Cytoadherence-linked asexual gene 9 (Clag9), a conserved Plasmodium protein expressed during the asexual blood stages, is involved in the cytoadherence of infected red blood cells (RBCs) to the endothelial lining of blood vessels. Here, we show that Plasmodium falciparum Clag9 (PfClag9) is a component of the PfClag9-RhopH complex that is involved in merozoite binding to human erythrocytes. To characterize PfClag9, we expressed four fragments of PfClag9, encompassing the entire protein.
KEYWORDS: Clag9, cytoadherence, Plasmodium, RhopH
ABSTRACT
Cytoadherence-linked asexual gene 9 (Clag9), a conserved Plasmodium protein expressed during the asexual blood stages, is involved in the cytoadherence of infected red blood cells (RBCs) to the endothelial lining of blood vessels. Here, we show that Plasmodium falciparum Clag9 (PfClag9) is a component of the PfClag9-RhopH complex that is involved in merozoite binding to human erythrocytes. To characterize PfClag9, we expressed four fragments of PfClag9, encompassing the entire protein. Immunostaining analysis using anti-PfClag9 antibodies showed expression and localization of PfClag9 at the apical end of the merozoites. Mass spectrometric analysis of merozoite extracts after immunoprecipitation using anti-PfClag9 antibody identified P. falciparum rhoptry-associated protein 1 (PfRAP1), PfRAP2, PfRAP3, PfRhopH2, and PfRhopH3 as associated proteins. The identified rhoptry proteins were expressed, and their association with PfClag9 domains was assessed by using protein-protein interaction tools. We further showed that PfClag9 binds human RBCs by interacting with the glycophorin A-band 3 receptor-coreceptor complex. In agreement with its cellular localization, PfClag9 was strongly recognized by antibodies generated during natural infection. Mice immunized with the C-terminal domain of PfClag9 were partially protected against a subsequent challenge infection with Plasmodium berghei, further supporting a biological role of PfClag9 during natural infection. Taken together, these results provide direct evidence for the existence of a PfRhopH-Clag9 complex on the Plasmodium merozoite surface that binds to human RBCs.
INTRODUCTION
Plasmodium falciparum malaria remains a leading cause of morbidity and death worldwide (1). Although the malaria burden has been reduced in the past decade through intervention strategies such as vector control and combination drug therapy (2), it is widely agreed that a highly effective malaria vaccine is needed to achieve the long-term goal of malaria elimination and eradication (3, 4). Asexual blood-stage forms of the parasite are responsible for clinical disease and death and therefore are desirable targets for protective immunity. The merozoite is a transient extracellular form of the asexual blood stage of P. falciparum and is highly specialized for erythrocyte invasion (5, 6). Electron microscopy and recent live imaging of the merozoite invasion process have provided insights into the sequence of events leading to merozoite invasion of red blood cells (RBCs) (7, 8). Invasion of erythrocytes is a complex and sequential process involving multiple merozoite surface antigens and their specific receptors on the RBC surface; however, to date few such interactions have been characterized in detail.
A high-molecular-weight (MW) rhoptry protein (RhopH) complex in P. falciparum and Plasmodium yoelii was previously reported to be important for erythrocyte binding and parasite growth (9–11). The cytoadherence-linked asexual gene (Clag) protein family, including Clag2, Clag3.1, Clag3.2, Clag8, and Clag9, forms part of this complex. While all members of the Clag family have identical intron-exon structures, except Clag9, others are highly divergent in their amino acid sequences within P. falciparum and between different Plasmodium spp. (12). Clag9, like the other family members, possesses 10 conserved Cys residues, of which 9 are located in the N-terminal region (9). A number of reports have suggested the presence of two separate P. falciparum RhopH (PfRhopH) complexes (12). One complex, PfRhopH1/Clag9, is involved in the binding of infected erythrocytes to host endothelial cells via CD36 (13), and two complexes, PfRhopH1/Clag3.1 and PfRhopH1/Clag3.2, are involved in merozoite interactions with erythrocytes (14). Moreover, it has also been speculated that Clag9 forms part of the PfEMP1/KHARP complex, which leads to cytoadherence (13). Collectively, it is speculated that these different P. falciparum Clag (PfClag) complexes help parasite survival by diversifying parasite-host interactions (12).
Here, we describe a PfRhopH complex containing Clag9 on the P. falciparum merozoite surface, consisting of PfRhopH3, PfRhopH2, P. falciparum rhoptry-associated protein 1 (PfRAP1), PfRAP2, and PfRAP3 proteins. Clag9 is recognized by sera from individuals living in different regions in which malaria is endemic. Further, we show that Clag9 binds human RBCs and that active immunization of mice with Clag9 C-terminal fragment delays death after a Plasmodium berghei challenge, thereby suggesting the vaccine potential of PfClag9.
RESULTS
Cloning, expression, and purification of PfClag9 protein fragments.
In silico analysis of the deduced amino acid sequence of PfClag9 revealed a putative signal peptide sequence followed by a protein domain containing four transmembrane regions (Fig. 1A). To characterize the PfClag9 protein, four protein fragments of PfClag9, excluding transmembrane domains, were expressed in Escherichia coli. Expression of the PfClag9a, PfClag9b, PfClag9c, and PfClag9d protein fragments was confirmed by SDS-PAGE and immunoblotting analysis with anti-His antibodies (see Fig. S1A in the supplemental material). The recombinant proteins were purified to near homogeneity under denaturing conditions by Ni-nitrilotriacetic acid (NTA) chromatography, followed by refolding in buffer containing reduced and oxidized glutathione. SDS-PAGE analysis of purified and refolded PfClag9 protein fragments identified a major band of the expected MW (Fig. 1B). Occasionally, lower-MW bands were also detected with the anti-His antibody, suggesting that they represent proteolytic cleavage products (Fig. 1B). Nonreducing PAGE analysis revealed several high-MW bands, corresponding to dimeric and multimeric forms (Fig. S1B). Specific antibodies against recombinant PfClag9 proteins were raised in mice and rabbits (see Fig. S9 in the supplemental material). All antisera recognized a single band of ∼150 kDa in a P. falciparum lysate, which corresponds well to the deduced molecular mass of native PfClag9 (Fig. 1C; also see Fig. S2A in the supplemental material). To determine the cellular localization of PfClag9, intact merozoites were stained with each of the four anti-PfClag9 antibodies raised in mice. All four mouse anti-PfClag9 antibody preparations stained merozoites at the apical end of the parasites (Fig. 1D; also see Fig. S2B).
FIG 1.
Expression of PfClag9 gene fragments and localization of PfClag9a on the merozoite surface. (A) Schematic representation of the architecture of the PfClag9 protein. Color codes describe the signal sequence, transmembrane domains (TM), and different segments of PfClag9 that were expressed in the present study. Numbers represent the amino acid positions. Numbers with c represent the positions of cysteine residues. (B) Coomassie-stained SDS-PAGE gels (left) and the corresponding immunoblots, using HRP-conjugated anti-His antibody (right), of the purified PfClag9 fragments, i.e., PfClag9a, PfClag9b, PfClag9c, and PfClag9d. The sizes of the MW markers are indicated. (C) Anti-PfClag9 antibodies recognizing an ∼150-kDa protein in asexual blood-stage lysates. Saponin-lysed, P. falciparum-infected erythrocytes were probed with rabbit anti-PfClag9a antibody. (D) IFA and confocal microscopy of P. falciparum-infected merozoites. P. falciparum parasites were probed using mouse anti-PfClag9a antibody, followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG secondary antibodies. PfRhopH3 was used as a marker for rhoptry protein. The parasite nuclei were counterstained with DAPI (blue), and slides were visualized with a confocal laser scanning microscope. As a negative control, preimmune antibodies were used to stain the fixed infected RBCs. BF, bright-field.
PfClag9 is part of the rhoptry protein complex.
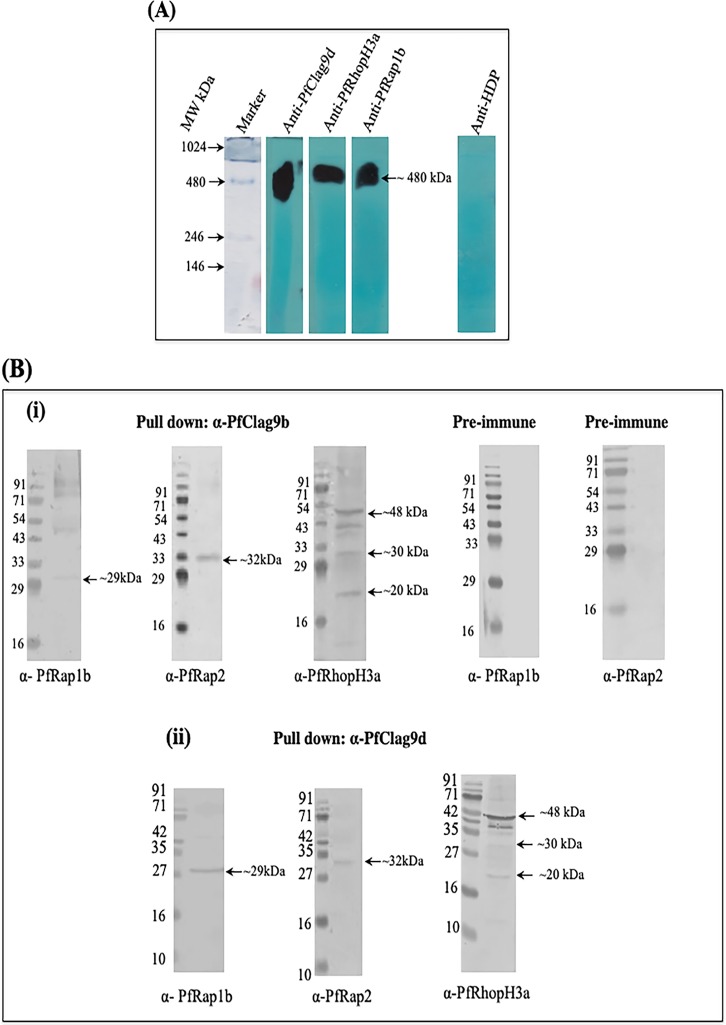
It has been proposed that P. falciparum harbors at least five different RhopH complexes, each containing a single RhopH1/Clag member; however, only two of these complexes have been partially characterized (15). To identify the PfRhopH-Clag complex containing PfClag9, a P. falciparum merozoite extract was immunoprecipitated with anti-PfClag9 antibodies, and individual protein components of the precipitates were identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. In addition to PfClag9 peptides, we identified peptides derived from PfRhopH2, PfRhopH3, PfRAP1, PfRAP2, and PfRAP3 (Table 1; also see Data Sets S1, S2, and S3 in the supplemental material), suggesting that PfClag9 is a part of the PfRhopH complex on the surface of P. falciparum merozoites. To confirm the existence of the PfRhopH-Clag9 complex at the schizont and merozoite stages, culture supernatants collected during invasion were separated on blue native PAGE (BN-PAGE) gels and analyzed by Western blotting using anti-PfRhopH3, anti-PfClag9, or anti-PfRAP1 antibodies. These three antibodies recognized a band of the same size (∼480 kDa) by Western blotting in the culture supernatants (Fig. 2A), while anti-heme detoxification protein (HDP) antibody (used as a negative control) did not stain this band, thus confirming the existence of a PfRhopH-PfRAP1-Clag9 complex. Colocalization studies subsequently performed on intact merozoites demonstrated strong colocalizations between PfClag9, PfRhopH, and PfRAP1, with Pearson’s coefficients of >0.5 (Fig. S3B). We next performed interaction analysis between the components of the PfRhopH complex and different fragments of PfClag9. To carry out interaction analysis, two fragments of PfRAP1, i.e., PfRAP1a (amino acids [aa] 25 to 350) and PfRAP1b (aa 350 to 612), PfRAP2 (aa 105 to 398), and two fragments of PfRhopH3, i.e., PfRhopH3a (aa 27 to 465) and PfRhopH3b (aa 617 to 865), were expressed as recombinant proteins. These proteins were purified, and antibodies were raised against them in mice and rabbits (Fig. S4A and B). Coimmunoprecipitation using anti-PfClag9, anti-PfRAP1, or anti-PfRhopH3 antibodies, followed by Western blotting, revealed high-affinity interactions between the PfRAP1 C-terminal fragment (PfRAP1b) and PfClag9d or PfClag9b fragments. Both the PfClag9d and PfClag9b fragments also showed interactions with PfRAP2 and the PfRhopH3 N-terminal fragment (PfRhopH3a) (Fig. 2B; also see Fig. S5A), supporting the notion that these proteins form a PfRhopH-Clag9 complex. These interactions were specific, as no such association was seen when coimmunoprecipitation was carried out using prebleed sera (Fig. 2B). In comparison, PfClag9a or PfClag9c fragments showed negligible interactions with the aforementioned rhoptry proteins. Further, a surface plasmon resonance (SPR) analysis was performed to assess the affinity of interactions between PfClag9 and P. falciparum rhoptry protein fragments. Recombinant PfRAP1b, PfRAP2, and PfRhopH3a proteins showed high-affinity interactions with PfClag9b, as well as PfClag9d fragments, with equilibrium dissociation constant (Kd) values of 1.73 × 10−10 M, 1.3 × 10−6 M, and 2.9 × 10−6 M, respectively, for PfClag9b and 8.26 × 10−10 M, 3.16 × 10−6 M, and 1.3 × 10−6 M for PfClag9d (Fig. S5B). These interactions were specific, as PfRAP1a and PfRhopH3b fragments showed low-affinity interactions with PfClag9b or PfClag9d fragments (Fig. S6A). PfRAP1b and PfRAP2 fragments showed low-affinity interactions with the PfClag9a fragment (Fig. S6B), thus confirming the involvement of PfClag9b and PfClag9d fragments in forming the PfRhopH complex. Taken together, these results support the notion that PfClag9 is a component of a protein complex on the P. falciparum merozoite surface.
TABLE 1.
LC-MS/MS identification of malarial proteins immunoprecipitated using anti-PfClag9a antibodies
| No. | Protein | PlasmoDB accession no. | Score | Coverage | No. of unique peptides | Peptide sequence |
|---|---|---|---|---|---|---|
| 1 | Cytoadherence-linked asexual protein 9 (Clag9) | PF3D7_0935800 | 244.03 | 12.99 | 14 | DLFSETSFLQTVYLLFK, SILDNDELYNSLSNLENLLLQTLEQDELK, FSQENDPVSK, EVVNDFFVIYK, IFNTNNLTHR, VVDPFNLFTNYFYFIK, YLNWQSILK, KEcNIYESDR, GIEFHDNNNYK, EcNIYESDR, FYIFVTK, ELYQNLVK, VVDPFnLFTnYFYFIK, YFEIGSLK |
| 2 | High-MW rhoptry protein 2 (RhopH2) | PF3D7_0929400 | 203.17 | 12.70 | 14 | LFVTEGTLEYLLLDK, NFFSELWQNIR, DcNVNQNFTETSK, LFEQIVDQIK, INIPEcFGPcTK, ADSDITYFVK, SLYGNNNNNNAGSESDVTLK, ScDISQYGIK, SVYYDDDVSLYR, NIVSDALTSEEIKR, LLTSYEYIDSIANNYFFLSEYK, EISEDLIK, QLDDEEIER, EYNEFLQDK |
| 3 | High-MW rhoptry protein 3 (RhopH3) | PF3D7_0905400 | 171.07 | 14.16 | 9 | LFFTYNFGDVEPQGK, VFTALYNFDSFIK, EDNSEIQcQNVR, NYLDSVQNLDTEcFK, ELSHNITDFSFK, NLYSTVEDEQR, LSTYTYSIFDK, GnGPDAGSFLDFVDEPEqFYWFVEHFLSVK, SWVSEFLK |
| 4 | Rhoptry-associated protein 2 (RAP2) | PF3D7_0501600 | 141.82 | 19.60 | 6 | ENYYNSDIAGPAR, DINPLFINDFILILNDK, YTEISVLNYVR, SEYYGTPDDLITSFFSIIK, SNPYFIVGSR, DYLGDFNK |
| 5 | Rhoptry-associated protein 3 (RAP3) | PF3D7_0501500 | 5.94 | 5.25 | 2 | VFIFNEINFFR, TDFLQDILEK |
| 6 | Rhoptry-associated protein 1 (RAP1) | PF3D7_1410400 | 3.98 | 2.17 | 2 | FLENQVK, LYEFENDLLK |
FIG 2.
PfClag9 is part of a PfRhopH complex. (A) Western blot analysis of invasion supernatants to determine the existence of a PfRhopH complex. HDP served as a negative control. (B) Coimmunoprecipitation. Recombinant PfRAP1b, PfRAP2, or PfRhopH3a was incubated with recombinant PfClag9b or PfClag9d and immunoprecipitated using anti-PfClag9b, anti-PfClag9d, or preimmune rabbit antibodies, and eluted proteins were probed with anti-RAP1b, anti-RAP2, and anti-RhopH3a mouse antibodies. Arrows indicate the bands for PfRAP1b, PfRAP2, and PfRhopH3a.
PfClag9a and PfClag9c fragments bind human RBCs via a GPA-band 3 complex.
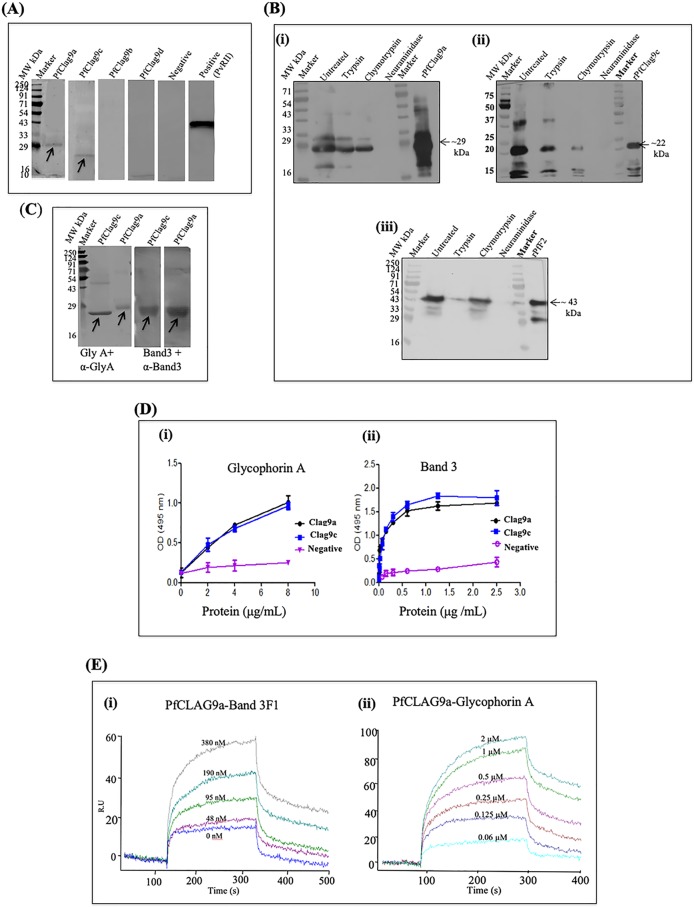
To assess whether PfClag9 is involved in merozoite invasion of human erythrocytes, we examined the binding of PfClag9 segments to human erythrocytes in an in vitro erythrocyte-binding assay. Briefly, four recombinant fragments of PfClag9, i.e., PfClag9a, PfClag9b, PfClag9c, and PfClag9d, were incubated with washed human erythrocytes, and bound proteins were analyzed by Western blotting using specific antibodies. As shown in Fig. 3A, the PfClag9a and PfClag9c protein fragments showed specific interactions with erythrocytes, while the PfClag9b and PfClag9d fragments did not show such binding activities. As expected, recombinant PvRII, a known erythrocyte binder, showed high levels of binding with erythrocytes in the same assays. We next tested the specificity of the interactions of erythrocytes and PfClag9a and PfClag9c fragments by treating human erythrocytes with trypsin, chymotrypsin, or neuraminidase. Pretreatment of erythrocytes with trypsin or chymotrypsin reduced the binding of PfClag9a or PfClag9c fragments to erythrocytes (Fig. 3B); however, pretreatment with neuraminidase, which preferentially cleaves α2-3-linked sialic acids of O-linked tetrasaccharides, completely abolished the binding of these PfClag9 fragments to human erythrocytes (Fig. 3B). These results suggested a sialic acid-dependent interaction of the PfClag9-RhopH complex with human erythrocytes.
FIG 3.
PfClag9 binds human erythrocytes via the GPA-band 3 complex. (A) Recombinant PfClag9 fragments, i.e., PfClag9a, PfClag9b, PfClag9c, and PfClag9d, were incubated with uninfected human erythrocytes, and bound proteins were eluted from the erythrocytes after centrifugation through oil. Immunoblot analysis shows the interaction of PfClag9a and PfClag9c with human erythrocytes, while Pfclag9b and PfClag9d failed to bind. PvRII was used as a positive control for the erythrocyte binding assay, while an internal protein (ClpQ) was used as a negative control. (B) PfClag9a (i) and PfClag9c (ii) bound erythrocytes after the treatment of erythrocytes with trypsin or chymotrypsin, while neuraminidase treatment abolished the binding, suggesting sialic acid-dependent binding of PfClag9 fragments to erythrocytes. The F2 domain of PfEBA-175 (PfF2) (iii) was used as a positive control. (C) Far Western blot analysis shows that PfClag9 fragments bind human erythrocytes through the GPA-band 3 complex. (D) ELISA was performed to confirm the interaction between the GPA-band 3 receptor-coreceptor complex and recombinant PfClag9a and PfClag9c fragments. As a control, HDP was used. GPA or band 3 was coated on the wells (100 ng/100 μl), and wells were incubated with different concentrations of PfClag9a, PfClag9c, or HDP. Dose-dependent binding between PfClag9 fragments and GPA, as well as band 3, was observed. (E) SPR analysis shows the interaction of recombinant PfClag9a with band 3 F1 or GPA.
Band 3 and glycophorin A (GPA) are the two most abundant RBC surface proteins, forming a tight stoichiometric complex on the RBC surface (16). Since PfClag9 binding to human RBCs was sialic acid dependent, we performed a far Western analysis to further investigate the interactions of PfClag9a and PfClag9c fragments with either GPA or the band 3 F1 fragment, both of which are exposed on the surface of RBCs. As seen in Fig. 3C, both PfClag9a and PfClag9c fragments interacted with both GPA and band 3 proteins. To confirm these interactions, an enzyme-linked immunosorbent assay (ELISA)-based binding assay in which increasing concentrations of soluble PfClag9a and PfClag9c were added to plates coated with GPA or band 3 proteins was performed. Bound protein was detected with the respective antiserum. Concentration-dependent binding of PfClag9a and PfClag9c was observed (Fig. 3D). Intracellular HDP did not show binding to either GPA or band 3, demonstrating the specificity of these interactions. Lastly, the affinity of PfClag9a for the GPA-band 3 complex was determined by SPR analysis. Increasing concentrations of PfClag9a were injected onto sensor chips with immobilized GPA or band 3; PfClag9 bound to both chips in a dose-dependent manner, with Kd values of 3.51 × 10−7 M and 4.53 × 10−4 M, respectively (Fig. 3E). Taken together, these results strongly suggested that PfClag9 binds to human erythrocytes through the interactions of the regions PfClag9a and PfClag9c with the GPA-band 3 complex.
Humoral immune responses to the PfClag9 fragments.
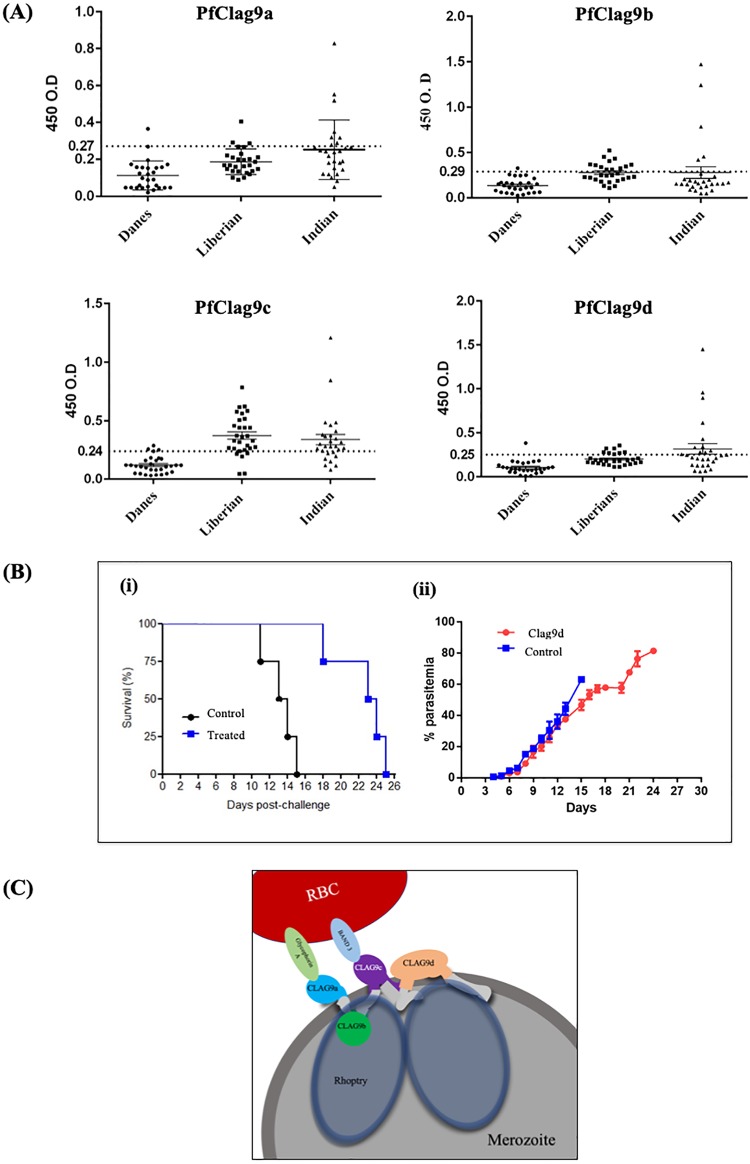
The antigenicity of PfClag9 was investigated using plasma samples collected from areas in Africa and India in which malaria is endemic. The PfClag9a, PfClag9b, PfClag9c, and PfClag9d fragments were frequently recognized by plasma samples from Liberia, Africa, with seropositivity rates of 10.71%, 46.42%, 85.71%, and 32.14%, respectively. Among Indian malaria-infected patients, the seropositivity rates were 28.57%, 17.85%, 71.43%, and 39.29%, respectively (Fig. 4A and Table 2; also see Fig. S10 in the supplemental material). Taken together, these results indicate that the PfClag9 C-terminal regions (PfClag9c and PfClag9d) contain the major epitopes for antibodies generated during natural P. falciparum infection (Fig. 4A).
FIG 4.
PfClag9 elicits protective immunity in P. berghei. (A) Naturally acquired humoral IgG immune responses to proteins of PfClag9 fragments. Human IgG antibodies against different fragments of PfClag9 were detected by ELISA in sera from naturally infected patients from Liberia and India. Sera from Denmark were used as negative controls. The positivity thresholds represented by dotted lines were determined from the mean reactivities (+2 standard deviations) of 28 serum samples from nonimmune Danish volunteers. (B) PfClag9d-immunized mice showed delayed death upon P. berghei challenge. Four mice were immunized with PfClag9d and challenged with 1 × 104 P. berghei (ANKA)-infected RBCs administered intraperitoneally. Four mice immunized with PBS with Freund’s adjuvant served as controls. (i) Kaplan-Meier curve of the overall survival of mice in each group. (ii) Parasitemia levels on different days after P. berghei challenge, in control and PfClag9d-immunized mice. The t test was performed on the parasitemia levels, and the P value was calculated to be 0.00075. (C) Model of the PfRhopH-Clag9 complex, based on protein-protein interactions and protein-erythrocyte interactions.
TABLE 2.
Number of seropositive serum samples identified with PfClag9a, PfClag9b, PfClag9c, and PfClag9d recombinant proteins in populations from areas in which malaria is endemic, as determined by ELISAa
| No. | Protein | No. of seropositive samples, of 28 serum samples |
|
|---|---|---|---|
| Liberian | Indian | ||
| 1 | PfClag9a | 3 | 8 |
| 2 | PfClag9b | 13 | 5 |
| 3 | PfClag9c | 24 | 20 |
| 4 | PfClag9d | 9 | 11 |
The seropositive thresholds were determined from the mean reactivities (+2 standard deviations) of 28 serum samples from nonimmune Danish volunteers.
Invasion inhibition assay.
The inhibitory potential of anti-PfClag9 antibodies regarding the invasion of Plasmodium merozoites into RBCs was evaluated. An invasion inhibition assay was performed with P. falciparum 3D7 parasites, using purified anti-PfClag9a, anti-PfClag9b, anti-PfClag9c, or anti-PfClag9d rabbit IgG at different concentrations. IgG purified from preimmune serum served as a negative control. None of the anti-PfClag9 IgGs showed inhibition of invasion, even at 10 mg/ml (Fig. S7B).
Active immunization with PfClag9d considerably delayed the death of mice after a lethal P. berghei challenge.
To determine whether PfClag9 has the potential to elicit protective immunity in vivo in the P. berghei challenge model, we immunized mice with the PfClag9d fragment, because it exhibits the greatest similarity (∼56%) between P. berghei Clag9 and PfClag9, in comparison with the other PfClag9 fragments (Fig. S7C). Two independent challenge studies were performed with recombinant PfClag9d-immunized mice. In the first experiment, four mice were immunized three times at 2-week intervals; in the second experiment, three mice were immunized with recombinant PfClag9d in Freund’s adjuvant. One week after the last immunization, the mice were challenged with 1 × 104 P. berghei-infected RBCs. All mice in the control group succumbed to infection at day 12 or earlier, while mice immunized with PfClag9d showed significantly delayed deaths, even though parasitemia levels were similar until day 12. After day 12 postinfection, recombinant PfClag9d-immunized mice showed a slow increase in parasitemia levels, in comparison with the control group (Fig. 4B; also see Fig. S8). In both experiments, a delayed onset of parasite growth was observed in PfClag9d-immunized mice, leading to increased survival times, compared to the control mice.
DISCUSSION
PfClag9, a member of the Clag family, has been implicated in cytoadherence, a process that involves the binding of infected erythrocytes to host endothelial cells (14). Of the five members of the PfClag family, PfClag9 is the most highly conserved (17). Besides cytoadherence, PfClag9 and other members of the PfClag family have been suggested to play roles in merozoite invasion and/or parasitophorous vacuole formation, as well as in increased erythrocyte permeability and nutrient uptake; however, limited experimental evidence exists for these roles (15). To characterize PfClag9, we expressed PfClag9 protein in a heterologous expression system. To date, there have been no reports of a full-length PfClag9 protein or its fragments being expressed in heterologous expression systems. Anti-PfClag9 antibodies generated previously were made by DNA vaccination or were raised against small peptides (13, 18). Since PfClag9 is a membrane- and cytoskeleton-associated protein with four putative transmembrane domains, we decided to clone and to express four PfClag9 fragments encompassing the entire PfClag9 gene but excluding the transmembrane domains. These PfClag9 segments were expressed in E. coli, and protocols for refolding of the recombinant protein fragments were developed. Immunostaining of asexual blood-stage parasites using anti-PfClag9 antibodies showed punctate staining typical of merozoite surface proteins (specifically, rhoptry proteins). This observation is in line with the previous findings that PfClag9 is localized to the rhoptry proteins in the merozoite stage (12, 17).
It has been suggested that different parasite clones express different subsets of RhopH complexes, consisting of a protein of the RhopH1/Clag family and RhopH2 and RhopH3 proteins, and each RhopH complex contains only a single RhopH1/Clag member, thus suggesting five different types of RhopH complexes in malaria parasites (15). The inability to genetically disrupt the RhopH2 and RhopH3 proteins further suggests that intact complexes are essential for parasite viability at asexual blood stages (19). To characterize individual components of the PfClag9-associated PfRhopH complex, we immunoprecipitated a P. falciparum merozoite lysate with anti-PfClag9 antibody and identified components of the precipitate with mass spectrometry-based methods. The results showed that PfClag9 forms part of a large complex with other rhoptry proteins, such as PfRhopH2, PfRhopH3, PfRAP2, PfRAP1, and PfRAP3. We also identified 14-3-3 protein (PlasmoDB accession no. PF3D7_0818200), along with rhoptry proteins, with high confidence. We did observe PfEMP1 and the 6-Cys protein P230 (PlasmoDB accession no. PF3D7_0209000) in the immunoprecipitates; however, their confidence values were low. A previous study showed that Clag9 is not essential for PfEMP1 expression on noncytoadherent P. falciparum parasites (3D7) (20). It is possible that PfClag9 exists in multiple protein complexes that are differentially expressed during the asexual blood stages. PfClag9 might be part of a high-MW rhoptry complex in merozoites and part of an adapter complex that traffics PfEMP1 to infected erythrocyte surfaces during the ring and trophozoite stages.
Having clarified that PfClag9 is expressed at the apical end of merozoites and is part of a large rhoptry complex, we next looked for its interaction with human RBCs, because a number of previous studies have shown that the RhopH complex binds to erythrocyte membranes and to synthetic lysosomes (15, 21–24). Analysis of the interactions of four PfClag9 fragments with human erythrocytes using an in vitro erythrocyte-binding assay showed that PfClag9a and PfClag9c fragments bound human erythrocytes. P. falciparum has multiple invasion pathways that use different receptor-ligand interactions. Two broadly defined alternate invasion pathways are involved in the invasion of erythrocytes, one that depends on host sialic acid interactions and another that is sialic acid independent (25). To identify the pathway(s) involving PfClag9, we analyzed the binding of PfClag9 protein fragments to human RBCs that had been treated with trypsin, chymotrypsin, or neuraminidase. Both the PfClag9a and PfClag9c fragments failed to bind neuraminidase-treated erythrocytes, thereby suggesting that PfClag9 binding is sialic acid dependent. GPA and band 3 are two abundant proteins on the RBC surface; they form a GPA-band 3 complex, which has been shown to bind the PfMSP1-RhopH3 complex (25, 26). We next tested the ability of PfClag9a and PfClag9c fragments to bind GPA and band 3 proteins by using different biochemical approaches. Both protein fragments bound to these RBC surface proteins with relatively high equilibrium constants. Since the interactions between PfClag9 fragments and the RBC surface are sialic acid dependent, it is possible that PfClag9 fragments bind GPA first and that this interaction brings the PfClag9 protein in close contact with band 3, thus strengthening the interaction. Based on the recognition of PfClag9 fragments by infected human sera and their interactions with human erythrocytes, we propose a model depicting the organization of PfClag9 on the merozoite surface (Fig. 4C).
Having assessed the role of PfClag9 in merozoite binding to human RBCs, we next assessed whether Clag9 is exposed to the human immune system during natural infections in two geographically distinct areas, in India and Liberia. Although all PfClag9 fragments were antigenic in these populations, higher seropositivity rates were observed for the PfClag9c and PfClag9d fragments, suggesting that these amino acid sequences contain major epitopes for naturally acquired antibodies. To understand the functional relevance of PfClag9 binding to RBCs and to determine whether the binding of specific antibodies might modify natural parasite infections, P. berghei challenge studies were performed in mice immunized with recombinant PfClag9d protein. Immunized mice remained healthy longer than unimmunized control mice, suggesting a protective role for PfClag9 in naturally acquired immunity.
In conclusion, we demonstrate that PfClag9 is part of a RhopH complex and is involved in binding to human erythrocytes in a sialic acid-dependent manner. The results further demonstrate that PfClag9 is a multifunctional protein that, in addition to having a role in cytoadherence, is involved in the binding of merozoites to erythrocytes through complex formation with other rhoptry proteins. Given that PfClag9 is nonmutable (27) and has multiple roles, together these results suggest that PfClag9 may be an important candidate for the discovery of novel antiparasitic strategies.
MATERIALS AND METHODS
Ethics statement.
All animal experiments conducted were approved by the Institutional Animals Ethics Committee of the International Centre for Genetic Engineering and Biotechnology (ICGEB) under approval no. ICGEB/PM/MAL/45. Written informed consent was obtained from patients for all Indian serum samples. Liberian and Danish serum samples are from the collection of Michael Theisen of the Statens Serum Institut.
Materials.
Recombinant GPA (product no. G7903) and anti-GPA (product no. G7900) were purchased from Sigma-Aldrich (India).
In silico sequence analysis of the PfClag9 protein.
In silico sequence analysis of the PfClag9 gene was performed with amino acid sequences of Clag9 from Plasmodium parasites. Schematic representations highlighting positions of the transmembrane domains and signal sequences were prepared by using three programs, i.e., SMART (28), Trmped (29), and SignalP 4.0 (30).
In vitro P. falciparum culture.
Plasmodium falciparum strain 3D7 was cultured on human erythrocytes (4% hematocrit) in RPMI 1640 medium (Invitrogen) supplemented with 10% O+ human serum, using the standard protocol described by Trager and Jensen (31). Parasite cultures were synchronized by two consecutive sorbitol treatments for 4 h, apart from following the protocol described by Lambros and Vanderberg (32).
Cloning, expression, and purification of PfClag9, PfRAP1, PfRAP2, and PfRhopH3 fragments and band 3 F1 and generation of antibodies against them.
Four fragments of the PfClag9 gene, i.e., PfClag9a (aa 22 to 245), PfClag9b (aa 266 to 445), PfClag9c (aa 472 to 673), and PfClag9d (aa 695 to 1021), PfRAP1a (aa 25 to 350), PfRAP1b (aa 350 to 620), PfRAP2 (aa 105 to 398), PfRhopH3a (aa 27 to 465), and PfRhopH3b (aa 617 to 865) were amplified from Plasmodium cDNA and cloned in the pET28b expression vector. The pET28b plasmids containing PfClag9a, PfRAP1a, PfRAP1b, PfRAP2, and PfRhopH3a gene segments were expressed in E. coli BL21(DE3) cells, while the pET28b plasmid with a PfClag9c gene fragment was expressed in E. coli BLR cells, by induction with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 5 h at 37°C. The pET28b plasmids carrying PfRhopH3b, PfClag9b, and PfClag9d genes were expressed in E. coli shuffle cells by induction with 0.4 mM IPTG for 8 h at 30°C. All of the proteins were expressed in inclusion bodies in E. coli. The inclusion bodies were solubilized in buffer (0.05 M Tris [pH 8.0], 0.15 M NaCl, 0.01 M dithiothreitol [DTT], 100 μg/ml lysozyme, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1% Triton X-100) containing 8 M urea, and recombinant proteins were purified to near homogeneity using Ni-NTA chromatography. Eluted protein fractions were analyzed on SDS-PAGE gels. Fractions containing ∼90% pure proteins were pooled and refolded by gently diluting the protein 40-fold in refolding buffer (0.05 M Tris [pH 8], 1 M urea, 1 mM EDTA, 0.5 M arginine, 0.4 mM Triton X-100, 1 mM reduced glutathione, 0.5 mM oxidized glutathione), with constant stirring, for 24 h at 4°C. The refolded proteins were concentrated, dialyzed against 0.05 M Tris (pH 8)-0.15 M NaCl, and stored at −80°C. The expressed proteins were run in SDS-PAGE gels and confirmed by Western blotting using anti-His monoclonal antibody (Sigma). Cloning and expression of band 3 F1 were performed as described previously (33). Polyclonal antisera against all of the purified recombinant protein fragments were generated in BALB/c mice, as well as in a New Zealand White rabbit strain, by a protocol described previously (34).
Antibody purification.
IgG purification for PfClag9a, PfClag9b, PfClag9c, and PfClag9d mouse and rabbit sera was performed using immobilized protein A/G resin from G-Biosciences. The protocol was performed according to the manual instructions.
Immunoblot analysis of Plasmodium falciparum merozoites.
To determine the specificity of anti-PfClag9 antibodies, we carried out immunoblotting analysis of a P. falciparum merozoite lysate. Briefly, P. falciparum merozoites were harvested, lysed with SDS sample buffer, and boiled at 98°C for 15 min. After removal of insoluble material by centrifugation (15,000 × g for 15 min), the parasite lysate was run on SDS-PAGE gels and proteins were transferred to nitrocellulose membranes. The membranes were probed with rabbit anti-PfClag9a (1:1,000 dilution), rabbit anti-PfClag9b (1:1,000 dilution), rabbit anti-PfClag9c (1:1,000 dilution), and rabbit anti-PfClag9d (1:1,000 dilution) antisera, followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:100,000). The membranes were developed with Pierce SuperSignal West Femto Substrate (product no. 34095; Pierce).
Complex identification in postinvasion culture supernatants.
Highly synchronized P. falciparum cultures were maintained at high parasitemia levels; after the invasion of the parasite into erythrocytes, the culture supernatant was collected by centrifugation at 1,000 × g for 5 min. The supernatants were further centrifuged at 15,000 × g for 10 min to remove insoluble debris and then were concentrated 20 times by using 3K Amicon filters and stored at −80°C in small aliquots. BN-PAGE analysis was carried out to separate various complexes present in the invasion supernatant, using the protocol described by Wittig et al. (35), with slight modifications. Briefly, proteins were mixed with BN-PAGE sample buffer (62.5 mM Tris-HCl [pH 6.8], 25% glycerol, 5% [wt/vol] Coomassie brilliant blue G-250), resolved in native PAGE gels (4% stacking/8% resolving gel) at 4°C, and then transferred to polyvinylidene difluoride (PVDF) membranes. To identify PfClag9-associated complexes, membranes were probed with rabbit anti-PfClag9d (1:10,000 dilution), anti-PfRhopH3b (1:10,000 dilution), anti-PfRAP1b (1:10,000 dilution), and anti-HDP (1:10,000 dilution) antisera, followed by incubation with HRP-conjugated goat anti-rabbit IgG secondary antibody (1:100,000). HDP was used as a negative control. The membranes were developed with Pierce SuperSignal West Femto substrate (product no. 34095; Pierce).
Indirect immunofluorescence assays.
Confocal laser scanning indirect immunofluorescence assays (IFAs) were performed with P. falciparum asexual blood stages. Briefly, P. falciparum merozoites were smeared on glass slides, air dried, and fixed for 30 min with prechilled methanol (−20°C). Fixed slides were air dried and blocked for 2 h at room temperature with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Slides were washed with PBST (PBS containing 0.05% Tween 20) and PBS. For the localization/colocalization studies, the following antibodies were used: mouse anti-PfClag9a (1:250 dilution), mouse anti-PfClag9b (1:250 dilution), mouse anti-PfClag9c (1:250 dilution), mouse anti-PfClag9d (1:250 dilution), rabbit anti-PfRhopH3b (1:500 dilution), and rabbit anti-PfRAP1b (1:500 dilution). The slides were incubated with the respective primary antibodies for 1 h at room temperature, washed with PBST, and incubated with the corresponding Alexa Fluor 488/Alexa Fluor 594-conjugated secondary antibodies for 1 h. The slides were then washed, mounted in ProLong Gold antifade reagent with 4′,6′-diamidino-2-phenylindole (DAPI) (Invitrogen), and viewed with a super resolution confocal microscope (N-SIM; Nikon, Japan). The images were processed using Nikon NIS-Elements AR v4.13.04 software. IMARIS images were created using IMARIS v4.0 software.
Immunoprecipitation.
Immunoprecipitation reactions were performed using the Pierce cross-link immunoprecipitation kit (Thermo Fisher Scientific Inc.), according to the manufacturer’s protocol. Merozoites were isolated from highly synchronized P. falciparum cultures. Merozoite lysates were prepared in immunoprecipitation lysis buffer (250 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 5% glycerol [pH 7.4]). For the immunoprecipitation, two batches of protein A/G-agarose were prepared. One batch was used to cross-link 20 μg of rabbit anti-PfClag9a antibody, while the second batch was incubated with an equal amount of preimmune rabbit antibody as a control. The beads were then incubated with parasite lysate (∼1 mg per reaction) at 4°C. After 12 h of incubation, protein A/G-agarose beads were washed with wash buffer, and bound proteins were eluted from the beads using elution buffer (Tris-glycine [pH 2.8]). The experiment was performed twice, and the respective preimmune antibodies were used as controls. Proteins in the immunoprecipitated samples were digested with the in-solution trypsin digestion method. Samples were first reduced with 10 mM DTT (final concentration) for 1 h at room temperature. After reduction, samples were alkylated with 40 mM iodoacetamide (Sigma-Aldrich) for 1 h at room temperature in the dark. Proteins were digested overnight at 37°C with trypsin added at a trypsin/protein ratio of 1:50 (wt/wt). After digestion, extracted peptides were acidified with 0.1% formic acid and analyzed with an Orbitrap Velos Pro mass spectrometer coupled with a Nano-LC 1000 system (Thermo Fisher Scientific). The peptides were separated on an Acclaim PepMap 100 C18 precolumn (2 cm, 75 μm) connected to an Acclaim PepMap 100 C18 column (15 cm, 50 μm). Peptides were separated with a 150-min gradient of 5% buffer B (0.1% formic acid in 95% acetonitrile) to 90% buffer B (with buffer A being 0.1% formic acid in 5% acetonitrile), at a flow rate of 0.3 μl/min. Peptides eluting from the column tip were electrosprayed directly into the mass spectrometer, with a spray voltage of 1.7 kV. Data acquisition was performed in a data-dependent mode to automatically switch between the first and second mass spectrometers. Full-scan mass spectra of intact peptides were acquired in the Fourier transform ion cyclotron resonance cell, with a resolution of 60,000. The top 20 ions were sequentially isolated and fragmented in the collision-induced dissociation cell, with a normalized collision energy of 35. Dynamic exclusion of ions previously sequenced within 30 s was applied. All unassigned charge states and singly charged ions were excluded from fragmentation. A minimum of 1,000 counts was required for selection for the second mass spectrometer.
The raw data were analyzed using Proteome Discoverer 1.4 using the SEQUEST algorithm, with Plasmodium database 10.0 downloaded from PlasmoDB (https://plasmodb.org/common/downloads/). All routine contaminants were also included in the database. Carbamidomethylation, deamidation, and oxidation were kept as variable modifications. Trypsin was used as the enzyme of choice, and two missed cleavages were allowed. A precursor tolerance of 20 ppm was set, whereas the tolerance for the second mass spectrometer was 0.6 Da. Percolator was used to validate the peptides, based on q values. A false-discovery rate of 5% was allowed, and only proteins identified with at least two peptides were selected for further experiments.
In vitro coimmunoprecipitation.
Briefly, 10 μg of PfRAP1b, PfRAP2, or PfRhopH3a was incubated for 2 h with 10 μg of PfClag9a, PfClag9b, PfClag9c, and PfClag9d, in separate reaction mixtures, in 100 μl binding buffer (50 mM phosphate buffer [pH 7.0], 75 mM NaCl, 2.5 mM EDTA [pH 8.0], 5 mM MgCl2, 0.1% NP-40, 10 mM DTT). The reaction mixture was further incubated for 2 h at 4°C with 20 μl of Pierce protein A/G-agarose beads (product no. 1861760; Pierce) cross-linked with 20 μg rabbit anti-PfClag9a, anti-PfClag9b, anti-PfClag9c, or anti-PfClag9d antibodies. The beads were then centrifuged at 1,000 × g for 5 min, washed with 200 μl of binding buffer containing 400 mM NaCl, and boiled for 5 min in SDS-PAGE sample buffer. Proteins were subsequently electrophoresed, immunoblotted, and probed with mouse anti-PfRAP1b, anti-PfRAP2, or anti-PfRhopH3a antisera, followed by HRP-conjugated goat anti-mouse IgG secondary antibody (1:3,000 dilution). The blots were developed using 3,3ʹ-diaminobenzidine as the substrate (Sigma-Aldrich).
ELISAs.
In vitro interactions between Clag9 and RBC surface receptors were examined by ELISAs, as described previously (36). Recombinant GPA or band 3 (2 μg/ml) was used for coating, while PfClag9a, PfClag9c, or P. falciparum MLH (negative control) was used as the interacting protein, in increasing concentrations. All experiments were performed in duplicate, and the mean ± standard error of the mean (SEM) was calculated.
Far Western assays.
Far Western assays were carried out according to the protocol described earlier (36). Recombinant PfClag9c or PfClag9a (1 to 5 μg) was used as the prey protein, while GPA or band 3 (2 μg/ml) was used as the bait protein.
SPR kinetic analysis.
The kinetics of binding of PfClag9 fragments to PfRAP1, PfRAP2, PfRhopH3, GPA, and band 3 were determined at 25°C with a Biacore 2000 SPR-based biosensor (Biacore AB) (0.15 M NaCl, 3 mM EDTA, 0.005% [vol/vol] surfactant P20), unless mentioned otherwise. Recombinant PfClag9a, PfClag9b, and PfClag9d were immobilized up to 2,000, 3,000, and 2,800 response units, respectively, in different flow cells of a CM5 sensor chip (GE Healthcare). For kinetic measurements, increasing concentrations of recombinant PfRAP1a, PfRAP1b, PfRAP2, PfRhopH3a, or PfRhopH3b were injected over immobilized PfClag9a, PfClag9b, and PfClag9d, as well as in the reference flow cell, at a flow rate of 20 μl/min. Increasing concentrations of recombinant GPA and band 3 F1 were injected over immobilized PfClag9a, as well as in the reference flow cell, at a flow rate of 20 μl/min. Reference-subtracted sensorgrams were analyzed using Biacore evaluation 4.1.1 software (GE Healthcare). To determine the kinetic parameters of the interactions, binding responses in the steady-state region of the sensorgrams were plotted against analyte concentrations and fitted to the standard 1:1 (Langmuir) bimolecular interaction, with simultaneous fitting of ka and kd. The apparent equilibrium dissociation constant (Kd) was calculated from the equation Kd = kd/ka, where kd is the dissociation rate constant and ka is the association rate constant.
Erythrocyte binding assays.
Erythrocyte binding assays were carried out as described previously (37). Briefly, 10 μg each of recombinant PfClag9 protein fragments was incubated with 100 μl of fresh packed RBCs for 1 h. The RBCs were separated from the supernatant by centrifugation through 600 μl of dibutylphthalate (Sigma) at 12,000 × g for 30 s. Proteins bound to the erythrocytes were eluted by incubation with 20 μl of 1.5 M NaCl in PBS at room temperature for 5 min. Eluted proteins were obtained after centrifugation at 12,000 × g and were mixed with an equal volume of 2× nonreducing sample buffer. The eluates were analyzed by immunoblotting with the respective antibodies. PvRII, a known erythrocyte binder, was used as a positive control. To determine whether PfClag9 binding to erythrocytes was through trypsin-, chymotrypsin-, or neuraminidase-sensitive receptors, binding studies were performed with RBCs treated with any of these enzymes, by protocols described previously (38). Briefly, 100 μl of packed RBCs was incubated for 1 h at 37°C with 1 mg of tosylsufonyl phenlanalanyl chloromethyl ketone-treated trypsin (Sigma) or bovine pancreas chymotrypsin (Sigma) in 10 ml of RPMI 1640 incomplete medium (pH 6.7), with rocking, and then washed twice with 10 ml of RPMI 1640 incomplete medium. For the trypsin-treated tubes, the erythrocytes were then treated for 10 min at room temperature with 1 mg of soybean trypsin inhibitor (Sigma) in 10 ml of RPMI 1640 incomplete medium, washed twice with 10 ml of RPMI 1640 incomplete medium, and then stored in incomplete RPMI 1640 medium at 4°C for a maximum of 1 day. For neuraminidase treatment, 100 μl of packed RBCs was incubated for 1 h at 37°C with 0.037 U of neuraminidase from Vibrio cholerae (Roche) in 5 ml of RPMI 1640 incomplete medium (pH 6.7), with rocking, and then washed twice with 5 ml of RPMI 1640 incomplete medium. The F2 domain of erythrocyte-binding antigen 175 (EBA-175) (PfF2) was used as a positive control for the enzyme treatment experiments (39).
Invasion inhibition assays.
Purified rabbit anti-PfClag9a, anti-PfClag9b, anti-PfClag9c, and anti-PfCLag9d IgGs were added to highly synchronized schizont-stage cultures (2% hematocrit and 1% parasitemia), at final concentrations of 1, 2, 5, and 8 mg/ml. Preimmune IgG was used as a negative control. The cultures were incubated for 40 h, for schizont rupture and merozoite invasion. Parasitemia levels were determined by flow cytometry. Percentage growth was calculated relative to the control. Means ± SEMs of duplicate measurements are presented.
Seroprevelance analysis.
ELISAs were performed to determine the seroreactivity of PfClag9a, PfClag9b, PfClag9c, and PfClag9d with sera from naturally infected malaria patients, as described earlier (40). Sera from 28 P. falciparum malaria patients in India and Liberia (hyperimmune) were used, while sera from 28 Danish volunteers were used as the negative controls. Briefly, 96-well, polystyrene, flat-bottom plates (Nunc-Maxisorp; Thermo-Scientific) were coated with 100 μl of 5 μg/ml PfClag9a, PfClag9b, PfClag9c, or PfClag9d protein in carbonate/bicarbonate buffer and incubated overnight at 4°C. After blocking, the wells were incubated for 1 h at room temperature with Indian, Liberian, or Danish serum samples (1:200 dilution), followed by incubation for 1 h at room temperature with HRP-conjugated goat anti-human IgG (1:3,000 dilution; Sigma). The bound antibody was detected with 3,3′,5,5′-tetramethylbenzidine (TMB) solution (Sigma). Plates were washed extensively with PBST between incubation periods. The optical density at 450 nm (OD450) was measured. The positivity thresholds were determined from the mean reactivities (+2 standard deviations) of 28 serum samples from nonimmune Danish volunteers.
Active immunization of mice and P. berghei challenge.
For active immunization, three and four BALB/c mice were immunized intraperitoneally with 50 μg of purified recombinant PfClag9d protein fragment per mouse, formulated with complete Freund’s adjuvant, for the first and second experiments, respectively. Booster doses were given on day 14 and day 28, with incomplete Freund’s adjuvant. Three mice that received only PBS with the respective adjuvant were used as controls for both experiments. On day 35, both immunized and control mice were challenged with 1 × 104 P. berghei (ANKA strain)-infected RBCs suspended in 100 μl PBS (pH 7.4). Parasitemia was monitored daily by microscopic examination of Giemsa-stained blood smears.
Statistical analysis.
Graphing and statistical analyses were performed with GraphPad Prism v7. Single-factor analysis of variance was performed using Microsoft Excel.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Department of Biotechnology, Ministry of Science and Technology, for supporting the laboratory under projects BT/01/CEIB/11/V/01 and BT/PR5267/MED/15/87/2012, supervised by P.M. The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
We thank Ashok Das and Rakesh Kumar Singh from ICGEB for their help and technical assistance.
We declare no competing financial interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2017. World malaria report 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CL, Smith DL, Hay SI, Cibulskis RE, Gething PW. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. 2016. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev 40:343–372. doi: 10.1093/femsre/fuw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanner M, Greenwood B, Whitty CJ, Ansah EK, Price RN, Dondorp AM, von Seidlein L, Baird JK, Beeson JG, Fowkes FJ, Hemingway J, Marsh K, Osier F. 2015. Malaria eradication and elimination: views on how to translate a vision into reality. BMC Med 13:167. doi: 10.1186/s12916-015-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller LH, Aikawa M, Dvorak JA. 1975. Malaria (Plasmodium knowlesi) merozoites: immunity and the surface coat. J Immunol 114:1237–1242. [PubMed] [Google Scholar]
- 6.Cowman AF, Tonkin CJ, Tham WH, Duraisingh MT. 2017. The molecular basis of erythrocyte invasion by malaria parasites. Cell Host Microbe 22:232–245. doi: 10.1016/j.chom.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Hadley T, Aikawa M, Miller LH. 1983. Plasmodium knowlesi: studies on invasion of rhesus erythrocytes by merozoites in the presence of protease inhibitors. Exp Parasitol 55:306–311. doi: 10.1016/0014-4894(83)90027-9. [DOI] [PubMed] [Google Scholar]
- 8.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. 2011. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 9:9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko O, Tsuboi T, Ling IT, Howell S, Shirano M, Tachibana M, Cao YM, Holder AA, Torii M. 2001. The high molecular mass rhoptry protein, RhopH1, is encoded by members of the clag multigene family in Plasmodium falciparum and Plasmodium yoelii. Mol Biochem Parasitol 118:223–231. doi: 10.1016/s0166-6851(01)00391-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko O. 2007. Erythrocyte invasion: vocabulary and grammar of the Plasmodium rhoptry. Parasitol Int 56:255–262. doi: 10.1016/j.parint.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Sherling ES, Knuepfer E, Brzostowski JA, Miller LH, Blackman MJ, van Ooij C. 2017. The Plasmodium falciparum rhoptry protein RhopH3 plays essential roles in host cell invasion and nutrient uptake. Elife 6:e23239. doi: 10.7554/eLife.23239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko O, Yim Lim BY, Iriko H, Ling IT, Otsuki H, Grainger M, Tsuboi T, Adams JH, Mattei D, Holder AA, Torii M. 2005. Apical expression of three RhopH1/Clag proteins as components of the Plasmodium falciparum RhopH complex. Mol Biochem Parasitol 143:20–28. doi: 10.1016/j.molbiopara.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Trenholme KR, Gardiner DL, Holt DC, Thomas EA, Cowman AF, Kemp DJ. 2000. clag9: a cytoadherence gene in Plasmodium falciparum essential for binding of parasitized erythrocytes to CD36. Proc Natl Acad Sci U S A 97:4029–4033. doi: 10.1073/pnas.040561197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt DC, Gardiner DL, Thomas EA, Mayo M, Bourke PF, Sutherland CJ, Carter R, Myers G, Kemp DJ, Trenholme KR. 1999. The cytoadherence linked asexual gene family of Plasmodium falciparum: are there roles other than cytoadherence? Int J Parasitol 29:939–944. doi: 10.1016/S0020-7519(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Thiruvengadam G, Desai SA. 2015. The conserved clag multigene family of malaria parasites: essential roles in host-pathogen interaction. Drug Resist Updat 18:47–54. doi: 10.1016/j.drup.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldwin MR, Li X, Hanada T, Liu SC, Chishti AH. 2015. Merozoite surface protein 1 recognition of host glycophorin A mediates malaria parasite invasion of red blood cells. Blood 125:2704–2711. doi: 10.1182/blood-2014-11-611707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling IT, Florens L, Dluzewski AR, Kaneko O, Grainger M, Yim Lim BYS, Tsuboi T, Hopkins JM, Johnson JR, Torii M, Bannister LH, Yates JR, Holder AA, Mattei D. 2004. The Plasmodium falciparum clag9 gene encodes a rhoptry protein that is transferred to the host erythrocyte upon invasion. Mol Microbiol 52:107–118. doi: 10.1111/j.1365-2958.2003.03969.x. [DOI] [PubMed] [Google Scholar]
- 18.Goel S, Valiyaveettil M, Achur RN, Goyal A, Mattei D, Salanti A, Trenholme KR, Gardiner DL, Gowda DC. 2010. Dual stage synthesis and crucial role of cytoadherence-linked asexual gene 9 in the surface expression of malaria parasite var proteins. Proc Natl Acad Sci U S A 107:16643–16648. doi: 10.1073/pnas.1002568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowman AF, Crabb BS. 2006. Invasion of red blood cells by malaria parasites. Cell 124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Nacer A, Roux E, Pomel S, Scheidig-Benatar C, Sakamoto H, Lafont F, Scherf A, Mattei D. 2011. Clag9 is not essential for PfEMP1 surface expression in non-cytoadherent Plasmodium falciparum parasites with a chromosome 9 deletion. PLoS One 6:e29039. doi: 10.1371/journal.pone.0029039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sam-Yellowe TY, Perkins ME. 1991. Interaction of the 140/130/110 kDa rhoptry protein complex of Plasmodium falciparum with the erythrocyte membrane and liposomes. Exp Parasitol 73:161–171. doi: 10.1016/0014-4894(91)90019-s. [DOI] [PubMed] [Google Scholar]
- 22.Sam-Yellowe TY, Shio H, Perkins ME. 1988. Secretion of Plasmodium falciparum rhoptry protein into the plasma membrane of host erythrocytes. J Cell Biol 106:1507–1513. doi: 10.1083/jcb.106.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rungruang T, Kaneko O, Murakami Y, Tsuboi T, Hamamoto H, Akimitsu N, Sekimizu K, Kinoshita T, Torii M. 2005. Erythrocyte surface glycosylphosphatidyl inositol anchored receptor for the malaria parasite. Mol Biochem Parasitol 140:13–21. doi: 10.1016/j.molbiopara.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Pinzon CG, Curtidor H, Garcia J, Vanegas M, Vizcaino C, Patarroyo MA, Patarroyo ME. 2010. Sequences of the Plasmodium falciparum cytoadherence-linked asexual protein 9 implicated in malaria parasite invasion to erythrocytes. Vaccine 28:2653–2663. doi: 10.1016/j.vaccine.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Baldwin M, Yamodo I, Ranjan R, Li X, Mines G, Marinkovic M, Hanada T, Oh SS, Chishti AH. 2014. Human erythrocyte band 3 functions as a receptor for the sialic acid-independent invasion of Plasmodium falciparum: role of the RhopH3-MSP1 complex. Biochim Biophys Acta 1843:2855–2870. doi: 10.1016/j.bbamcr.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salinas ND, Tolia NH. 2016. Red cell receptors as access points for malaria infection. Curr Opin Hematol 23:215–223. doi: 10.1097/MOH.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR, Udenze K, Bronner IF, Casandra D, Mayho M, Brown J, Li S, Swanson J, Rayner JC, Jiang RHY, Adams JH. 2018. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 360:eaap7847. doi: 10.1126/science.aap7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letunic I, Doerks T, Bork P. 2015. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res 43:D257–D260. doi: 10.1093/nar/gku949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalbfleisch T, Cambon A, Wattenberg BW. 2007. A bioinformatics approach to identifying tail-anchored proteins in the human genome. Traffic 8:1687–1694. doi: 10.1111/j.1600-0854.2007.00661.x. [DOI] [PubMed] [Google Scholar]
- 30.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 31.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 32.Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 33.Alam MS, Choudhary V, Zeeshan M, Tyagi RK, Rathore S, Sharma YD. 2015. Interaction of Plasmodium vivax tryptophan-rich antigen PvTRAg38 with band 3 on human erythrocyte surface facilitates parasite growth. J Biol Chem 290:20257–20272. doi: 10.1074/jbc.M115.644906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sachdeva S, Mohmmed A, Dasaradhi PV, Crabb BS, Katyal A, Malhotra P, Chauhan VS. 2006. Immunogenicity and protective efficacy of Escherichia coli expressed Plasmodium falciparum merozoite surface protein-142 using human compatible adjuvants. Vaccine 24:2007–2016. doi: 10.1016/j.vaccine.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 35.Wittig I, Braun H-P, Schagger H. 2006. Blue native PAGE. Nat Protoc 1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 36.Paul G, Deshmukh A, Kaur I, Rathore S, Dabral S, Panda A, Singh SK, Mohmmed A, Theisen M, Malhotra P. 2017. A novel Pfs38 protein complex on the surface of Plasmodium falciparum blood-stage merozoites. Malar J 16:79. doi: 10.1186/s12936-017-1716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh S, Pandey K, Chattopadhayay R, Yazdani SS, Lynn A, Bharadwaj A, Ranjan A, Chitnis C. 2001. Biochemical, biophysical, and functional characterization of bacterially expressed and refolded receptor binding domain of Plasmodium vivax Duffy-binding protein. J Biol Chem 276:17111–17116. doi: 10.1074/jbc.M101531200. [DOI] [PubMed] [Google Scholar]
- 38.Gaur D, Storry JR, Reid ME, Barnwell JW, Miller LH. 2003. Plasmodium falciparum is able to invade erythrocytes through a trypsin-resistant pathway independent of glycophorin B. Infect Immun 71:6742–6746. doi: 10.1128/iai.71.12.6742-6746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandey KC, Singh S, Pattnaik P, Pillai CR, Pillai U, Lynn A, Jain SK, Chitnis CE. 2002. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol Biochem Parasitol 123:23–33. doi: 10.1016/s0166-6851(02)00122-6. [DOI] [PubMed] [Google Scholar]
- 40.Courtin D, Oesterholt M, Huismans H, Kusi K, Milet J, Badaut C, Gaye O, Roeffen W, Remarque EJ, Sauerwein R, Garcia A, Luty AJF. 2009. The quantity and quality of African children’s IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS One 4:e7590. doi: 10.1371/journal.pone.0007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.