2010). TBE-31 has been investigated in cancer model systems and has been found to have strong antioxidant and anti-inflammatory effects which are thought to contribute to its anticancer activity (Onyango et al., 2014; Knatko et al., 2015; Chan et al., 2016). It has also been shown to reduce lipid peroxidation and improve mitochondrial imbalance in Friedrich’s ataxia (Abeti et al., 2015).
Conclusions
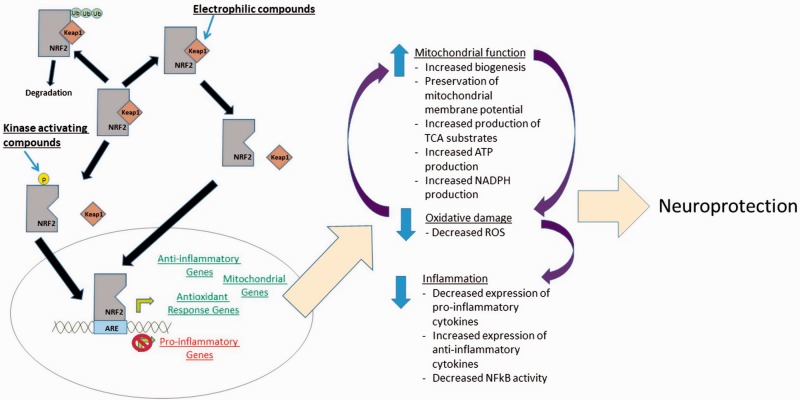
It is increasingly clear that NRF2 could represent a viable therapeutic target in neurological conditions. Its activation attenuates many processes involved in neurodegenerative disorders including mitochondrial dysfunction, oxidative stress, and neuroinflammation (Figure 2). NRF2-activating compounds are already FDA approved for use MS, and clinical testing is underway in Friedrich’s ataxia, but the literature suggests that such activators could be warranted in many other neurodegenerative conditions as well.
Figure 2.
Summary of neuroprotective effects of NRF2 activation.
NRF2 = nuclear factor erythroid 2-related factor 2; ROS = reactive oxygen species; ARE = antioxidant response element.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Mikah S. Brandes https://orcid.org/0000-0003-0892-1608
Nora E. Gray https://orcid.org/0000-0001-6848-859X
References
- Abdullah A., Kitteringham N. R., Jenkins R. E., Goldring C., Higgins L., Yamamoto M., Hayes J., Park B. K. (2012). Analysis of the role of Nrf2 in the expression of liver proteins in mice using two-dimensional gel-based proteomics. Pharmacol Rep, 64(3), 680–697. [DOI] [PubMed] [Google Scholar]
- Abeti R., Baccaro A., Esteras N., Giunti P. (2018). Novel Nrf2-inducer prevents mitochondrial defects and oxidative stress in Friedreich's ataxia models. Front Cell Neurosci, 12, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeti R., Parkinson M. H., Hargreaves I. P., Angelova P. R., Sandi C., Pook M. A., Giunti P., Abramov A. Y. (2016). Mitochondrial energy imbalance and lipid peroxidation cause cell death in Friedreich's ataxia. Cell Death Dis, 7, e2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeti R., Uzun E., Renganathan I., Honda T., Pook M. A., Giunti P. (2015). Targeting lipid peroxidation and mitochondrial imbalance in Friedreich's ataxia. Pharmacol Res, 99, 344–350. [DOI] [PubMed] [Google Scholar]
- Agarwal N. B., Jain S., Agarwal N. K., Mediratta P. K., Sharma K. K. (2011). Modulation of pentylenetetrazole-induced kindling and oxidative stress by curcumin in mice. Phytomedicine, 18(8–9), 756–759. [DOI] [PubMed] [Google Scholar]
- Agyeman A. S., Chaerkady R., Shaw P. G., Davidson N. E., Visvanathan K., Pandey A., Kensler T. W. (2012). Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat, 132(1), 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2017). Alzheimer’s disease facts and figures. Alzheimer's and Dementia, 13, 325–373. [DOI] [PubMed] [Google Scholar]
- Anzovino A., Chiang S., Brown B. E., Hawkins C. L., Richardson D. R., Huang M. L. (2017). Molecular alterations in a mouse cardiac model of Friedreich ataxia: An impaired Nrf2 response mediated via upregulation of Keap1 and activation of the Gsk3beta axis. Am J Pathol, 187(12), 2858–2875. [DOI] [PubMed] [Google Scholar]
- Aranca T. V., Jones T. M., Shaw J. D., Staffetti J. S., Ashizawa T., Kuo S. H., Fogel B. L., Wilmot G. R., Perlman S. L., Onyike C. U., Ying S. H., Zesiewicz T. A. (2016). Emerging therapies in Friedreich's ataxia. Neurodegener Dis Manag, 6(1), 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. (2015). Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis, 30(3), 747–754. [DOI] [PubMed] [Google Scholar]
- Bahn G., Jo D. G. (2019). Therapeutic approaches to Alzheimer's disease through modulation of NRF2. Neuromolecular Med, 21(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Behl C. (2005). Oxidative stress in Alzheimer’s disease: Implications for prevention and therapy. Subcell Biochem, 38, 65–78. [DOI] [PubMed] [Google Scholar]
- Bellezza I., Tucci A., Galli F., Grottelli S., Mierla A. L., Pilolli F., Minelli A. (2012). Inhibition of NF-kappaB nuclear translocation via HO-1 activation underlies alpha-tocopheryl succinate toxicity. J Nutr Biochem, 23(12), 1583–1591. [DOI] [PubMed] [Google Scholar]
- Bhardwaj M., Kumar A. (2016). Neuroprotective effect of lycopene against PTZ-induced kindling seizures in mice: Possible behavioural, biochemical and mitochondrial dysfunction. Phytother Res, 30(2), 306–313. [DOI] [PubMed] [Google Scholar]
- Bhat S., Acharya U. R., Hagiwara Y., Dadmehr N., Adeli H. (2018). Parkinson's disease: Cause factors, measurable indicators, and early diagnosis. Comput Biol Med, 102, 234–241. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M.et al. (2008). A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J Exp Med, 205(8), 1869–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettler U., Sommerfeld K., Volz N., Pahlke G., Teller N., Somoza V., Lang R., Hofmann T., Marko D. (2011). Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. J Nutr Biochem, 22(5), 426–440. [DOI] [PubMed] [Google Scholar]
- Branca C., Ferreira E., Nguyen T. V., Doyle K., Caccamo A., Oddo S. (2017). Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum Mol Genet, 26(24), 4823–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne P., Chandraratna D., Angood C., Tremlett H., Baker C., Taylor B. V., Thompson A. J. (2014). Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology, 83(11), 1022–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne S. E. (2008). Mitochondria and Huntington's disease pathogenesis: Insight from genetic and chemical models. Ann N Y Acad Sci, 1147, 358–382. [DOI] [PubMed] [Google Scholar]
- Browne S. E., Beal M. F. (2006). Oxidative damage in Huntington’s disease pathogenesis. Antioxid Redox Signal, 8(11–12), 2061–2073. [DOI] [PubMed] [Google Scholar]
- Browne S. E., Bowling A. C., MacGarvey U., Baik M. J., Berger S. C., Muqit M. M., Bird E. D., Beal M. F. (1997). Oxidative damage and metabolic dysfunction in Huntington's disease: Selective vulnerability of the basal ganglia. Ann Neurol, 41(5), 646–653. [DOI] [PubMed] [Google Scholar]
- Buendia I., Michalska P., Navarro E., Gameiro I., Egea J., Leon R. (2016). Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther, 157, 84–104. [DOI] [PubMed] [Google Scholar]
- Calkins M. J., Jakel R. J., Johnson D. A., Chan K., Kan Y. W., Johnson J. A. (2005). Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A, 102(1), 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolo M., Casili G., Lanza M., Filippone A., Paterniti I., Cuzzocrea S., Esposito E., (2018). Multiple mechanisms of dimethyl fumarate in amyloid β-induced neurotoxicity in human neuronal cells. J Cell Mol Med, 22(2), 1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A., Cutter G., Fox R. J., Xiao J., Lewin J. B., Edwards M. R. (2017). Comparative effectiveness of delayed-release dimethyl fumarate versus glatiramer acetate in multiple sclerosis patients: Results of a matching-adjusted indirect comparison. J Comp Eff Res, 6(4), 313–323. [DOI] [PubMed] [Google Scholar]
- Chan E., Saito A., Honda T., Di Guglielmo G. M. (2016). The acetylenic tricyclic bis(cyano enone), TBE-31, targets microtubule dynamics and cell polarity in migrating cells. Biochim Biophys Acta, 1863(4), 638–649. [DOI] [PubMed] [Google Scholar]
- Chandran R., Kim T., Mehta S. L., Udho E., Chanana V., Cengiz P., Kim H., Kim C., Vemuganti R. (2018). A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J Cereb Blood Flow Metab, 38(10), 1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. H., Chen Y. C., Wu Y. R., Lee W. F., Chen C. M. (2012). Downregulation of genes involved in metabolism and oxidative stress in the peripheral leukocytes of Huntington's disease patients. PLoS One, 7(9), e46492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary P., Marracci G., Galipeau D., Pocius E., Morris B., Bourdette D. (2015). Lipoic acid reduces inflammation in a mouse focal cortical experimental autoimmune encephalomyelitis model. J Neuroimmunol, 289, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checker R. L. G., Thoh M., Sharma D., Sandu S. K. (2015). Sulforaphane, a naturally occurring isothiocyanate, exhibits anti-inflammatory effects by targeting GSK3β/Nrf-2 and NF-κB pathways in T cells. J Funct Foods, 19, 426–438. [Google Scholar]
- Chen C. M., Wu Y. R., Cheng M. L., Liu J. L., Lee Y. M., Lee P. W., Soong B. W., Chiu D. T. (2007). Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington's disease patients. Biochem Biophys Res Commun, 359(2), 335–340. [DOI] [PubMed] [Google Scholar]
- Chen K., Gunter K., Maines M. D. (2000). Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem, 75(1), 304–313. [DOI] [PubMed] [Google Scholar]
- Chen P. C., Vargas M. R., Pani A. K., Smeyne R. J., Johnson D. A., Kan Y. W., Johnson J. A. (2009). Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A, 106(8), 2933–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Du J. K., Hu X., Yu Q., Li D. X., Wang C. N., Zhu X. Y., Liu Y. J. (2017). Protective effects of resveratrol on mitochondrial function in the hippocampus improves inflammation-induced depressive-like behavior. Physiol Behav, 182, 54–61. [DOI] [PubMed] [Google Scholar]
- Chen X., Xi Z., Liang H., Sun Y., Zhong Z., Wang B., Bian L., Sun Q. (2019). Melatonin prevents mice cortical astrocytes from hemin-induced toxicity through activating PKCalpha/Nrf2/HO-1 signaling in vitro. Front Neurosci, 13, 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Kong R. H., Zhang L. M., Zhang J. N. (2012). Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br J Pharmacol, 167(4), 699–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M. C., Nicol C. J., Cheng Y. C. (2018). Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem Int, 115, 1–10. [DOI] [PubMed] [Google Scholar]
- Cho H. Y., Gladwell W., Wang X., Chorley B., Bell D., Reddy S. P., Kleeberger S. R. (2010). Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med, 182(2), 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang Y. C., Chen S. D., Hsu C. Y., Chen S. F., Chen N. C., Jou S. B. (2019). Resveratrol promotes mitochondrial biogenesis and protects against seizure-induced neuronal cell damage in the hippocampus following status epilepticus by activation of the PGC-1alpha signaling pathway. Int J Mol Sci, 20(4), 998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles L. D., Tuite P. J., Oz G., Mishra U. R., Kartha R. V., Sullivan K. M., Cloyd J. C., Terpstra M. (2018). Repeated-dose oral N-acetylcysteine in Parkinson's disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J Clin Pharmacol, 58(2), 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. R., Lyon C. J., Xia X., Liu J. Z., Tangirala R. K., Yin F., Boyadjian R., Bikineyeva A., Pratico D., Harrison D. G., Hsueh W. A. (2009). Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res, 104(6), e42–e54. [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. (2008). Multiple sclerosis. Lancet, 372(9648), 1502–1517. [DOI] [PubMed] [Google Scholar]
- Cook N. R., Albert C. M., Gaziano J. M., Zaharris E., MacFadyen J., Danielson E., Buring J. E., Manson J. E. (2007). A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med, 167(15), 1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J. G., Li Y. Y., Zhao Y., Bhattacharjee S., Lukiw W. J. (2010). Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-kappaB in stressed human astroglial cells and in Alzheimer disease. J Biol Chem, 285(50), 38951–38960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga M. K., Madhuchhanda, Mishra T. K., Mohan A. (1997). Lipid peroxide, beta-carotene and alpha-tocopherol in ischaemic stroke and effect of exogenous vitamin E supplementation on outcome. J Assoc Physicians India, 45(11), 843–846. [PubMed] [Google Scholar]
- Dai Y., Zhang H., Zhang J., Yan M. (2018). Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-kappaB pathway. Chem Biol Interact, 284, 32–40. [DOI] [PubMed] [Google Scholar]
- Damiano M., Galvan L., Deglon N., Brouillet E. (2010). Mitochondria in Huntington's disease. Biochim Biophys Acta, 1802(1), 52–61. [DOI] [PubMed] [Google Scholar]
- Darwish H., Haddad R., Osman S., Ghassan S., Yamout B., Tamim H., Khoury S. (2017). Effect of vitamin D replacement on cognition in multiple sclerosis patients. Sci Rep, 7, 45926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alcantara G. F., Simoes-Neto E., da Cruz G. M., Nobre M. E., Neves K. R., de Andrade G. M., Brito G. A., Viana G. S. (2017). Curcumin reverses neurochemical, histological and immuno-histochemical alterations in the model of global brain ischemia. J Tradit Complement Med, 7(1), 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks E. D. (2016). Dimethyl fumarate: A review in relapsing-remitting MS. Drugs, 76(2), 243–254. [DOI] [PubMed] [Google Scholar]
- del Hoyo P., Garcia-Redondo A., de Bustos F., Molina J. A., Sayed Y., Alonso-Navarro H., Caballero L., Arenas J., Jimenez-Jimenez F. J. (2006). Oxidative stress in skin fibroblasts cultures of patients with Huntington's disease. Neurochem Res, 31(9), 1103–1109. [DOI] [PubMed] [Google Scholar]
- Demirovic D., Rattan S. I. (2011). Curcumin induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology, 12(5), 437–444. [DOI] [PubMed] [Google Scholar]
- de Oliveira M. R., de Bittencourt Brasil F., Furstenau C. R. (2018). Sulforaphane promotes mitochondrial protection in SH-SY5Y cells exposed to hydrogen peroxide by an Nrf2-dependent mechanism. Mol Neurobiol, 55(6), 4777–4787. [DOI] [PubMed] [Google Scholar]
- Di Bona D., Scapagnini G., Candore G., Castiglia L., Colonna-Romano G., Duro G., Nuzzo D., Iemolo F., Lio D., Pellicano M., Scafidi V., Caruso C., Vasto S. (2010). Immune-inflammatory responses and oxidative stress in Alzheimer’s disease: Therapeutic implications. Curr Pharm Des, 16(6), 684–691. [DOI] [PubMed] [Google Scholar]
- Di Filippo M., Chiasserini D., Tozzi A., Picconi B., Calabresi P. (2010). Mitochondria and the link between neuroinflammation and neurodegeneration. J Alzheimers Dis, 20(Suppl 2), S369–S379. [DOI] [PubMed] [Google Scholar]
- Ding Y., Ren D., Xu H., Liu W., Liu T., Li L., Li J., Li Y., Wen A. (2017). Antioxidant and pro-angiogenic effects of corilagin in rat cerebral ischemia via Nrf2 activation. Oncotarget, 8(70), 114816–114828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Abramov A. Y. (2015). The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med, 88(Pt B), 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Holtzclaw W. D., Cole R. N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. (2002). Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A, 99(18), 11908–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Talalay P., Sharkey J., Zhang Y., Holtzclaw W. D., Wang X. J., David E., Schiavoni K. H., Finlayson S., Mierke D. F., Honda T. (2010). An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with Keap1. J Biol Chem, 285(44), 33747–33755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doknark S., Mingmalairak S., Vattanajun A., Tantisira B., Tantisira M. (2014). Study of ameliorating effects of ethanolic extract of Centella asiatica on learning and memory deficit in animal models. J Med Assoc Thai, 97(Suppl 2), S68–S76. [PubMed] [Google Scholar]
- Dong W., Yang B., Wang L., Li B., Guo X., Zhang M., Jiang Z., Fu J., Pi J., Guan D., Zhao R. (2018). Curcumin plays neuroprotective roles against traumatic brain injury partly via Nrf2 signaling. Toxicol Appl Pharmacol, 346, 28–36. [DOI] [PubMed] [Google Scholar]
- Duan W., Zhang R., Guo Y., Jiang Y., Huang Y., Jiang H., Li C. (2009). Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. In Vitro Cell Dev Biol Anim, 45(7), 388–397. [DOI] [PubMed] [Google Scholar]
- Duran R., Barrero F. J., Morales B., Luna J. D., Ramirez M., Vives F. (2010). Oxidative stress and plasma aminopeptidase activity in Huntington's disease. J Neural Transm (Vienna), 117(3), 325–332. [DOI] [PubMed] [Google Scholar]
- Dysken M. W.et al. (2014). Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA, 311(1), 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N., Geczy C. L., Halliday G. M. (2015). Inflammation is genetically implicated in Parkinson's disease. Neuroscience, 302, 89–102. [DOI] [PubMed] [Google Scholar]
- Eftekharzadeh B., Maghsoudi N., Khodagholi F. (2010). Stabilization of transcription factor Nrf2 by tBHQ prevents oxidative stress-induced amyloid beta formation in NT2N neurons. Biochimie, 92(3), 245–253. [DOI] [PubMed] [Google Scholar]
- Ellrichmann G., Petrasch-Parwez E., Lee D. H., Reick C., Arning L., Saft C., Gold R., Linker R. A. (2011). Efficacy of fumaric acid esters in the R6/2 and YAC128 models of Huntington's disease. PLoS One, 6(1), e16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteras N., Dinkova-Kostova A. T., Abramov A. Y. (2016). Nrf2 activation in the treatment of neurodegenerative diseases: A focus on its role in mitochondrial bioenergetics and function. Biol Chem, 397(5), 383–400. [DOI] [PubMed] [Google Scholar]
- Fan J., Chen Q., Wei L., Zhou X., Wang R., Zhang H. (2018). Asiatic acid ameliorates CCl4-induced liver fibrosis in rats: Involvement of Nrf2/ARE, NF-kappaB/IkappaBalpha, and JAK1/STAT3 signaling pathways. Drug Des Devel Ther, 12, 3595–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez O., Giovannoni G., Fox R. J., Gold R., Phillips J. T., Potts J., Okwuokenye M., Marantz J. L. (2017). Efficacy and safety of delayed-release dimethyl fumarate for relapsing-remitting multiple sclerosis in prior interferon users: An integrated analysis of DEFINE and CONFIRM. Clin Ther, 39(8), 1671–1679. [DOI] [PubMed] [Google Scholar]
- Fischer M. T., Sharma R., Lim J. L., Haider L., Frischer J. M., Drexhage J., Mahad D., Bradl M., van Horssen J., Lassmann H. (2012). NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain, 135(Pt 3), 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora S., Gupta R. (2007). Beneficial effects of Centella asiatica aqueous extract against arsenic-induced oxidative stress and essential metal status in rats. Phytother Res, 21(10), 980–988. [DOI] [PubMed] [Google Scholar]
- Fox R. J., Miller D. H., Phillips J. T., Hutchinson M., Havrdova E., Kita M., Yang M., Raghupathi K., Novas M., Sweetser M. T., Viglietta V., Dawson K. T., & Confirm Study Investigators. (2012). Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med, 367(12), 1087–1097. [DOI] [PubMed] [Google Scholar]
- Fu M.-H., Wu C.-W., Lee Y.-C., Hung C.-Y., Chen I.-C., Wu K. L. H. (2018). Nrf2 activation attenuates the early suppression of mitochondrial respiration due to the α-synuclein overexpression. Biomedical J, 41(3), 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballah H. H., Zakaria S. S., Elbatsh M. M., Tahoon N. M. (2016). Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson's disease. Chem Biol Interact, 251, 10–16. [DOI] [PubMed] [Google Scholar]
- Galasko D., Montine T. J. (2010). Biomarkers of oxidative damage and inflammation in Alzheimer's disease. Biomark Med, 4(1), 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallai V., Sarchielli P., Trequattrini A., Franceschini M., Floridi A., Firenze C., Alberti A., Di Benedetto D., Stragliotto E. (1995). Cytokine secretion and eicosanoid production in the peripheral blood mononuclear cells of MS patients undergoing dietary supplementation with n-3 polyunsaturated fatty acids. J Neuroimmunol, 56(2), 143–153. [DOI] [PubMed] [Google Scholar]
- Gao Y., Chu S. F., Li J. P., Zhang Z., Yan J. Q., Wen Z. L., Xia C. Y., Mou Z., Wang Z. Z., He W. B., Guo X. F., Wei G. N., Chen N. H. (2015). Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington's disease. Acta Pharmacol Sin, 36(3), 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Fu R., Wang J., Yang X., Wen L., Feng J. (2018). Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via Nrf2/HO-1 pathway. Pharm Biol, 56(1), 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genestra M. (2007). Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal, 19(9), 1807–1819. [DOI] [PubMed] [Google Scholar]
- Giordano G., White C. C., Mohar I., Kavanagh T. J., Costa L. G. (2007). Glutathione levels modulate domoic acid induced apoptosis in mouse cerebellar granule cells. Toxicol Sci, 100(2), 433–444. [DOI] [PubMed] [Google Scholar]
- Gold R., Kappos L., Arnold D. L., Bar-Or A., Giovannoni G., Selmaj K., Tornatore C., Sweetser M. T., Yang M., Sheikh S. I., Dawson K. T., DEFINE Study Investigators. (2012). Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med, 367(12), 1098–1107. [DOI] [PubMed] [Google Scholar]
- Gopal S., Mikulskis A., Gold R., Fox R. J., Dawson K. T., Amaravadi L. (2017). Evidence of activation of the Nrf2 pathway in multiple sclerosis patients treated with delayed-release dimethyl fumarate in the Phase 3 DEFINE and CONFIRM studies. Mult Scler, 23(14), 1875–1883. [DOI] [PubMed] [Google Scholar]
- Goris A., Williams-Gray C. H., Clark G. R., Foltynie T., Lewis S. J., Brown J., Ban M., Spillantini M. G., Compston A., Burn D. J., Chinnery P. F., Barker R. A., Sawcer S. J. (2007). Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson's disease. Ann Neurol, 62(2), 145–153. [DOI] [PubMed] [Google Scholar]
- Goswami A., Dikshit P., Mishra A., Mulherkar S., Nukina N., Jana N. R. (2006). Oxidative stress promotes mutant huntingtin aggregation and mutant huntingtin-dependent cell death by mimicking proteasomal malfunction. Biochem Biophys Res Commun, 342(1), 184–190. [DOI] [PubMed] [Google Scholar]
- Gray N., Morré J., Kelley J., Maier C. S., Stevens J. F., Quinn J. F., Soumyanath A., (2014). Caffeoylquinic acids in Centella asiatica protect against amyloid-β toxicity. J Alzheimer's Dis, 40, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N., Sampath H., Zweig J. A., Quinn J. F., Soumyanath A. (2015). Centella asiatica attenuates amyloid-β-induced oxidative stress and mitochondrial dysfunction. J Alzheimer's Dis, 45(3), 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N., Zweig J. A., Caruso M., Martin M. D., Zhu J. Y., Quinn J. F., Soumayanath A. (2018. a). Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice. Brain Behav, 93, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N., Zweig J. A., Caruso M., Zhu J. Y., Wright K. M., Quinn J. F., Soumyanath A. (2018. b). Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in β-amyloid overexpressing mice. Mol Cell Neurosci, 93, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N., Zweig J. A., Matthews D. G., Caruso M., Quinn J. F., Soumyanath A. (2017. a). Centella asiatica attenuates mitochondrial dysfunction and oxidative stress in Aβ-exposed hippocampal neurons. Oxid Med Cell Longev, 2017, 7023091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N., Zweig J. A., Murchison C., Caruso M., Matthews D. G., Kawamoto C., Harris C. J., Quinn J. F., Soumyanath A. (2017. b). Centella asiatica attenuates Aβ-induced neurodegenerative spine loss and dendritic simplification. Neursci Lett, 646, 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T., Fiskum G. (2010). Brain mitochondria from rats treated with sulforaphane are resistant to redox-regulated permeability transition. J Bioenerg Biomembr, 42(6), 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T., Shafer J., Fiskum G. (2011). Sulforaphane inhibits mitochondrial permeability transition and oxidative stress. Free Radic Biol Med, 51(12), 2164–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubandru M., Margina D., Tsitsimpikou C., Goutzourelas N., Tsarouhas K., Ilie M., Tsatsakis A. M., Kouretas D. (2013). Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food Chem Toxic, 61, 209–214. [DOI] [PubMed] [Google Scholar]
- Gupta Y., Veerendra Kumar M., Srivastava A. (2003). Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav, 74(3), 579–585. [DOI] [PubMed] [Google Scholar]
- Haleagrahara N., Ponnusamy K. (2010). Neuroprotective effect of Centella asiatica extract (CAE) on experimentally induced Parkinsonism in aged Sprague Dawley rats. J Toxicol Sci, 35(1), 41–47. [DOI] [PubMed] [Google Scholar]
- Halliday G. M., McRitchie D. A., Macdonald V., Double K. L., Trent R. J., McCusker E. (1998). Regional specificity of brain atrophy in Huntington's disease. Exp Neurol, 154(2), 663–672. [DOI] [PubMed] [Google Scholar]
- Hammer A., Waschbisch A., Kuhbandner K., Bayas A., Lee D. H., Duscha A., Haghikia A., Gold R., Linker R. A. (2018). The NRF2 pathway as potential biomarker for dimethyl fumarate treatment in multiple sclerosis. Ann Clin Transl Neurol, 5(6), 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Wang M., Jing X., Shi H., Ren M., Lou H. (2014). (-)- Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem Res, 39(7), 1292–1299. [DOI] [PubMed] [Google Scholar]
- Han J. M., Lee Y. J., Lee S. Y., Kim E. M., Moon Y., Kim H. W., Hwang O. (2007). Protective effect of sulforaphane against dopaminergic cell death. J Pharmacol Exp Ther, 321(1), 249–256. [DOI] [PubMed] [Google Scholar]
- Harari O. A., Liao J. K. (2010). NF-kappaB and innate immunity in ischemic stroke. Ann N Y Acad Sci, 1207, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattingen E., Magerkurth J., Pilatus U., Mozer A., Seifried C., Steinmetz H., Zanella F., Hilker R. (2009). Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson's disease. Brain, 132(Pt 12), 3285–3297. [DOI] [PubMed] [Google Scholar]
- Hayashi G., Jasoliya M., Sahdeo S., Saccà F., Pane C., Filla A., Marsili A., Puorro G., Lanzillo R., Brescia Morra V., Cortopassi G. (2017). Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum Mol Genet, 26(15), 2864–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Yan H., Ni H., Liang W., Jin W. (2019). Expression of nuclear factor erythroid 2-related factor 2 following traumatic brain injury in the human brain. Neuroreport, 30(5), 344–349. [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group. (2002). MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet, 360(9326), 23–33. [DOI] [PubMed] [Google Scholar]
- Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R. L., Atwood C. S., Johnson A. B., Kress Y., Vinters H. V., Tabaton M., Shimohama S., Cash A. D., Siedlak S. L., Harris P. L., Jones P. K., Petersen R. B., Perry G., Smith M. A. (2001). Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci, 21, 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E. C., Vyas S., Hunot S. (2012). Neuroinflammation in Parkinson's disease. Parkinsonism Relat Disord, 18(Suppl 1), S210–S212. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Nguyen T., Pickett C. B. (2002). Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem, 277(45), 42769–42774. [DOI] [PubMed] [Google Scholar]
- Huang K., Gao X., Wei W. (2017. a). The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-beta1 expressions in rat glomerular mesangial cells. Exp Cell Res, 361(1), 63–72. [DOI] [PubMed] [Google Scholar]
- Huang T., Hao D. L., Wu B. N., Mao L. L., Zhang J. (2017. b). Uric acid demonstrates neuroprotective effect on Parkinson's disease mice through Nrf2-ARE signaling pathway. Biochem Biohys Res Commun, 493(4), 1443–1449. [DOI] [PubMed] [Google Scholar]
- Ishii T., Itoh K., Ruiz E., Leake D. S., Unoki H., Yamamoto M., Mann G. E. (2004). Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: Activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res, 94(5), 609–616. [DOI] [PubMed] [Google Scholar]
- Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. (1997). An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun, 236(2), 313–322. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tabuchi M., Mizuguchi N., Imano M., Tsubaki M., Nishida S., Hashimoto S., Matsuo K., Nakayama T., Ito A., Munakata H., Satou T. (2013). Neuroprotective effect of (-)-epigallocatechin-3-gallate in rats when administered pre- or post-traumatic brain injury. J Neural Transm (Vienna), 120(5), 767–783. [DOI] [PubMed] [Google Scholar]
- Jakel R. J., Townsend J. A., Kraft A. D., Johnson J. A. (2007). Nrf2-mediated protection against 6-hydroxydopamine. Brain Res, 1144, 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M., Cho I. H. (2016). Sulforaphane ameliorates 3-nitropropionic acid-induced striatal toxicity by activating the Keap1-Nrf2-ARE pathway and inhibiting the MAPKs and NF-kappaB pathways. Mol Neurobiol, 53(4), 2619–2635. [DOI] [PubMed] [Google Scholar]
- Jauch E. C., Jauch E. C., Saver J. L., Adams H. P., Jr., Bruno A., Connors J. J., Demaerschalk B. M., Khatri P., McMullan P. W., Jr., Qureshi A. I., Rosenfield K., Scott P. A., Summers D. R., Wang D. Z., Wintermark M., Yonas H., American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease, & Council on Clinical Cardiology. (2013). Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 44(3), 870–947. [DOI] [PubMed] [Google Scholar]
- Jazwa A., Rojo A. I., Innamorato N. G., Hesse M., Fernandez-Ruiz J., Cuadrado A. (2011). Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental Parkinsonism. Antioxid Redox Signal, 14(12), 2347–2360. [DOI] [PubMed] [Google Scholar]
- Jiang J. Z., Ye J., Jin G. Y., Piao H. M., Cui H., Zheng M. Y., Yang J. S., Che N., Choi Y. H., Li L. C., Yan G. H. (2017). Asiaticoside mitigates the allergic inflammation by abrogating the degranulation of mast cells. J Agric Food Chem, 65(37), 8128–8135. [DOI] [PubMed] [Google Scholar]
- Jimenez-Sanchez M., Licitra F., Underwood B. R., Rubinsztein D. C. (2017). Huntington's disease: Mechanisms of pathogenesis and therapeutic strategies. Cold Spring Harb Perspect Med, 7(7), a024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. N., Yu Y. V., Gundemir S., Jo C., Cui M., Tieu K., Johnson G. V. (2013). Impaired mitochondrial dynamics and Nrf2 signaling contribute to compromised responses to oxidative stress in striatal cells expressing full-length mutant huntingtin. PLoS One, 8(3), e57932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Amirahmadi S., Ward C., Fabry Z., Johnson J. A. (2010). The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci, 114(2), 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A., Beal M. F. (2012). Antioxidants in Huntington's disease. Biochim Biophys Acta, 1822(5), 664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo M. S., Kim W. D., Lee K. Y., Kim J. H., Koo J. H., Kim S. G. (2016). AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol, 36(14), 1931–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar M. A., Villalba H., Prasad S., Liles T., Sifat A. E., Sajja R. K., Abbruscato T. J., Cucullo L. (2017). Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is metformin a viable countermeasure? Redox Biol, 13, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar V., Kaur I. P. (2011). Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem Toxicol, 49(11), 2906–2913. [DOI] [PubMed] [Google Scholar]
- Kalinowska-Lyszczarz A., Szczucinski A., Pawlak M. A., Losy J. (2011). Clinical study on CXCL13, CCL17, CCL20 and IL-17 as immune cell migration navigators in relapsing-remitting multiple sclerosis patients. J Neurol Sci, 300(1–2), 81–85. [DOI] [PubMed] [Google Scholar]
- Kanninen K., Kanninen K., Malm T. M., Jyrkkänen H. K., Goldsteins G., Keksa-Goldsteine V., Tanila H., Yamamoto M., Ylä-Herttuala S., Levonen A. L., Koistinaho J. (2008). Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci, 39(3), 302–313. [DOI] [PubMed] [Google Scholar]
- Kappos L., Gold R., Miller D. H., Macmanus D. G., Havrdova E., Limmroth V., Polman C. H., Schmierer K., Yousry T. A., Yang M., Eraksoy M., Meluzinova E., Rektor I., Dawson K. T., Sandrock A. W., O'Neill G. N., & BG-12 Phase II Study Investigators. (2008). Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: A multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet, 372(9648), 1463–1472. [DOI] [PubMed] [Google Scholar]
- Katila N., Bhurtel S., Shadfar S., Srivastav S., Neupane S., Ojha U., Jeong G. S., Choi D. Y. (2017). Metformin lowers alpha-synuclein phosphorylation and upregulates neurotrophic factor in the MPTP mouse model of Parkinson's disease. Neuropharmacology, 125, 396–407. [DOI] [PubMed] [Google Scholar]
- Khaibullin T., Ivanova V., Martynova E., Cherepnev G., Khabirov F., Granatov E., Rizvanov A., Khaiboullina S. (2017). Elevated levels of proinflammatory cytokines in cerebrospinal fluid of multiple sclerosis patients. Front Immunol, 8, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili M., Azimi A., Izadi V., Eghtesadi S., Mirshafiey A., Sahraian M. A., Motevalian A., Norouzi A., Sanoobar M., Eskandari G., Farhoudi M., Amani F. (2014). Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: A double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation, 21(6), 291–296. [DOI] [PubMed] [Google Scholar]
- Khan A., Ali T., Rehman S. U., Khan M. S., Alam S. I., Ikram M., Muhammad T., Saeed K., Badshah H., Kim M. O. (2018). Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front Pharmacol, 9, 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. V., Kim H. Y., Ehrlich H. Y., Choi S. Y., Kim D. J., Kim Y. (2013). Amelioration of Alzheimer's disease by neuroprotective effect of sulforaphane in animal model. Amyloid, 20(1), 7–12. [DOI] [PubMed] [Google Scholar]
- Kim J., Moody J. P., Edgerly C. K., Bordiuk O. L., Cormier K., Smith K., Beal M. F., Ferrante R. J. (2010). Mitochondrial loss, dysfunction and altered dynamics in Huntington's disease. Hum Mol Genet, 19(20), 3919–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac N., Relja M., Klepac R., Hecimovic S., Babic T., Trkulja V. (2007). Oxidative stress parameters in plasma of Huntington's disease patients, asymptomatic Huntington's disease gene carriers and healthy subjects: a cross-sectional study. J Neurol, 254(12), 1676–1683. [DOI] [PubMed] [Google Scholar]
- Knatko E. V., Ibbotson S. H., Zhang Y., Higgins M., Fahey J. W., Talalay P., Dawe R. S., Ferguson J., Huang J. T., Clarke R., Zheng S., Saito A., Kalra S., Benedict A. L., Honda T., Proby C. M., Dinkova-Kostova A. T. (2015). Nrf2 activation protects against solar-simulated ultraviolet radiation in mice and humans. Cancer Prev Res (Phila), 8(6), 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Kang M. I., Watai Y., Tong K. I., Shibata T., Uchida K., Yamamoto M. (2006). Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol, 26(1), 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E. H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., Yamamoto M. (2016). Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun, 7, 11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchaki E., Afarini M., Abolhassani J., Mirhosseini N., Bahmani F., Masoud S. A., Asemi Z. (2018). High-dose omega-3 fatty acid plus vitamin D3 supplementation affects clinical symptoms and metabolic status of patients with multiple sclerosis: A randomized controlled clinical trial. J Nutr, 148(8), 1380–1386. [DOI] [PubMed] [Google Scholar]
- Ku H. J., Park J. W. (2017). Downregulation of IDH2 exacerbates H2O2-mediated cell death and hypertrophy. Redox Rep, 22(1), 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. P., Sharma S. K., M., Nagarajan K., Dixit P. K. M. (2016). Effects of lycopene and sodium valproate on pentylenetetrazol-induced kindling in mice. Iran J Med Sci, 41(5), 430–436. [PMC free article] [PubMed] [Google Scholar]
- Kunz A., Abe T., Hochrainer K., Shimamura M., Anrather J., Racchumi G., Zhou P., Iadecola C. (2008). Nuclear factor-kappaB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J Neurosci, 28(7), 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze R., Urrutia A., Hoffmann A., Liu H., Helluy X., Pham M., Reischl S., Korff T., Marti H. H. (2015). Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp Neurol, 266, 99–111. [DOI] [PubMed] [Google Scholar]
- Kwak M. K., Cho J. M., Huang B., Shin S., Kensler T. W. (2007). Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic Biol Med, 43(5), 809–817. [DOI] [PubMed] [Google Scholar]
- Lai L., Wang M., Martin O. J., Leone T. C., Vega R. B., Han X., Kelly D. P. (2014). A role for peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis. J Biol Chem, 289(4), 2250–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabee C. M., Desai S., Agasing A., Georgescu C., Wren J. D., Axtell R. C., Plafker S. M. (2016). Loss of Nrf2 exacerbates the visual deficits and optic neuritis elicited by experimental autoimmune encephalomyelitis. Mol Vis, 22, 1503–1513. [PMC free article] [PubMed] [Google Scholar]
- La Rosa P., Russo M., D'Amico J., Petrillo S., Aquilano K., Lettieri-Barbato D., Turchi R., Bertini E. S., Piemonte F. (2019). Nrf2 induction re-establishes a proper neuronal differentiation program in Friedreich's ataxia neural stem cells. Front Cell Neurosci, 13, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres-Becker I., Garcia-Yague A. J., Scannevin R. H., Casarejos M. J., Kugler S., Rabano A., Cuadrado A. (2016). Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson's disease. Antioxid Redox Signal, 25(2), 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. F., Kuo H. P., Liu M., Chou C. K., Xia W., Du Y., Shen J., Chen C. T., Huo L., Hsu M. C., Li C. W., Ding Q., Liao T. L., Lai C. C., Lin A. C., Chang Y. H., Tsai S. F., Li L. Y., Hung M. C. (2009. a). KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell, 36(1), 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M., Shih A. Y., Murphy T. H., Johnson J. A. (2003). NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem, 278(39), 37948–37956. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Lee Y. K., Ban J. O., Ha T. Y., Yun Y. P., Han S. B., Oh K. W., Hong J. T. (2009. b). Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J Nutr, 139(10), 1987–1993. [DOI] [PubMed] [Google Scholar]
- Lee M. K., Kim S. R., Sung S. H., Lim D., Kim H., Choi H., Park H. K., Je S., Ki Y. C. (2000). Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity. Res Commun Mol Pathol Pharmacol, 108(1–2), 75–86. [PubMed] [Google Scholar]
- Lei X., Lei L., Zhang Z., Cheng Y. (2016). Neuroprotective effects of lycopene pretreatment on transient global cerebral ischemia-reperfusion in rats: The role of the Nrf2/HO1 signaling pathway. Mol Med Rep, 13(1), 412–418. [DOI] [PubMed] [Google Scholar]
- Li K., Besse E. K., Ha D., Kovtunovych G., Rouault T. A. (2008). Iron-dependent regulation of frataxin expression: Implications for treatment of Friedreich ataxia. Hum Mol Genet, 17(15), 2265–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhang X., Cui L., Wang L., Liu H., Ji H., Du Y. (2013). Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res, 1497, 32–39. [DOI] [PubMed] [Google Scholar]
- Li W., Suwanwela N. C., Patumraj S. (2016). Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res, 106, 117–127. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang H. (2017). Soybean isoflavones ameliorate ischemic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Food Funct, 8(8), 2935–2944. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhao X., Hu Y., Sun H., He Z., Yuan J., Cai H., Sun Y., Huang X., Kong W., Kong W. (2018). Age-associated decline in Nrf2 signaling and associated mtDNA damage may be involved in the degeneration of the auditory cortex: Implications for central presbycusis. Int J Mol Med, 42(6), 3371–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N., Dupuis J. H., Yada R. Y., Kitts D. D. (2019). Chlorogenic acid isomers directly interact with Keap 1-Nrf2 signaling in Caco-2 cells. Mol Cell Biochem, 457(1–2), 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. H., Kim K. M., Kim S. W., Hwang O., Choi H. J. (2008). Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: Novel cytoprotective mechanism against oxidative damage. Pharmacol Res, 57(5), 325–331. [DOI] [PubMed] [Google Scholar]
- Linker R. A., Lee D. H., Ryan S., A. M. van Dam, Conrad R., Bista P., Zeng W., Hronowsky X., Buko A., Chollate S., Ellrichmann G., Bruck W., Dawson K., Goelz S., Wiese S., Scannevin R. H., Lukashev M., Gold R. (2011). Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain, 134(Pt 3), 678–692. [DOI] [PubMed] [Google Scholar]
- Liu C. B., Wang R., Yi Y. F., Gao Z., Chen Y. Z. (2018). Lycopene mitigates beta-amyloid induced inflammatory response and inhibits NF-kappaB signaling at the choroid plexus in early stages of Alzheimer's disease rats. J Nutr Biochem, 53, 66–71. [DOI] [PubMed] [Google Scholar]
- Liu L., Vollmer M. K., Kelly M. G., Fernandez V. M., Fernandez T. G., Kim H., Dore S. (2019. a). Reactive gliosis contributes to Nrf2-dependent neuroprotection by pretreatment with dimethyl fumarate or Korean red ginseng against hypoxic-ischemia: Focus on hippocampal injury. Mol Neurobiol. Advance online publication. doi: 10.1007/s12035-019-01760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Jin Z., Xu Z., Yang H., Li L., Li G., Li F., Gu S., Zong S., Zhou J., Cao L., Wang Z., Xiao W. (2019. b). Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones, 24(2), 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Li G., Tang H., Pan R., Wang H., Jin F., Yan X., Xing Y., Chen G., Fu Y., Dong J. (2019. c). Madecassoside ameliorates lipopolysaccharide-induced neurotoxicity in rats by activating the Nrf2-HO-1 pathway. Neurosci Lett, 709, 134386. [DOI] [PubMed] [Google Scholar]
- Liu Y., Hettinger C. L., Zhang D., Rezvani K., Wang X., Wang H. (2014). Sulforaphane enhances proteasomal and autophagic activities in mice and is a potential therapeutic reagent for Huntington's disease. J Neurochem, 129(3), 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Qiu J., Wang Z., You W., Wu L., Ji C., Chen G. (2015). Dimethylfumarate alleviates early brain injury and secondary cognitive deficits after experimental subarachnoid hemorrhage via activation of Keap1-Nrf2-ARE system. J Neurosurg, 123(4), 915–923. [DOI] [PubMed] [Google Scholar]
- Longbrake E. E., Ramsbottom M. J., Cantoni C., Ghezzi L., Cross A. H., Piccio L. (2016). Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Mult Scler, 22(8), 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell M., Markesbery W. R. (2007). Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res, 85(2007), 3036–3040. [DOI] [PubMed] [Google Scholar]
- Lozano R.et al. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet, 380(9859), 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupoli F., Vannocci T., Longo G., Niccolai N., Pastore A. (2018). The role of oxidative stress in Friedreich’s ataxia. FEBS Lett, 592(5), 718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C., Maharjan S., Wang Q., Sun Y., Han X., Wang S., Mao Z., Xin Y., Zhang B. (2017). Alpha-lipoic acid promotes neurological recovery after ischemic stroke by activating the Nrf2/HO-1 pathway to attenuate oxidative damage. Cell Physiol Biochem, 43(3), 1273–1287. [DOI] [PubMed] [Google Scholar]
- Ma L., Cao T. T., Kandpal G., Warren L., Fred Hess J., Seabrook G. R., Ray W. J. (2010). Genome-wide microarray analysis of the differential neuroprotective effects of antioxidants in neuroblastoma cells overexpressing the familial Parkinson's disease alpha-synuclein A53T mutation. Neurochem Res, 35(1), 130–142. [DOI] [PubMed] [Google Scholar]
- Majkutewicz I., Kurowska E., Podlacha M., Myslinska D., Grembecka B., Rucinski J., Pierzynowska K., Wrona D. (2018). Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer's disease. Brain Res, 1686, 19–33. [DOI] [PubMed] [Google Scholar]
- Majkutewicz I., Kurowska E., Podlacha M., Myślińska D., Grembecka B., Ruciński J., Plucińska K., Jerzemowska G., Wrona D. (2016). Dimethyl fumarate attenuates intracerebroventricular streptozotocin-induced spatial memory impairment and hippocampal neurodegeneration in rats. Behav Brain Res, 308, 24–37. [DOI] [PubMed] [Google Scholar]
- Meng F., Wang J., Ding F., Xie Y., Zhang Y., Zhu J. (2017). Neuroprotective effect of matrine on MPTP-induced Parkinson's disease and on Nrf2 expression. Oncol Lett, 13(1), 296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z., Li H. Y., Si C. Y., Liu Y. Z., Teng S. (2019). Asiatic acid inhibits cardiac fibrosis throughNrf2/HO-1 and TGF-beta1/Smads signaling pathways in spontaneous hypertension rats. Int Immunopharmacol, 74, 105712. [DOI] [PubMed] [Google Scholar]
- Moi P., Chan K., Asunis I., Cao A., Kan Y. W. (1994). Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A, 91(21), 9926–9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes Diaz G., Fraussen J., Van Wijmeersch B., Hupperts R., Somers V. (2018. a). Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci Rep, 8(1), 8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes Diaz G., Hupperts R., Fraussen J., Somers V. (2018. b). Dimethyl fumarate treatment in multiple sclerosis: Recent advances in clinical and immunological studies. Autoimmun Rev, 17(12), 1240–1250. [DOI] [PubMed] [Google Scholar]
- Moon D. O., Kim M. O., Kang S. H., Choi Y. H., Kim G. Y. (2009). Sulforaphane suppresses TNF-alpha-mediated activation of NF-kappaB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett, 274(1), 132–142. [DOI] [PubMed] [Google Scholar]
- Moon H. E., Paek S. H. (2015). Mitochondrial dysfunction in Parkinson's disease. Exp Neurobiol, 24(2), 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B., Ezerina D., Amoako T. N., Riemer J., Seedorf M., Dick T. P. (2013). Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nat Chem Biol, 9(2), 119–125. [DOI] [PubMed] [Google Scholar]
- Mori T., Ishii T., Akagawa M., Nakamura Y., Nakayama T. (2010). Covalent binding of tea catechins to protein thiols: the relationship between stability and electrophilic reactivity. Biosci Biotechnol Biochem, 74(12), 2451–2456. [DOI] [PubMed] [Google Scholar]
- Morini M., Roccatagliata L., Dell'Eva R., Pedemonte E., Furlan R., Minghelli S., Giunti D., Pfeffer U., Marchese M., Noonan D., Mancardi G., Albini A., Uccelli A. (2004). Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J Neuroimmunol, 148(1–2), 146–153. [DOI] [PubMed] [Google Scholar]
- Motohashi H., Yamamoto M. (2004). Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med, 10(11), 549–557. [DOI] [PubMed] [Google Scholar]
- Mudgal J., Nampoothiri M., Basu Mallik S., Kinra M., Hall S., Grant G., Anoopkumar-Dukie S., Rao C. M., Arora D. (2019). Possible involvement of metformin in downregulation of neuroinflammation and associated behavioural changes in mice. Inflammopharmacology, 27(5), 941–948. [DOI] [PubMed] [Google Scholar]
- Muller S., Behnen M., Bieber K., Moller S., Hellberg L., Witte M., Hansel M., Zillikens D., Solbach W., Laskay T., Ludwig R. J. (2016). Dimethylfumarate impairs neutrophil functions. J Invest Dermatol, 136(1), 117–126. [DOI] [PubMed] [Google Scholar]
- Murray C. J.et al. (2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet, 380(9859), 2197–2223. [DOI] [PubMed] [Google Scholar]
- Narayanan S. V., Dave K. R., Saul I., Perez-Pinzon M. A. (2015). Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2-related factor 2. Stroke, 46(6), 1626–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A., Boveris A. (2009). Brain mitochondrial dysfunction and oxidative damage in Parkinson's disease. J Bioenerg Biomembr, 41(6), 517–521. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Nioi P., Pickett C. B. (2009). The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem, 284(20), 13291–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel A., Kohlhaas M., Maack C. (2014). Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol, 73, 26–33. [DOI] [PubMed] [Google Scholar]
- Noh H., Jeon J., Seo H. (2014). Systemic injection of LPS induces region-specific neuroinflammation and mitochondrial dysfunction in normal mouse brain. Neurochem Int, 69, 35–40. [DOI] [PubMed] [Google Scholar]
- Nouhi F., Tusi S. K., Abdi A., Khodagholi F. (2011). Dietary supplementation with tBHQ, an Nrf2 stabilizer molecule, confers neuroprotection against apoptosis in amyloid beta-injected rat. Neurochem Res, 36(5), 870–878. [DOI] [PubMed] [Google Scholar]
- Oksanen M., Hyotylainen I., Trontti K., Rolova T., Wojciechowski S., Koskuvi M., Viitanen M., Levonen A. L., Hovatta I., Roybon L., Lehtonen S., Kanninen K. M., Hamalainen R. H., Koistinaho J. (2019). NF-E2-related factor 2 activation boosts antioxidant defenses and ameliorates inflammatory and amyloid properties in human Presenilin-1 mutated Alzheimer's disease astrocytes. Glia. Advance online publication. doi:10.1002/glia.23741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mealey G. B., Berry W. L., Plafker S. M. (2017). Sulforaphane is a Nrf2-independent inhibitor of mitochondrial fission. Redox Biol, 11, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontaneda D., Nicholas J., Carraro M., Zhou J., Hou Q., Babb J., Riester K., Mendoza J. P., Livingston T., Jhaveri M. (2019). Comparative effectiveness of dimethyl fumarate versus fingolimod and teriflunomide among MS patients switching from first-generation platform therapies in the US. Mult Scler Relat Disord, 27, 101–111. [DOI] [PubMed] [Google Scholar]
- Onyango E. O., Fu L., Gribble G. W. (2014). Synthesis of a dicyano abietane, a key intermediate for the anti-inflammatory agent TBE-31. Org Lett, 16(1), 322–324. [DOI] [PubMed] [Google Scholar]
- Ou Z., Kong X., Sun X., He X., Zhang L., Gong Z., Huang J., Xu B., Long D., Li J., Li Q., Xu L., Xuan A. (2018). Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun, 69, 351–363. [DOI] [PubMed] [Google Scholar]
- Paupe V., Dassa E. P., Goncalves S., Auchere F., Lonn M., Holmgren A., Rustin P. (2009). Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia. PLoS One, 4(1), e4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N., Gerhard A., Tai Y. F., Ho A. K., Turkheimer F., Barker R. A., Brooks D. J., Piccini P. (2006). Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology, 66(11), 1638–1643. [DOI] [PubMed] [Google Scholar]
- Peng K., Tao Y., Zhang J., Wang J., Ye F., Dan G., Zhao Y., Cai Y., Zhao J., Wu Q., Zou Z., Cao J., Sai Y. (2016). Resveratrol regulates mitochondrial biogenesis and fission/fusion to attenuate rotenone-induced neurotoxicity. Oxid Med Cell Longev, 2016, 6705621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo S., Piermarini E., Pastore A., Vasco G., Schirinzi T., Carrozzo R., Bertini E., Piemonte F. (2017). Nrf2-inducers counteract neurodegeneration in Frataxin-silenced motor neurons: Disclosing new therapeutic targets for Friedreich's ataxia. Int J Mol Sci, 18(10), 2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Z., Zhang L. F., Cui Y. J., Chang Y. M., Jiang C. W., Meng Z. Z., Xu P., Liu H. Y., Wang D. Y., Cao X. B. (2015). The protective effects of salidroside from exhaustive exercise-induced heart injury by enhancing the PGC-1 alpha -NRF1/NRF2 pathway and mitochondrial respiratory function in rats. Oxid Med Cell Longev, 2015, 876825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polek B., Roach M. J., Andrews W. T., Ehling M., Salek S. (2013). Burden of Friedreich's ataxia to the patients and healthcare systems in the United States and Canada. Front Pharmacol, 4, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M., Pavese N., Tai Y. F., Kiferle L., Mason S. L., Brooks D. J., Tabrizi S. J., Barker R. A., Piccini P. (2011). Microglial activation in regions related to cognitive function predicts disease onset in Huntington's disease: a multimodal imaging study. Hum Brain Mapp, 32(2), 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep H., Diya J. B., Shashikumar S., Rajanikant G. K. (2012). Oxidative stress–assassin behind the ischemic stroke. Folia Neuropathol, 50(3), 219–230. [DOI] [PubMed] [Google Scholar]
- Prakash A., Kumar A. (2013). Mitoprotective effect of Centella asiatica against aluminum-induced neurotoxicity in rats: Possible relevance to its anti-oxidant and anti-apoptosis mechanism. Neurol Sci, 34(8), 1403–1409. [DOI] [PubMed] [Google Scholar]
- Prasad S., Sajja R. K., Kaisar M. A., Park J. H., Villalba H., Liles T., Abbruscato T., Cucullo L. (2017). Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol, 12, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prema A., Janakiraman U., Manivasagam T., Thenmozhi A. J. (2015). Neuroprotective effect of lycopene against MPTP induced experimental Parkinson's disease in mice. Neurosci Lett, 599, 12–19. [DOI] [PubMed] [Google Scholar]
- Prosperini L.et al. (2018). Fingolimod vs dimethyl fumarate in multiple sclerosis: A real-world propensity score-matched study. Neurology, 91(2), e153–e161. [DOI] [PubMed] [Google Scholar]
- Qi X., Lewin A. S., Sun L., Hauswirth W. W., Guy J. (2006). Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J Biol Chem, 281(42), 31950–31962. [DOI] [PubMed] [Google Scholar]
- Qu M., Jiang Z., Liao Y., Song Z., Nan X. (2011). Protective effects of lycopene against amyloid beta-induced neurotoxicity in cultured rat cortical neurons. Neurosci Lett, 505(3), 286–290. [DOI] [PubMed] [Google Scholar]
- Qu M., Li L., Chen C., Li M., Pei L., Chu F., Yang J., Yu Z., Wang D., Zhou Z. (2016). Lycopene prevents amyloid [beta]-induced mitochondrial oxidative stress and dysfunctions in cultured rat cortical neurons. Neurochem Res, 41(6), 1354–1364. [DOI] [PubMed] [Google Scholar]
- Quinti L.et al. (2017). KEAP1-modifying small molecule reveals muted NRF2 signaling responses in neural stem cells from Huntington's disease patients. Proc Natl Acad Sci U S A, 114(23), E4676–E4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. M., Sykiotis G. P., Nishimura M., Bodmer R., Bohmann D. (2013). Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell, 12(4), 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan M., Weinstock-Guttman B., Nguyen L. T., Badgett D., Miller C., Patrick K., Brownscheidle C., Jacobs L. (2001). In vivo gene expression revealed by cDNA arrays: The pattern in relapsing-remitting multiple sclerosis patients compared with normal subjects. J Neuroimmunol, 116(2), 213–219. [DOI] [PubMed] [Google Scholar]
- Ramsey C. P., Glass C. A., Montgomery M. B., Lindl K. A., Ritson G. P., Chia L. A., Hamilton R. L., Chu C. T., Jordan-Sciutto K. L. (2007). Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol, 66(1), 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reata Pharmaceuticals. (2019). Reata announces positive topline results from the MOXIe registrational trial of omaveloxolone in patients with Friedreich’s ataxia. Los Angeles, CA: Globe News Wire. [Google Scholar]
- Rehman S. U., Ali T., Alam S. I., Ullah R., Zeb A., Lee K. W., Rutten B. P. F., Kim M. O. (2019). Ferulic acid rescues LPS-induced neurotoxicity via modulation of the TLR4 receptor in the mouse hippocampus. Mol Neurobiol, 56(4), 2774–2790. [DOI] [PubMed] [Google Scholar]
- Ren L., Zhan P., Wang Q., Wang C., Liu Y., Yu Z., Zhang S. (2019). Curcumin upregulates the Nrf2 system by repressing inflammatory signaling-mediated Keap1 expression in insulin-resistant conditions. Biochem Biophys Res Commun, 514(3), 691–698. [DOI] [PubMed] [Google Scholar]
- Robledinos-Anton N., R. Fernandez-Gines, Manda G., Cuadrado A. (2019). Activators and Inhibitors of NRF2: A review of their potential for clinical development. Oxid Med Cell Longev, 2019, 9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo R., R. Fernandez-Gajardo, Gutierrez R., Matamala J. M., Carrasco R., Miranda-Merchak A., Feuerhake W. (2013). Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol Disord Drug Targets, 12(5), 698–714. [DOI] [PubMed] [Google Scholar]
- Rojo A. I., Innamorato N. G., Martin-Moreno A. M., De Ceballos M. L., Yamamoto M., Cuadrado A. (2010). Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia, 58(5), 588–598. [DOI] [PubMed] [Google Scholar]
- Rojo A. I., Pajares M., Garcia-Yague A. J., Buendia I., Van Leuven F., Yamamoto M., Lopez M. G., Cuadrado A. (2018). Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol, 18, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo A. I., Pajares M., Rada P., Nunez A., Nevado-Holgado A. J., Killik R., Van Leuven F., Ribe E., Lovestone S., Yamamoto M., Cuadrado A. (2017). NRF2 deficiency replicates transcriptomic changes in Alzheimer's patients and worsens APP and TAU pathology. Redox Biol, 13, 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddawi-Konefka R., Seelige R., Gross E. T., Levy E., Searles S. C., Washington A., Jr., Santosa E. K., Liu B., O'Sullivan T. E., Harismendy O., Bui J. D. (2016). Nrf2 induces IL-17D to mediate tumor and virus surveillance. Cell Rep, 16(9), 2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghian M., Mastrolia V., Rezaei Haddad A., Mosley A., Mullali G., Schiza D., Sajic M., Hargreaves I., Heales S., Duchen M. R., Smith K. J. (2016). Mitochondrial dysfunction is an important cause of neurological deficits in an inflammatory model of multiple sclerosis. Sci Rep, 6, 33249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidu N. E. B., Kavian N., Leroy K., Jacob C., Nicco C., Batteux F., Alexandre J. (2019). Dimethyl fumarate, a two-edged drug: Current status and future directions. Med Res Rev, 39(5), 1923–1952. [DOI] [PubMed] [Google Scholar]
- Sandberg M., Patil J., D'Angelo B., Weber S. G., Mallard C. (2014). NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology, 79, 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M., Ernesto C., Thomas R. G., Klauber M. R., Schafer K., Grundman M., Woodbury P., Growdon J., Cotman C. W., Pfeiffer E., Schneider L. S., Thal L. J. (1997). A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's disease cooperative study. N Engl J Med, 336(17), 1216–1222. [DOI] [PubMed] [Google Scholar]
- Santos R., Lefevre S., Sliwa D., Seguin A., Camadro J. M., Lesuisse E. (2010). Friedreich ataxia: Molecular mechanisms, redox considerations, and therapeutic opportunities. Antioxid Redox Signal, 13(5), 651–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Okamoto S. I., Cui J., Watanabe Y., Furuta K., Suzuki M., Tohyama K., Lipton S. A. (2006). Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci U S A, 103(3), 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira A. H. (2008). Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol, 7(1), 97–109. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Clark J. B., Jenner P., Marsden C. D. (1990). Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem, 54(3), 823–827. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. (1989). Mitochondrial complex I deficiency in Parkinson's disease. Lancet, 1(8649), 1269. [DOI] [PubMed] [Google Scholar]
- Schipper H., Liberman A., Stopa E. G. (1998). Neural heme oxygenase-1 expression in idiopathic Parkinson's disease. Exp Neurol, 150(1), 60–68. [DOI] [PubMed] [Google Scholar]
- Schrag M., Mueller C., Zabel M., Crofton A., Kirsch W. M., Ghribi O., Squitti R., Perry G. (2013). Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Neurobiol Dis, 59, 100–110. [DOI] [PubMed] [Google Scholar]
- Schulze-Topphoff U., Varrin-Doyer M., Pekarek K., Spencer C. M., Shetty A., Sagan S. A., Cree B. A., Sobel R. A., Wipke B. T., Steinman L., Scannevin R. H., Zamvil S. S. (2016). Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci U S A, 113(17), 4777–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl S. E., Potashkin J. A. (2011). The promise of neuroprotective agents in Parkinson's disease. Front Neurol, 2, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semnani M., Mashayekhi F., Azarnia M., Salehi Z. (2017). Effects of green tea epigallocatechin-3-gallate on the proteolipid protein and oligodendrocyte transcription factor 1 messenger RNA gene expression in a mouse model of multiple sclerosis. Folia Neuropathol, 55(3), 199–205. [DOI] [PubMed] [Google Scholar]
- Sen C. K., Sashwati R., Packer L. (1999). Fas mediated apoptosis of human Jurkat T-cells: intracellular events and potentiation by redox-active alpha-lipoic acid. Cell Death Differ, 6(5), 481–491. [DOI] [PubMed] [Google Scholar]
- Shah Z. A., Li R. C., Ahmad A. S., Kensler T. W., Yamamoto M., Biswal S., Dore S. (2010). The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab, 30(12), 1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Qiu W., Xiao G., Cheng J., Zhang N. (2018). Resveratrol attenuates cognitive deficits of traumatic brain injury by activating p38 signaling in the brain. Med Sci Monit, 24, 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A. Y., Imbeault S., Barakauskas V., Erb H., Jiang L., Li P., Murphy T. H. (2005. a). Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem, 280(24), 22925–22936. [DOI] [PubMed] [Google Scholar]
- Shih A. Y., Li P., Murphy T. H. (2005. b). A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci, 25(44), 10321–10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomol G., Muralidhara. (2008. a). Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and its in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine, 15(11), 971–984. [DOI] [PubMed] [Google Scholar]
- Shinomol G., Muralidhara. (2008. b). Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicology, 29(6), 948–957. [DOI] [PubMed] [Google Scholar]
- Shinto L., Quinn J., Montine T., Dodge H. H., Woodward W., Baldauf-Wagner S., Waichunas D., Bumgarner L., Bourdette D., Silbert L., Kaye J. (2014). A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer's disease. J Alzheimers Dis, 38(1), 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivandzade F., Prasad S., Bhalerao A., Cucullo L. (2019). NRF2 and NF-B interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol, 21, 101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Martin K. A., Calabresi P. A., Bhargava P. (2017). Dimethyl fumarate alters B-cell memory and cytokine production in MS patients. Ann Clin Transl Neurol, 4(5), 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N. Y., Lee Y. H., Na H. K., Baek J. H., Surh Y. J. (2018). Leptin induces SIRT1 expression through activation of NF-E2-related factor 2: Implications for obesity-associated colon carcinogenesis. Biochem Pharmacol, 153, 282–291. [DOI] [PubMed] [Google Scholar]
- Sorolla M. A., Reverter-Branchat G., Tamarit J., Ferrer I., Ros J., Cabiscol E. (2008). Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic Biol Med, 45(5), 667–678. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Alfieri A., Siow R. C., Mann G. E., Fraser P. A. (2013). Temporal and spatial distribution of Nrf2 in rat brain following stroke: quantification of nuclear to cytoplasmic Nrf2 content using a novel immunohistochemical technique. J Physiol, 591(14), 3525–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack C., Ho D., Wille E., Calingasan N. Y., Williams C., Liby K., Sporn M., Dumont M., Beal M. F. (2010). Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington's disease. Free Radic Biol Med, 49(2), 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D., Yin T., Smalstig E. B., Hsu M. A., Panetta J., Little S., Clemens J. (2000). Transcription factor nuclear factor-kappa B is activated in neurons after focal cerebral ischemia. J Cereb Blood Flow Metab, 20(3), 592–603. [DOI] [PubMed] [Google Scholar]
- Suh J. H., Shenvi S. V., Dixon B. M., Liu H., Jaiswal A. K., Liu R. M., Hagen T. M. (2004). Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A, 101(10), 3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumari-Ramesh S., Alleyne C. H., Jr.(2016). Post-injury administration of Tert-butylhydroquinone attenuates acute neurological injury after intracerebral hemorrhage in mice. J Mol Neurosci, 58(4), 525–531. [DOI] [PubMed] [Google Scholar]
- Tabassum R., Vaibhav K., Shrivastava P., Khan A., Ejaz Ahmed M., Javed H., Islam F., Ahmad S., Saeed Siddiqui M., Safhi M., Islam F. (2013). Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats. Neurol Sci, 34, 925–933. [DOI] [PubMed] [Google Scholar]
- Taghizadeh M., Tamtaji O. R., Dadgostar E., Daneshvar Kakhaki R., Bahmani F., Abolhassani J., Aarabi M. H., Kouchaki E., Memarzadeh M. R., Asemi Z. (2017). The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson's disease: A randomized, double-blind, placebo-controlled trial. Neurochem Int, 108, 183–189. [DOI] [PubMed] [Google Scholar]
- Tai Y. F., Pavese N., Gerhard A., Tabrizi S. J., Barker R. A., Brooks D. J., Piccini P. (2007). Microglial activation in presymptomatic Huntington's disease gene carriers. Brain, 130(Pt 7), 1759–1766. [DOI] [PubMed] [Google Scholar]
- Takaya K., Suzuki T., Motohashi H., Onodera K., Satomi S., Kensler T. W., Yamamoto M. (2012). Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med, 53(4), 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanito M., Agbaga M. P., Anderson R. E. (2007). Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med, 42(12), 1838–1850. [DOI] [PubMed] [Google Scholar]
- Thimmulappa R. K., Mai K. H., Srisuma S., Kensler T. W., Yamamoto M., Biswal S. (2002). Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res, 62(18), 5196–5203. [PubMed] [Google Scholar]
- Treumer F., Zhu K., Glaser R., Mrowietz U. (2003). Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol, 121(6), 1383–1388. [DOI] [PubMed] [Google Scholar]
- Trimmer P. A., Swerdlow R. H., Parks J. K., Keeney P., Bennett J. P., Jr., Miller S. W., Davis R. E., Parker W. D., Jr.(2000). Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines. Exp Neurol, 162(1), 37–50. [DOI] [PubMed] [Google Scholar]
- Trist B. G., Hare D. J., Double K. L. (2019). Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell, e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufekci K. U., E. Civi Bayin, Genc S.,& Genc K. (2011). The Nrf2/ARE pathway: A promising target to counteract mitochondrial dysfunction in Parkinson's disease. Parkinson’s Dis, 2011, 314082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankan P. (2013). Prevalence gradients of Friedreich's ataxia and R1b haplotype in Europe co-localize, suggesting a common Palaeolithic origin in the Franco-Cantabrian ice age refuge. J Neurochem, 126(Suppl 1), 11–20. [DOI] [PubMed] [Google Scholar]
- Vargas M. R., Johnson J. A. (2009). The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med, 11, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial G., Detaille D., Guigas B. (2019). Role of mitochondria in the mechanism(s) of action of metformin. Front Endocrinol (Lausanne), 10, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Otter M., Landgren S., Nilsson S., Celojevic D., Bergstrom P., Hakansson A., Nissbrandt H., Drozdzik M., Bialecka M., Kurzawski M., Blennow K., Nilsson M., Hammarsten O., Zetterberg H. (2010). Association of Nrf2-encoding NFE2L2 haplotypes with Parkinson's disease. BMC Med Genet, 11, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel J. P., Myers R. H., Stevens T. J., Ferrante R. J., Bird E. D., Richardson E. P., Jr.(1985). Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol, 44(6), 559–577. [DOI] [PubMed] [Google Scholar]
- Wang B., Zhu X., Kim Y., Li J., Huang S., Saleem S., Li R. C., Xu Y., Dore S., Cao W. (2012). Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med, 52(5), 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. F., Cui Z. W., Zhong Z. H., Sun Y. H., Sun Q. F., Yang G. Y., Bian L. G. (2015). Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression. Acta Pharmacol Sin, 36(8), 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zou Q., Suo Y., Tan X., Yuan T., Liu Z., Liu X. (2019). Lycopene ameliorates systemic inflammation-induced synaptic dysfunction via improving insulin resistance and mitochondrial dysfunction in the liver-brain axis. Food Funct, 10(4), 2125–2137. [DOI] [PubMed] [Google Scholar]
- Wang L., Li B., Quan M. Y., Li L., Chen Y., Tan G. J., Zhang J., Liu X. P., Guo L. (2017. a). Mechanism of oxidative stress p38MAPK-SGK1 signaling axis in experimental autoimmune encephalomyelitis (EAE). Oncotarget, 8(26), 42808–42816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Li W. X., Dai S. X., Guo Y. C., Han F. F., Zheng J. J., Li G. H., Huang J. F. (2017. b). Meta-analysis of Parkinson's disease and Alzheimer's disease revealed commonly impaired pathways and dysregulation of NRF2-dependent genes. J Alzheimers Dis, 56(4), 1525–1539. [DOI] [PubMed] [Google Scholar]
- Wang W., Wu Y., Zhang G., Fang H., Wang H., Zang H., Xie T., Wang W. (2014). Activation of Nrf2-ARE signal pathway protects the brain from damage induced by epileptic seizure. Brain Res, 1544, 54–61. [DOI] [PubMed] [Google Scholar]
- Wasserman W. W., Fahl W. E. (1997). Functional antioxidant responsive elements. Proc Natl Acad Sci U S A, 94(10), 5361–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Li X., Li X., Liu Q., Cheng Y. (2018). Oxidative stress in Parkinson's disease: A systematic review and meta-analysis. Front Mol Neurosci, 11, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller C., Oxlade N., Dobbs S. M., Dobbs R. J., Charlett A., Bjarnason I. T. (2005). Role of inflammation in gastrointestinal tract in aetiology and pathogenesis of idiopathic parkinsonism. FEMS Immunol Med Microbiol, 44(2), 129–135. [DOI] [PubMed] [Google Scholar]
- Williamson T. P., Johnson D. A., Johnson J. A. (2012). Activation of the Nrf2-ARE pathway by siRNA knockdown of Keap1 reduces oxidative stress and provides partial protection from MPTP-mediated neurotoxicity. Neurotoxicology, 33(3), 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Liu R., Dai W., Jie Y., Yu G., Fan X., Huang Q. (2015). Lycopene attenuates early brain injury and inflammation following subarachnoid hemorrhage in rats. Int J Clin Exp Med, 8(8), 14316–14322. [PMC free article] [PubMed] [Google Scholar]
- Wu D., (2016). Green tea EGCG, T-cell function, and T-cell-mediated autoimmune encephalomyelitis. J Investig Med, 64(8), 1213–1219. [DOI] [PubMed] [Google Scholar]
- Wu Y. L., Chang J. C., Lin W. Y., Li C. C., Hsieh M., Chen H. W., Wang T. S., Wu W. T., Liu C. S., Liu K. L. (2018). Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway. Free Radic Biol Med, 115, 309–317. [DOI] [PubMed] [Google Scholar]
- Xia D., Zhai X., Wang H., Chen Z., Fu C., Zhu M. (2019). Alpha lipoic acid inhibits oxidative stress-induced apoptosis by modulating of Nrf2 signalling pathway after traumatic brain injury. J Cell Mol Med, 23(6), 4088–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Song D., Cheng Y., Hu Y., Wang F., Lu Z., Wang Y. (2019). Biogenic nanoselenium particles activate Nrf2-ARE pathway by phosphorylating p38, ERK1/2, and AKT on IPEC-J2 cells. J Cell Physiol, 234(7), 11227–11234. [DOI] [PubMed] [Google Scholar]
- Xu Q., Kanthasamy A. G., Reddy M. B. (2018). Epigallocatechin gallate protects against TNFalpha- or H2O2-induced apoptosis by modulating iron related proteins in a cell culture model. Int J Vitam Nutr Res, 88(3–4), 158–165. [DOI] [PubMed] [Google Scholar]
- Yang B., Xu Y., Hu Y., Luo Y., Lu X., Tsui C. K., Lu L., Liang X. (2016). Madecassic acid protects against hypoxia-induced oxidative stress in retinal microvascular endothelial cells via ROS-mediated endoplasmic reticulum stress. Biomed Pharmacother, 84, 845–852. [DOI] [PubMed] [Google Scholar]
- Yang G. Z., Wang Z. J., Bai F., Qin X. J., Cao J., Lv J. Y., Zhang M. S. (2015). Epigallocatechin-3-gallate protects HUVECs from PM2.5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules, 20(4), 6626–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Calingasan N. Y., Thomas B., Chaturvedi R. K., Kiaei M., Wille E. J., Liby K. T., Williams C., Royce D., Risingsong R., Musiek E. S., Morrow J. D., Sporn M., Beal M. F. (2009). Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS One, 4(6), e5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. B., Chen W. W., Chen H. P., Cai S. X., Lin J. D., Qiu L. Z. (2018). MiR-155 aggravated septic liver injury by oxidative stress-mediated ER stress and mitochondrial dysfunction via targeting Nrf-2. Exp Mol Pathol, 105(3), 387–394. [DOI] [PubMed] [Google Scholar]
- Yao J., Irwin R. W., Zhao L., Nilsen J., Hamilton R. T., Brinton R. D. (2009). Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci, 106(34), 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Miao W., Liu Z., Han W., Shi K., Shen Y., Li H., Liu Q., Fu Y., Huang D., Shi F. D. (2016). Dimethyl fumarate and monomethyl fumarate promote post-ischemic recovery in mice. Transl Stroke Res, 7(6), 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo I. H., Kim M. J., Kim J., Sung J. J., Park S. T., Ahn S. W. (2019). The anti-inflammatory effect of sulforaphane in mice with experimental autoimmune encephalomyelitis. J Korean Med Sci, 34(28), e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Wang W., Pang W., Xiao Z., Jiang Y., Hong Y. (2017). Dietary lycopene supplementation improves cognitive performances in tau transgenic mice expressing P301L mutation via inhibiting oxidative stress and tau hyperphosphorylation. J Alzheimers Dis, 57(2), 475–482. [DOI] [PubMed] [Google Scholar]
- Zabel M., Nackenoff A., Kirsch W. M., Harrison F. E., Perry G., Schrag M. (2018). Markers of oxidative damage to lipids, nucleic acids and proteins and antioxidant enzymes activities in Alzheimer's disease brain: A meta-analysis in human pathological specimens. Free Radic Biol Med, 115, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitone S. A., Abo-Elmatty D. M., Shaalan A. A. (2012). Acetyl-L-carnitine and alpha-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson's disease therapy. Pharmacol Biochem Behav, 100(3), 347–360. [DOI] [PubMed] [Google Scholar]
- Zhang J., Tucker L. D., DongYan, Lu Y., Yang L., Wu C., Li Y., Zhang Q. (2018). Tert-butylhydroquinone post-treatment attenuates neonatal hypoxic-ischemic brain damage in rats. Neurochem Int, 116, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wu C., Zhao S., Yuan D., Lian G., Wang X., Wang L., Yang J. (2010). Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-kappaB signaling pathways in N9 microglia induced by lipopolysaccharide. Int Immunopharmacol, 10(3), 331–338. [DOI] [PubMed] [Google Scholar]
- Zhang S. M., Hernan M. A., Chen H., Spiegelman D., Willett W. C., Ascherio A. (2002). Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology, 59(8), 1161–1169. [DOI] [PubMed] [Google Scholar]
- Zhang Y. K., Wu K. C., Klaassen C. D. (2013). Genetic activation of Nrf2 protects against fasting-induced oxidative stress in livers of mice. PLoS One, 8(3), e59122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Ren B., Guo R., Zhang W., Ma S., Yao Y., Yuan T., Liu Z., Liu X. (2017. a). Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-kappaB transcriptional pathway. Food Chem Toxicol, 109(Pt 1), 505–516. [DOI] [PubMed] [Google Scholar]
- Zhao H., Zhao X., Liu L., Zhang H., Xuan M., Guo Z., Wang H., Liu C. (2017. b). Neurochemical effects of the R form of alpha-lipoic acid and its neuroprotective mechanism in cellular models of Parkinson's disease. Int J Biochem Cell Biol, 87, 86–94. [DOI] [PubMed] [Google Scholar]
- Zhao J., Kobori N., Aronowski J., Dash P. K. (2006). Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett, 393(2–3), 108–112. [DOI] [PubMed] [Google Scholar]
- Zhao J., Moore A. N., Clifton G. L., Dash P. K. (2005). Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res, 82(4), 499–506. [DOI] [PubMed] [Google Scholar]
- Zhao J., Moore A. N., Redell J. B., Dash P. K. (2007). Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci, 27(38), 10240–10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K., Dai X., Xiao N., Wu X., Wei Z., Fang W., Zhu Y., Zhang J., Chen X. (2017). Curcumin ameliorates memory decline via inhibiting BACE1 expression and beta-amyloid pathology in 5× FAD transgenic mice. Mol Neurobiol, 54, 1967–1977. [DOI] [PubMed] [Google Scholar]
- Zuo L., Zhou T., Pannell B. K., Ziegler A. C., Best T. M. (2015). Biological and physiological role of reactive oxygen species–the good, the bad and the ugly. Acta Physiol (Oxf), 214(3), 329–348. [DOI] [PubMed] [Google Scholar]