Abstract
Background:
Patients with cirrhosis and coronary artery disease (CAD) are at high risk for morbidity during surgical revascularization so they are often referred for complex percutaneous coronary intervention (PCI). Percutaneous coronary intervention in the cirrhotic population also has inherent risks; however, quantifiable data on long-term outcomes are lacking.
Methods:
Patients with angiographically significant CAD and cirrhosis were identified from the catheterization lab databases of the University of Pennsylvania Health System between 2007 and 2015. Outcomes were obtained from the medical record and telephonic contact with patients/families.
Results:
Percutaneous coronary intervention was successfully performed in 42 patients (51 PCIs). Twenty-nine patients with significant CAD were managed medically (36 angiograms). The primary outcome (a composite of mortality, subsequent revascularization, and myocardial infarction) was not significantly different between the 2 groups during a follow-up period at 1 year (PCI: 50%, Control: 40%, P = .383). In the PCI group, a composite adverse outcome rate that included acute kidney injury (AKI), severe bleed, and peri-procedural stroke was elevated (40%), with severe bleeding occurring after 23% of PCI events and post-procedural AKI occurring after 26% of events. The medical management group had significantly fewer total matched adverse outcomes (17% vs 40% in the PCI group, P = .03), with severe bleeding occurring after 11% of events and AKI occurring after 6% of events. Increased risk of adverse events following PCI was associated with severity of liver disease by Child-Pugh class.
Conclusions:
Percutaneous coronary intervention in patients with cirrhosis is associated with an elevated risk of adverse events, including severe bleeding and AKI.
Keywords: PCI, cirrhosis, dual antiplatelet therapy
Introduction
Patients with cirrhosis or end-stage liver disease often have co-existing coronary artery disease (CAD) with a similar or higher prevalence than the general population.1-7 Individuals with pre-cirrhotic pathology, particularly nonalcoholic steatohepatitis (NASH) or nonalcoholic fatty liver disease (NAFLD), also have an elevated risk of developing CAD.8,9 When an indication for coronary revascularization arises, patients with advanced liver disease are often deemed poor surgical revascularization candidates given their risk of death, post-operative liver failure, and/or bleeding, and thus they are often referred for high-risk percutaneous coronary intervention (PCI).5,10-13 However, PCI in the cirrhotic population has its own inherent risks, including but not limited to: acute kidney injury (AKI) secondary to dynamic renal perfusion, and increased bleeding while on dual antiplatelet therapy (DAPT) due to coagulopathies, cytopenias, and consequences of portal hypertension such as varices and gastritis.
In addition, there exists a cohort of patients with advanced liver disease who may be candidates for orthotopic liver transplantation. The evaluation and battery of testing necessary to assess a patient’s candidacy for solid organ transplantation is extensive and includes screening for occult cardiovascular conditions.14,15 In many centers, patients with CAD may be denied listing for liver transplantation unless they first undergo coronary revascularization. This common uninvestigated strategy is based on the intention of mitigating the risk of perioperative ischemia or infarction as well as treating a comorbid condition that may shorten life expectancy independent of cirrhosis.5,7,16,17
The safety and efficacy of PCI in the cirrhotic population are not well known, and there are currently no clear hepatology or transplant practice guidelines addressing revascularization. While a few retrospective studies investigating the safety and short-term/in-hospital outcomes of PCI in cirrhotics have been reported,1,4,18-20 long-term outcome data are scant. Furthermore, the incidence of bleeding attributable to PCI and antiplatelet therapy, acute renal failure, and outcomes of patients who receive transplant following PCI remain poorly characterized. We sought to undertake an exploratory analysis of single-center patient-level outcome data to evaluate the short- and long-term outcomes and adverse events related to PCI in patients with cirrhosis.
Methods
Patients with liver disease who underwent coronary angiography at the Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center between January 2007 and December 2015 were identified. Corroborating patient-level electronic medical record data were obtained using proprietary natural language search software. Liver disease was identified using the search terms “cirrhosis,” “liver,” “hepatic,” or “MELD” (Model for End-Stage Liver Disease). From the populated list, patient charts were individually abstracted by authors DYL and MDS to confirm the combined diagnosis of cirrhosis/end-stage liver disease and angiographically significant CAD diagnosed during coronary angiography (Figure 1). Patients with other pathologies including acute liver injury or hepatic tumors without specific mention of cirrhosis were excluded, as were patients with previous liver transplantation unless they had documented end-stage allograft dysfunction. Angiographically significant obstructive CAD was defined as a greater than or equal to 50% stenosis in the left main coronary artery or greater than or equal to 70% stenosis in any other major epicardial coronary artery or major branch, as determined by visual estimation of the performing board-certified interventional cardiologist.
Figure 1.
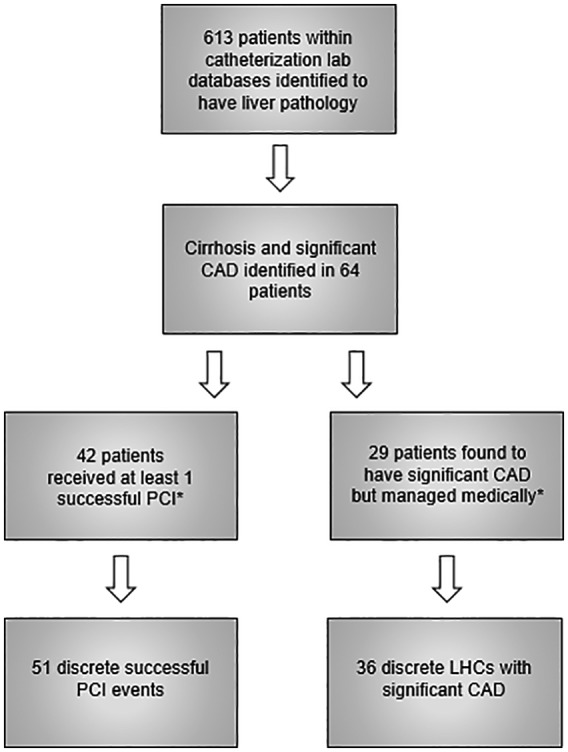
Identification of patients with angiographically significant CAD and cirrhosis (time interval: 2007-2015).
CAD indicates coronary artery disease; LHC, left heart catheterization; PCI, percutaneous coronary intervention. *Seven patients were at some point members of both these groups during the specified time interval. See text for details.
Patient medical records were individually reviewed in their entirety. If the medical record was incomplete or if patients were lost to follow-up, patients or next-of-kin were contacted directly by phone. Follow-up chart review was also performed at the Philadelphia Veterans Affairs (VA) Medical Center for a small subset of patients. Based on treatment strategy, 2 cohorts of cirrhotic patients were examined: Cohort A—patients with obstructive CAD treated with PCI, and Cohort B—patients with obstructive CAD managed medically without PCI. Given that some patients had multiple angiograms at discreet and often distant time points, comparisons of adverse events and cardiovascular outcomes were based on individual PCI or diagnostic angiography episodes; as such, a small subset of patients may be included in both groups at distinct distant time points. Staged PCI or repeat PCI/catheterization performed for the same indication within the same hospitalization was considered part of 1 single episode.
Patients were further stratified based on the severity of their liver disease (Child-Pugh classification).11,12 Acute kidney injury was defined as a documented increase in serum creatinine by 0.3 mg/dL or a 50% increase over baseline. Bleeding events were defined as the Bleeding Academic Research Consortium (BARC) definition type 2 or above.21 Severe bleeding events were defined as BARC type 3B or above. Significant procedural site hematomas were defined as any hematoma meeting BARC type 2 or above criteria. Events involving transfusions or hospitalizations for anemia without a documented overt bleed were not counted as significant bleeding events. For staged procedures, contrast load was averaged across the interventions, and radial/femoral access was defined as the access used during the initial intervention.
The primary outcome was a composite of major adverse cardiovascular events (MACE) including death, myocardial infarction, or need for repeat revascularization. Secondary outcomes included a composite adverse event rate that included AKI, severe bleeding events (BARC 3B or above), and post-procedural stroke, as well as individual components of the composite outcome. Short-term peri-procedural outcomes included peri-procedural AKI, stroke, and site complications, whereas long-term outcomes were MACE and major bleeding events. Follow-up was obtained for up to 1 year, or until death or last contact with the patient. Transplant status was followed for more than 1 year until either death, listing for transplant, or transplantation.
Results are reported as un-adjusted outcome data with statistical comparisons on baseline demographics, cardiovascular outcomes, and adverse event rates using the 2-sided Fisher’s exact testing for categorical variables or 2-sided t-test for continuous variables. The study protocol was approved by the institutional review board (IRB) of the University of Pennsylvania (#818083) and the Philadelphia VA (#01626). Informed consent was waived per the IRB protocol for the electronic medical data review, and verbal informed consent was obtained for telephonic contact as per IRB protocol.
Results
We identified 613 patients with liver pathology, 64 of whom carried diagnosis of cirrhosis and angiographically significant CAD (Figure 1). Forty-two patients underwent 51 discreet PCI episodes (Cohort A). Twenty-nine patients had at least 1 coronary angiogram with significant CAD subsequently managed medically, resulting in 36 such events (Cohort B).
Patient demographics are listed in Table 1. No significant differences were observed in the baseline characteristics between the groups, although there was a numerically higher rate of insulin-dependent diabetes in those treated with PCI. Average contrast use was higher in the PCI group (PCI: 178.97 mL, medical management: 94.03 mL, P < .01), and average SYNTAX scores were slightly higher in the medical management group (PCI: 17.73, medical management: 23.97, P = .04). Groups were also stratified based on CAD complexity and Child-Pugh class (Table 2). Left main or multivessel CAD were found with similar frequency in both groups (PCI: 68.6%, medical management: 75.0%, P = .52). Coronary intervention (N = 51) used bare metal stents in 55% of cases, drug-eluting stents in 39%, and balloon angioplasty alone in 8%. A numerically higher proportion of Child-Pugh class A patients received drug-eluting stents (56%, 9/16) compared with Child-Pugh class B and C patients (31%, 10/32, P = .12). Of those undergoing PCI, 51% were performed on an urgent or emergent basis while 25% were performed during evaluation for liver transplant candidacy (Table 3). In the medical management cohort, 33% of angiograms were performed on an urgent or emergent basis, and 33% were performed for liver transplant evaluation.
Table 1.
Baseline characteristics.
| PCI events n = 51 discrete events |
Significant CAD without PCI n = 36 discrete events |
P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 62.25 ± 1.05 | 62.69 ± 1.16 | .78 |
| Gender | 40 M, 11 F | 33 M, 3 F | .14 |
| Body mass index | 29.1 ± 0.84 | 29.80 ± 0.80 | .55 |
| Medical history | |||
| Systolic heart failure (EF ⩽ 45%) | 15 (29%) | 11 (31%) | 1.00 |
| Peripheral vascular disease | 15 (29%) | 12 (34%) | .64 |
| Stroke/TIA | 5 (10%) | 1 (3%) | .39 |
| COPD | 8 (16%) | 5 (14%) | 1.00 |
| Hypertension | 44 (86%) | 29 (83%) | .76 |
| Hyperlipidemia | 37 (73%) | 20 (63%) | .36 |
| Insulin-dependent diabetes | 27 (53%) | 10 (31%) | .07 |
| Chronic kidney disease | 18 (38%) | 8 (24%) | .23 |
| Anticoagulation | 2 (4%) | 3 (9%) | .64 |
| Current/former smoker | 44 (86%) | 30 (88%) | 1.00 |
| Cirrhosis etiology | |||
| Hepatitis B | 4 (8%) | 0 (0%) | .14 |
| Hepatitis C | 24 (47%) | 17 (50%) | .83 |
| Alcohol | 2 (4%) | 4 (12%) | .23 |
| NASH | 14 (27%) | 6 (19%) | .45 |
| Multifactorial | 4 (8%) | 1 (3%) | .40 |
| Unknown/Other | 3 (6%) | 6 (17%) | .15 |
| History of decompensations | |||
| Ascites | 27 (54%) | 16 (49%) | .66 |
| Bleeding varices | 8 (16%) | 4 (11%) | .75 |
| Hepatic encephalopathy | 19 (38%) | 12 (36%) | 1.00 |
| Hepatocellular carcinoma | 11 (22%) | 12 (33%) | .23 |
| Lab data | |||
| Thrombocytopenia | 33 (65%) | 25 (74%) | .48 |
| Platelet counts ( 1000/µL) | 129.20 ± 9.76 | 114.44 ± 11.22 | .32 |
| Albumin (g/dL) | 3.05 ± 0.09 | 3.18 ± 0.12 | .39 |
| Creatinine (mg/dL) | 1.79 ± 0.27 | 1.36 ± 0.17 | .19 |
| Total bilirubin (mg/dL) | 1.56 ± 0.19 | 1.72 ± 0.22 | .58 |
| INR | 1.27 ± 0.03 | 1.28 ± 0.05 | .99 |
| MELD | 13.70 ± 0.71 | 13.18 ± 0.92 | .66 |
| MELD-Na | 15.22 ± 0.79 | 13.91 ± 0.96 | .29 |
| Procedural characteristics | |||
| Contrast use | 178.97 ± 11.34 | 94.03 ± 7.90 | <.01 |
| SYNTAX score | 17.73 ± 1.65 | 23.97 ± 2.75 | .04 |
| Access | 45 femoral, 6 radial | 27 femoral, 9 radial | .15 |
| Child-Pugh class | |||
| A | 16 (31%) | 13 (36%) | .98 |
| B | 26 (51%) | 17 (47%) | |
| C | 6 (12%) | 4 (11%) | |
| Unknown | 3 (6%) | 2 (6%) | |
Data are presented as percentages for categorical variables or as mean ± standard error of the mean (SEM) for continuous variables. Total n for each row may be 1 to 3 less than 51 or 36 if variables were unobtainable or missing for certain patients—percentages, means, and SEM were adjusted for such.
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease score; NASH, nonalcoholic steatohepatitis; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Table 2.
Anatomic characteristics of coronary lesions.
| Child-Pugh class | 1-Vessel disease | 2-Vessel disease | 3-Vessel disease | Left main with or without multivessel disease |
|---|---|---|---|---|
| PCI events (n = 51) | 16 | 10 | 17 | 8 |
| A | 5 | 4 | 7 | 0 |
| B | 6 | 5 | 8 | 7 |
| C | 3 | 1 | 1 | 1 |
| Unknown | 2 | 0 | 1 | 0 |
| Significant CAD without PCI (n = 36) | 9 | 11 | 11 | 5 |
| A | 5 | 4 | 4 | 0 |
| B | 3 | 4 | 6 | 4 |
| C | 1 | 3 | 0 | 0 |
| Unknown | 0 | 0 | 1 | 1 |
Abbreviations: CAD, coronary artery disease; PCI, percutaneous coronary intervention.
Table 3.
PCI and transplant outcomes.
| PCI | Significant CAD without PCI | |
|---|---|---|
| No. of patients | n = 42 | n = 29 |
| Urgent/emergent procedure | 26/51 (51%) | 12/36 (33%) |
| Elective procedure | 25/51 (49%) | 24/36 (67%) |
| Elective procedure for transplant evaluation | 13/51 (25%) | 12/36 (33%) |
| Staged PCI | 15/51 (29%) | |
| Failed PCI | 2 | |
| CABG | 2 | |
| Listed for liver transplant | 11 | 9 |
| Complete revascularization of listed patients | 7 (64%) | 0 (0%) |
| Transplanted | 4 | 6 |
| Complete revascularization of transplanted patients | 3 (75%) | 0 (0%) |
| Peri-transplant cardiovascular events | 1 (25%) | 0 |
Abbreviations: CABG, coronary artery bypass graft; CAD, coronary artery disease; PCI, percutaneous coronary intervention.
Three-month and 1-year mortality were associated with severity of liver disease (Table 4). Mortality in the PCI group, compared with the medical therapy group, was greater at 3 months (27% vs 8%, P = .048) and at 1 year (42% vs 14%, P = .008). The rates of subsequent revascularization (13% vs 20%, P = .377) and myocardial infarction (23% vs 17%, P = .591) did not differ statistically. The composite outcome rate of mortality, need for repeat revascularization, and myocardial infarction were similar (50% vs 40%, P = .383).
Table 4.
Mortality and composite major adverse cardiac events.
| 3-month mortality | 1-year mortality | Revascularization | Myocardial infarction | Composite | |
|---|---|---|---|---|---|
| PCI | 13/48 (27%) | 20/48 (42%) | 6/48 (13%) | 11/48 (23%) | 24/48 (50%) |
| Child-Pugh class A | 3/15 (20%) | 4/15 (27%) | 3/15 (20%) | 2/15 (13%) | 5/15 (33%) |
| Child-Pugh class B | 6/26 (23%) | 11/26 (42%) | 3/26 (12%) | 6/26 (23%) | 14/26 (54%) |
| Child-Pugh class C | 3/5 (60%) | 4/5 (80%) | 0/5 (0%) | 2/5 (40%) | 4/5 (80%) |
| Unknown | 1/2 (50%) | 1/2 (50%) | 0/2 (0%) | 1/2 (50%) | 1/2 (50%) |
| Significant CAD without PCI | 3/36 (8%) | 5/35 (14%) | 7/35 (20%) | 6/35 (17%) | 14/35 (40%) |
| Child-Pugh class A | 0/13 (0%) | 0/12 (0%) | 3/13 (23%) | 0/13 (0%) | 3/13 (23%) |
| Child-Pugh class B | 3/17 (18%) | 5/17 (29%) | 3/17 (18%) | 6/17 (35%) | 10/17 (59%) |
| Child-Pugh class C | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) |
| Unknown | 0/2 (0%) | 0/2 (0%) | 1/1 (100%) | 0/1 (0%) | 1/1 (100%) |
| P-value | .048 | .008 | .377 | .591 | .383 |
Discrepancies in total numbers between 3-month and 1-year survivals are due to loss to follow-up. Composite score is the combined rate of mortality, myocardial infarction, and need for revascularization in the follow-up period.
Abbreviations: CAD, coronary artery disease; PCI, percutaneous coronary intervention.
In the PCI group, the composite adverse event rate of stroke, AKI, and severe bleeding episodes at 1-year follow-up was high at 40%. Severe bleeding (BARC 3B and above) while on DAPT was observed after 23% of the PCI events and post-procedural AKI was observed after 26%. Total bleeding events (BARC 2 and higher) approached 46%, including a 10% rate of access site complications under the same definition (Table 5). Four PCI patients, all with pre-existing renal dysfunction (glomerular filtration rate [GFR] < 60), developed AKI requiring renal replacement therapy. The medical management group had a significantly lower composite adverse event rate of peri-procedural stroke, AKI, and severe bleeding (17% vs 40%, P = .032) and a lower rate of AKI (6% vs 26%, P = .02). While the overall rate of total major and minor bleeding events was similar between the PCI and the medical management groups (46% vs 40%, P = .658), the rate of severe bleeding events was numerically twice as high in the PCI group (23% vs 11%, P = .25). Adverse event rates in the PCI group were associated with Child-Pugh class (class A: 2/15 or 13%, classes B and C: 16/31 or 52%, P = .02) but not MELD score (MELD < 15: 10/32 or 31%, MELD ⩾ 15: 9/16 or 56%, P = .12).
Table 5.
Adverse events related to PCI.
| Child-Pugh class | Post-procedural AKIa | Post-procedural stroke | Bleeding events (BARC 2+) | Severe bleeding events (BARC 3B+) | Site complications | Composite adverse events of stroke, AKI, severe bleed |
|---|---|---|---|---|---|---|
| PCI | 12/47 (26%) | 0/51 (0%) | 22/48 (46%) | 11/48 (23%) | 5/51 (10%) | 19/48 (40%) |
| A | 1/15 (7%) | 0 | 6/15 (40%) | 1/15 (7%) | 2/16 (13%) | 2/15 (13%) |
| B | 9/24 (38%) | 0 | 13/26 (50%) | 8/26 (31%) | 1/26 (4%) | 14/26 (54%) |
| C | 1/6 (17%) | 0 | 3/5 (60%) | 2/5 (40%) | 2/6 (33%) | 2/5 (40%) |
| Unknown | 1/2 (50%) | 0 | 0/2 (0%) | 0/2 (0%) | 0/3 (0%) | 1/2 (50%) |
| Significant CAD without PCI | 2/35 (6%) | 0/36 (0%) | 14/35 (40%) | 4/35 (11%) | 0/36 (0%) | 6/35 (17%) |
| A | 0/13 (0%) | 0 | 3/13 (23%) | 0/13 (0%) | 0/13 (0%) | 0/13 (0%) |
| B | 2/17 (12%) | 0 | 7/17 (41%) | 1/17 (6%) | 0/17 (0%) | 3/17 (18%) |
| C | 0/4 (0%) | 0 | 4/4 (100%) | 3/4 (75%) | 0/4 (0%) | 3/4 (75%) |
| Unknown | 0/1 (0%) | 0 | 0/1 (0%) | 0/1 (0%) | 0/2 (0%) | 0/1 (0%) |
| P-value | .020 | .658 | .251 | .074 | .032 |
Follow-up interval for adverse events was until death, loss to follow-up, 1 year, or crossover to PCI. Patients who were lost to follow-up were removed from the analysis of adverse events unless the events occurred during follow-up availability. Other adverse events not listed in the table include aspiration pneumonia and toe gangrene.
Patients who already had end-stage renal disease on dialysis were excluded from the analysis of AKI.
Total bleeding events—PCI group: 41 total (14 BARC 2, 14 BARC 3a, 10 BARC 3b, 2 BARC 3c, 1 BARC 5b); Significant CAD without PCI group—22 total (11 BARC 2, 7 BARC 3a, 3 BARC 3b, 1 BARC 3c).21
Abbreviations: AKI, acute kidney injury; BARC, Bleeding Academic Research Consortium; CAD, coronary artery disease; PCI, percutaneous coronary intervention.
When stratified by procedural indication (Table 6 and Supplemental Table I), procedures done for acute coronary syndrome (ACS) generally had poorer outcomes compared with procedures done for elective reasons (stable angina, pre-operative evaluation, transplant evaluation), with the primary outcome occurring after 59% of ACS procedures and 31% of elective procedures (P = .015). The composite adverse event outcome was also numerically higher, occurring after 39% of ACS procedures and 21% of elective procedures (P = .095). However, stratification by procedure indication did not change the composite and adverse outcome findings presented in Tables 4 and 5, as there was no difference in the primary outcome between the PCI and no-PCI groups for both ACS and elective procedures (Table 6) and a numerically higher adverse event rate in the PCI group for both ACS and elective procedures (Supplemental Table I).
Table 6.
Major adverse cardiac events stratified by procedural indication.
| 3-month mortality | 1-year mortality | Revascularization | Myocardial infarction | Composite | |
|---|---|---|---|---|---|
| ACS | 13/44 (30%) | 19/44 (43%) | 8/44 (18%) | 13/44 (30%) | 26/44 (59%)* |
| PCI | 11/30 (37%) | 16/30 (53%) | 5/30 (17%) | 8/30 (27%) | 18/30 (60%) |
| Significant CAD without PCI | 2/14 (14%) | 3/14 (21%) | 3/14 (21%) | 5/14 (36%) | 8/14 (57%) |
| P-value | .170 | .058 | .695 | .724 | 1.000 |
| Elective | 3/40 (8%) | 6/39 (15%) | 5/39 (13%) | 4/39 (10%) | 12/39 (31%)* |
| PCI | 2/18 (11%) | 4/18 (22%) | 1/18 (6%) | 3/18 (17%) | 6/18 (33%) |
| Significant CAD without PCI | 1/22 (5%) | 2/21 (10%) | 4/21 (19%) | 1/21 (5%) | 6/21 (29%) |
| P-value | .579 | .387 | .349 | .318 | 1.000 |
Discrepancies in total numbers are due to loss of follow-up. Composite score is the combined rate of mortality, myocardial infarction, and need for revascularization in the follow-up period.
Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; PCI, percutaneous coronary intervention.
P = .015 for comparison of composite outcome between ACS and elective intervention.
Overall, 11 patients in the PCI group were candidates for liver transplant, of which only 7 underwent pre-operative complete percutaneous revascularization; 4 ultimately received orthotopic liver transplantation (Table 3). In the medical management group, 9 patients were candidates for transplant, of which 6 received liver transplantation. While all transplanted patients were free of unrevascularized left main or proximal left anterior descending CAD at the time of surgery, most of the patients with CAD were successfully operated on despite incomplete revascularization. An isolated and minor type 2 non-ST elevation myocardial infarction occurred in the PCI group, managed expectantly (Table 3).
Discussion
To our knowledge, this is the first study to evaluate long-term PCI outcomes of patients with cirrhosis in the United States using event-level data. The total composite outcome (myocardial infarction, repeat revascularization, and mortality) was not statistically different between patients treated with PCI and those without PCI (despite a higher complexity of CAD as measured by SYNTAX score within the non-PCI cohort). Importantly, however, there was an increased adverse event rate including AKI and severe bleeding in those treated with PCI and subsequent long-term DAPT therapy, with the rate correlated to cirrhosis severity as defined by Child-Pugh class.
In the context of cirrhosis, patients with pre-existing CAD generally have poorer outcomes with liver transplantation,5,16,17 with multivessel CAD predicting increased mortality and length of stay after transplantation.22 The 2013 American Association for the Study of Liver Diseases (AASLD) and the American Society of Transplantation guidelines recommended cardiac evaluation with stress echocardiography in all adult liver transplant candidates, with cardiac catheterization and revascularization as clinically indicated if significant CAD was detected.14 The scientific statement from the American Heart Association (AHA) and the American College of Cardiology (ACC) suggests that noninvasive stress testing may be considered for transplant candidates who have multiple risk factors for CAD.15 Cirrhotics found to have comorbid multivessel CAD have limited options, unable to undergo transplant due to cardiac risk while simultaneously having prohibitive risk for surgical revascularization.11,12 Many are referred for high-risk multivessel PCI. Such patients frequently are asymptomatic or have stable angina; a population which, in the absence of liver disease, does not derive a survival benefit from PCI.23
Studies of PCI in this patient population have mostly been retrospective, focusing on short-term outcomes, with little data on the completeness of revascularization or long-term outcomes.1,4,18-20,24 Our data support and extend the findings of a recent retrospective study evaluating the in-hospital and short-term outcomes of PCI in patients with end-stage liver disease, which concluded that although PCI remains relatively safe, it is riskier than for the general population.1 The largest long-term study to date was a retrospective study of 233 Japanese patients, which suggested that complete revascularization was not associated with better survival outcomes given a high rate of non-cardiovascular mortality in cirrhotics.24 Our findings in an American population are similar and provide unique insights into the long-term risk of adverse events while also suggesting that successful transplantation is possible in selected patients, both following successful PCI and also when PCI is not performed.
Adverse event rates of PCI were high, with occurrence of the composite event rate in up to 40% and occurrence of AKI and severe bleeding in 26% and 23% of PCIs, respectively, and greater than that observed in patients who were medically managed. This difference was mostly driven by the rate of AKI, and notably, 4 patients required initiation of renal replacement therapy after their PCI. The cirrhotic patient population, characterized by dynamic renal perfusion and a high prevalence of baseline chronic kidney disease, may be exquisitely sensitive to the higher quantities of iodinated contrast exposure during PCI. Furthermore, many patients have complex multivessel disease resulting in staged and/or long multivessel PCI procedures compared with the general population, further increasing their contrast exposure.
Prior studies assessing in-hospital and short-term outcomes of PCI in cirrhotics show increased hemorrhagic and transfusion rates of 7% to 15%.1,18 In our study, long-term bleeding rates during up to 1-year follow-up were greater, with a 46% rate of combined major and minor bleeding events. Moreover, bleeding risk increased (40%) even without PCI in patients with cirrhosis given their coagulopathy and portal hypertension. However, despite the similarity in total bleeding rate, the rate of severe bleeding rate was numerically doubled after PCI, suggesting that PCI and subsequent use of DAPT convert minor bleeding events into major bleeding events.
Our results, while only hypothesis-generating, showed an increased mortality rate and an overall equivalence in composite cardiovascular outcomes in cirrhotic patients treated with PCI compared with medical therapy. The increased mortality may be attributable to the revascularization procedure itself, the untoward and cumulative risks that come thereafter, or from unmeasured confounders. Finally, despite the lack of complete revascularization in most of the patients receiving transplant, there was only 1 minor peri-procedural cardiovascular event, managed conservatively. Further large-scale studies will be needed to truly address this issue, especially given the increase in major adverse events related to PCI.
Prior studies have noted a correlation between the severity and complexity of CAD as measured by the SYNTAX score and the severity of liver disease as measured by the NAFLD fibrosis score.25 However, we did not find a convincing correlation between the SYNTAX score and the severity of liver disease as measured by Child-Pugh class within the overall population of our study (Supplemental Table II), potentially due to the low number of patients with NASH cirrhosis and high number with hepatitis C (Table 1).
Finally, despite the lack of complete revascularization in most of the patients receiving transplant, there was only 1 minor peri-procedural cardiovascular event requiring no intervention. Although only hypothesis-forming, it suggests that complete revascularization may not be absolutely necessary prior to clearance for transplant, and that a multidisciplinary team of cardiologists, hepatologists, and transplant surgeons were effective at risk stratifying patients based on their individualized coronary anatomy and cardiac, bleeding, hepatic, and renal risks. For example, it is possible that left main or proximal left anterior descending coronary artery may be the only vessels necessarily requiring pre-operative revascularization. A prior multicenter retrospective review demonstrated that the presence of obstructive CAD did not affect post-liver transplant survival when current CAD treatment strategies were employed, although that study was not designed to detect adverse events while awaiting transplant.26 Further large-scale multicenter studies will be needed to address this issue, given the high rate of major adverse events related to PCI.
Limitations of our study include the limitations of a retrospective analysis at a single institution. Comparisons between mortality and transplant outcomes between the PCI and the medical management groups are limited given the small sample size and heterogeneity of the patient population. Furthermore, a small subset of patients was lost to follow-up despite efforts to contact them directly. Finally, our exploratory analysis resulted in sample sizes too small for propensity matching. Strengths of our study include a comprehensive review of patient-level data and up to 1-year follow-up. Furthermore, the ability to identify and manually classify individual events rather than using registry data and diagnosis codes led to a more accurate assessment of these events and their severity.
Conclusions
In conclusion, PCI in patients with cirrhosis is associated with an increased 1-year adverse event rate including severe bleeding and renal injury related to the underlying severity of liver disease without a corresponding decrease in the rate of composite major adverse cardiovascular outcomes. Large-scale, multi-institutional, prospective studies or registry analysis of long-term outcomes of PCI in patients with cirrhosis are needed to provide additional guidance to practice.
Supplemental Material
Supplemental material, cic_901491 for One-Year Outcomes of Percutaneous Coronary Intervention in Patients with End-Stage Liver Disease by Daniel Y Lu, Matthew D Saybolt, Daniel H Kiss, William H Matthai, Kimberly A Forde, Jay Giri and Robert L Wilensky in Clinical Medicine Insights: Cardiology
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conception, design, data analysis, and drafting of the manuscript were performed by DYL, MDS, and RLW. Chart review was performed by DYL and MDS. Critical revision of the manuscript and final approval were performed by all listed authors.
ORCID iD: Daniel Y Lu  https://orcid.org/0000-0001-9824-3956
https://orcid.org/0000-0001-9824-3956
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Singh V, Patel NJ, Rodriguez AP, et al. Percutaneous coronary intervention in patients with end-stage liver disease. Am J Cardiol. 2016;117:1729-1734. [DOI] [PubMed] [Google Scholar]
- 2. Tiukinhoy-Laing SD, Rossi JS, Bayram M, et al. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am J Cardiol. 2006;98:178-181. [DOI] [PubMed] [Google Scholar]
- 3. Ehtisham J, Altieri M, Salame E, Saloux E, Ollivier I, Hamon M. Coronary artery disease in orthotopic liver transplantation: pretransplant assessment and management. Liver Transpl. 2010;16:550-557. [DOI] [PubMed] [Google Scholar]
- 4. Azarbal B, Poommipanit P, Arbit B, et al. Feasibility and safety of percutaneous coronary intervention in patients with end-stage liver disease referred for liver transplantation. Liver Transpl. 2011;17:809-813. [DOI] [PubMed] [Google Scholar]
- 5. Keeffe BG, Valantine H, Keeffe EB. Detection and treatment of coronary artery disease in liver transplant candidates. Liver Transpl. 2001;7:755-761. [DOI] [PubMed] [Google Scholar]
- 6. Ali A, Bhardwaj HL, Heuman DM, Jovin IS. Coronary events in patients undergoing orthotopic liver transplantation: perioperative evaluation and management. Clin Transplant. 2013;27:E207-E215. [DOI] [PubMed] [Google Scholar]
- 7. Carey WD, Dumot JA, Pimentel RR, et al. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation. 1995;59:859-864. [PubMed] [Google Scholar]
- 8. Edens MA, Kuipers F, Stolk RP. Non-alcoholic fatty liver disease is associated with cardiovascular disease risk markers. Obes Rev. 2009;10:412-419. [DOI] [PubMed] [Google Scholar]
- 9. Kadayifci A, Tan V, Ursell PC, Merriman RB, Bass NM. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: a comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J Hepatol. 2008;49:595-599. [DOI] [PubMed] [Google Scholar]
- 10. Csikesz NG, Nguyen LN, Tseng JF, Shah SA. Nationwide volume and mortality after elective surgery in cirrhotic patients. J Am Coll Surg. 2009;208:96-103. [DOI] [PubMed] [Google Scholar]
- 11. Filsoufi F, Salzberg SP, Rahmanian PB, et al. Early and late outcome of cardiac surgery in patients with liver cirrhosis. Liver Transpl. 2007;13:990-995. [DOI] [PubMed] [Google Scholar]
- 12. Suman A, Barnes DS, Zein NN, Levinthal GN, Connor JT, Carey WD. Predicting outcome after cardiac surgery in patients with cirrhosis: a comparison of Child-Pugh and MELD scores. Clin Gastroenterol Hepatol. 2004;2:719-723. [DOI] [PubMed] [Google Scholar]
- 13. Shaheen AA, Kaplan GG, Hubbard JN, Myers RP. Morbidity and mortality following coronary artery bypass graft surgery in patients with cirrhosis: a population-based study. Liver Int. 2009;29:1141-1151. [DOI] [PubMed] [Google Scholar]
- 14. Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165. [DOI] [PubMed] [Google Scholar]
- 15. Lentine KL, Costa SP, Weir MR, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2012;60:434-480. [DOI] [PubMed] [Google Scholar]
- 16. Diedrich DA, Findlay JY, Harrison BA, Rosen CB. Influence of coronary artery disease on outcomes after liver transplantation. Transplant Proc. 2008;40:3554-3557. [DOI] [PubMed] [Google Scholar]
- 17. Plotkin JS, Scott VL, Pinna A, Dobsch BP, De Wolf AM, Kang Y. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg. 1996;2:426-430. [DOI] [PubMed] [Google Scholar]
- 18. Sharma M, Yong C, Majure D, et al. Safety of cardiac catheterization in patients with end-stage liver disease awaiting liver transplantation. Am J Cardiol. 2009;103:742-746. [DOI] [PubMed] [Google Scholar]
- 19. Jacobs E, Singh V, Damluji A, et al. Safety of transradial cardiac catheterization in patients with end-stage liver disease. Catheter Cardiovasc Interv. 2014;83:360-366. [DOI] [PubMed] [Google Scholar]
- 20. Pillarisetti J, Patel P, Duthuluru S, et al. Cardiac catheterization in patients with end-stage liver disease: safety and outcomes. Catheter Cardiovasc Interv. 2011;77:45-48. [DOI] [PubMed] [Google Scholar]
- 21. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747. [DOI] [PubMed] [Google Scholar]
- 22. Yong CM, Sharma M, Ochoa V, et al. Multivessel coronary artery disease predicts mortality, length of stay, and pressor requirements after liver transplantation. Liver Transpl. 2010;16:1242-1248. [DOI] [PubMed] [Google Scholar]
- 23. Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-1516. [DOI] [PubMed] [Google Scholar]
- 24. Marui A, Kimura T, Tanaka S, et al. Coronary revascularization in patients with liver cirrhosis. Ann Thorac Surg. 2011;91:1393-1399. [DOI] [PubMed] [Google Scholar]
- 25. Turan Y. The nonalcoholic fatty liver disease fibrosis score is related to epicardial fat thickness and complexity of coronary artery disease. Angiology. 2020;71:77-82. [DOI] [PubMed] [Google Scholar]
- 26. Wray C, Scovotti JC, Tobis J, et al. Liver transplantation outcome in patients with angiographically proven coronary artery disease: a multi-institutional study. Am J Transplant. 2013;13:184-191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, cic_901491 for One-Year Outcomes of Percutaneous Coronary Intervention in Patients with End-Stage Liver Disease by Daniel Y Lu, Matthew D Saybolt, Daniel H Kiss, William H Matthai, Kimberly A Forde, Jay Giri and Robert L Wilensky in Clinical Medicine Insights: Cardiology


