The pulmonary immune response protects healthy individuals against Pneumocystis pneumonia (PcP). However, the immune response also drives immunopathogenesis in patients who develop severe PcP, and it is generally accepted that optimal treatment requires combination strategies that promote fungal killing and also provide effective immunomodulation. The anti-inflammatory drug sulfasalazine programs macrophages for enhanced Pneumocystis phagocytosis and also suppresses PcP-related immunopathogenesis.
KEYWORDS: Pneumocystis, antifungal therapy, immunomodulation, immunopathogenesis, pulmonary infection, respiratory pathogens
ABSTRACT
The pulmonary immune response protects healthy individuals against Pneumocystis pneumonia (PcP). However, the immune response also drives immunopathogenesis in patients who develop severe PcP, and it is generally accepted that optimal treatment requires combination strategies that promote fungal killing and also provide effective immunomodulation. The anti-inflammatory drug sulfasalazine programs macrophages for enhanced Pneumocystis phagocytosis and also suppresses PcP-related immunopathogenesis. Anti-Pneumocystis antibody opsonizes Pneumocystis organisms for greater phagocytosis and may also mask antigens that drive immunopathogenesis. Thus, we hypothesized that combining antibody and sulfasalazine would have the dual benefit of enhancing fungal clearance while dampening immunopathogenesis and allow the rescue of severe PcP. To model a clinically relevant treatment scenario in mice, therapeutic interventions were withheld until clear symptoms of pneumonia were evident. When administered individually, both passive antibody and sulfasalazine improved pulmonary function and enhanced Pneumocystis clearance to similar degrees. However, combination treatment with antibody and sulfasalazine produced a more rapid improvement, with recovery of body weight, a dramatic improvement in pulmonary function, reduced lung inflammation, and the rapid clearance of the Pneumocystis organisms. Accelerated fungal clearance in the combination treatment group was associated with a significant increase in macrophage phagocytosis of Pneumocystis. Both passive antibody and sulfasalazine resulted in the suppression of Th1 cytokines and a marked increase in lung macrophages displaying an alternatively activated phenotype, which were enhanced by combination treatment. Our data support the concept that passive antibody and sulfasalazine could be an effective and specific adjunctive therapy for PcP, with the potential to accelerate fungal clearance while attenuating PcP-associated immunopathogenesis.
INTRODUCTION
Pneumocystis jirovecii is an opportunistic pathogen that causes life-threatening pneumonia (Pneumocystis pneumonia [PcP]) in immunocompromised patients. The morbidity and mortality secondary to PcP are significant when one considers that, according to the Centers for Disease Control and Prevention (CDC), the incidence of PcP is 9% for hospitalized patients with HIV/AIDS and 1% for solid-organ transplant patients, with an overall incidence of 40 cases per 1,000 person-years in these populations in the United States (1). With the increasing utilization of immunomodulatory agents, the pool of patients at risk of developing PcP will likely increase (2). For example, soon after the introduction of rituximab, a monoclonal antibody (MAb) that targets the CD20 antigen on B lymphocytes, reports of PcP associated with B cell depletion began to appear in the literature (3). Even though there are established treatments for PcP, mortality remains high and has changed little in the past several decades (1, 4). A major contributor to the pathogenesis of PcP that leads to respiratory failure and, ultimately, death is the immune response elicited by this fungal pathogen (5–9), and we hypothesize that effective control of immunopathogenesis is the critical factor that current treatment regimens fail to adequately address. Therapies that effectively target PcP-related immunopathogenesis are likely to be a necessary feature of treatments able to reduce the persistently high mortality rates among patients that develop severe PcP. Current treatments involve the use of high-dose corticosteroids (CS) for the purpose of suppressing immunopathogenesis. However, CS do not always provide a benefit for PcP patients, and their effectiveness remains unclear (10–14). Given the pleiotropic effects of CS, it seems likely that off-target effects may counteract some of the expected anti-inflammatory benefits during PcP and that more specific targeting of PcP-related immunopathogenesis, such as with specific antibody, would improve clinical outcomes.
Alveolar macrophages are the main effector cells for the removal of Pneumocystis from the lungs and also regulate pulmonary inflammation and lung repair (15–17). Thus, we hypothesized that targeting macrophage function would enhance fungal clearance while simultaneously removing the antigenic stimulus that drives immunopathogenesis. Studies of macrophage biology have demonstrated them to be complex cells whose function varies based on phenotype. Classically activated macrophages (CAMs), or M1 macrophages, have an inflammatory phenotype in response to exposure to lipopolysaccharide (LPS) and interferon gamma (IFN-γ). In contrast, alternatively activated macrophages (AAMs), or M2 macrophages, are proresolution and/or anti-inflammatory and can be programmed via multiple mechanisms, including exposure to interleukin-4 (IL-4) and IL-13 or antigen-antibody immune complexes (18, 19). Importantly, M2 macrophages appear to be potent effector cells for Pneumocystis killing (15, 20, 21) but are not absolutely necessary to eradicate Pneumocystis (22).
The opsonization of microorganisms facilitates the recognition and clearance of pathogens by phagocytes. Different classes of proteins act as specific or nonspecific opsonins. The role of opsonins in the clearance of fungi has not been well studied; however, there is some experimental support for their importance. For example, the fungal pathogen Candida albicans was shown to be more efficiently phagocytosed in the presence of mannose-binding lectin (MBL) than under conditions when the opsonin MBL was absent (23). Two opsonins shown to affect the clearance of Pneumocystis are complement and antibody (24, 25). Standardized assays to measure the phagocytosis of Pneumocystis have only recently been developed, and as a result, there is only limited experimental support for antibody acting in concert with macrophages to clear Pneumocystis (15, 24–26). In addition to promoting Pneumocystis clearance through opsonization, anti-Pneumocystis antibody may also provide a benefit during PcP treatment by masking or removing the Pneumocystis antigens that drive immunopathogenesis. Furthermore, antibody has also been shown to have nonspecific immunomodulatory effects, as exemplified by its use in diseases like idiopathic thrombocytopenic purpura or Kawasaki disease (27–32). This effect of antibody or immunoglobulin (Ig) might prove valuable in the management of PcP because of the prominent immunopathogenic component.
We have previously reported that passive antibody provides protection against PcP when used prophylactically (25, 33, 34). Similarly, sulfasalazine (SSZ) also appears to be effective for prophylaxis against Pneumocystis in mice and humans (15, 35–37). However, the potential utility of passive antibody or SSZ as treatment options for moderate to severe PcP has not been evaluated. Both antibody and SSZ have the potential to enhance Pneumocystis clearance while suppressing inflammation and PcP-related immunopathogenesis. We report here that combination treatment with passive antibody and SSZ induces a dramatic recovery of mice suffering from severe PcP, which is characterized by a shift in macrophage polarization, increased macrophage-mediated phagocytosis of Pneumocystis, and reduced immunopathogenesis.
RESULTS
Combination treatment with anti-Pneumocystis antibody and SSZ produces dramatic recovery from severe PcP.
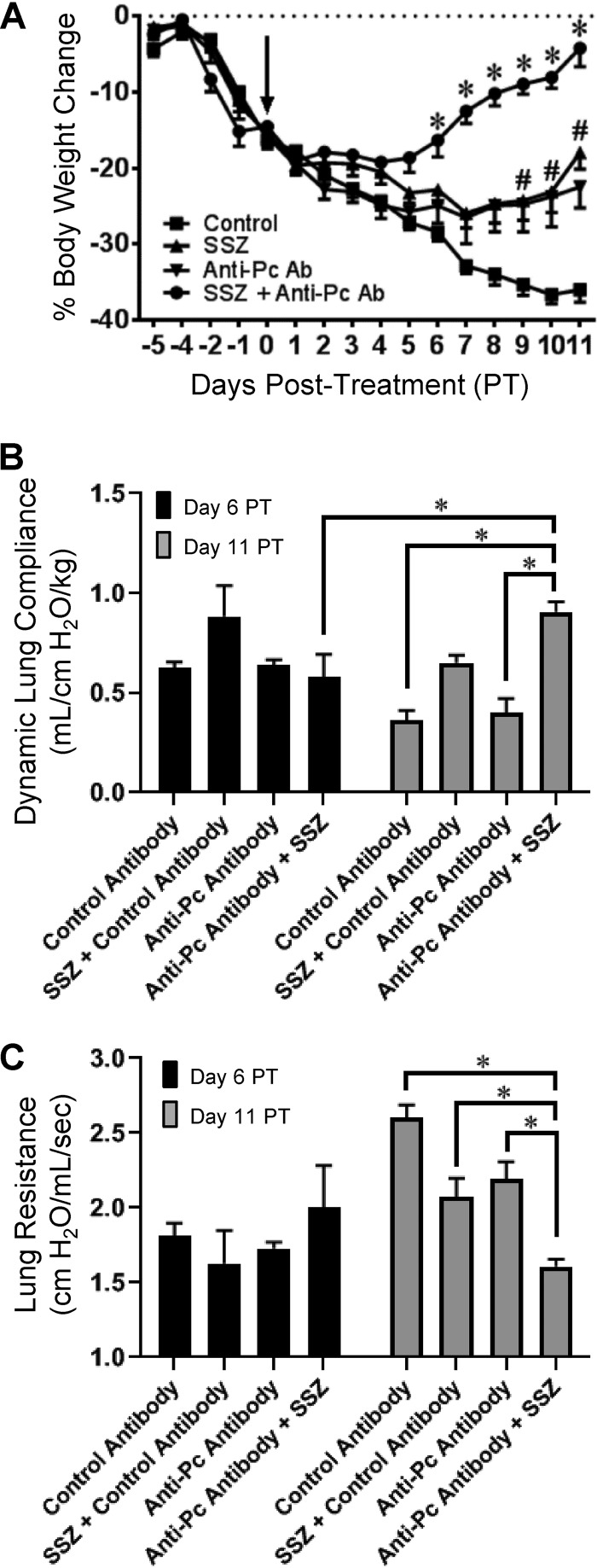
Mice displaying obvious clinical signs of PcP-related immune reconstitution inflammatory syndrome (IRIS), including a greater than 10% body weight loss and a greater than 25% increase in respiratory rate, were treated with nonspecific control antibody, passive anti-Pneumocystis antibody, SSZ, or a combination of anti-Pneumocystis antibody plus SSZ. As expected, control mice displayed characteristic symptoms of severe PcP over the course of the study, including dramatic weight loss, reduced lung compliance, and increased lung resistance (Fig. 1A to C). However, mice treated individually with Pneumocystis-specific passive antibody or SSZ demonstrated enhanced recovery from PcP compared to control untreated mice. The improvement was clearly demonstrated by an accelerated recovery of body weight (Fig. 1A). Furthermore, the beneficial effects of the individual treatments were markedly enhanced by combination treatment with both specific anti-Pneumocystis antibody and sulfasalazine. In contrast to untreated mice with PcP, mice treated with the combination therapy began to recover weight by day 6 posttreatment and had nearly completely recovered the lost body weight by day 11 posttreatment (Fig. 1A). None of the experimental groups showed a statistically significant change in pulmonary function measurements at day 6 posttreatment. However, by day 11 posttreatment, dramatic improvements in pulmonary function measurements were observed in the combination treatment group relative to the measurements for control mice with PcP. The combination therapy group had a greater than 39% higher lung compliance and 62% reduced lung resistance compared to results for untreated mice (Fig. 1B and C). In our experience, these pulmonary function measurements have proven to be reliable indicators of the severity of PcP in this model, thereby lending physiological significance to the observed results. These data demonstrate that, when used as a treatment for severe PcP, anti-Pneumocystis antibody plus SSZ induces a rapid recovery from the severe respiratory impairment associated with PcP.
FIG 1.
Accelerated recovery from PcP in mice treated with anti-Pneumocystis (anti-Pc) antibody, SSZ, or a combination of both. Pneumocystis-infected SCID mice were immune reconstituted with wild-type splenocytes. (A) Treatments were started at 8 days postreconstitution (arrow), when the mice displayed obvious signs of PCP (a >10% body weight loss and a >25% increase in respiratory rate). Body weight loss was used to noninvasively monitor the progression of disease following treatment. For body weight loss, values are the mean ± 1 standard error measurement (SEM). *, P < 0.05 for mice in the SSZ plus anti-Pneumocystis antibody (Ab) combination treatment group compared to the results for mice in the control treatment group at the same time; #, P < 0.05 for mice in the SSZ or anti-Pneumocystis antibody treatment group compared to the results for mice in the control treatment group at the same time. For time points up to and including day 6 posttreatment (PT), data are for ≥11 mice per group. For time points later than day 6 posttreatment, data are for ≥6 mice per group. (B and C) Pulmonary function, as assessed by dynamic lung compliance (B) and lung resistance (C), was measured at 6 and 11 days posttreatment. Values are the mean ± 1 standard error measurement. *, P < 0.05 between groups, as indicated. At the day 6 time point, data are for 4 mice per group. At the day 11 time point, data are for 5 to 7 mice per group.
Anti-Pneumocystis antibody and SSZ accelerate fungal clearance.
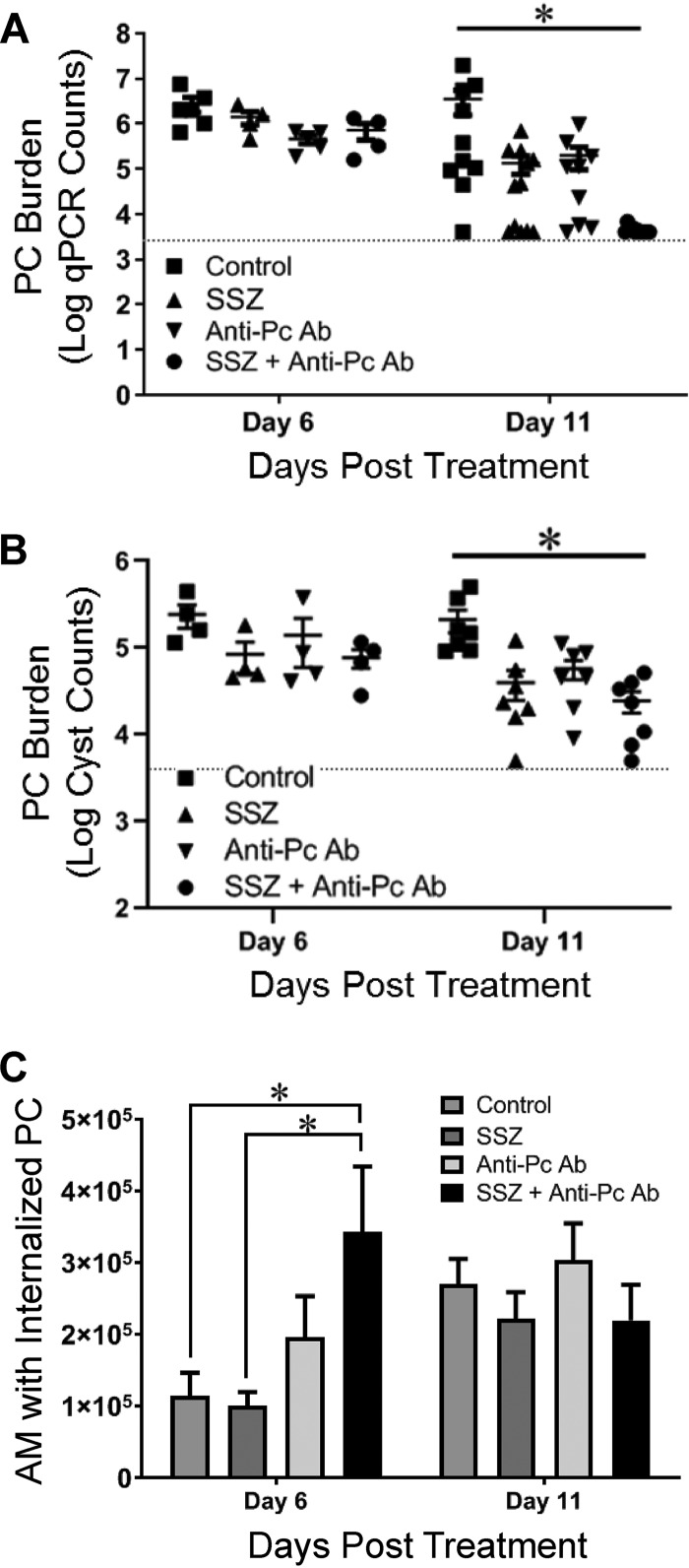
To determine whether anti-Pneumocystis antibody or SSZ promoted Pneumocystis clearance from the lungs during a clinical treatment scenario, total fungal lung burdens were determined by quantitative real-time PCR (qPCR) (38), and lung cyst burdens were determined by Gomori’s methenamine silver (GMS) staining, as described previously (39). At day 6 posttreatment, the anti-Pneumocystis antibody, SSZ, and combination treatment groups had a trend toward fewer total organisms and cyst forms than control mice, but the differences were not statistically significant (Fig. 2A and B). However, by day 11 posttreatment the anti-Pneumocystis antibody- and SSZ-treated groups had a 1- to 2-log reduction in the total lung Pneumocystis burden relative to the control group (Fig. 2A). Furthermore, the anti-Pneumocystis antibody-plus-SSZ combination treatment group displayed a greater than 3-log average reduction in total lung Pneumocystis burden compared to that for the untreated control mice with PcP (Fig. 2A). Several mice in the combination treatment group had an undetectable Pneumocystis burden, while the burden in the remaining mice was very near the limit of detection, indicating the nearly complete clearance of Pneumocystis in the combination treatment group. A similar pattern of accelerated clearance was found when the Pneumocystis burden was assessed by the use of microscopic cyst counts, although the cyst counts were lower than the total Pneumocystis burden determined by qPCR (Fig. 2B). These results demonstrate that anti-Pneumocystis antibody plus SSZ promotes the rapid clearance of an established Pneumocystis infection.
FIG 2.
Accelerated alveolar macrophage (AM)-mediated fungal clearance in anti-Pneumocystis antibody- and SSZ-treated mice. (A and B) The lung Pneumocystis (PC) burden in experimental mice was measured at 6 and 11 days posttreatment by two distinct methods: quantitative real-time PCR (qPCR) of the single-copy Pneumocystis kexin gene in lung homogenates (A) and direct Gomori’s methenamine silver staining of cysts in lung homogenate preparations (B). The limit of detection for each assay is indicated by a dotted line. Values are the mean ± 1 SEM. *, P < 0.05 between the control group and the SSZ-plus-anti-Pneumocystis antibody-treated group. At the day 6 time point, data are for 4 mice per group. At the day 11 time point, data are for ≥10 mice per group for qPCR analyses and 7 mice per group for cyst counts. (C) Macrophage phagocytosis of Pneumocystis was quantified by multispectral imaging flow cytometry, as we have described previously (15). Values are the mean ± 1 SEM. *, P < 0.05 between the indicated groups. At the day 6 time point, data are for 4 mice per group. At the day 11 time point, data are for 5 to 7 per group.
Accelerated Pneumocystis clearance in mice treated with anti-Pneumocystis antibody plus SSZ is associated with the earlier induction of macrophage phagocytosis.
Based on prior work, we hypothesized that anti-Pneumocystis antibody and SSZ would accelerate the kinetics of Pneumocystis clearance by promoting lung macrophage phagocytosis of Pneumocystis. To quantify Pneumocystis phagocytosis by macrophages, we used an imaging flow cytometer-based method that we previously described (15). Mice with PcP treated with either anti-Pneumocystis antibody or SSZ did not show a significant increase in macrophage phagocytosis of Pneumocystis at day 6 posttreatment compared to results for control mice (Fig. 2C). However, mice with PcP treated with the combination of anti-Pneumocystis antibody plus SSZ showed a 3-fold increase in the number of lung macrophages with ingested Pneumocystis organisms at this time (Fig. 2C). There was no difference between the groups at day 11 posttreatment. All control mice with PcP and mice treated with either anti-Pneumocystis antibody or SSZ showed an increase in the number of macrophages with ingested Pneumocystis between day 6 and day 11 posttreatment. In contrast, mice with PcP treated with the combination of anti-Pneumocystis antibody plus SSZ showed a decrease in the number of macrophages with ingested Pneumocystis over this time (Fig. 2C). This was likely due to the high rate of Pneumocystis clearance in this group, which left fewer Pneumocystis organisms in the lungs by day 11. These data indicate that an early enhancement of macrophage phagocytosis of Pneumocystis leads to the accelerated fungal clearance observed in the anti-Pneumocystis antibody-plus-SSZ-treated group.
Anti-Pneumocystis antibody and SSZ alter the lung cytokine environment.
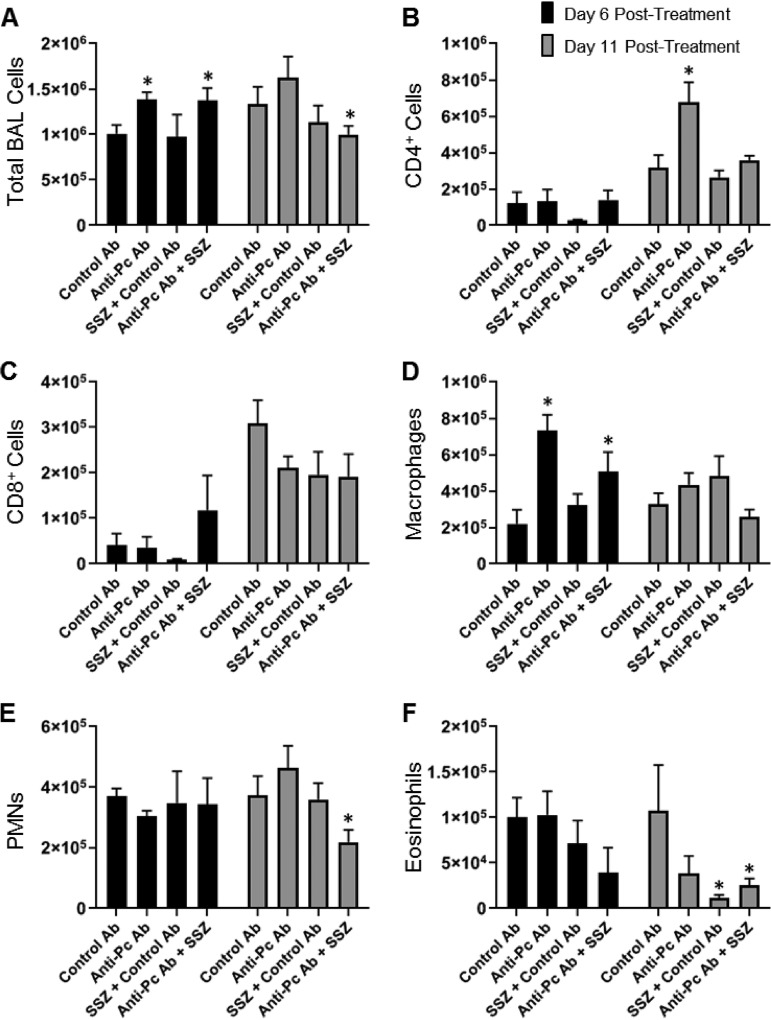
To determine whether the combination of anti-Pneumocystis antibody and SSZ improves PcP by reducing the number of immune cells in the lungs, cells were recovered from the bronchoalveolar lavage (BAL) fluid of experimental mice. At day 6 posttreatment, few significant differences in the number of BAL fluid cells recovered were noted between the treatment groups (Fig. 3). However, there was a significant increase in the total number of BAL fluid cells recovered from the anti-Pneumocystis antibody and anti-Pneumocystis antibody plus SSZ groups at this time (Fig. 3A). The increase in the total number of cells in the anti-Pneumocystis antibody-treated groups was almost entirely attributed to an increased number of macrophages in these groups (Fig. 3D). No differences in the number of BAL fluid total lymphocytes, polymorphonuclear leukocytes (PMNs), CD4+ T cells, or CD8+ T cells were observed at this time (Fig. 3). In contrast, by day 11 posttreatment, the mice treated with anti-Pneumocystis antibody plus SSZ had fewer total BAL fluid cells than control mice (Fig. 3A). The reduced number of total BAL fluid cells recovered was almost entirely accounted for by fewer PMNs in the BAL fluid of anti-Pneumocystis antibody-plus-SSZ-treated mice than in the BAL fluid of the controls (Fig. 3E). Thus, with the exception of PMNs, we found few differences in the cellular composition of the alveoli in treated and untreated mice that would explain the beneficial effects of anti-Pneumocystis antibody plus SSZ. We have previously reported that PMNs do not contribute significantly to PcP-related immunopathogenesis but are faithful markers of the severity of PcP (40). Thus, the reduced numbers of PMNs in the anti-Pneumocystis antibody-plus-SSZ-treated group support our findings of improved lung function and less severe disease in the combination treatment group.
FIG 3.
Effect of anti-Pneumocystis antibody and SSZ treatment on lung immune cell populations during PcP. Bronchoalveolar lavage was performed on experimental mice at 6 and 11 days postreconstitution. Total cells (A), CD4+ T cells (B), CD8+ T cells (C), macrophages (D), PMNs (E), and eosinophils (F) were enumerated by Diff-Quik staining and flow cytometry. Values are the mean ± 1 SEM. *, P < 0.05 between the indicated group and the control group at the same time point. At the day 6 time point, data are for 4 mice per group. At the day 11 time point, data are for 5 to 7 mice per group.
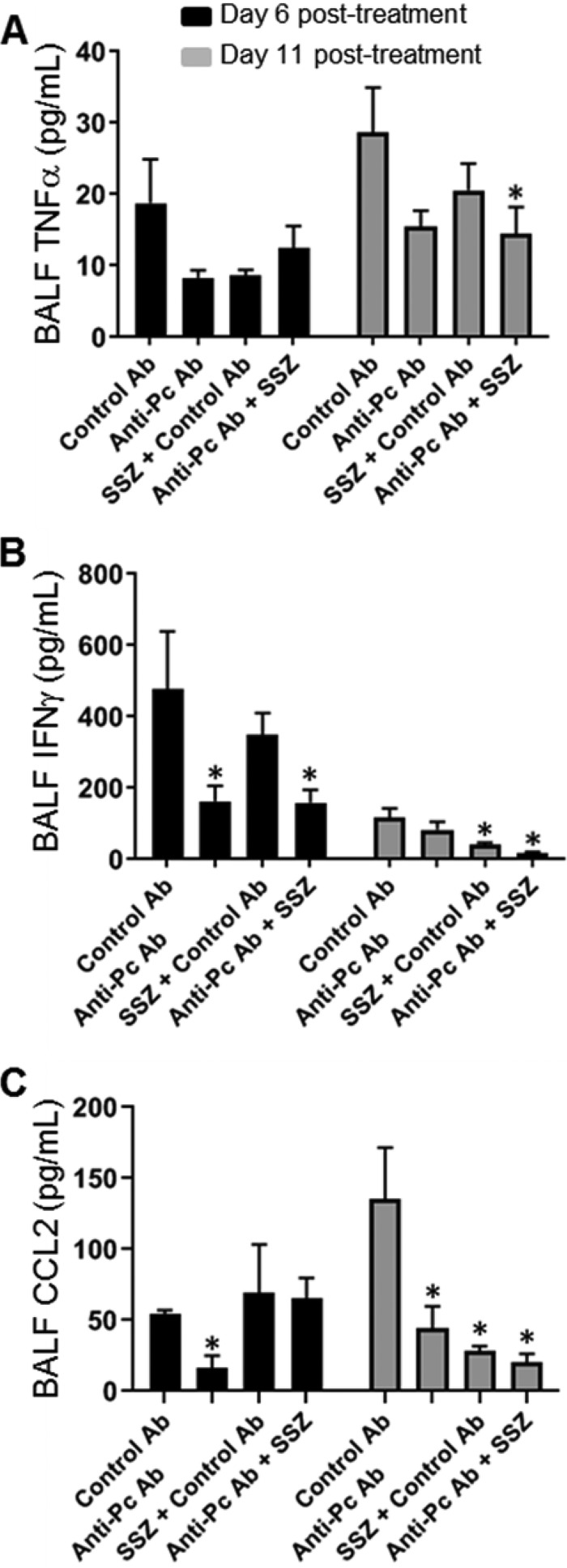
It is likely that the lack of change in immune cell numbers over the short course of treatment is related to the fact that we withheld the initiation of treatment until after the animals were already experiencing the immunopathological consequences of PcP. Therefore, we hypothesized that the beneficial effects of anti-Pneumocystis antibody plus SSZ were related to immune modulation, rather than a reduction in immune cell numbers in the lung. Several cytokines and chemokines known to be involved in the host immune response to PcP were measured in the BAL fluid of experimental mice. Tumor necrosis factor alpha (TNF-α) is an important proinflammatory cytokine with a direct role in promoting PcP-related immunopathogenesis (38, 41). By day 11 posttreatment, anti-Pneumocystis antibody-plus-SSZ-treated mice demonstrated a 50% reduction in the amount of TNF-α in the lung compared to that in untreated mice, which coincided with the better lung function in this group (Fig. 4A). Elevated lung levels of the Th1 cytokine IFN-γ are also associated with PcP-related immunopathogenesis (8, 15). IFN-γ levels were significantly reduced at both day 6 and day 11 posttreatment in the anti-Pneumocystis antibody-plus-SSZ-treated group compared to that in the untreated control group with PcP (Fig. 4B). IL-4 and IL-5 levels in the BAL fluid of experimental mice were also measured, but no differences were found (data not shown). CCL2 is a chemokine that regulates monocyte/macrophage recruitment and polarization. CCL2 is produced by lung epithelial cells during Pneumocystis infection (42–44) and is associated with PcP-related immunopathogenesis (15, 42). CCL2 levels were significantly reduced in all animals treated with anti-Pneumocystis antibody and/or SSZ at 11 days posttreatment (Fig. 4C).
FIG 4.
Effect of anti-Pneumocystis antibody and SSZ treatment on lung Th1 cytokine levels during PcP. Bronchoalveolar lavage (BAL) was performed on experimental mice at 6 and 11 days posttreatment. TNF-α, IFN-γ, and CCL2 levels were measured in BAL fluid (BALF) by ELISA. Values are the mean ± 1 SEM. *, P < 0.05 between the indicated group and the control group at the same time point. At the day 6 time point, data are for 4 mice per group. At the day 11 time point, data are for 6 to 10 mice per group.
Anti-Pneumocystis antibody and SSZ promote an M2 macrophage phenotype.
The finding that the Th1 cytokines IFN-γ and TNF-α were downregulated by anti-Pneumocystis antibody plus SSZ treatment indicated that suppression of the Th1 response may be beneficial for recovery from PcP. Polarization status regulates the antifungal effects of macrophages, and prior studies have indicated that M2 macrophages are effectors for Pneumocystis clearance and may also dampen lung inflammation. To test the effect of anti-Pneumocystis antibody and SSZ on the macrophage phenotype in the environment of the Pneumocystis-infected lung, we administered these agents or control nonspecific antibody to mice with obvious signs of PcP-related IRIS and harvested alveolar macrophages 3 days later. Administration of sulfasalazine resulted in a 16-fold increase in the M2/M1 macrophage ratio in the alveolar space compared to that in control mice with PcP, while administration of anti-Pneumocystis antibody resulted in an approximately 33-fold increase in the M2/M1 ratio (Table 1). Combination treatment with anti-Pneumocystis antibody and SSZ resulted in an even more pronounced shift in the macrophage phenotype, demonstrating a 47-fold increase in the M2/M1 ratio present in the lung compared to that in control mice with PcP. The beneficial effects of anti-Pneumocystis antibody and SSZ on PcP in a treatment scenario are associated with a reduction in lung Th1 cytokine levels and an increase in M2 macrophage polarization.
TABLE 1.
Effect of administration of anti-Pneumocystis antibody or sulfasalazine on the ratio of alveolar macrophages of the M2 to the M1 phenotype in the lung
| Treatment | Phenotype (no. of macrophagesa) |
Ratio |
||
|---|---|---|---|---|
| M1 | M2 | M2/M1 | Fold increaseb | |
| Control antibody | 290 | 10 | 0.03 | 1 |
| SSZ | 200 | 100 | 0.5 | 16.6 |
| Anti-Pneumocystis antibody | 149 | 151 | 0.99 | 33.0 |
| SSZ + anti-Pneumocystis antibody | 125 | 175 | 1.4 | 46.6 |
Number of macrophages per 300 macrophages counted.
Fold increase in the M2/M1 ratio compared to that achieved with the control antibody.
DISCUSSION
PcP is characterized by severe hypoxia and respiratory failure, and it is now clear that immune-mediated inflammatory lung injury is a major component of the pathogenesis of PcP (5, 6, 9, 15, 45). Despite the availability of effective antibiotics, the mortality rates among patients developing severe PcP remain high, likely due to the inability to adequately control immunopathogenesis during treatment. Corticosteroids are typically used to suppress inflammation, but recent studies suggest that the broadly acting agents do not provide a significant benefit to patients (10–14). Therefore, alternative strategies to suppress immunopathogenesis while eradicating the infection are plainly needed. Our prior studies found that the immunomodulatory drug sulfasalazine dramatically reduces the severity of PcP if it is administered before the onset of the pathogenic pulmonary immune response (15). Unexpectedly, SSZ accelerated the clearance of Pneumocystis through enhancement of the macrophage-mediated phagocytosis of Pneumocystis. We have also reported that passively administered anti-Pneumocystis antibody protects immunodeficient mice against Pneumocystis infection (24). Although these agents have been used prophylactically to prevent the onset of complications of PcP, they have not been tested in treatment scenarios. In the current study, we found that when used as a treatment for active PcP, the combination of anti-Pneumocystis antibody and SSZ reduced the severity of PcP-related respiratory impairment, accelerated Pneumocystis clearance, and promoted recovery. These findings indicate that appropriate immunomodulation has the potential to improve outcomes in patients with PcP.
In our prior studies of PcP-related immunopathogenesis, we found that depleting specific T cell subsets or blocking T cell recruitment before the onset of the Pneumocystis-driven immune response was highly effective for preventing immunopathogenesis. However, the suppression of immunopathogenesis in mice treated with anti-Pneumocystis antibody plus SSZ after the onset of PcP was not associated with a drastic reduction in T cell numbers in the lung (Fig. 3). The likely explanation for this finding is that we initiated treatment after the pathogenic immune response was already ongoing. Thus, we suspect that passive antibody and SSZ did not act by reducing the magnitude of the T cell population participating in the pulmonary immune response but did so by modulating it in a manner that promoted fungal clearance while suppressing lung injury. In support of this conclusion, we found significantly lower levels of the Th1 cytokines TNF-α and IFN-γ in the lungs of anti-Pneumocystis antibody-plus-SSZ-treated mice with PcP than in those of the control mice. The reduced levels of Th1 cytokines coincided with an increase in the proportion of alternatively activated M2 macrophages in the lungs of mice treated with anti-Pneumocystis antibody and/or SSZ. It is now recognized that macrophages can vary in their biological characteristics based on their mode of activation. CAMs, or M1 macrophages, are proinflammatory and are polarized by exposure to LPS, TNF-α, and IFN-γ. AAMs, or M2 macrophages, exhibit anti-inflammatory and proresolution activity and can be polarized by exposure to IL-4/IL-13 or antigen-antibody complexes (18, 19). In addition, recent studies have indicated that M2 macrophages promote the clearance of Pneumocystis from the lungs (15, 16, 20). Thus, treatments that bring about a shift in the macrophage phenotype to an M2 or anti-inflammatory phenotype would be expected to benefit PcP treatment by suppressing inflammation and immunopathogenesis while enhancing fungal clearance, as we have now reported for combination treatment with anti-Pneumocystis antibody plus SSZ treatment.
The precise role for antibody in the immune response to fungal infections is incompletely understood. We hypothesize that the beneficial effects of passive antibody for the treatment of PcP are likely multifaceted. Antibody likely masks immunopathogenic epitopes and clears the Pneumocystis antigens that drive PcP immunopathogenesis, while also opsonizing Pneumocystis for enhanced fungal clearance. Furthermore, the presentation of antigen complexed with antibody results in the activation of macrophage M2 polarization pathways, which may add to the beneficial effects of the antibody by increasing phagocytic potential and initiating anti-inflammatory programs. We have previously shown that SSZ enhances antibody-independent macrophage phagocytosis of Pneumocystis (15). We suspect that the addition of specific antibody further enhances Pneumocystis phagocytosis by SSZ-stimulated lung macrophages, explaining the accelerated clearance in combination-treated mice. Indeed, we found that the combination of anti-Pneumocystis antibody plus SSZ enhances macrophage phagocytosis of Pneumocystis in the lung (Fig. 2). Specific antifungal antibodies have been shown to increase phagocytosis in a model of Candida fungal infection (46). It seems likely that specific anti-Pneumocystis antibodies help opsonize Pneumocystis in a similar manner and that this effect is enhanced with the addition of sulfasalazine. In our model, we waited for the Pneumocystis infection to create significant illness prior to starting treatment. For cryptococcal fungal infections, there has been demonstration of a modified outcome with passive administration of specific fungal antibodies to capsular glucuronoxylomannan prior to infection (47, 48). In that experiment, monoclonal antibodies were given up to 24 h prior to infection, and mice that received antibody had a lower fungal burden. Another study from the same group evaluated more specific groups of anticryptococcal antibodies and found that passive administration prior to infection decreased the fungal burden, except for a few antibodies that led to worse infection with increased mortality (49). In the same study, C3 complement-deficient mice had improved protection with specific anticryptococcal antibodies compared to mice with normal complement, demonstrating the role that complement plays with antibody for protection against some fungal pathogens. This was, in fact, what we observed. While both specific anti-Pneumocystis antibody and SSZ produced some treatment benefit in mice with PcP, the combination of the two treatment modalities showed a much more pronounced effect. That the benefit noted in our experiment was due to the effect of specific anti-Pneumocystis antibodies and not to some nonspecific immune-modulatory effect from nonspecific high-dose immune globulin is supported by the observation that the irrelevant antibody-treated mice in the control group displayed none of the beneficial effects noted in the specific anti-Pneumocystis antibody-treated group. An important feature of our experimental approach is that we waited for the mice with PcP infection to become clinically symptomatic. This more closely mimics how patients with PcP present for medical care. Additional studies will be necessary to determine whether there is a relationship between the specific antigen or antigens targeted for opsonization and the beneficial effects that we observed.
In a previous study, we demonstrated that sulfasalazine reduced PcP-related immunopathogenesis, while it enhanced macrophage phagocytosis of Pneumocystis (15). SSZ has been shown to exert immunomodulatory effects via the blockade of inflammatory transcription factor nuclear factor kappa B (NF-κB) (50), and NF-κB-dependent proinflammatory responses to Pneumocystis have been reported for both alveolar macrophages and lung epithelial cells (51–53). NF-κB also regulates the polarization program of T cells, B cells, and macrophages, and we hypothesize that NF-κB inhibition is a major mechanism by which SSZ attenuates immunopathogenesis in our treatment model of PcP. It is possible that the additive effects of SSZ on inflammation and pathogen clearance in our model could be caused by the elicitation of a proresolution, M2 phenotype in alveolar macrophages. Although it is still a matter of some debate (54), the ability of macrophages to differentiate into transcriptionally distinct functional states (e.g., M1, M2a, M2b, etc.) in response to different cytokines and other environmental cues is well documented (55, 56). While M1 macrophages are associated with proinflammatory and immune-activating stimuli (i.e., IFN-γ, TNF-α, LPS), alternatively activated (M2) macrophages are skewed toward promoting the resolution of immune responses by producing IL-10 while suppressing proinflammatory mediators like IFN-γ, TNF-α, and IL-12. Interestingly, M2-polarized macrophages have also been reported to show increased levels of the β-glucan receptor Dectin-1 (57, 58), increased complement-mediated phagocytosis (59), and increased phagosomal degradation activity (60). Consistent with this idea, the bioactive metabolite of SSZ, 5-aminosalicylic acid, is an activating ligand for the pro-M2 transcription factor peroxisome proliferator-activated receptor gamma (61). Thus, it is possible that anti-Pneumocystis antibody and SSZ together elicit a specific M2-like program in alveolar macrophages that not only results in the reversal of immunopathogenesis but may also augment the phagocytic clearance of Pneumocystis by alveolar macrophages either directly (via Dectin-1 or other c-type lectins [62–65]) or indirectly (via increased antibody-mediated clearance).
In conclusion, this is the first time that passively administered anti-Pneumocystis antibody plus SSZ has been used to treat mice with existing PcP. This combination treatment achieved a dramatic degree of improvement in the severity of PcP. Further studies need to be completed to evaluate the specific mechanisms by which anti-Pneumocystis antibody and SSZ function in concert to attain this level of rescue. Importantly, the physiological improvement and decreased fungal burden were achieved without standard-of-care antibiotic treatment with trimethoprim-sulfamethoxazole (TMP-SMX). Future studies will need to determine whether the inclusion of TMP-SMX affects this treatment regimen. These findings open the possibility that PcP treatment can be significantly improved with better and more specific immunomodulatory strategies to limit immunopathogenesis and enhance fungal clearance.
MATERIALS AND METHODS
Mouse model of Pneumocystis pneumonia.
Severe combined immune-deficient (SCID) mice on a C.B-17 background were maintained in our specific-pathogen-free colony at the University of Rochester Medical Center. For these studies, 6- to 8-week-old female mice were infected with Pneumocystis by intratracheal (i.t.) inoculation of 1 × 105 Pneumocystis cysts and then also cohoused with other Pneumocystis-infected SCID mice to ensure infection. The mice were fed acidified water and sterile food. Immune reconstitution (IR) was initiated 3 weeks after infection by giving mice an intraperitoneal (i.p.) injection of 5 × 107 splenocytes from wild-type C.B-17 mice as described previously (6, 15). All animals involved were treated ethically according to the guidelines from the Association for Assessment and Accreditation of Laboratory Care International (AAALAC International), and the protocols used were approved by the University of Rochester Committee on Animal Resources.
Anti-Pneumocystis antibody and sulfasalazine administration.
The anti-Pneumocystis antibodies have been developed and characterized by our laboratories (Table 2) (25, 66, 67). We pooled several Pneumocystis-specific IgG and IgM antibodies and injected mice i.p. with this antibody combination (0.1 mg of each antibody per dose) every 3 days after immune reconstitution. Control mice were given an equal amount of nonspecific whole-mouse immunoglobulin purified from naive animals (ChromPure; Jackson ImmunoResearch Laboratories, Inc.). Sulfasalazine (SSZ; Sigma, St. Louis, MO) was prepared fresh daily and given via i.p. injections at a dose of 200 mg/kg of body weight, as described previously (15). Each group within an experiment contained at least 6 mice, and each experiment was replicated 1 to 2 times. For the experiments assessing the effect of treatment with anti-Pneumocystis monoclonal antibody on the macrophage phenotype, control mice received an equivalent amount of a mouse MAb raised against endotoxin or the Haemophilus influenzae type b capsular polysaccharide, both of which were produced in our laboratories.
TABLE 2.
Specific anti-Pneumocystis monoclonal antibody pool
Physiological assessment of pulmonary function.
Lung resistance and compliance were measured on live ventilated mice using a Harvard rodent ventilator (Harvard Apparatus, Holliston, MA) and a whole-body plethysmograph (Buxco Electronics Inc., Wilmington, NC) as previously described (41). Briefly, mice were anesthetized, a tracheostomy was performed, and a 20-gauge cannula was inserted 3 mm into an anterior nick in the exposed trachea. To ensure that the mice survived the procedure, spontaneous respirations were observed before proceeding. The mice were immediately placed into a plethysmograph (Buxco Electronics Inc.) and connected to a Harvard rodent ventilator (Harvard Apparatus). The mice were ventilated with a tidal volume of 0.01 ml/g of body weight at a rate of 150 breaths per minute. Respiratory flow and pressure measurements were taken from the plethysmograph chamber. Dynamic lung compliance was calculated in milliliters per centimeter of H2O from the flow and pressure signals and then normalized for body weight. Resistance values were calculated in centimeters of H2O per milliliter per second from the same signals. Pulmonary function was normalized to body weight. The Biosystems XA software package (Buxco Electronics Inc.) was used to collect and analyze the data.
Determination of Pneumocystis lung burden.
The Pneumocystis lung burden in lung homogenates from mice was assessed using Gomori’s methenamine silver (GMS) staining of cyst forms (39) and real-time quantitative PCR (qPCR) to quantify the total Pneumocystis burden, as described previously (41). Primers specific for the single-copy Pneumocystis kexin gene (68) and a Bio-Rad CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA) were used for qPCR determination of the Pneumocystis burden, as described previously (41).
Cytokine analyses in BAL fluid.
Bronchoalveolar lavage (BAL) was performed on whole lung with four, 1-ml volumes of ice-cold 1× Hanks’ balanced salt solution (HBSS). The concentrations of the cytokines TNF-α, IFN-γ, CCL2, IL-4, IL-5, and IL-17 were measured with DuoSet enzyme-linked immunosorbent assays (ELISAs; R&D, Minneapolis, MN) per the manufacturer’s instructions. The ELISAs were performed on cell-free supernatant from BAL fluid to measure cytokine protein concentrations.
Flow cytometry.
Standard surface flow cytometry was performed for the following markers: CD3, CD4, CD8, CD11c, and GR-1 (BD Biosciences, San Diego, CA). The cellular fractions obtained by bronchoalveolar lavage were incubated with Fc Block (BD Biosciences), followed by incubation with fluorescently labeled antibodies that recognize the markers listed above. Fluorescence-minus-one (FMO) controls were used to set the gates for each antibody stain in the panel. At least 10,000 cells were routinely analyzed for each Pneumocystis-infected mouse, and at least 5,000 cells were analyzed for uninfected control mice. Multispectral imaging flow cytometry (ImageStream; Amnis Corporation) was used as we have described previously (15) to quantify the number of CD11c+ alveolar macrophages in experimental mice that contained internalized Pneumocystis. Internalized Pneumocystis organisms were identified in permeabilized and fixed macrophages using a pool of specific anti-Pneumocystis MAbs created in our laboratories (15). Briefly, the lungs were lavaged, and the recovered cells were washed with phosphate-buffered saline (PBS) and incubated with mouse Fc Block (BD Biosciences). The cells were then surface stained with anti-CD11c-phycoerythrin (clone HL3; BD Biosciences) and washed with PBA (0.5% bovine serum albumin and 0.05% sodium azide in PBS). The cells were permeabilized with BD Cytofix/Cytoperm fixation and permeabilization solution and incubated with a pool of anti-Pneumocystis MAbs (MAbs 4F11, 2B5, 3D6, 1F1, and 1F5). Following a wash step, the cells were incubated with Alexa Fluor 647-conjugated goat anti-mouse IgG (H+L; Invitrogen Molecular Probes, OR). Stained cells were washed, pelleted, and resuspended in 50 μl of ice-cold 1% paraformaldehyde in PBS. Samples were stored at 4°C in the dark until analyzed. At least 20,000 event image files were collected. Alveolar macrophages without internalized Pneumocystis treated in the same manner were used as internal controls to set the threshold for the fluorescence detection of Pneumocystis. In addition, isotype controls were used to confirm the binding specificity of the anti-Pneumocystis antibodies. Data were analyzed using ImageStream data exploration and analysis software (IDEAS; Amnis Corporation).
Effect of antibody on alveolar macrophage phenotype.
Pneumocystis-infected SCID mice underwent immune reconstitution (IR) with normal splenocytes as described above. Groups of mice received nonspecific mouse immunoglobulin, anti-Pneumocystis antibodies, SSZ, or anti-Pneumocystis antibodies plus SSZ. The antibodies were given as described above on days 1 and 3 post-IR, and SSZ was given on days 1, 2, and 3 post-IR. The mice were sacrificed on day 3 postreconstitution, and bronchoalveolar lavage was performed. Aliquots containing 5 × 104 cells were cytospun onto glass slides and fixed with 3% paraformaldehyde in PBS for 15 min at room temperature. The cells were permeabilized with 0.02% Triton X-100 in PBS for 15 min at room temperature, rinsed 3 times with PBS, and then blocked with species-specific 5% normal serum in 0.05% Tween 20 in PBS (PBS-T) for 1 h at room temperature. Anti-inducible nitric oxide synthase (anti-iNOS) antibody (Abcam) and anti-YM-1 antibody (Stemcell) were used to distinguish M1 and M2 macrophages. iNOS was used as a prototypical marker of M1 macrophages, and YM-1 was used as a marker of M2 macrophages (16). DAPI (4′,6-diamidino-2-phenylindole; Molecular Probes) diluted 1:36 in PBS for 5 min at room temperature and then rinsed 3 times in PBS was used to identify macrophage nuclei. Slides were mounted with antifade solution (Vectashield; Vector Laboratories, CA). A Nikon Eclipse E400 fluorescence microscope was used for photomicroscopy. Control alveolar macrophages from uninfected SCID mice were incubated with the same antibodies and used to set the threshold for the fluorescence detection of iNOS and YM-1 in the experimental samples. All photographs for a given protein were taken with identical exposure settings.
Statistical analysis.
Differences between experimental groups were analyzed using analysis of variance with Bonferroni’s multiple-comparison posttest. Differences were considered significant at P values of <0.05. All data were analyzed using GraphPad Prism (version 8) software (GraphPad Software, San Diego, CA). For statistical purposes, the value used for the Pneumocystis burden of animals with zero detectable Pneumocystis organisms either by cyst counts or by qPCR was equivalent to the limit of detection of the assay.
REFERENCES
- 1.Morris A, Lundgren JD, Masur H, Walzer PD, Hanson DL, Frederick T, Huang L, Beard CB, Kaplan JE. 2004. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis 10:1713–1720. doi: 10.3201/eid1010.030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roux A, Gonzalez F, Roux M, Mehrad M, Menotti J, Zahar JR, Tadros VX, Azoulay E, Brillet PY, Vincent F. 2014. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med Mal Infect 44:185–198. doi: 10.1016/j.medmal.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Garrido I, Carmona EM, Specks U, Limper AH. 2013. Pneumocystis pneumonia in patients treated with rituximab. Chest 144:258–265. doi: 10.1378/chest.12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gigliotti F, Wright TW. 2012. Pneumocystis: where does it live? PLoS Pathog 8:e1003025. doi: 10.1371/journal.ppat.1003025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gigliotti F, Wright TW. 2005. Immunopathogenesis of Pneumocystis carinii pneumonia. Expert Rev Mol Med 7:1–16. doi: 10.1017/S1462399405010203. [DOI] [PubMed] [Google Scholar]
- 6.Wright TW, Gigliotti F, Finkelstein JN, McBride JT, An CL, Harmsen AG. 1999. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J Clin Invest 104:1307–1317. doi: 10.1172/JCI6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bello-Irizarry SN, Wang J, Johnston CJ, Gigliotti F, Wright TW. 2014. MyD88 signaling regulates both host defense and immunopathogenesis during Pneumocystis infection. J Immunol 192:282–292. doi: 10.4049/jimmunol.1301431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhagwat SP, Gigliotti F, Xu H, Wright TW. 2006. Contribution of T cell subsets to the pathophysiology of Pneumocystis-related immunorestitution disease. Am J Physiol Lung Cell Mol Physiol 291:L1256–L1266. doi: 10.1152/ajplung.00079.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limper AH, Offord KP, Smith TF, Martin WJ II. 1989. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis 140:1204–1209. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 10.Wieruszewski PM, Barreto JN, Frazee E, Daniels CE, Tosh PK, Dierkhising RA, Mara KC, Limper AH. 2018. Early corticosteroids for Pneumocystis pneumonia in adults without HIV are not associated with better outcome. Chest 154:636–644. doi: 10.1016/j.chest.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Wieruszewski PM, Barreto EF, Barreto JN, Yadav H, Tosh PK, Mara KC, Limper AH. 27 February 2019. Preadmission corticosteroid therapy and the risk of respiratory failure in adults without HIV presenting with Pneumocystis pneumonia. J Intensive Care Med doi: 10.1177/0885066619834242. [DOI] [PubMed] [Google Scholar]
- 12.Fujikura Y, Manabe T, Kawana A, Kohno S. 2017. Adjunctive corticosteroids for Pneumocystis jirovecii pneumonia in non-HIV-infected patients: a systematic review and meta-analysis of observational studies. Arch Bronconeumol 53:55–61. doi: 10.1016/j.arbres.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Lemiale V, Debrumetz A, Delannoy A, Alberti C, Azoulay E. 2013. Adjunctive steroid in HIV-negative patients with severe Pneumocystis pneumonia. Respir Res 14:87. doi: 10.1186/1465-9921-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walzer PD, Evans HE, Copas AJ, Edwards SG, Grant AD, Miller RF. 2008. Early predictors of mortality from Pneumocystis jirovecii pneumonia in HIV-infected patients: 1985–2006. Clin Infect Dis 46:625–633. doi: 10.1086/526778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Gigliotti F, Bhagwat SP, George TC, Wright TW. 2010. Immune modulation with sulfasalazine attenuates immunopathogenesis but enhances macrophage-mediated fungal clearance during Pneumocystis pneumonia. PLoS Pathog 6:e1001058. doi: 10.1371/journal.ppat.1001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhagwat SP, Gigliotti F, Wang J, Wang Z, Notter RH, Murphy PS, Rivera-Escalera F, Malone J, Jordan MB, Elliott MR, Wright TW. 2018. Intrinsic programming of alveolar macrophages for protective antifungal innate immunity against Pneumocystis infection. Front Immunol 9:2131. doi: 10.3389/fimmu.2018.02131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limper AH, Standing JE, Hoyte JS. 1996. The role of alveolar macrophages in Pneumocystis carinii elimination from the lower respiratory tract. J Eukaryot Microbiol 43:12S. doi: 10.1111/j.1550-7408.1996.tb04953.x. [DOI] [PubMed] [Google Scholar]
- 18.Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E. 2015. Phenotypic, functional and plasticity features of classical and alternatively activated human macrophages. Am J Respir Cell Mol Biol 53:676–678. doi: 10.1165/rcmb.2015-0012OC. [DOI] [PubMed] [Google Scholar]
- 19.Arango Duque G, Descoteaux A. 2014. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson MP, Christmann BS, Werner JL, Metz AE, Trevor JL, Lowell CA, Steele C. 2011. IL-33 and M2a alveolar macrophages promote lung defense against the atypical fungal pathogen Pneumocystis murina. J Immunol 186:2372–2381. doi: 10.4049/jimmunol.1002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson MP, Christmann BS, Dunaway CW, Morris A, Steele C. 2012. Experimental Pneumocystis lung infection promotes M2a alveolar macrophage-derived MMP12 production. Am J Physiol Lung Cell Mol Physiol 303:L469–L475. doi: 10.1152/ajplung.00158.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZQ, Wang J, Hoy Z, Keegan A, Bhagwat S, Gigliotti F, Wright TW. 2015. Neither classical nor alternative macrophage activation is required for Pneumocystis clearance during immune reconstitution inflammatory syndrome. Infect Immun 83:4594–4603. doi: 10.1128/IAI.00763-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwer N, Dolman KM, van Houdt M, Sta M, Roos D, Kuijpers TW. 2008. Mannose-binding lectin (MBL) facilitates opsonophagocytosis of yeasts but not of bacteria despite MBL binding. J Immunol 180:4124–4132. doi: 10.4049/jimmunol.180.6.4124. [DOI] [PubMed] [Google Scholar]
- 24.Wells J, Haidaris CG, Wright TW, Gigliotti F. 2006. Complement and Fc function are required for optimal antibody prophylaxis against Pneumocystis carinii pneumonia. Infect Immun 74:390–393. doi: 10.1128/IAI.74.1.390-393.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gigliotti F, Haidaris CG, Wright TW, Harmsen AG. 2002. Passive intranasal monoclonal antibody prophylaxis against murine Pneumocystis carinii pneumonia. Infect Immun 70:1069–1074. doi: 10.1128/iai.70.3.1069-1074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limper AH, Hoyte JS, Standing JE. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J Clin Invest 99:2110–2117. doi: 10.1172/JCI119384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luzi G, Bongiorno F, Paparo Barbaro S, Bruno G. 2009. Intravenous IgG: biological modulating molecules. J Biol Regul Homeost Agents 23:1–9. [PubMed] [Google Scholar]
- 28.Gupta M, Noel GJ, Schaefer M, Friedman D, Bussel J, Johann-Liang R. 2001. Cytokine modulation with immune gamma-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J Clin Immunol 21:193–199. doi: 10.1023/a:1011039216251. [DOI] [PubMed] [Google Scholar]
- 29.Kazatchkine M, Mouthon L, Kaveri SV. 2000. Immunomodulatory effects of intravenous immunoglobulins. Ann Med Interne (Paris) 151(Suppl 1):1S13–1S18. [PubMed] [Google Scholar]
- 30.Wolf HM, Eibl MM. 1996. Immunomodulatory effect of immunoglobulins. Clin Exp Rheumatol 14(Suppl 15):S17–S25. [PubMed] [Google Scholar]
- 31.Mazer BD, Al-Tamemi S, Yu JW, Hamid Q. 2005. Immune supplementation and immune modulation with intravenous immunoglobulin. J Allergy Clin Immunol 116:941–944. doi: 10.1016/j.jaci.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Samuelsson A, Towers TL, Ravetch JV. 2001. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 33.Gigliotti F, Hughes WT. 1988. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Invest 81:1666–1668. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Empey KM, Hollifield M, Schuer K, Gigliotti F, Garvy BA. 2004. Passive immunization of neonatal mice against Pneumocystis carinii f. sp. muris enhances control of infection without stimulating inflammation. Infect Immun 72:6211–6220. doi: 10.1128/IAI.72.11.6211-6220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushina K, Hirata A, Hayashi N, Takenaka S, Ito H, Ogura T, Fujisawa Y, Imamura M, Yamashita N, Kujime R, Nakahashi S, Kameda H. 2016. Possible preventive effect of salazosulfapyridine against development of Pneumocystis pneumonia in methotrexate-receiving patients with rheumatoid arthritis. Mod Rheumatol 26:976–978. doi: 10.3109/14397595.2015.1118196. [DOI] [PubMed] [Google Scholar]
- 36.Nunokawa T, Yokogawa N, Shimada K, Sugii S, Nishino J, Gosho M, Wagatsuma Y, Tohma S. 2019. Prophylactic effect of sulfasalazine against Pneumocystis pneumonia in patients with rheumatoid arthritis: a nested case-control study. Semin Arthritis Rheum 48:573–578. doi: 10.1016/j.semarthrit.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Nunokawa T, Yokogawa N, Shimada K, Sugii S. 2019. Effect of sulfasalazine use on the presence of Pneumocystis organisms in the lung among patients with rheumatoid arthritis: a test-negative design case-control study with PCR tests. Mod Rheumatol 29:436–440. doi: 10.1080/14397595.2018.1465647. [DOI] [PubMed] [Google Scholar]
- 38.Pryhuber GS, Huyck HL, Bhagwat S, O'Reilly MA, Finkelstein JN, Gigliotti F, Wright TW. 2008. Parenchymal cell TNF receptors contribute to inflammatory cell recruitment and respiratory failure in Pneumocystis carinii-induced pneumonia. J Immunol 181:1409–1419. doi: 10.4049/jimmunol.181.2.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Disney MD, Stephenson R, Wright TW, Haidaris CG, Turner DH, Gigliotti F. 2005. Activity of Hoechst 33258 against Pneumocystis carinii f. sp. muris, Candida albicans, and Candida dubliniensis. Antimicrob Agents Chemother 49:1326–1330. doi: 10.1128/AAC.49.4.1326-1330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swain SD, Wright TW, Degel PM, Gigliotti F, Harmsen AG. 2004. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect Immun 72:5722–5732. doi: 10.1128/IAI.72.10.5722-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright TW, Pryhuber GS, Chess PR, Wang Z, Notter RH, Gigliotti F. 2004. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J Immunol 172:2511–2521. doi: 10.4049/jimmunol.172.4.2511. [DOI] [PubMed] [Google Scholar]
- 42.Wright TW, Johnston CJ, Harmsen AG, Finkelstein JN. 1999. Chemokine gene expression during Pneumocystis carinii-driven pulmonary inflammation. Infect Immun 67:3452–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bello-Irizarry SN, Wang J, Olsen K, Gigliotti F, Wright TW. 2012. The alveolar epithelial cell chemokine response to Pneumocystis requires adaptor molecule MyD88 and interleukin-1 receptor but not Toll-like receptor 2 or 4. Infect Immun 80:3912–3920. doi: 10.1128/IAI.00708-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Gigliotti F, Bhagwat SP, Maggirwar SB, Wright TW. 2007. Pneumocystis stimulates MCP-1 production by alveolar epithelial cells through a JNK-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 292:L1495–L1505. doi: 10.1152/ajplung.00452.2006. [DOI] [PubMed] [Google Scholar]
- 45.Hahn PY, Limper AH. 2003. The role of inflammation in respiratory impairment during Pneumocystis carinii pneumonia. Semin Respir Infect 18:40–47. doi: 10.1053/srin.2003.50004. [DOI] [PubMed] [Google Scholar]
- 46.Wellington M, Bliss JM, Haidaris CG. 2003. Enhanced phagocytosis of Candida species mediated by opsonization with a recombinant human antibody single-chain variable fragment. Infect Immun 71:7228–7231. doi: 10.1128/iai.71.12.7228-7231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukherjee S, Lee S, Mukherjee J, Scharff MD, Casadevall A. 1994. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun 62:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukherjee J, Scharff MD, Casadevall A. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun 60:4534–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro S, Beenhouwer DO, Feldmesser M, Taborda C, Carroll MC, Casadevall A, Scharff MD. 2002. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun 70:2598–2604. doi: 10.1128/iai.70.5.2598-2604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahl C, Liptay S, Adler G, Schmid RM. 1998. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest 101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Gigliotti F, Maggirwar S, Johnston C, Finkelstein JN, Wright TW. 2005. Pneumocystis carinii activates the NF-kappaB signaling pathway in alveolar epithelial cells. Infect Immun 73:2766–2777. doi: 10.1128/IAI.73.5.2766-2777.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Nazario N, Rangel-Moreno J, O'Reilly MA, Pasparakis M, Gigliotti F, Wright TW. 2013. Selective ablation of lung epithelial IKK2 impairs pulmonary Th17 responses and delays the clearance of Pneumocystis. J Immunol 191:4720–4730. doi: 10.4049/jimmunol.1301679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman OA, Standing JE, Limper AH. 1993. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J Immunol 150:3932–3940. [PubMed] [Google Scholar]
- 54.Martinez FO, Gordon S. 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Labonte AC, Tosello-Trampont AC, Hahn YS. 2014. The role of macrophage polarization in infectious and inflammatory diseases. Mol Cells 37:275–285. doi: 10.14348/molcells.2014.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roszer T. 2015. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. 2005. Macrophage receptors and immune recognition. Annu Rev Immunol 23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 58.Gales A, Conduche A, Bernad J, Lefevre L, Olagnier D, Beraud M, Martin-Blondel G, Linas MD, Auwerx J, Coste A, Pipy B. 2010. PPARgamma controls Dectin-1 expression required for host antifungal defense against Candida albicans. PLoS Pathog 6:e1000714. doi: 10.1371/journal.ppat.1000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freeman SA, Grinstein S. 2014. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev 262:193–215. doi: 10.1111/imr.12212. [DOI] [PubMed] [Google Scholar]
- 60.Balce DR, Li B, Allan ER, Rybicka JM, Krohn RM, Yates RM. 2011. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood 118:4199–4208. doi: 10.1182/blood-2011-01-328906. [DOI] [PubMed] [Google Scholar]
- 61.Rousseaux C, Lefebvre B, Dubuquoy L, Lefebvre P, Romano O, Auwerx J, Metzger D, Wahli W, Desvergne B, Naccari GC, Chavatte P, Farce A, Bulois P, Cortot A, Colombel JF, Desreumaux P. 2005. Intestinal antiinflammatory effect of 5-amiNOSalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med 201:1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steele C, Marrero L, Swain S, Harmsen AG, Zheng M, Brown GD, Gordon S, Shellito JE, Kolls JK. 2003. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor. J Exp Med 198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kottom TJ, Hebrink DM, Jenson PE, Marsolek PL, Wuthrich M, Wang H, Klein B, Yamasaki S, Limper AH. 2018. Dectin-2 Is a C-type lectin receptor that recognizes Pneumocystis and participates in innate immune responses. Am J Respir Cell Mol Biol 58:232–240. doi: 10.1165/rcmb.2016-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kottom TJ, Hebrink DM, Jenson PE, Nandakumar V, Wuthrich M, Wang H, Klein B, Yamasaki S, Lepenies B, Limper AH. 2017. The interaction of Pneumocystis with the C-type lectin receptor Mincle exerts a significant role in host defense against infection. J Immunol 198:3515–3525. doi: 10.4049/jimmunol.1600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoving JC. 2018. Pneumocystis and interactions with host immune receptors. PLoS Pathog 14:e1006807. doi: 10.1371/journal.ppat.1006807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gigliotti F, Stokes DC, Cheatham AB, Davis DS, Hughes WT. 1986. Development of murine monoclonal antibodies to Pneumocystis carinii. J Infect Dis 154:315–322. doi: 10.1093/infdis/154.2.315. [DOI] [PubMed] [Google Scholar]
- 67.Wells J, Gigliotti F, Simpson-Haidaris PJ, Haidaris CG. 2004. Epitope mapping of a protective monoclonal antibody against Pneumocystis carinii with shared reactivity to Streptococcus pneumoniae surface antigen PspA. Infect Immun 72:1548–1556. doi: 10.1128/iai.72.3.1548-1556.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee LH, Gigliotti F, Wright TW, Simpson-Haidaris PJ, Weinberg GA, Haidaris CG. 2000. Molecular characterization of KEX1, a kexin-like protease in mouse Pneumocystis carinii. Gene 242:141–150. doi: 10.1016/s0378-1119(99)00533-8. [DOI] [PubMed] [Google Scholar]