Abstract
Objective
Socket prosthesis attachment is the current gold standard for limb amputees. Osseointegrated implantation is a novel technique that has many proposed advantages over the current gold standard. Clear advantages for its use over socket prosthetic attachment has been well established in literature. It decreases socket problems as pinching, pressure points, chronic skin problems and frequent socket change due to atrophy of muscles.
Methods
We reviewed primary research articles documenting complication rates and outcome measures in patients with osseointegrated prosthesis implantation after limb amputation.
Results
Nine studies were identified with a total of 211–242 patients. Clinical, radiographic, and functional outcomes, as well as complications were considered. The mean duration of follow-up was greater than 12 months in all studies.
Conclusions
Osseointegration is an effective alternative to socket prosthesis in transfemoral amputees. Transtibial and upper extremity implants are underreported in the literature and clear indication for their effectiveness over socket prosthesis does not exist. Minor complications are most common, such as soft tissue infections, and may be mitigated in the future by improvements in surgical technique and implant design.
The level of evidence is 3.
Keywords: Osseointegration, Transfemoral, Transtibial, Amputation
1. Background
Lower extremity amputation creates a significant impact on the patient's functional capabilities and quality of life.1,2 Prolonged rehabilitation is required for fitting in a traditional suspended socket prosthesis. The higher the level of amputation, the more difficult the fit for the socket prosthesis.3,4 It has been reported that up to one-third of transfemoral amputation treated with socket prostheses have chronic skin problems associated with the socket of the prosthesis, which can have a negative impact on mobility and quality of life.5, 6, 7, 8 While new materials and socket designs have been developed, skin problems remain a burden for these patients, as the skin in weight-bearing areas undergoes considerable friction and pressure during ambulation.
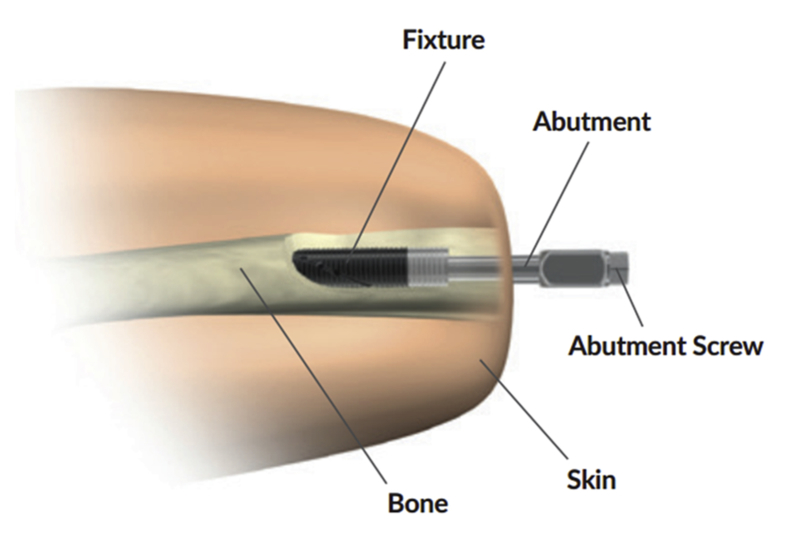
Osseointegration (OI) is a technique in which the artificial limb is anchored into the bone in an attempt to mitigate these problems (Fig. 1).9 While this technique is well established in dentistry, it has only recently begun to be used in the field of extremity amputation.10, 11, 12, 13 This technique has many proposed advantages, including direct prosthesis control, improved stability, increased walking ability, improved functional capacity, and an increase in the overall quality of life.13, 14, 15 (see Fig. 2)
Fig. 1.
The Osseointegrated Prostheses for the Rehabilitation of Amputees (OPRA) Implant System. It includes three main parts: an implanted fixture, an abutment, and an abutment screw. The first operation implants the fixture into the residual bone. The percutaneous parts (the abutment and the sbutment screw) are installed into the fixture six months later in a second operation. Replacements to these latter two components can be made if needed. A percutaneous area where the implant protrudes from the residual limb is created during the second operation. This figure is reproduced with permission from Brånemark R, Berlin O, Hagberg K et al. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: A prospective study of 51 patients. Bone Joint J 2014; 96-B:106–113.
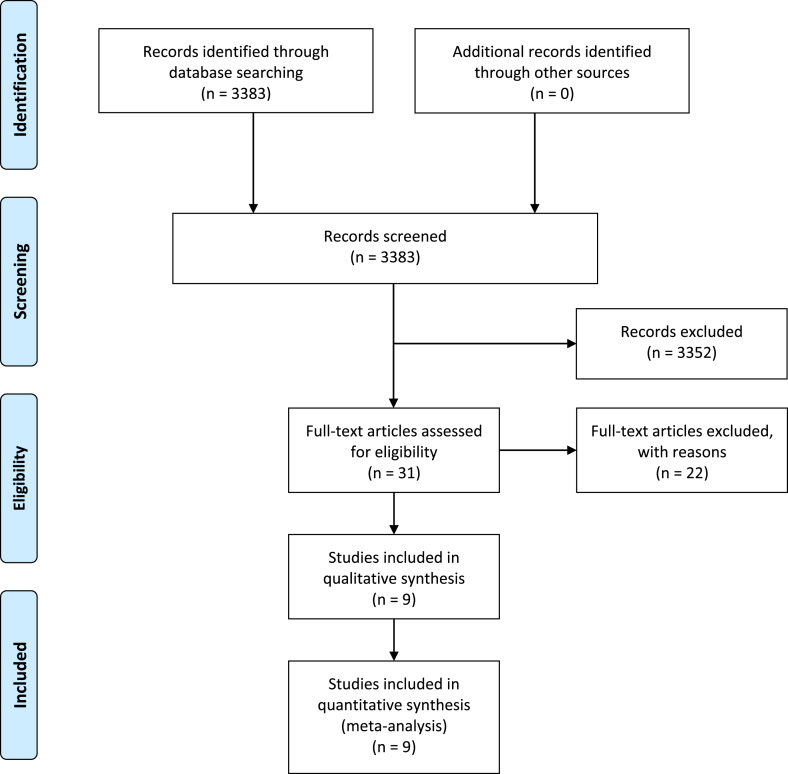
Fig. 2.
Flow diagram depicting study selection algorithm (created using PRISMA 2009 Flow diagram, version 2.1.3).
The purpose of this review is to examine the positive outcomes and complication rates of OI as an appropriate treatment for both upper and lower limb amputation.
2. Methods
This systematic review was conducted according to the guidelines described in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols 2015 (PRISMA-P 2015).16 Controlled and non-controlled follow-up studies, provided that the latter included both baseline and follow-up data, were included. The focus was on clinical outcomes measures and reported complication rates, thus results collected were in patients’ natural contexts. Data collected in a laboratory setting or controlled studies that included a population without amputation as a control group were excluded. The study population included lower limb and upper extremity prosthetic users over 18 years of age; trans-tibial (below-knee) prosthesis; trans-femoral (above-knee) prosthesis; trans-radial prosthesis; trans-ulnar prosthesis; or trans-humeral (above-elbow) prosthesis. The control intervention (if applicable) should include either some other prosthesis system or no prosthesis system.
We considered studies focusing primarily on activity and/or participation and/or quality of life outcomes and/or reported complications with the osseointegrated implant and/or cost outcomes.
2.1. Protocol
Methods for this systematic review were prespecified in a protocol which was uploaded to PROSPERO in May 2019.
2.2. Search strategy
All published studies evaluating clinical and adverse outcomes of OI in transfemoral, transtibial, or upper extremity amputations were selected. The authors selected keywords and synonyms based on the inclusion and exclusion criteria. The search was performed without language restrictions during May 2019 in the following databases: Cochrane Central Register of Controlled Trials, PubMed, CINAHL, EMBASE, Web of Science, and OVID MEDLINE. The search strategy was as follows: (osseointegrat* OR osseo-integrat* OR bone-anchored prosthe*) AND (amput*). The references of relevant articles were also reviewed for additional publications. Articles were not restricted based on language, study design, publication status or date.
2.3. Study selection
Two authors (CG and EP) independently went through titles and abstracts of the identified studies and excluded duplicates and studies that were not in accordance with the inclusion criteria. Potential studies identified were read in full text. Inclusion and exclusion criteria identified. Descriptions of design, methods, results (Table 1) are displayed as a result of the data extraction process.
Table 1.
Summary of included studies.
| Studies of Outcomes in Osseointegration Prosthesis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Title | Study Design | Years of data collection | # Patients | # Implants | Site of Amputation | Cause of Amputation | Mean Follow-up | Results | Complications (not explicitly found in results) |
| Nebergall (2012) | Stable fixation of OI system for above knee amputees | Prospective case series | 1999–2007 (OPRA) | 51 | 55 | Transfemoral (unilateral only) | Trauma, n = 23(%) Tumor, n = 11(%) Other, n = 5(%) | 24 mo |
|
|
|
|
|||||||||
|
|
|||||||||
| Branemark (2014) | A novel OI percutaneous prosthetic system | Prospective case series | 1999–2007 (OPRA) | 51 | 55 | Transfemoral | Trauma, n = 33(65%) Tumor, n = 12(24%) | 24 mo |
|
|
|
|
|||||||||
|
|
|||||||||
| ||||||||||
| ||||||||||
| ||||||||||
| Hagberg (2014) | Outcome of percutaneous OI prosthesis | Prospective case series | 1999–2007 (OPRA) | 39 | 39 | Transfemoral (unilateral only) | Trauma, n = 23(%) Tumor, n = 11(%) Other, n = 5(%) | 24 mo |
|
|
|
||||||||||
| Hansson (2018) | Patients with unilateral transfemoral amputation treated with percutaneous OI prosthesis | Prospective case series | 1999–2007 (OPRA) | 39 | 39 | Transfemoral (unilateral only) | Trauma, n = 23(%) Tumor, n = 11(%) Other, n = 5(%) | 24 mo |
|
|
| ||||||||||
| ||||||||||
| ||||||||||
| Tillander (2010) | OI titanium implants | Prospective case series | 2005–2008 | 39 (possible overlap with OPRA) | 45 | Transfemoral, n = 33 Transtibial, n = 1 Transradial, n = 4, | n/a (Trauma and Tumor) | 36 mo |
|
|
| Transulnar, n = 4 Transhumeral, n = 3 |
|
|||||||||
| Tillander (2017) | Osteomyelitis risk in patients with TFA treated with OI | Retrospective case series | 1990–2010 | 96 (51 OPRA + others) | 102 | Transfemoral | Trauma, n = 71(%) Tumor, n = 20(%) Ischemia, n = 5 Infection, n = 5(%) Other, n = 1(%) | 7.9 yr (range 1.5–19.6) |
|
|
|
||||||||||
| Juhnke (2015) | Fifteen years of experience with ILP | Retrospective cohort | 1999–2013 | 69 (30 in group 1; 39 in group 2) | 73 | Transfemoral | Trauma, n = 51(74%) Tumor, n = 7(10%) Infection, n = 3(4%) Burn, n = 1 Other, n = 5(%) | Group 1: |
|
|
| 74 (range 6–144) |
|
|
||||||||
| Group 2: 32 (range 1–59) |
|
|
||||||||
|
|
|||||||||
| ||||||||||
| ||||||||||
| Muderis (2016) | Direct skeletal attachment prosthesis for the amputee athlete | Retrospective case series | 1999–2013 | 112 (includes all patients from Juhnke study) | 120 | Transfemoral | Trauma, n = 88 Tumor, n = 12 Other, n = 20 | 12 |
|
|
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
| Al Muderis (2016) | Safety of OI Implants for TFA | Prospective case series | 2009–2013 | 86 | 91 | Transfemoral | Trauma, n = 65(76%) Tumor, n = 11(13%) Infection, n = 8(9%) Congenital, n = 1(1%) Other, n = 1(1%) | 34 (range 24–71) |
|
|
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
*PF (physical function); RP (role-physical); PCS: physical component summary.
2.4. Assessment of study quality
The finally included studies were read and assessed for study quality by all four authors. A modified criteria list adopted from a previously defined method was used to assess the internal validity of the included studies.17 The assessment included a yes/no option to describe the following: description of the study population; inclusion/exclusion criteria described; sufficient study size (>10 patient years); follow-up > 12 months; dropouts < 20%; dropouts described; outcome measures and data presentation congruent with the study aims; confounder adjusted in the analysis; and psychometric properties of the instruments reported. Another published review has tested this modified version.18 The yes/no question was transformed into 1/0, making it possible to generate a score representing the internal validity of the included studies (max internal validity score = 10) (Table 2).
Table 2.
Summary of internal validity assessment of included studies (modified from Borghouts et al., 1998).a
| Author (Year) | Selection of population described | Inclusion and exclusion criteria described | Study size > 10 patient years | Follow-up > 12 months | Dropouts < 20% | Description of dropouts | Outcome measures congruent with aims | Data presentation congruent with aims |
Confounders adjusted in the analysis | Psychometric propertiesof instruments reported |
Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nebergall (2012) | 0 | 0 | 1 | 1 | 1 | NA | 1 | 1 | 0 | 1 | 6 |
| Branemark (2014) | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Hagberg (2014) | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Hansson (2018) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 8 |
| Tillander (2010) | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 5 |
| Tillander (2017) | 0 | 0 | 1 | 1 | NA | NA | 1 | 1 | 0 | 1 | 5 |
| Juhnke (2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 9 |
| Muderis (2016) | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 0 | 1 | 7 |
| Al Muderis (2016) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | NA | 9 |
If the study fulfilled the criteria, it was assigned ‘1’, if not ‘0’. NA, Not applicable.
A modified version of the criteria described by Shekelle et al. was used to assess the external validity and applicability of the included studies.19 We replaced the question “treatment benefits in relation to adverse effects” with another one considered to be more applicable to our study – effect gain >10%. In summary, the assessment is comprised of yes/no transformed into 1/0 (maximum external validity score = 4) to describe the following: study participants and interventions described in detail; clinically relevant outcomes measured and reported; and size of the effect clinically important (Table 3).
Table 3.
Summary of external validity and applicability assessment of included studies (modified from Shekelle et al., 1994).
| Author (Year) | Study participants described in detail |
Intervention described in detail | Clinically relevant outcomes measured and reported |
Size of the effect clinically important (at least 10% gain) |
Total score |
|---|---|---|---|---|---|
| Nebergall (2012) | 0 | 1 | 0 | 0 | 1 |
| Branemark (2014) | 1 | 1 | 1 | 1 | 4 |
| Hagberg (2014) | 1 | 1 | 1 | 1 | 4 |
| Hansson (2018) | 1 | 1 | 1 | 1 | 4 |
| Tillander (2010) | 0 | 1 | 0 | 0 | 1 |
| Tillander (2017) | 1 | 1 | 1 | 0 | 3 |
| Juhnke (2015) | 1 | 1 | 1 | 0 | 3 |
| Muderis (2016) | 1 | 1 | 1 | 1 | 4 |
| Al Muderis (2016) | 1 | 1 | 1 | 0 | 3 |
3. Results
The initial search process resulted in 3383 titles (Fig. 2). After screening titles and abstracts, 3352 were excluded due to not fulfilling the inclusion title or being duplicate records. The remaining 31 reports were retrieved in full text. 22 studies were excluded for failing to meet inclusion criteria. Nine studies published between 2010 and 2018 were eligible for review. These included six prospective case series, two retrospective case series, and one retrospective cohort study. Six of the nine studies included data from overlapping study populations, and the remaining three studies included data from another set of overlapping study populations. Between 211 and 242 patients, including 234 to 267 implants, were analyzed. The mean durations of follow up were all greater than 12 months.
3.1. Infection
Seven studies20, 21, 22, 23, 24, 25, 26, 27 reported infection as a complication. The infection rate ranged between 18% and 63%; with most infections being superficial and easily treated with antibiotics.20,22,26 A study by Muderis et al. of 112 patients and 120 implants found considerable reduction in surgical revision rates due to infection from 29% to 7% after improved implant design.21 The overall infection rate was not reported in that study. Tillander et al. reported a 20% ten-year cumulative risk for osteomyelitis and a 10% ten-year cumulative risk for implant removal due to osteomyelitis, but also did not remark upon the overall infection rate.25
3.2. Fracture
Three studies20,22,24 reported fracture as an adverse event. The periprosthetic fracture rate ranged from 0% to 7%, while the overall fracture rate ranged from 3% to 10%.20,22,24 Juhnke et al. reported a revision rate based on periprosthetic or pertrochanteric fractures of 7.2% but did not report the overall fracture rate.24 Al Muderis et al. reported a 3% incidence of femur fracture sustained proximal to the tip of the implant.20 Branemark et al. reported no periprosthetic fractures but did not comment on whether the fractures in other locations were related to prosthetic use.22
3.3. Q-TFA
Three studies21, 22, 23 reported results from Questionnaire for Persons with a Transfemoral Amputation (Q-TFA). All three studies noted that Q-TFA scores were significantly improved following OI implantation in their cohorts. Muderis et al. reported a mean increase in the Q-TFA of 35.8 points in the Australian arm of their study.21 Branemark et al. found a mean prosthetic use score increase of 32 points, with an overall improvement in 69% of patients.22 Hagberg et al. also documented an overall improvement in prosthetic use with the subset of patients in the Branemark study with only unilateral transfemoral amputations.23
3.4. SF-36
Two studies22,23 reported results from the 36-Item Short Form Health Survey (SF-36). Both studies reported a significant increase in the physical function and physical component summary sections of the SF-3622,23.
3.5. Mechanical complications
Four studies20,22,23,27 reported mechanical complications as an adverse event. Branemark et al. and Al Muderis et al. reported device breakage rates of 8% and 31%, respectively.20,22 Nebergall et al. and Hagberg et al. reported implant removal rates due to loosening of 7% and 3%, respectively.23,27
3.6. Economic impact
One study28 reported the economic impact of OI implantation. Hansson et al. reported QALY measures when comparing OI prosthesis to traditional socket prosthesis using the Osseointegrated Prostheses for the Rehabilitation of Amputees (OPRA) implant. Their study found the incremental cost per QALY gained was €83,374 Euros for OI prosthesis compared to socket prosthesis.28
4. Discussion
Infection as an adverse event was the most frequently reported measure across all cohorts, with six studies reporting this complication. Treatment modalities for infections varied across the literature. Al Muderis et al. established a grading system for infections in their cohort which drove their treatment protocol; the authors report treating grade 1 A infections with oral antibiotics, whereas grade 2C infections were treated with surgical debridement.20 The majority of studies included in our review did not establish a grading system beyond a superficial or deep classification; however, the literature seems to support that superficial (or “soft tissue”) infections were treated with antibiotics, whereas deep infections were treated with implant removal and/or surgical debridement. Tillander et al. was the only study to examine osteomyelitis in OI implants. 63% of patients with osteomyelitis were treated with explantation, whereas the remaining 37% were treated with IV antibiotics.25 While the overall infection rate has a wide range across the literature, the incidence of deep or complicated infections requiring reoperation or resulting in sepsis appears to be low. Skin and soft tissue complications occur in up to one third of patients with socket prosthesis, but there are no high-quality studies comparing these complications in socket versus OI prosthetics.5 The prolonged contact with the prosthesis exposes the stump to a number of other painful skin conditions, including hyperhidrosis, ulcerations, dermatitis, and bullous diseases.29 Recent literature suggests that special coatings over the implant can be used to not only facilitate skin-implant integration but also prevent periprosthetic joint infection by preventing the formation of bacterial biofilms.30 Juhnke et al. for example, used a titanium coated implant in conjunction with a well-defined wound hygiene protocol; none of these patients underwent reoperation for infectious complications.24
Periprosthetic fracture was reported in a few studies; however, the etiology was not always clearly stated. Al Muderis et al. reported 3/91 (3.3%) periprosthetic fractures due to trauma sustained after surgery.20 Juhnke et al. described a periprosthetic fracture rate of 5%–10%, but the etiologies of these fractures were not included.24 Branemark et al. described four patients who underwent transfemoral amputation who suffered five unrelated fractures, but no periprosthetic fracture; it is unclear if these fractures were due to imbalance or other prosthesis-related issues.22 The fracture rate overall appears low, from 0 to 10% from these three studies. Evaluation of etiology of these fractures as well as any confounding variables (smoking, diabetes, etc.) would be helpful in risk reduction for future implants. This would be a difficult analysis, however, as the incidence of periprosthetic fracture is so low.
Several studies demonstrated strong evidence supporting positive outcome measures for OI. Q-TFA scores were the most commonly reported clinical outcome, with three studies reporting Q-TFA scores. The literature supports the trend of Q-TFA scores increasing after OI implantation in transfemoral amputees, however, not all studies reported every Q-TFA measure.21, 22, 23 Both of the studies that reported the SF-36 scores for patient self-assessment for quality of life demonstrated considerably increased scores following OI implantation.22,23 While advancements in socket prosthesis have allowed increased prosthetic use, the overall satisfaction rate is low due to residual limb skin problems and discomfort.3 Hansson et al. investigated the quality-adjusted life-years (QALY) for patients receiving transfemoral OI and found that it results in an improved quality of life, though at a relatively high-cost.28 The cost per QALY would be substantially reduced, however, when considering socket prosthesis patients who have a decline in quality of life over time.28
The primary limitation to this review is a lack of standardization of outcome measurement. For example, functional outcomes were measured according to several different scoring modalities. Even in the most commonly used scoring system, the Q-TFA, the various components of the Q-TFA were not always reported. While the overall trend of clinical results shows promising results for improved quality of life, this review is unable to pool the results into one large cohort of patients. We also recommend adopting a grading system for periprosthetic infections, similar to that described by Al Muderis et al..20 Understanding the severity of infections and the level of intervention required to treat them is crucial in evaluating the risks of osseointegration.
5. Conclusion
This review revealed that for patients with transfemoral amputations, osseointegration is an effective alternative to socket prosthesis. Transtibial and upper extremity implants are underreported in the literature, preventing the authors of this review from drawing definitive conclusions. Major complications, such as deep infections, reoperation, and periprosthetic fracture, are rare. Minor complications are most common, such as soft tissue infections, and may be mitigated in the future by improvements in surgical technique and implant design.
Osseointegration in transfemoral, transtibial, and upper extremity amputations results in a significant improvement in quality of life and high implant survival. Unfortunately, most studies included in this review are level IV therapeutic studies. The use of osseointegrated implants would be bolstered by further comparative studies with traditional socket prosthetics.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Financial disclosure
None reported.
References
- 1.Livingston D.H., Keenan D., Kim D., Elcavage J., Malangoni M.A. Extent of disability following traumatic extremity amputation. J Trauma. 1994;37(3):495–499. doi: 10.1097/00005373-199409000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Ephraim P.L., MacKenzie E.J., Wegener S.T., Dillingham T.R., Pezzin L.E. Environmental barriers experienced by amputees: the craig hospital inventory of environmental factors-short Form. Arch Phys Med Rehabil. 2006;87(3):328–333. doi: 10.1016/j.apmr.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Dillingham T.R., Pezzin L.E., MacKenzie E.J., Burgess A.R. Use and satisfaction with prosthetic devices among persons with trauma-related amputations: a long-term outcome study. Am J Phys Med Rehabil. 2001;80(8):563–571. doi: 10.1097/00002060-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hagberg K., Branemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25(3):186–194. doi: 10.1080/03093640108726601. [DOI] [PubMed] [Google Scholar]
- 5.Lyon C.C., Kulkarni J., Zimerson E., Van Ross E., Beck M.H. Skin disorders in amputees. J Am Acad Dermatol. 2000;42(3):501–507. doi: 10.1016/s0190-9622(00)90227-5. [DOI] [PubMed] [Google Scholar]
- 6.Meulenbelt H.E., Geertzen J.H., Jonkman M.F., Dijkstra P.U. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90(1):74–81. doi: 10.1016/j.apmr.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Demet K., Martinet N., Guillemin F., Paysant J., Andre J.M. Health related quality of life and related factors in 539 persons with amputation of upper and lower limb. Disabil Rehabil. 2003;25(9):480–486. doi: 10.1080/0963828031000090434. [DOI] [PubMed] [Google Scholar]
- 8.Pezzin L.E., Dillingham T.R., MacKenzie E.J. Rehabilitation and the long-term outcomes of persons with trauma-related amputations. Arch Phys Med Rehabil. 2000;81(3):292–300. doi: 10.1016/s0003-9993(00)90074-1. [DOI] [PubMed] [Google Scholar]
- 9.Van de Meent H., Hopman M.T., Frolke J.P. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013;94(11):2174–2178. doi: 10.1016/j.apmr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Branemark P.I., Hansson B.O., Adell R. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. [PubMed] [Google Scholar]
- 11.Branemark R., Branemark P.I., Rydevik B., Myers R.R. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38(2):175–181. [PubMed] [Google Scholar]
- 12.Aschoff H.H., Kennon R.E., Keggi J.M., Rubin L.E. Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: an endo-exo device. J Bone Joint Surg Am. 2010;92(Suppl 2):180–186. doi: 10.2106/JBJS.J.00806. [DOI] [PubMed] [Google Scholar]
- 13.Hagberg K., Branemark R., Gunterberg B., Rydevik B. Osseointegrated trans-femoral amputation prostheses: prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthet Orthot Int. 2008;32(1):29–41. doi: 10.1080/03093640701553922. [DOI] [PubMed] [Google Scholar]
- 14.Frossard L., Stevenson N., Smeathers J. Monitoring of the load regime applied on the osseointegrated fixation of a trans-femoral amputee: a tool for evidence-based practice. Prosthet Orthot Int. 2008;32(1):68–78. doi: 10.1080/03093640701676319. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg M., Hagberg K., Bullington J. My prosthesis as a part of me: a qualitative analysis of living with an osseointegrated prosthetic limb. Prosthet Orthot Int. 2011;35(2):207–214. doi: 10.1177/0309364611409795. [DOI] [PubMed] [Google Scholar]
- 16.Moher D., Shamseer L., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borghouts J.A., Koes B.W., Bouter L.M. The clinical course and prognostic factors of non-specific neck pain: a systematic review. Pain. 1998;77(1):1–13. doi: 10.1016/S0304-3959(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 18.Salminen A.L., Brandt A., Samuelsson K., Toytari O., Malmivaara A. Mobility devices to promote activity and participation: a systematic review. J Rehabil Med. 2009;41(9):697–706. doi: 10.2340/16501977-0427. [DOI] [PubMed] [Google Scholar]
- 19.Shekelle P.G., Andersson G., Bombardier C. A brief introduction to the critical reading of the clinical literature. Spine. 1994;19(18 Suppl):2028S–2031S. doi: 10.1097/00007632-199409151-00002. [DOI] [PubMed] [Google Scholar]
- 20.Al Muderis M., Khemka A., Lord S.J., Van de Meent H., Frolke J.P. Safety of osseointegrated implants for transfemoral amputees: a two-center prospective cohort study. J Bone Joint Surg Am. 2016;98(11):900–909. doi: 10.2106/JBJS.15.00808. [DOI] [PubMed] [Google Scholar]
- 21.Al Muderis M., Aschoff HH., Bosley B., Raz G., Gerdesmeyer L., Burkett B. Direct skeletal attachment prosthesis for the amputee athlete: the unknown potential. Sports Eng. 2016 Sep 1;19(3):141–145. [Google Scholar]
- 22.Branemark R., Berlin O., Hagberg K., Bergh P., Gunterberg B., Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective study of 51 patients. Bone Joint Lett J. 2014;96-B(1):106–113. doi: 10.1302/0301-620X.96B1.31905. [DOI] [PubMed] [Google Scholar]
- 23.Hagberg K., Hansson E., Branemark R. Outcome of percutaneous osseointegrated prostheses for patients with unilateral transfemoral amputation at two-year follow-up. Arch Phys Med Rehabil. 2014;95(11):2120–2127. doi: 10.1016/j.apmr.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Juhnke D.L., Beck J.P., Jeyapalina S., Aschoff H.H. Fifteen years of experience with Integral-Leg-Prosthesis: cohort study of artificial limb attachment system. J Rehabil Res Dev. 2015;52(4):407–420. doi: 10.1682/JRRD.2014.11.0280. [DOI] [PubMed] [Google Scholar]
- 25.Tillander J., Hagberg K., Berlin O., Hagberg L., Branemark R. Osteomyelitis risk in patients with transfemoral amputations treated with osseointegration prostheses. Clin Orthop Relat Res. 2017;475(12):3100–3108. doi: 10.1007/s11999-017-5507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tillander J., Hagberg K., Hagberg L., Branemark R. Osseointegrated titanium implants for limb prostheses attachments: infectious complications. Clin Orthop Relat Res. 2010;468(10):2781–2788. doi: 10.1007/s11999-010-1370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nebergall A., Bragdon C., Antonellis A., Karrholm J., Branemark R., Malchau H. Stable fixation of an osseointegated implant system for above-the-knee amputees: titel RSA and radiographic evaluation of migration and bone remodeling in 55 cases. Acta Orthop. 2012;83(2):121–128. doi: 10.3109/17453674.2012.678799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansson E., Hagberg K., Cawson M., Brodtkorb T.H. Patients with unilateral transfemoral amputation treated with a percutaneous osseointegrated prosthesis. Bone Joint Lett J. 2018;100-B(4):527–534. doi: 10.1302/0301-620X.100B4.BJJ-2017-0968.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meulenbelt H.E., Dijkstra P.U., Jonkman M.F., Geertzen J.H. Skin problems in lower limb amputees: a systematic review. Disabil Rehabil. 2006;28(10):603–608. doi: 10.1080/09638280500277032. [DOI] [PubMed] [Google Scholar]
- 30.Raphel J., Holodniy M., Goodman S.B., Heilshorn S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials. 2016;84:301–314. doi: 10.1016/j.biomaterials.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]