Abstract
Background
Vitamin D (Vit D) function in asthma progression has been studied well. The effects of genetic variations in Vit D pathway molecules have been also studied, although the results are contradicted. In the present study, for the first time we examined the Vit D pathway molecules included serum Vit D and vitamin D‐binding protein (VDBP) and also genetic variations in the vitamin D receptor (VDR) and VDBP in a Kurdish population with asthma.
Methods
An enzyme‐linked immunosorbent assay (ELISA) method was used to measure the serum Vit D and VDBP. VDR rs1544410 and rs2228570 and VDBP rs7041 were assessed by polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP).
Results
The serum level of Vit D significantly decreased in asthmatic patients versus controls (16.26 ± 6.76 vs 23.05 ± 10.57 ng/mL, P value = .001). We observed an indirect correlation between Vit D and clinical findings. We also found an increased level of serum VDBP in patients as compared to the controls (1044.6 ± 310.82 vs 545.95 ± 121.73 µg/mL, P value < .0001). Besides, the risk of asthma progression was increased in patients with the VDR rs2228570 CC and VDBP rs7041 GG genotypes (OR = 3.56, P = .0382 and OR = 2.58, P = .01, respectively).
Conclusion
In summary, our results explain the influence of the genetic variations in VDR and VDBP in addition to Vit D and VDBP serum concentrations on asthma susceptibility in the Kurdish population.
Keywords: 1,25‐dihydroxyvitamin D3; VDBP rs7041; VDR rs2228570; vitamin D receptor; vitamin D‐binding protein
1. INTRODUCTION
Asthma is a chronic inflammation of lungs that characterized by obstruction and hyper‐responsiveness of airways and causes clinical symptoms including shortness of breath, chest tightness or pain, and coughing during night or early in the morning.1 In addition to environmental factor effects, it has been also proved that genetic background affected the pathogenesis of asthma.2 Previous studies showed that a decrease in 1,25‐dihydroxycholecalciferol (1,25‐dihydroxyvitamin D3, Vit D) concentration progresses asthma symptoms due to the immune‐modulatory effects of Vit D.3, 4, 5 It has been previously revealed that the anti‐asthmatic effect of Vit D was applied via inhibiting the T‐cell IL4 production and suppression of Th2 immune response.5, 6, 7, 8 Besides, decreased plasma levels of Vit D result in severe asthma attacks and it has been demonstrated that Vit D supplement prevents the attacks and improves the symptoms.9
The correct function of Vit D is applied via proper transport in circulation by vitamin D‐binding protein (VDBP) and interaction with vitamin D response elements (VDRE) on DNA by making a triple complex with vitamin D receptor (VDR). The protective role of Vit D against asthma comes from the hypothesis that VDR over‐expresses in immune system cells especially in antigen‐presenting cells (APCs) and activated T cells.10 Genetic polymorphisms in the VDR gene effect on Vit D bind to the receptor and thus can reduce its function. Among all reported single nucleotide polymorphisms (SNPs), the association of the rs2228570 (FokI) and rs1544410 (BsmI) with the development of allergic diseases has been well studied.11 Both of those SNPs are located in VDR gene promoter and affect the VDR protein function. Despite the large numbers of studies, previous data showed the inconsistent results about the role of VDR SNPs in the progression of asthma in different populations.12, 13, 14, 15, 16, 17, 18
In addition to VDR, VDBP is bind to Vit D and has an important role in the plasma transportation of Vit D metabolites. The GC gene encoded VDBP and one of the most polymorphic sites in this gene is rs7041, which is located in codon 420 and is responsible for reducing the biological function of VDBP. The role of rs7041 SNP on progression of some respiratory diseases has been previously studied 19; however, there is a paucity of evidence with regard to the relation of those polymorphism with the development of asthma.19, 20, 21
As it was mentioned, the potential involvement of VDR in the progression of asthma has been previously investigated, although the results are controversy. Clinical heterogeneity and criteria for the selection of studied subjects are among the most important reasons in creating contradictory results. Besides, the potential role of VDBP rs7041 on asthma susceptibility is not well understood. Our previous study clearly revealed that alteration in Vit D metabolism including decline serum Vit D concentration, genetic variations in VDR and VDBP genes, and higher levels of serum VDBP increases the risk of chronic urticaria.22 Therefore, the current study was conducted to evaluate the possible association between VDR BsmI, FokI, VDBP HaeIII SNPs, and also Vit D and VDBP concentrations with asthma susceptibility in a Kurdish population.
2. MATERIAL AND METHODS
2.1. Participants
From April 2017 to August 2018, a total of 110 consecutive asthmatic patients who were admitted to the Kurdistan Asthma and Allergy Clinic and also 110 age‐ and sex‐matched healthy subjects were included in this study. The Global Initiative for Asthma (GINA) guidelines were used for the diagnosis of asthma in patients and confirmed by spirometry.23 We measured the forced expiratory volume in 1 s (FEV1) (% of predicted), the forced vital capacity (FVC), and FEV1/FVC ratio,24 and the best values were chosen for further analysis. Based on the severity of symptoms, we then classified the patients into mild, moderate, and severe persistent clusters. The control group selected from the people who came to a clinical laboratory for routine checkup after answer to a checklist and became sure that they did not have any health problem such as allergic disease, immunologic disorders, rheumatologic diseases, and history of chronic or recently acute infection. The patients were allergic asthmatics without any exposure to cigarette and without any history of other forms of allergy. All individuals were from Kurd ethnicity. All participants in the study provided their written consent prior to the study, and the study protocol was approved by the ethical committee of Kurdistan University of Medical Sciences.
2.2. Serum Vit D and VDBP level measurement
Five milliliter blood specimen was obtained from studied subjects through venipuncture. The serum was then separated and kept in −20°C pending evaluation. Two enzyme‐linked immunosorbent assay (ELISA) kits were used for the determination of the serum Vit D (catalog number 7725‐300, Monobind Inc.) and VDBP (catalog number CK‐E10771, Eastbiopharm, China) concentrations and the resulting absorbance read on a Stat Fax 2100 Microplate Reader (Awareness Technology Inc.) apparatus at 450 nm. The results were then expressed as ng/mL for Vit D and µg/mL for VDBP. The limit of detection (LOD) for Vit D and VDBP ELISA kits was 0.67 ng/mL and 5.41 µg/mL, respectively. According to the manufacturer's data sheets, the within‐ and between‐assay precisions were 4.95%, 5.63% and <10%, <12%, for Vit D and VDBP ELISA kits, respectively. Vit D deficiency, insufficiency, and sufficiency were defined as <10, between 10 and 30, and 30‐100 ng/mL.
2.3. Polymorphism genotyping
Whole blood was used for DNA extraction using a DNG‐Plus DNA extraction kit (Sinaclon). Briefly, blood specimens were lysed and DNA selectively precipitated. DNA quality and quantity were evaluated electrophoretically and photometrically. Identification of SNPs in samples was performed using a PCR‐RFLP method. The PCR condition and the primers used for PCR were based on our previous study.22 The PCR products were further used for genotype analysis using RFLP method. Briefly, the product size for VDR rs1544410, VDR rs2228570, and VDBP rs7041 was 248 bp, 341 bp, and 809 bp, respectively. The VDR rs1544410 was cut by FspI restriction enzyme and produced three different DNA bands on gel electrophoresis: homozygote GG (175 + 73 bp), heterozygote GA (248 + 175+73 bp), and homozygote AA (248 bp) (Figure S1). The results for VDR rs2228570 and VDBP rs7041 were as follows (Figure S1):
VDR rs2228570 (BseGI): homozygote TT (289 + 59 bp), heterozygote TC (341 + 289+59 bp), and homozygote CC (341bp).
VDBP rs7041 (BsuRI): homozygote TT (809 bp), heterozygote TG (809 + 577+232 bp), and homozygote GG (577 + 232 bp).
2.4. Statistical analysis
To study the frequencies of alleles and genotypes and also to evaluate the deviation of genotype frequencies from Hardy‐Weinberg equilibrium, a chi‐square test was used. The association between studied variables with risk of disease were assessed by 2 × 2 contingency table, and the odds ratio (OR) and confidence interval (CI) were evaluated. If there was a normality distribution, the parametric tests were used and data were shown as mean ± standard deviation. The independent‐samples t test or Mann‐Whitney statistical test was used for studying the possible differences between studied groups. The Spearman correlation coefficient was performed to evaluate the association between two variables. P value <.05 was considered as statistically significant. All statistical analysis was performed by SPSS 16 software (SPSS Inc.).
3. RESULTS
3.1. Demographic and clinical characteristics
The present study included 110 asthmatic patients and 110 healthy individuals. The male/female frequencies were 49 (44.54%)/61 (55.46%) for the case group and 41 (37.27%)/69 (62.73%) for the control group (P = .14). The age of subjects in mean ± SD was 31.34 ± 7.81 and 28.73 ± 6.89 years, for the patient and normal groups, respectively (P = .203). No significant differences were found between groups for sex and age of subjects. The pulmonary function characteristics were measured, and the corresponding values were as follows: FEV1 (%predicted) = 70.04 ± 20.86, FVC = 69.32 ± 19.31 and FEV1/FVC = 71.99 ± 4.75. With regard to asthma severity, 35 patients (36.8%) had mild symptoms, 24 patients (25.3%) showed moderate symptoms, and 36 patients (37.9%) had severe symptoms.
3.2. Measurement of Vit D and VDBP in serum samples
Concentrations of Vit D in the patient and control groups were 16.26 ± 6.76 and 23.05 ± 10.57 ng/mL, respectively. Figure 1A shows that the serum Vit D significantly decreased in asthmatic patients compared to healthy subjects (P = .001). Vitamin D deficiency or insufficiency was presented in 71.42% of patients, while the corresponding value for the healthy group was 40.32% (P < .0001). The results indicated that decline in Vit D is a risk factor for the progression of asthma (OR = 3.04, 95% CI = 1.32‐7.00, Z statistic = 2.6, P = .0092). Differences of serum Vit D concentration between males and females were not statistically significant, although female subjects had a bit lower Vit D content (P = .19; 21.81 ± 11.4 vs 19.12 ± 8.27 ng/mL). Furthermore, our data did not show a significant association between age of subjects with serum Vit D level (P = .36). On the other hand, serum VDBP concentration increased in patients compared to controls (1044.6 ± 310.82 and 545.95 ± 121.73 µg/mL, respectively), and the difference between groups for VDBP was statistically significant (P < .0001; Figure 1B). Our results also revealed a direct correlation between high level of serum VDBP and development of asthma (Spearman's ρ = 0.61, P = .001).
Figure 1.
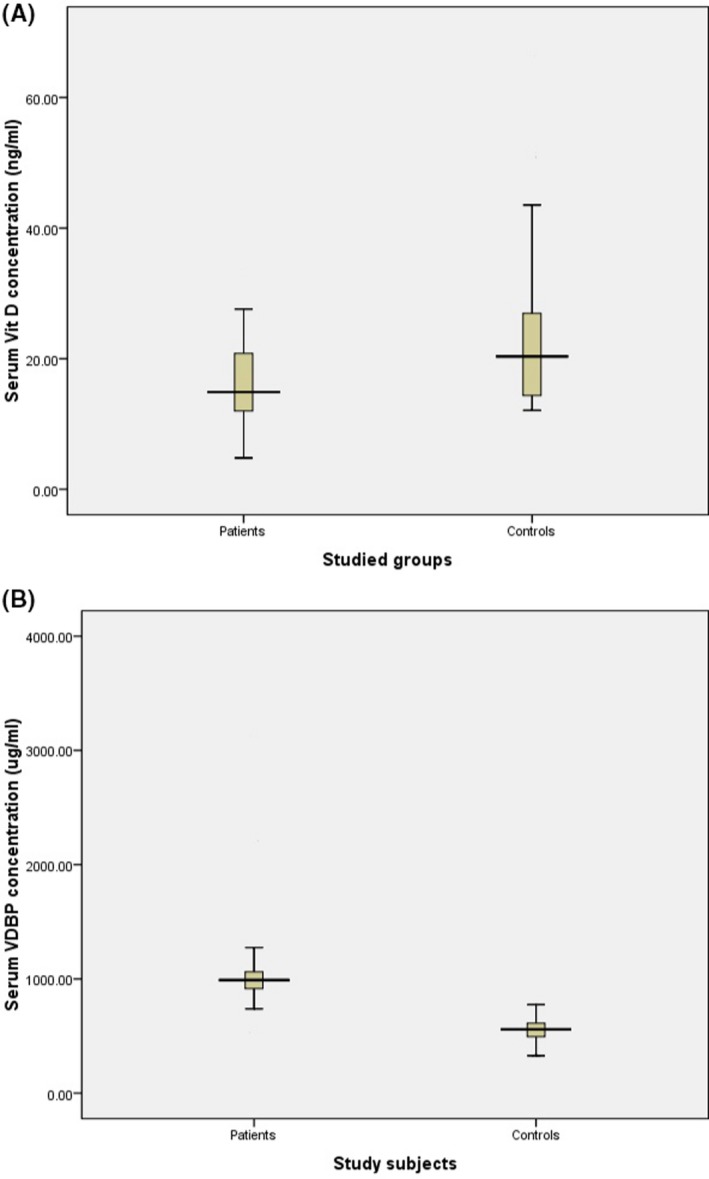
Concentration of Vit D and VDBP in studied subjects. 1A, Vit D is decreased in asthmatic patients compared to controls (P = .001). 1B, VDBP is significantly increased in asthmatic patients compared to controls (P < .0001)
Linear regression analysis showed that rise in serum Vit D directly linked to higher FEV1% in asthmatic patients (r 2 = .79, P < .0001; Figure 2A). Our results also showed a direct correlation between Vit D with FVC (r 2 = .78, P < .0001) and Vit D with FEV1%/FVC (r 2 = .22, P = .001; Figure 2B,C). Furthermore, determination of serum Vit D based on severity of asthma showed a significant decline of Vit D in cases with severe symptoms compared to mild and moderate symptom groups (Figure 2D). In addition, no significant correlation was found between serum VDBP with clinical outcomes including FEV1%, FVC, FEV1%/FVC, asthma severity, and also the age of studied subjects (data not shown).
Figure 2.
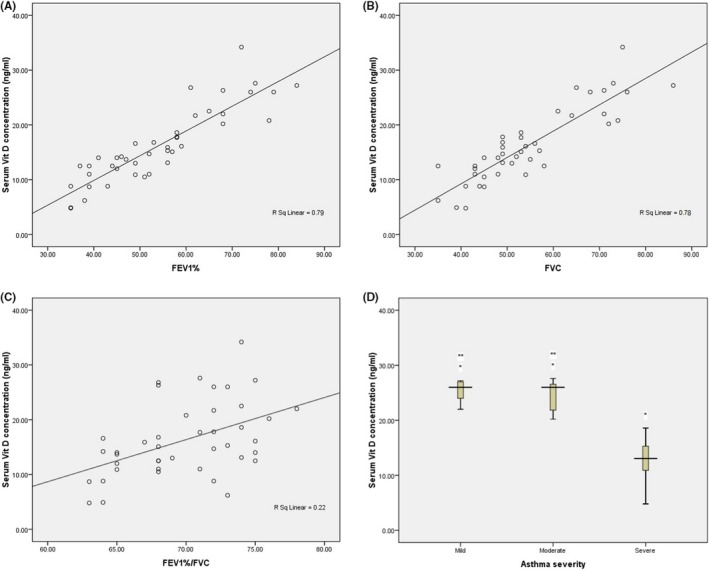
Correlation of serum Vit D with clinical findings of studied subjects. 2A, Positive correlation between serum vitamin D levels and percent predicted forced expiratory volume in 1 s (FEV1%) (r 2 = .79, P < .0001). 2B, Effect of serum levels of vitamin D on the forced vital capacity (FVC) was also significant (r 2 = .78, P < .0001). 2C, Correlation coefficient between serum vitamin D and FEV1%/FVC among patients with asthma (r 2 = 0.22, P = .001). 2D, Concentration of serum vitamin D was statistically significant between patients with mild (25.24 ± 2.21 ng/mL) symptoms compared to severe (12.72 ± 3.66 ng/mL) symptoms (*P < .0001). Patients with moderate (24.92 ± 4.06 ng/mL) symptoms also showed a significant difference for serum vitamin D concentration when compared to patients with severe symptoms (P < .0001). No significant difference was observed between mild and moderate groups for serum vitamin D concentration (**P = .8)
3.3. Genotype analysis
The distribution of genotype and allele frequencies for VDR rs1544410, VDR rs2228570, and VDBP rs7041 SNPs in patients with the asthma and control groups is shown in Table 1. All of the studied SNPs including VDR rs1544410, VDR rs2228570, and VDBP rs7041 were consistent with Hardy‐Weinberg equilibrium (P > .05).
Table 1.
Genotypes and alleles frequencies in the patient and control groups
| Genotype frequencies | Asthmatic patients (N = 110) | Controls (N = 110) | P Value | OR (95% CI) | Allele frequencies (%) | Asthmatic patients (%) | Controls (%) | P Value | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VDR rs1544410 | GG | 65 (59.09%) | 59 (53.64%) | .2 | Reference | VDR rs1544410 | G | 74.55 | 73.18 | .81 | 0.90 (0.48‐1.7) |
| GA | 34 (30.91%) | 43 (39.09%) | .2 | 0.72 (0.41‐1.27) | |||||||
| AA | 11 (10%) | 8 (7.87%) | .6 | 1.25 (0.47‐3.31) | A | 25.45 | 26.82 | .81 | |||
| VDR rs2228570 | TT | 51 (46.36%) | 66 (60%) | .04 | Reference | VDR rs2228570 | T | 68.18 | 78.18 | <.05 | 1.67 (0.89‐3.14) |
| TC | 48 (43.64%) | 40 (36.36%) | .27 | 1.55 (0.89‐2.71) | |||||||
| CC | 11 (10%) | 4 (3.64%) | .03 | 3.56 (1.07‐11.83) | C | 31.82 | 21.82 | <.05 | |||
| VDBP rs7041 | TT | 21 (19.1%) | 29 (26.36%) | .2 | Reference | VDBP rs7041 | T | 40 | 52.73 | .07 | 1.69 (0.97‐2.96) |
| TG | 46 (41.8%) | 58 (52.73%) | .11 | 1.1 (0.55‐2.17) | |||||||
| GG | 43 (39.1%) | 23 (20.91%) | .003 | 2.58 (1.21‐5.5) | G | 60 | 47.27 | .07 | |||
The VDR rs1544410 GG genotype was more frequent in patients compared to control, although the difference was not significant (P = .21). Similar results were seen for GA (P = .21) and AA (P = .58) genotypes. The frequency of G and A alleles had no significant differences between patient and healthy subjects (P = .81), although the frequency of G allele was significantly higher in both groups versus the A allele (P < .0001). The clinical properties of studied subjects did not show any significant difference for VDR rs1544410 SNP. However, serum concentration of VDBP has increased in GG genotype compared to heterozygote GA and homozygote AA genotypes (1269.2 ± 737.67, 1026.4 ± 229.82, and 989.96 ± 130.23 µg/mL, respectively) (P = .03; Figure 3A). Similar results were seen for serum VDBP in patients with GG genotype compared to the AA + GA group (P = .001, Table S1).
Figure 3.
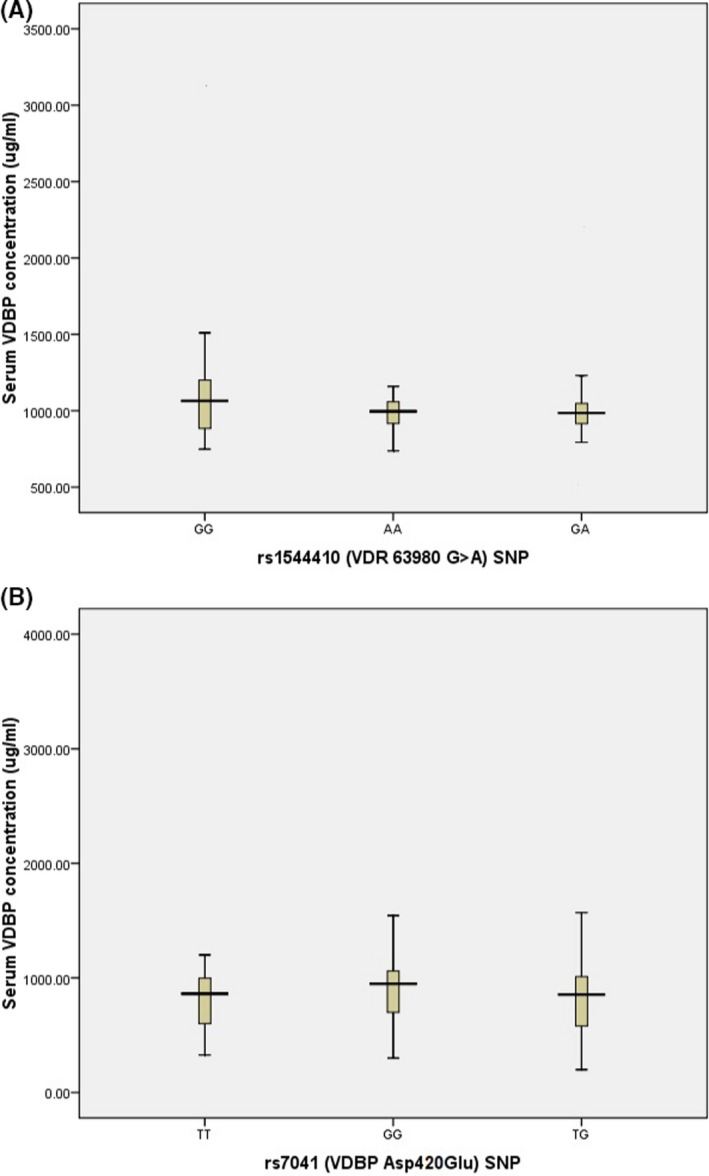
Serum VDBP concentration in different genotypes: 3A, VDBP level (in μg/mL unit) starting with the VDR rs1544410 AA genotype (989.96 ± 130.23 µg/mL) and increasing over about 1026.4 ± 229.82 µg/mL (nearly similar value) for the GA genotype up to about 1269.2 ± 737.67 µg/mL for the GG group with acceptable significance between GG genotype with AA and GA genotypes (P = .03). 3B, VDBP level (in μg/mL unit) was the lowest in the VDBP TG rs7041 (794.58 ± 226.99 µg/ml) and followed by the TT genotype about 816.41 ± 274.03 µg/mL (nearly similar value) and reached to maximum level in GG genotype to 974.79 ± 471.62 µg/mL, and a one‐way ANOVA analysis showed that there is a significant difference between GG genotype with TT and TG genotypes for serum VDBP level (P = .02)
Genotype analysis of VDR rs2228570 showed the higher prevalence for TT genotype in both cases and controls compared to CC genotype. The TT genotype was also higher in control subjects compared to patients with asthma (P = .0424), while the CC genotype had significantly higher frequency in patients as compared to controls (P = .0307). Similar significant differences were also observed when compared to two groups based on T and C alleles (P < .05). Our data showed that CC genotype has a potential role in the progression of asthma (OR = 3.56, 95% CI = 1.07 to 11.83, P = .0383). However, we observed that VDR rs2228570 is not a risk factor for asthma development in allele level (OR = 1.67, 95% CI = 0.89 to 3.14, P = .11). Furthermore, analysis of studied groups did not show any correlation between VDR rs2228570 SNP and clinical findings of asthmatic patients.
The rate of the GG‐rs7041 was increased in the patient group compared to healthy subjects (39.1% vs 20.91%, P = .003). In addition, GG genotype was a potential risk factor for asthma development (OR = 2.58, 95% CI = 1.21‐5.5, P = .014). Our data showed that VDBP‐rs7041 has no significant association with clinical findings included serum Vit D, FEV1%, FVC, and FEV1/FVC. However, serum VDBP concentration was observed to be increased in GG genotype compared to TT and TG genotype (974.79 ± 471.62, 816.41 ± 274.03, and 794.58 ± 226.99 µg/mL, respectively) (P = .02, Figure 3B). In addition, the VDBP serum concentration was found to be increased in patients with GG genotype when compared to the TT + TG group (P = .028, Table S1). We also found that the GG‐rs7041 was a potential risk factor for asthma development compared to TT + TG (OR = 2.43, 95% CI = 1.33‐4.42, P = .004; Table S2).
4. DISCUSSION
Here, we demonstrated that serum Vit D level declined in patients with asthma. We also observed the higher concentration of VDBP in those patients. A significant indirect correlation was found between serum Vit D and VDBP. Decline in Vit D levels linked to clinical findings included FEV1%, FVC, FEV/FVC, and asthma severity, while increased levels of the serum VDBP did not link with those characteristics. We also evaluated the frequency of VDR rs1544410, rs2228570, and VDBP rs7041 SNPs and the progressive role of these variations in asthma patients. The CC genotype of the VDR rs2228570 and the GG genotype of VDBP rs7041 were observed to be significantly increased in patients compared to controls and this increase associated with asthma development. To the best of our knowledge, our study is the first one that concurrently assessed the role of Vit D pathway including VDR and VDBP in the progression of asthma in the Kurd ethnicity.
The effect of Vit D on reducing the asthma exacerbation has been previously well studied. Vit D concentration inversely correlates with asthma progression and pulmonary function. Furthermore, Vit D supplements showed a protective role among patients with asthma.25 The immune‐modulatory effects of Vit D applies through direct targeting of immune system cells included B cells, T cells, dendritic cells, and macrophages.26, 27 Similarly, our data indicated decrease of Vit D in patients and we also observed a direct correlation between Vit D and clinical findings in the studied subjects.
It has been previously showed that higher level of VDBP decreases the bioactivity of Vit D. Gupta et al 28 showed that VDBP increased in biological fluids of children with severe therapy‐resistant asthma and the association between rises in BAL‐VDBP with asthma control was significant. More recently, Jiang et al 29 revealed that VDBP increased in patients with steroid‐resistant asthma as compared to steroid‐sensitive asthma. They proposed that serum VDBP may apply as a useful biomarker for predicting the resistance to steroid in asthma patients. In line with these studies, we found that serum VDBP was increasing in asthma patients, although our results did not show any link between VDBP and clinical characteristics.
It has been shown that variations in VDR gene may impact on progression of asthma. Tizaoui et al 30 studied a total of eight previous case‐control studies and showed a significant association between homozygous wild type of rs1544410 with risk of asthma (OR = 2.017, 95% CI = 1.236‐3.851, P = .017), although it seems that the VDR rs2228570 is not a probable risk factor for development of asthma in the codominant model (OR = 1.187, 95% CI = 0.975‐1.446, P = .088). They proposed that study features may influence on the association between rs2228570 and asthma susceptibility. More recently, Zhao et al 31 investigated the possible correlation between polymorphisms in VDR gene and susceptibility to childhood asthma. The highest significant odds ratio was seen in the case of ApaI polymorphism in homozygous and allele models (1.674 and 1.221, respectively). They were also found that Asian ethnicity with ApaI SNP has a higher risk to the progression of asthma. With regard to rs2228570 and rs1544410 polymorphisms, they showed that VDR rs1544410 may be significantly associated with progression of asthma in homozygous (OR = 1.462, 95% CI = 1.016‐2.105, P = .041) and allele level (OR = 1.181, 95% CI = 1.006‐1.386, P = .042) in Caucasian population. Similarly, VDR rs2228570 was also a risk factor for childhood asthma susceptibility in dominant (OR = 1.281, 95% CI = 1.055‐1.555, P = .012) and allele (OR = 1.591, 95% CI = 1.052‐2.405, P = .028) models in Caucasian patients. In contrast, Hou et al 32 did not find any correlation between VDR rs1544410 with childhood bronchial asthma. In our study, no association was found between VDR rs1544410 loci with asthma susceptibility in the Kurdish population. However, we found that VDR rs2228570 polymorphism should be considered as a potential risk factor for the progression of asthma in the Kurd ethnicity.
There was a paucity of studies that investigated the impact of VDBP genetic variations on asthma susceptibility. The effect of VDBP SNPs on progression of COPD has been previously investigated.20 Those results revealed that GC1f and GC2 alleles may be linked to sputum hypersecretion in COPD patients. In another study, Ismail et al 33 observed that homozygous GG genotype of the VDBP rs2282679 may be linked to asthma susceptibility and clinical findings in asthmatic patients including lung functions, asthma severity, and concentration of IgE and Vit D. In addition, Randolph et al 34 showed that carriers of C allele of the VDBP rs7041 had a higher risk to the progression of respiratory syncytial virus bronchiolitis and later asthma development (OR = 1.12, 95% CI = 1.02‐1.4, P = .03). Li et al 21 also showed that compared to GC1, patients with GC2 haplotype are more susceptible for the development of asthma. Contrarily, VDBP GC1s have been proposed as a protective factor for the progression of asthma when compared to GC1f/1f genotype in the Hispanic population.35 More recently, Fawzy et al 36 observed that GG genotype and G allele of the VDBP rs7041 are a potential risk factor for asthma development, while rs4588 AA genotype and A allele had a protection role in that study. In line with previous results, our study revealed a provocative effect for GG genotype of VDBP rs7041 in asthma development of the Kurdish population.
Our study had several limitations that must be noted; first limitation is linked to the numbers of studied SNPs. We only evaluated two VDR SNPs and one VDBP polymorphism, while there are more SNPs that could influence on disease progression. Second, we could not rule out the effects of environmental factors on studied subjects. Finally, we only sampled single blood specimen and subsequently measured Vit D and VDBP in those samples; hence, the role of biological variations must be considered.
In conclusion, we have identified that decrease of Vit D is associated with high level of VDBP and those phenomenon involve in the pathogenesis of asthma. Besides, we found that VDR rs2228570 and VDBP rs7041 correlated to asthma exacerbation and should be considered as potential genetic factors in asthma progression in the Kurdish population.
CONFLICT OF INTEREST
Dr R Nasiri‐Kalmarzi declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Dr M Abdi has received research grants from Kurdistan University of Medical Sciences. Mr J Hosseini declares that he has no conflict of interest. Mrs S Tavana declares that she has no conflict of interest. Dr A Mokarizadeh declares that he has no conflict of interest. Mr R Rahbari declares that he has no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors contributed equally in this work.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of Kurdistan University of Medical Sciences and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Supporting information
ACKNOWLEDGMENTS
The authors wish to thank all patients and health stuffs who participated in this study. Financial support from Kurdistan University of Medical Sciences is highly appreciated.
Nasiri‐Kalmarzi R, Abdi M, Hosseini J, Tavana S, Mokarizadeh A, Rahbari R. Association of vitamin D genetic pathway with asthma susceptibility in the Kurdish population. J Clin Lab Anal. 2020;34:e23039 10.1002/jcla.23039
Funding information
This work was supported by a research grant from Kurdistan University of Medical Sciences (Grant/Award Number: “1394/358”).
REFERENCES
- 1. Boulet LP, FitzGerald JM, Levy ML, et al. A guide to the translation of the Global Initiative for Asthma (GINA) strategy into improved care. Eur Respir J. 2012;39(5):1220‐1229. [DOI] [PubMed] [Google Scholar]
- 2. Tian HQ, Cheng L. The role of vitamin D in allergic rhinitis. Asia Pac Allergy. 2017;7(2):65‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shifren A, Witt C, Christie C, Castro M. Mechanisms of remodeling in asthmatic airways. J Allergy (Cairo). 2012;2012:316049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta‐analysis. J Allergy Clin Immunol. 2011;127(3): 724.e30‐733.e30. [DOI] [PubMed] [Google Scholar]
- 5. Ali NS, Nanji K. A review on the role of vitamin D in asthma. Cureus. 2017;9(5):e1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlberg C, Seuter S, Heikkinen S. The first genome‐wide view of vitamin D receptor locations and their mechanistic implications. Anticancer Res. 2012;32(1):271‐282. [PubMed] [Google Scholar]
- 7. Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126(1):52 e5‐58 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482‐496. [DOI] [PubMed] [Google Scholar]
- 9. Martineau AR, Cates CJ, Urashima M, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;5(9):CD011511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25‐dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374(2):334‐338. [DOI] [PubMed] [Google Scholar]
- 11. Yazici D, Yavuz D, Tarcin O, Sancak S, Deyneli O, Akalin S. Vitamin D receptor gene ApaI, TaqI, FokI and BsmI polymorphisms in a group of Turkish patients with Hashimoto's thyroiditis. Minerva Endocrinol. 2013;38(2):195‐201. [PubMed] [Google Scholar]
- 12. Bosse Y, Lemire M, Poon AH, et al. Asthma and genes encoding components of the vitamin D pathway. Respir Res. 2009;24(10):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fang WL, Gao LB, Liang WB, et al. Association analysis of vitamin D receptor gene polymorphisms in chinese population with asthma. Iran J Allergy Asthma Immunol. 2009;8(3):141‐147. [PubMed] [Google Scholar]
- 14. Poon AH, Laprise C, Lemire M, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170(9):967‐973. [DOI] [PubMed] [Google Scholar]
- 15. Raby BA, Lazarus R, Silverman EK, et al. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170(10):1057‐1065. [DOI] [PubMed] [Google Scholar]
- 16. Saadi A, Gao G, Li H, Wei C, Gong Y, Liu Q. Association study between vitamin D receptor gene polymorphisms and asthma in the Chinese Han population: a case‐control study. BMC Med Genet. 2009;21(10):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vollmert C, Illig T, Altmuller J, et al. Single nucleotide polymorphism screening and association analysis–exclusion of integrin beta 7 and vitamin D receptor (chromosome 12q) as candidate genes for asthma. Clin Exp Allergy. 2004;34(12):1841‐1850. [DOI] [PubMed] [Google Scholar]
- 18. Wjst M. Variants in the vitamin D receptor gene and asthma. BMC Genet. 2005;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chishimba L, Thickett DR, Stockley RA, Wood AM. The vitamin D axis in the lung: a key role for vitamin D‐binding protein. Thorax. 2010;65(5):456‐462. [DOI] [PubMed] [Google Scholar]
- 20. Laufs J, Andrason H, Sigvaldason A, et al. Association of vitamin D binding protein variants with chronic mucus hypersecretion in Iceland. Am J Pharmacogenomics. 2004;4(1):63‐68. [DOI] [PubMed] [Google Scholar]
- 21. Li F, Jiang L, Willis‐Owen SA, Zhang Y, Gao J. Vitamin D binding protein variants associate with asthma susceptibility in the Chinese Han population. BMC Med Genet. 2011;12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nasiri‐Kalmarzi R, Abdi M, Hosseini J, Babaei E, Mokarizadeh A, Vahabzadeh Z. Evaluation of 1,25‐dihydroxyvitamin D3 pathway in patients with chronic urticaria. QJM. 2018;111(3):161‐169. [DOI] [PubMed] [Google Scholar]
- 23. Bateman E, Hurd S, Barnes P, et al. Global strategy for asthma management and prevention: GINA executive summar. Eur Respir J. 2018;51(2):143‐178. [DOI] [PubMed] [Google Scholar]
- 24. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 25. Litonjua AA. Vitamin D and childhood asthma: causation and contribution to disease activity. Curr Opin Allergy Clin Immunol. 2019;19(2):126‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adorini L, Penna G, Giarratana N, et al. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J Steroid Biochem Mol Biol. 2004;89‐90(1‐5):437‐441. [DOI] [PubMed] [Google Scholar]
- 27. Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25‐dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181‐1183. [DOI] [PubMed] [Google Scholar]
- 28. Gupta A, Dimeloe S, Richards DF, Bush A, Saglani S, Hawrylowicz CM. Vitamin D binding protein and asthma severity in children. J Allergy Clin Immunol. 2012;129(6):1669‐1671. [DOI] [PubMed] [Google Scholar]
- 29. Jiang H, Chi X, Zhang X, Wang J. Increased serum VDBP as a risk predictor for steroid resistance in asthma patients. Respir Med. 2016;114:111‐116. [DOI] [PubMed] [Google Scholar]
- 30. Tizaoui K, Berraies A, Hamdi B, Kaabachi W, Hamzaoui K, Hamzaoui A. Association of vitamin D receptor gene polymorphisms with asthma risk: systematic review and updated meta‐analysis of case‐control studies. Lung. 2014;192(6):955‐965. [DOI] [PubMed] [Google Scholar]
- 31. Zhao DD, Yu DD, Ren QQ, Dong B, Zhao F, Sun YH. Association of vitamin D receptor gene polymorphisms with susceptibility to childhood asthma: a meta‐analysis. Pediatr Pulmonol. 2017;52(4):423‐429. [DOI] [PubMed] [Google Scholar]
- 32. Hou C, Zhu X, Chang X. Correlation of vitamin D receptor with bronchial asthma in children. Exp Ther Med. 2018;15(3):2773‐2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ismail MF, Elnady HG, Fouda EM. Genetic variants in vitamin D pathway in Egyptian asthmatic children: a pilot study. Hum Immunol. 2013;74(12):1659‐1664. [DOI] [PubMed] [Google Scholar]
- 34. Randolph AG, Yip WK, Falkenstein‐Hagander K, et al. Vitamin D‐binding protein haplotype is associated with hospitalization for RSV bronchiolitis. Clin Exp Allergy. 2014;44(2):231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Navas‐Nazario A, Li FY, Shabanova V, et al. Effect of vitamin D‐binding protein genotype on the development of asthma in children. Ann Allergy Asthma Immunol. 2014;112(6):519‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fawzy MS, Elgazzaz MG, Ibrahim A, Hussein MH, Khashana MS, Toraih EA. Association of group‐specific component exon 11 polymorphisms with bronchial asthma in children and adolescents. Scand J Immunol. 2019;89(3):e12740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


