The core circadian regulator CCA1 is a direct repressor of brassinosteroid-inactivating genes BAS1 and SOB7, and interacts with another repressor, ATAF2. Their differential suppressing effects are regulated by light.
Keywords: Arabidopsis thaliana, ATAF2, BAS1, brassinosteroids, CCA1, cytochrome P450, hypocotyl growth, SOB7, transcription factor
Abstract
Brassinosteroids (BRs) are a group of steroid hormones regulating plant growth and development. Since BRs do not undergo transport among plant tissues, their metabolism is tightly regulated by transcription factors (TFs) and feedback loops. BAS1 (CYP734A1, formerly CYP72B1) and SOB7 (CYP72C1) are two BR-inactivating cytochrome P450s identified in Arabidopsis thaliana. We previously found that a TF ATAF2 (ANAC081) suppresses BAS1 and SOB7 expression by binding to the Evening Element (EE) and CIRCADIAN CLOCK ASSOCIATED 1 (CCA1)-binding site (CBS) on their promoters. Both the EE and CBS are known binding targets of the circadian regulatory protein CCA1. Here, we confirm that CCA1 binds the EE and CBS motifs on BAS1 and SOB7 promoters, respectively. Elevated accumulations of BAS1 and SOB7 transcripts in the CCA1 null mutant cca1-1 indicate that CCA1 is a repressor of their expression. When compared with either cca1-1 or the ATAF2 null mutant ataf2-2, the cca1-1 ataf2-2 double mutant shows higher SOB7 transcript accumulations and a stronger BR-insensitive phenotype of hypocotyl elongation in white light. CCA1 interacts with ATAF2 at both DNA–protein and protein–protein levels. ATAF2, BAS1, and SOB7 are all circadian regulated with distinct expression patterns. These results demonstrate that CCA1 and ATAF2 differentially suppress BAS1- and SOB7-mediated BR inactivation.
Introduction
Brassinosteroids (BRs) are a class of polyhydroxysteroid hormones that regulate plant growth (J.Y. Zhu et al., 2013), stress tolerance (Nolan et al., 2017), and disease resistance (Belkhadir et al., 2012). BRs do not undergo transport processes within the plant body (Symons and Reid, 2004; Savaldi-Goldstein et al., 2007; Symons et al., 2008). Their biosynthesis and catabolism are tightly regulated in different plant tissues and developmental stages (Zhao and Li, 2012). In the model plant Arabidopsis thaliana, several transcription factors (TFs) have been identified as regulators of the BR biosynthetic genes DWARF4 (DWF4), CONSTITUTIVE PHOTOMORPHOGENIC DWARF (CPD), and BRASSINOSTEROID-6-OXIDASE 2 (BR6ox2). A TCP-family TF TCP1 (Guo et al., 2010) and a NAC-family TF JUNGBRUNNEN1 (JUB1) (Shahnejat-Bushehri et al., 2016) activate and suppress the expression of DWF4, respectively. COGWHEEL1 (COG1), a Dof-type TF, binds to the promoters of two phytochrome-interacting-factor (PIF)-encoding genes PIF4 and PIF5 to promote their expression (Wei et al., 2017). PIF4 and PIF5 are two basic helix–loop–helix (bHLH) TFs that directly promote the expression of DWF4 and BR6ox2 (Wei et al., 2017). Two homologous TFs CESTA (CES) and BR Enhanced Expression 1 (BEE1) interact with each other and promote the expression of CPD by directly binding a G-box motif in its promoter (Poppenberger et al., 2011).
In Arabidopsis, the transcription of key BR biosynthetic and catabolic genes is feedback regulated to maintain hormone homeostasis (Tanaka et al., 2005). PHYB ACTIVATION TAGGED SUPPRESSOR 1 (BAS1, CYP734A1, formerly CYP72B1) and SUPPRESSOR OF PHYB-4 7 (SOB7, CYP72C1) are two BR-inactivating cytochrome P450s (P450s) that are subject to transcriptional feedback regulation loops (Neff et al., 1999; Turk et al., 2003; 2005; Thornton et al., 2010). Overexpression of BAS1, SOB7, or their orthologs from other plant species confers a BR-deficient dwarf phenotype in Arabidopsis (Neff et al., 1999; Turk et al., 2005; Thornton et al., 2011).
Three TFs are known to be the transcriptional regulators of BAS1 or SOB7. LATERAL ORGAN BOUNDARIES (LOB) directly binds the promoter of BAS1 and activates its expression (Bell et al., 2012). The auxin response factor 7 (ARF7) can bind to the E-box motifs of the BAS1 promoter and suppress its expression (Youn et al., 2016). We previously reported that the NAC TF ATAF2 (ANAC081) can bind to the promoters of both BAS1 and SOB7 as a repressor (Peng et al., 2015). ATAF2 is also known to regulate disease resistance (Delessert et al., 2005; X. Wang et al., 2009; Wang and Culver, 2012), abiotic stress tolerance (Takasaki et al., 2015), and auxin biosynthesis (Huh et al., 2012). ATAF2 can act as either an activator or a repressor depending on growth conditions or promoter context (Delessert et al., 2005; X. Wang et al., 2009; Nagahage et al., 2018).
ATAF2 binds the Evening Element (EE; AAAATATCT or its reverse complement sequence) and the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1)-binding site (CBS; AAAAATCT or its reverse complement sequence) on BAS1 and SOB7 promoters (Peng et al., 2015). The EE sequence has one extra ‘T’ when compared with that of the CBS, and both are known as the binding targets of the core circadian clock regulatory protein CCA1 (Wang and Tobin, 1998; Michael and McClung, 2002; Harmer and Kay, 2005). CCA1 is a MYB TF initially identified as an activator of Lhcb1*3, which encodes a light-harvesting Chl a/b protein (Wang et al., 1997). Similar to ATAF2, CCA1 can act as either an activator (Fujiwara et al., 2008) or a repressor (Li et al., 2011) of downstream genes under different circumstances.
In this research, we confirmed that CCA1 binds the EE and CBS elements of BAS1 and SOB7 promoters, respectively. Like ATAF2, CCA1 is also a repressor of BAS1 and SOB7 expression. CCA1 interacts with ATAF2 at both DNA–protein and protein–protein levels. The suppressing effect of CCA1 and ATAF2 on SOB7 expression can be either additive or redundant depending on the light or dark growth conditions for Arabidopsis seedlings. ATAF2, BAS1, and SOB7 are all circadian regulated with distinct expression patterns. Our findings provide novel insight into the connection between BR homeostasis and circadian clock regulatory pathways.
Materials and methods
Plant materials and growth conditions
All Arabidopsis plants used in this study are in the Columbia (Col-0) background. The cca1-1 mutant in the Col-0 background (CS67781) and the ataf2-2 mutant (SALK_015750) were obtained from the Arabidopsis Biological Resource Center (ABRC). The cca1-1 mutant in Col-0 was created by backcrossing the original cca1-1 mutant in the Wassilewskija (Ws) background (Green and Tobin, 1999) six times into Col-0 (Yakir et al., 2009). Primers for characterizing cca1-1 were described previously (Green and Tobin, 1999). Primers for characterizing ataf2-2 were designed using the web tool provided by the Salk Institute (http://signal.salk.edu/tdnaprimers.2.html). cca1-1, ataf2-2, and cca1-1 ataf2-2 were all verified as gene knockout mutants via quantitative reverse transcription–PCR (qRT–PCR; see Supplementary Fig. S1 at JXB online). The pBAS1:BAS1-GUS and pSOB7:SOB7-GUS constructs and the histochemical β-glucuronidase (GUS) staining procedures were described previously (Sandhu et al., 2012; Peng et al., 2015). The pATAF2::GUS construct harbors a transcriptional fusion of GUS and a 2 kb ATAF2 promoter (X. Wang et al., 2009). Plant GUS staining images were photographed using a Leica MZ10 F modular stereo microscope and a Leica DFC295 digital microscope color camera. For transgenic events, homozygous single-locus T-DNA insertion lines were selected for crossing and further analysis. Unless otherwise stated, all seeds were surface-sterilized by ethanol, plated on half-strength Linsmaier and Skoog medium with 10 g l–1 phytagel (Sigma-Aldrich, made in USA) and 15 g l–1 sucrose, stratified at 4 °C in the dark for 4 d, treated by red light for 1–2 h to induce germination, and grown in growth chambers at 25 °C in the dark, 80 μmol m−2 s−1 continuous white light (red:far-red light ratio 1:1), or 12 h 80 μmol m−2 s−1 light and a 12 h dark photoperiod depending on the experiment. Unless otherwise stated, 4-day-old seedlings were used for total RNA extraction and hypocotyl measurements. For circadian analysis of gene expression, seedlings were grown in a 12 h light and 12 h dark photoperiod for 7 d before RNA samples were extracted at 4 h intervals. Seedlings continued to grow in the same photoperiod during the 2 day RNA extraction schedule. For seed collection, seedlings were transferred to the greenhouse and grown at 22 °C in a photoperiod of 16 h of light and 8 h of dark. Seeds for all physiological and molecular assays are from plants grown at the same time under the same conditions.
Yeast one-hybrid and two-hybrid assays
The Gateway-compatible yeast one-hybrid (Y1H) system used in this research was developed by Deplancke et al. (2006). The promoter DNA fragments (baits) were amplified using primer pairs with adaptor sequences of the attB4 and attB1R sites, respectively (Deplancke et al., 2004). The baits were cloned into pDONR-P4-P1R (Invitrogen) via BP reactions (Gateway BP Clonase II, Invitrogen). The resulting pDONR–bait constructs were used for LR reactions with Y1H destination vector pMW#2 (Gateway LR Clonase II, Invitrogen). pMW#2 contains the Gateway cassette of attR4 and attL1 recombination sites and a HIS3 (pMW#2) reporter gene. The resulting pMW#2-bait constructs were linearized by digestion with XhoI. Then DNA bait::HIS3 (pMW#2-bait) sequences were integrated via homologous recombination into the mutant HIS3 locus of the yeast strain YM4271 developed for Y1H analysis. The successful integrations of baits in yeast genomes were verified by PCR using the combinations of bait- and vector-specific primers (Deplancke et al., 2006). The self-activation of HIS3 was tested by yeast tolerance to gradient concentrations (0–80 mM) of 3-AT (3-aminotriazole; a competitive inhibitor of the His3p enzyme). After self-activation tests of HIS3 reporters, the yeast bait clones with the lowest background of reporter activity (self-activation) were selected and used to test their interactions with the preys. The sequences of baits pBAS1-EE, pSOB7-CBS, pBAS1-CBS1, pBAS1-EEm, and pSOB7-CBSm were described previously (Peng et al., 2015). The sequence of bait pATAF2-CBS is given in Supplementary Table S1. The full-length cDNA clone of CCA1 (C105127; AT2G46830.2) was obtained from the ABRC. CCA1 was cloned into the Gateway-compatible prey vector pACT2-GW (pACT2-GW-CCA1) and its interaction with the baits mentioned above was tested. An empty prey vector was used as a negative control. The procedure for the yeast two-hybrid (Y2H) assay was described previously (Zhao et al., 2013). ATAF2 cDNA was cloned into the Gateway-compatible bait vector pBTM116-D9 (pBTM116-D9-ATAF2). The prey construct pACT2-GW-CCA1 was used to transform yeast strain A. After testing for self-activation, the resulting clone was used for transformation of the bait construct pBTM116-D9-ATAF2. The empty bait vector was used as a negative control. The CCA1–ATAF2 interaction was tested by yeast tolerance to 3-AT and ability to grow in SDIV medium deprived of uracil, histidine, leucine, and tryptophan. The PCR-amplified sequences in all constructs used in this research were verified by sequencing.
EMSA and pull-down assay
Maltose-binding protein- (MBP) tagged CCA1 (MBP–CCA1), hexa-histidine-tagged CCA1 (His-CCA1), and hexa-histidine-tagged ATAF2 (His-ATAF2) were each expressed in the Escherichia coli strain Rosetta. All tags are fused at the N-terminus. Escherichia coli cell cultures were lysed via freeze–thaw followed by sonication in phosphate-buffered saline (PBS) containing lysozyme and Benzonase nuclease. MBP–CCA1 and MBP were purified using amylose resin (New England Biolabs). His-CCA1 and His-ATAF2 were purified using HisPur Ni-NTA resin (Thermo Fisher Scientific). To facilitate the successive binding experiments, maltose or imidazole was removed from purified MBP–CCA1, MBP, His-CCA1, or His-ATAF2 via four rounds of dialysis using the Slide-A-Lyzer mini dialysis device (Thermo Fisher Scientific). EMSA was carried out using the fluorescence-based EMSA kit from Invitrogen. DNA probes and protein–DNA complexes were separated by non-denaturing PAGE. DNA bands were stained by SYBR Green and scanned using the Bio-Rad ChemiDoc Touch imaging system. For the pull-down assay, the mixture of MBP–CCA1 and His-ATAF2 was incubated overnight at 4 °C with end over end mixing, and then loaded onto the amylose resin. After washing away unbound proteins, the bound proteins were eluted using elution buffer containing 10 mM maltose. As the negative control, the mixture of MBP and His-ATAF2 was subjected to identical procedures. The eluted samples were analyzed by SDS–PAGE and stained using Coomassie brilliant blue R-250.
Transcript analysis
Transcript accumulations of BAS1, SOB7, ATAF2, and CCA1 were measured by qRT–PCR. Total RNA was extracted from 4-day-old seedlings using the RNeasy Plant Kit (Qiagen). The DNase I Digestion Set (Sigma) was used to perform on-column elimination of genomic DNA contamination. First-strand cDNA was synthesized using the iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad). Quantitative PCRs (qPCRs) were performed using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) and the CFX96 Real-Time System (Bio-Rad). The Bio-Rad CFX Manager software was used to analyze and compare data using the ΔΔCT method. Relative expression levels of target genes were determined by normalizing to the transcript levels of POLYUBIQUITIN 10 (UBQ10). Each data point represents nine replicates (three biological replicates×three technical replicates). qPCR primers for UBQ10, ATAF2, BAS1, and SOB7 were described previously (Peng et al., 2015). qPCR primers for CCA1 are 5'-TCGAAAGACGGGAAGTGGAACG-3' and 5'-GTCGATCTTCATTGGCCATCTCAG-3'. All qPCR primers were designed using QuantPrime (https://quantprime.mpimp-golm.mpg.de/;Arvidsson et al., 2008).
Hypocotyl and root measurements
Seed plating and hypocotyl measurement were described previously (Favero et al., 2016; 2017). For brassinolide (BL) treatment assays, seeds were put on BL-containing plates from the beginning of the experiments. The same volume of ethanol was used to dissolve gradient concentrations of BL and added to the media including the non-BL control. All 4-day-old seedlings were scanned/photographed and measured using NIH ImageJ (C.A. Schneider et al., 2012). Each data point represents the result of the 30 tallest seedlings. Seven-day-old seedlings grown on vertical plates under continuous white light were used for primary root length measurement. Each data point represents the result of the 10 longest roots. All experiments were repeated three times. Each independent experiment showed a similar trend of differences.
Statistical analysis
One-way ANOVA with Tukey’s HSD test was used to determine the significance of differences among multiple hypocotyl measurement or qPCR data sets. Two-tailed Student’s t-test was used to compare two groups of qPCR data. The P-value significance level was set as 0.01.
Accession numbers
Arabidopsis Genome Initiative numbers for the genes used in this study are as follows: CCA1 (AT2G46830), ATAF2 (AT5G08790), BAS1 (AT2G26710), SOB7 (AT1G17060), and UBQ10 (AT4G05320).
Results
CCA1 binds the EE and CBS motifs on BAS1 and SOB7 promoters, respectively
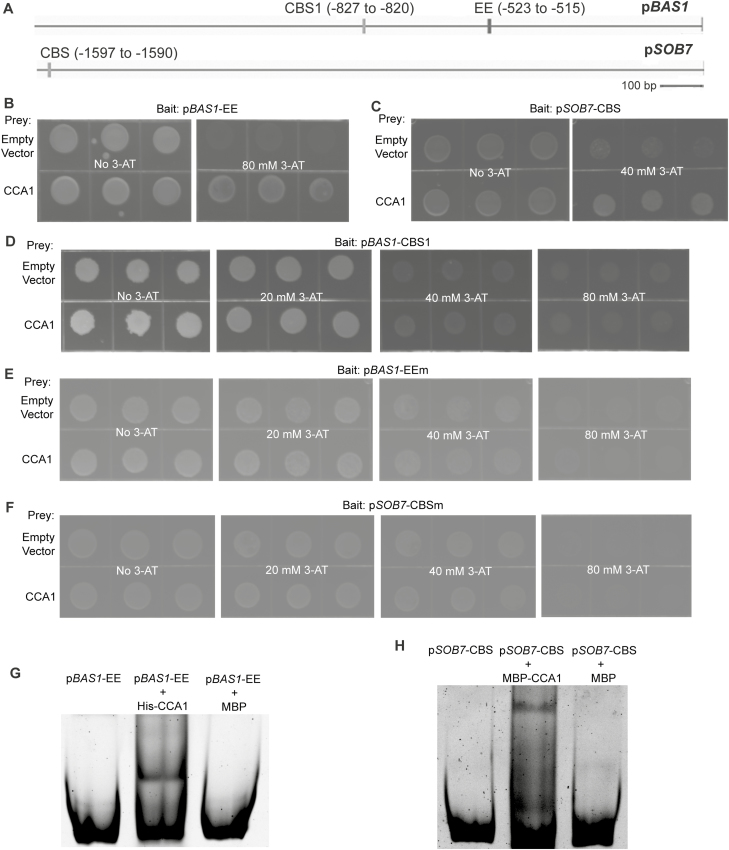
ATAF2 binds three EE- and CBS-containing fragments of the BAS1 and SOB7 promoters (Fig. 1A), namely pBAS1-EE (−731 to −504), pBAS1-CBS1 (−844 to −786), and pSOB7-CBS (−1623 to −1524) (Peng et al., 2015). Since both EE and CBS elements are known binding targets of CCA1 (Pan et al., 2009), we tested, via targeted Y1H assays, the capability of CCA1 to bind to the three BAS1 and SOB7 promoter fragments mentioned above. CCA1 was confirmed to interact with pBAS1-EE (Fig. 1B) and pSOB7-CBS (Fig. 1C). Unlike ATAF2, CCA1 did not bind pBAS1-CBS1 in our assay (Fig. 1D).
Fig. 1.
CCA1 binds the EE and CBS motifs on BAS1 and SOB7 promoters, respectively. (A) The BAS1 promoter harbors both EE and CBS motifs, while only one CBS motif exists in the SOB7 promoter. CCA1 interacted with pBAS1-EE (B) and pSOB7-CBS (C), but not with pBAS1-CBS1 (D) in targeted Y1H assays. CCA1 did not interact with pBAS1-EEm (E) or pSOB7-CBS (F), in which the EE or CBS motifs have been mutated, respectively. Interactions of CCA1 with pBAS1-EEm (G) and pSOB7-CBS (H) were confirmed by EMSA. For each Y1H interaction tested, the indicated bait sequence was integrated into the mutant HIS3 locus of the yeast strain YM4271. The bait-integrated yeast clone with the lowest self-activation was transformed with the indicated prey construct and empty prey vector (negative control), and then plated on selection medium supplemented with 3-AT at the concentrations shown. Yeast clones were grown at 28 °C for 3–4 d. Three independent clones were shown for each sample. For EMSA, His–CCA1 and MBP–CCA1 were incubated with pBAS1-EE and pSOB7-CBS, respectively, and separated by non-denaturing PAGE. DNA probes and protein–DNA complexes were stained by SYBR Green. MBP was used as a negative control.
Two EE/CBS-mutated fragments (Peng et al., 2015), pBAS1-EEm (EE was mutated from AAAATATCT to AACATATCT) and pSOB7-CBSm (CBS was mutated from AGATTTTT to AGATTCTT), were used to test whether the interactions between CCA1 and pBAS1-EE/pSOB7-CBS were mediated by the EE and CBS motifs, respectively. Both pBAS1-EEm (Fig. 1E) and pSOB7-CBSm (Fig. 1F) lost their capacity to bind to CCA1 in targeted Y1H assays, indicating that the EE and CBS motifs are responsible for the binding of CCA1 to BAS1 and SOB7 promoters, respectively.
The binding of CCA1 to pBAS1-EE (Fig. 1G) and pSOB7-CBS (Fig. 1H) was further confirmed by EMSA. In our system, the MBP tag seemed to interfere with the capacity of CCA1 to bind to pBAS1-EE. Therefore, we showed the binding of His-CCA1 to pBAS1-EE instead (Fig. 1G). MBP cannot bind to pBAS1-EE (Fig. 1G), which indicated that the interaction between pBAS1-EE and CCA1 is protein selective. MBP–CCA1 can bind pSOB7-CBS whereas MBP cannot (Fig. 1H), which demonstrated that the specificity of binding comes from CCA1.
CCA1 is a repressor of BAS1–GUS and SOB7–GUS activity
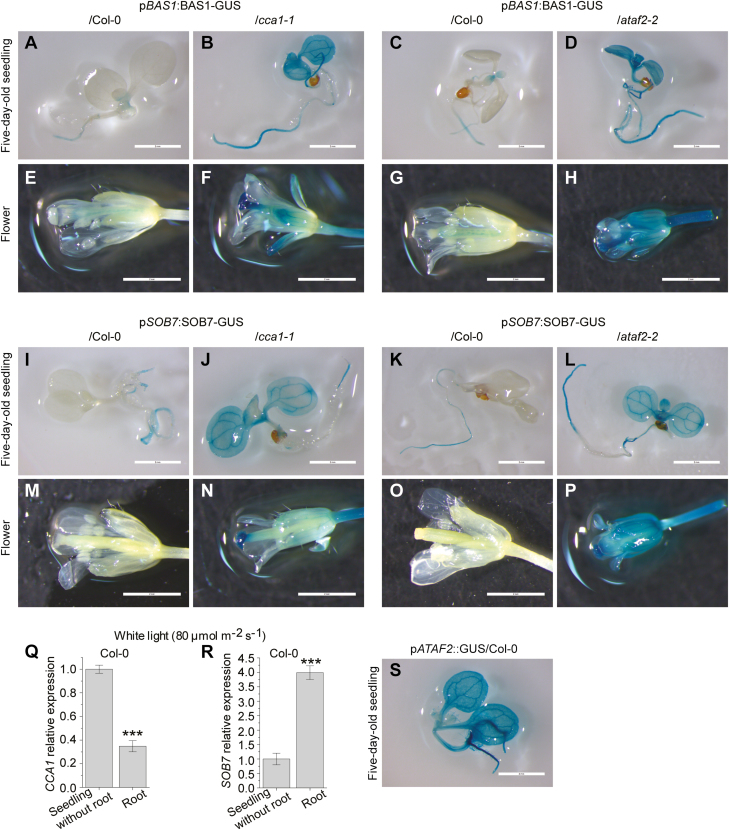
To test the effects of CCA1 on BAS1 and SOB7 activity, two constructs pBAS1:BAS1-GUS and pSOB7:SOB7-GUS (genomic DNA translational fusions with 1.6 kb and 2.1 kb of their native promoters, respectively; Sandhu et al., 2012; Peng et al., 2015) were used to transform the CCA1 loss-of-function mutant cca1-1. Approximately 25% of the T1 primary transformants of both pBAS1:BAS1-GUS/cca1-1 (Fig. 2A) and pSOB7:SOB7-GUS/cca1-1 (Fig. 2B) conferred a severe dwarf phenotype associated with BR deficiency (BR-dwarf). Similar BR-dwarf transformants were observed when expressing the two constructs in the ATAF2 loss-of-function mutant ataf2-2, while none of the pBAS1:BAS1-GUS and pSOB7:SOB7-GUS transgenic plants in the Col-0 background showed dwarfism (Peng et al., 2015). The results indicate that like ATAF2, CCA1 may also suppress the expression and activity of BAS1 and SOB7.
Fig. 2.
CCA1 is a repressor of BAS1 and SOB7 expression. Ectopic expression of pBAS1:BAS1-GUS (A) and pSOB7:SOB7-GUS (B) in the cca1-1 background caused BR deficiency-associated dwarfism in about a quarter of T1 plants of both transgenic events. Single-locus T-DNA insertional pBAS1:BAS1-GUS/cca1-1 and pSOB7:SOB7-GUS/cca1-1 homozygous T3 lines were selected from BR-dwarf plants and crossed with Col-0, respectively. Homozygous pBAS1:BAS1-GUS (C) and pSOB7:SOB7-GUS (D) sibling lines in cca1-1 and Col-0 backgrounds were selected from the two F2 segregation populations for comparison of morphology. F3 homozygous segregants of pBAS1:BAS1-GUS/Col-0 (E), pBAS1:BAS1-GUS/cca1-1 (E), pSOB7:SOB7-GUS/Col-0 (F), and pSOB7:SOB7-GUS/cca1-1 (F) retained their morphologically normal or BR-dwarf phenotypes, respectively.
To compare the activity of pBAS1:BAS1-GUS or pSOB7:SOB7-GUS in wild-type (Col-0) and cca1-1 backgrounds with identical insertion sites in the Arabidopsis genome, we adopted a cross-segregation approach previously applied to ataf2-2 (Peng et al., 2015). Homozygous T3 plants were isolated from the BR-dwarf pBAS1:BAS1-GUS/cca1-1 and pSOB7:SOB7-GUS/cca1-1 lines with T-DNA inserted at a single locus. Those homozygous T-DNA insertional plants were crossed with Col-0, and the F2 segregants were genotyped. Many pBAS1:BAS1-GUS/cca1-1 and pSOB7:SOB7-GUS/cca1-1 F2 segregants retained the BR-dwarf phenotype, whereas all pBAS1:BAS1-GUS/Col-0 and pSOB7:SOB7-GUS/Col-0 siblings were morphologically normal (Fig. 2C, D). F3 homozygous segregants of pBAS1:BAS1-GUS/Col-0 (Fig. 2E), pBAS1:BAS1-GUS/cca1-1 (Fig. 2E), pSOB7:SOB7-GUS/Col-0 (Fig. 2F), and pSOB7:SOB7-GUS/cca1-1 (Fig. 2F) retained their morphologically normal or BR-dwarf phenotypes, respectively. The results confirmed that the BR-dwarf phenotype of pBAS1:BAS1-GUS/cca1-1 and pSOB7:SOB7-GUS/cca1-1 transgenic plants were caused by the disruption of CCA1.
CCA1 modulates the tissue-specific protein accumulation patterns of BAS1–GUS and SOB7–GUS
Both BAS1–GUS and SOB7–GUS have specific accumulation patterns that limit their presence in certain tissues of seedlings and plant organs (Sandhu et al., 2012). BAS1–GUS accumulates in seedling roots, the shoot apex, and certain leaf regions, whereas SOB7–GUS activity can only be observed in the root tip and elongation zone (Peng et al., 2015). Using CCA1–GUS transgenic lines, Pruneda-Paz et al. (2009) revealed that CCA1 exhibits expression throughout the whole seedling except the roots. Based on our previous results (Figs 1, 2), CCA1 may act as a tissue-specific repressor of BAS1 and SOB7. To test this hypothesis, we performed GUS staining on F3 homozygous segregants of pBAS1:BAS1-GUS/Col-0, pBAS1:BAS1-GUS/cca1-1, pSOB7:SOB7-GUS/Col-0, and pSOB7:SOB7-GUS/cca1-1 (Fig. 3; Supplementary Fig. S2). Five-day-old seedlings, cauline and rosette leaves, as well as flowers and siliques were stained (Fig. 3; Supplementary Fig. S2). To compare the effects of CCA1 and ATAF2 in modulating BAS1–GUS and SOB7–GUS accumulation, seedlings and flowers of corresponding F3 homozygous segregants in Col-0 and ataf2-2 backgrounds (Peng et al., 2015) were also stained at the same time (Fig. 3). The results showed that BAS1 and SOB7 expression expanded to more tissues with the disruption of CCA1. In a cca1-1 background, both BAS1–GUS and SOB7–GUS fusion signals were dramatically expanded and enhanced in seedlings, leaves, flowers, and siliques when compared with their expression patterns in the wild type (Col-0) (Fig. 3; Supplementary Fig. S2). Compared with CCA1, the disruption of ATAF2 led to even broader expression of BAS1–GUS and SOB7–GUS in seedlings and flowers (Fig. 3A–P).
Fig. 3.
CCA1 and ATAF2 modulate the tissue-specific expression patterns of BAS1 and SOB7 in seedlings and flowers. The roles of CCA1 and ATAF2 in restricting BAS1 and SOB7 expression within certain tissues of seedlings and flowers were demonstrated by GUS analysis on F3 homozygous segregants of pBAS1:BAS1-GUS/Col-0 and pBAS1:BAS1-GUS/cca1-1, pBAS1:BAS1-GUS/Col-0 and pBAS1:BAS1-GUS/ataf2-2, pSOB7:SOB7-GUS/Col-0 and pSOB7:SOB7-GUS/cca1-1, and pSOB7:SOB7-GUS/Col-0 and pSOB7:SOB7-GUS/ataf2-2 (A–P). (Q) CCA1 showed significantly reduced transcript accumulation in seedling roots when compared with seedlings without roots. (R) SOB7 was preferably expressed in seedling roots. Five-day-old seedlings grown at 25 °C in 80 μmol m−2 s−1 continuous white light were used for GUS staining and RNA extraction. Scale bars=2 cm. Each qRT–PCR value is the mean of results from three biological replicates×three technical replicates (n=9). Error bars denote the SE. Two-tailed Student’s t-test was used to determine the significance of differences. ***P<0.001.
CCA1 showed significantly reduced transcript accumulation in roots when compared with the rest of the seedling (Fig. 3Q), which is consistent with previous observations (Pruneda-Paz et al., 2009). In contrast, SOB7 was preferably expressed in seedling root (Fig. 3R). The relatively low expression of CCA1 in roots may at least partially allow SOB7 to be expressed in these tissues. In contrast, the presence of CCA1 in other tissues may contribute to the inhibition of SOB7 expression. Unlike CCA1, pATAF2::GUS showed universal expression in seedlings (Supplementary Fig. 3S), which is consistent with the broad expansion of pBAS1:BAS1-GUS and pSOB7:SOB7-GUS in the ataf2-2 genetic background (Peng et al., 2015; Fig. 3D, H, L, P).
CCA1 and ATAF2 differentially suppress the transcript accumulation of BAS1 and SOB7
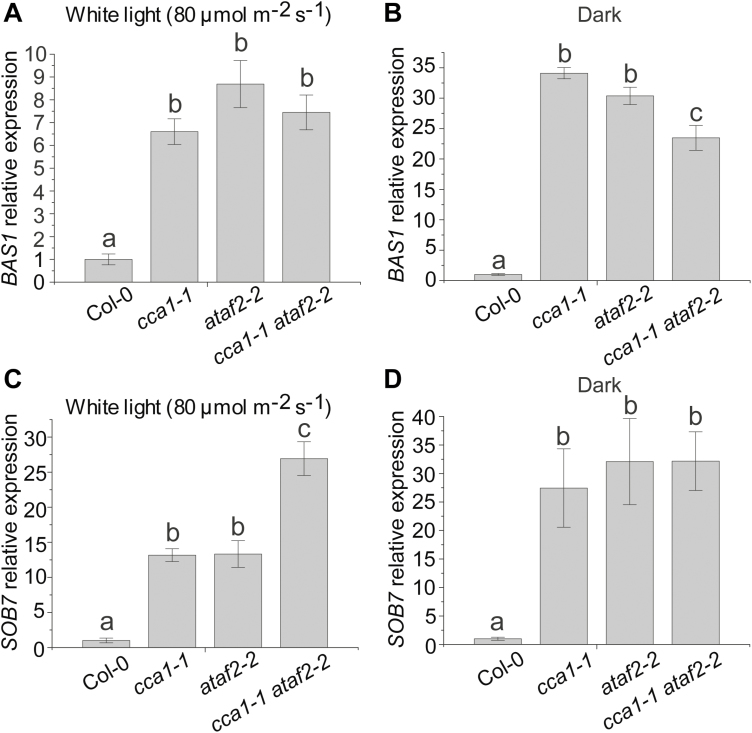
Since CCA1 and ATAF2 have similar functions in suppressing BAS1–GUS and SOB7–GUS accumulation, we made the cca1-1 ataf2-2 double mutant and compared it with the single mutants and the wild type with regard to BAS1 and SOB7 transcript accumulation. For 4-day-old seedlings grown at 25 °C in 80 μmol m−2 s−1 continuous white light or darkness, cca1-1, ataf2-2, and the cca1-1 ataf2-2 double mutant showed similarly elevated BAS1 expression when compared with the wild type (Col-0) with the exception that cca1-1 ataf2-2 showed lower BAS1 transcript accumulation than either single mutant in the dark (Fig. 4A, B), demonstrating that the removal of either CCA1 or ATAF2 can significantly de-repress BAS1 transcript accumulation. In contrast, in white light, the cca1-1 ataf2-2 double mutant conferred significantly higher SOB7 transcript accumulation than either cca1-1 or ataf2-2 single mutants (Fig. 4C). However, in darkness, the genetic impact of CCA1 or ATAF2 on SOB7 transcript accumulation is similar and has no additive effect (Fig. 4D). These results indicate that CCA1 and ATAF2 can additively suppress SOB7 transcript accumulation in white light but not in darkness.
Fig. 4.
CCA1 and ATAF2 differentially suppress the transcript accumulation of BAS1 and SOB7. For 4-day-old seedlings grown at 25 °C in 80 μmol m−2 s−1 continuous white light (A) or darkness (B), cca1-1, ataf2-2, and the cca1-1 ataf2-2 double mutant showed similarly elevated BAS1 expression when compared with the wild type (Col-0) except that cca1-1 ataf2-2 showed even lower BAS1 transcript accumulation than either single mutant in the dark. In contrast, in white light, the cca1-1 ataf2-2 double mutant conferred significantly higher SOB7 transcript accumulation than either cca1-1 or ataf2-2 single mutants (C). However, in darkness, the genetic impact of CCA1 or ATAF2 on SOB7 transcript accumulation is similar and has no additive effect (D). Each qRT–PCR value is the mean of results from three biological replicates×three technical replicates (n=9). Error bars denote the SE. One-way ANOVA with Tukey’s HSD test was used to determine the significance of differences. Groups with significant differences were labeled by different letters.
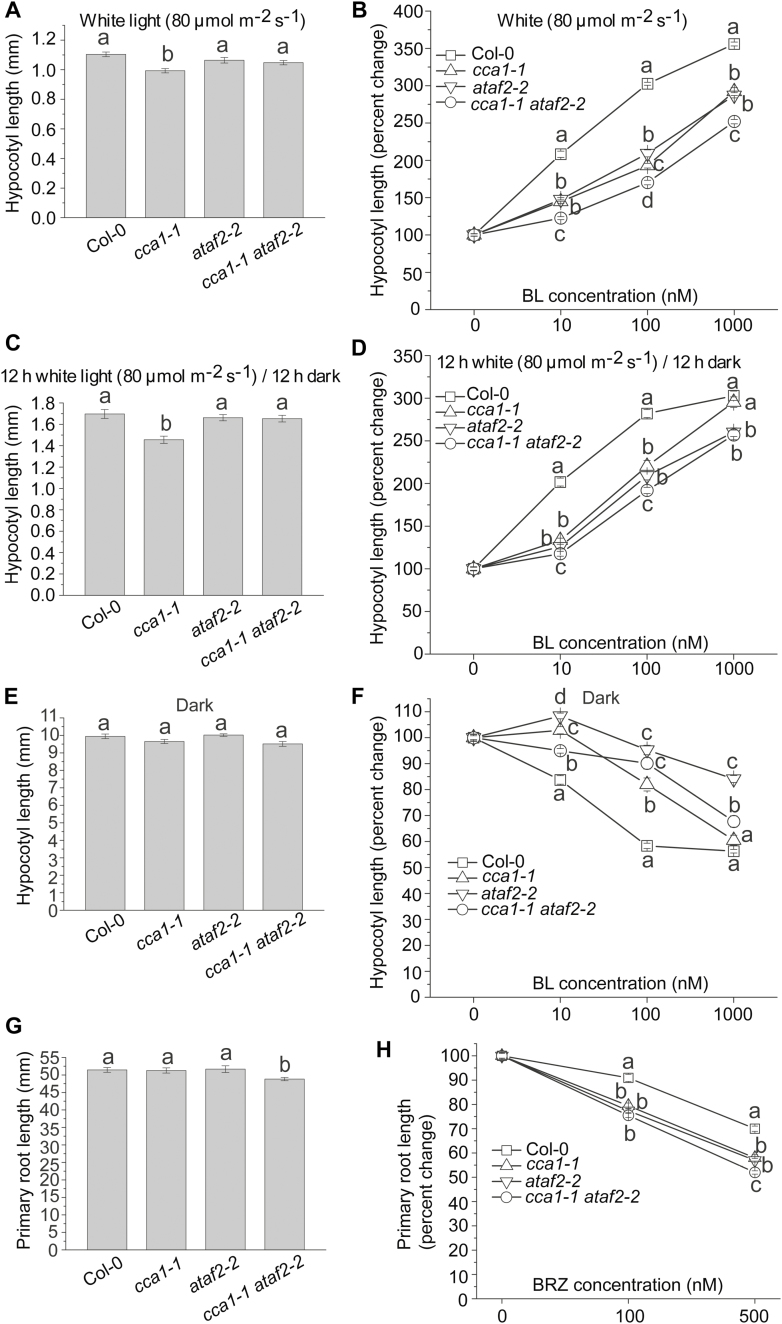
Both CCA1 and ATAF2 impact seedling responsiveness to exogenous BL and the BR biosynthesis inhibitor brassinazole
Since BRs promote hypocotyl growth in white light but have the opposite, suppressing, effect under darkness (Turk et al., 2003; Peng et al., 2015), Arabidopsis seedlings with elevated BAS1 or SOB7 expression are less responsive to exogenous BRs when compared with the wild type (Turk et al., 2005; Peng et al., 2015). To test the BR sensitivity of Col-0, cca1-1, ataf2-2, and cca1-1 ataf2-2 seedlings of all four genotypes were grown on media with gradient concentrations of BL (0, 10, 100, and 1000 nM) for 4 d in 80 μmol m−2 s−1 of continuous white light, 12 h/12 h light and dark photoperiod, and darkness. Since cca1-1 had slightly shorter hypocotyls than Col-0 even without BL treatment (Fig. 5A), seedling hypocotyl lengths in response to exogenous BL were described as percentage changes instead of their absolute values (Fig. 5B). The hypocotyl growth of both cca1-1 and ataf2-2 was less responsive to BL treatments when compared with that of Col-0 (Fig. 5B). cca1-1 ataf2-2 was even more insensitive to BL than the two single mutants (Fig. 5B). Based on our previous gene expression results (Fig. 4), the additive effect of CCA1 and ATAF2 in regulating BR-responsive hypocotyl growth in light is due to their collaborative suppression of SOB7 expression. When seedlings were grown under the 12 h 80 μmol m−2 s−1 light and 12 h dark photoperiod (Fig. 5C), cca1-1 ataf2-2 was less sensitive to BL than the two single mutants under 10 nM or 100 nM BL treatment (Fig. 5D). However, when under the toxic dose of 1000 nM BL treatment, cca1-1 ataf2-2 and ataf2-2 showed similar levels of BL insensitivity whereas cca1-1 and Col-0 were almost equally sensitive to BL (Fig. 5D). In the dark, cca1-1 ataf2-2 did not show higher BL insensitivity than either cca1-1 or ataf2-2 (Fig. 5E, F). The results indicated that compared with ATAF2, CCA1 may be more sensitive to dark and high-concentration BR conditions regarding its regulatory role in maintaining BR homeostasis.
Fig. 5.
Both CCA1 and ATAF2 impact seedling responsiveness to exogenous BL and the BR biosynthesis inhibitor BRZ. (A) cca1-1 had slightly shorter hypocotyls than Col-0, ataf2-2, and cca1-1 ataf2-2 when grown under continuous white light. (B) The hypocotyl growth of both cca1-1 and ataf2-2 was less responsive to BL treatments when compared with that of Col-0. cca1-1 ataf2-2 was even more insensitive to BL than the two single mutants. When seedlings were grown under the 12 h 80 μmol m−2 s−1 light and 12 h dark photoperiod, cca1-1 also had slightly shorter hypocotyls than Col-0, ataf2-2, and cca1-1 ataf2-2 (C), and cca1-1 ataf2-2 was more insensitive to BL than the two single mutants under 10 nM or 100 nM BL treatment (D). (E) Col-0, cca1-1, ataf2-2, and cca1-1 ataf2-2 showed similar hypocotyl lengths when grown in the dark. (F) cca1-1 ataf2-2 did not show higher BL insensitivity than either cca1-1 or ataf2-2 in the dark. (G) cca1-1 ataf2-2 seedlings had slightly shorter primary roots than Col-0, cca1-1, and ataf2-2. (H) cca1-1, ataf2-2, and cca1-1 ataf2-2 all showed significantly higher sensitivity to exogenous BRZ treatments than Col-0, with cca1-1 ataf2-2 being even more sensitive than either single mutant with the treatment of 500 nM BRZ. Four-day-old seedlings grown at 25 °C in 80 μmol m−2 s−1 continuous white light, in a 12 h/12 h light/dark photoperiod, and in darkness were used for hypocotyl measurements. Each data point of hypocotyl length represents the average of measurements from 30 seedlings (n=30). Seven-day-old seedlings grown on vertical plates under continuous white light were used for primary root length measurement. Each data point of root length represents the result of 10 roots (n=10). Error bars denote the SE. One-way ANOVA with Tukey’s HSD test was used to determine the significance of differences. Groups with significant differences were labeled by different letters.
With regard to root growth, cca1-1 ataf2-2 seedlings had significantly shorter primary roots than Col-0, cca1-1, and ataf2-2 (Fig. 5G). When testing the growth response of primary roots to the BR biosynthesis inhibitor brassinazole (BRZ; Asami et al., 2000), cca1-1, ataf2-2, and cca1-1 ataf2-2 all showed significantly higher sensitivity to exogenous BRZ treatments than Col-0, with cca1-1 ataf2-2 being more sensitive than either single mutant with the treatment of 500 nM BRZ (Fig. 5H).
CCA1 is not feedback regulated by BRs
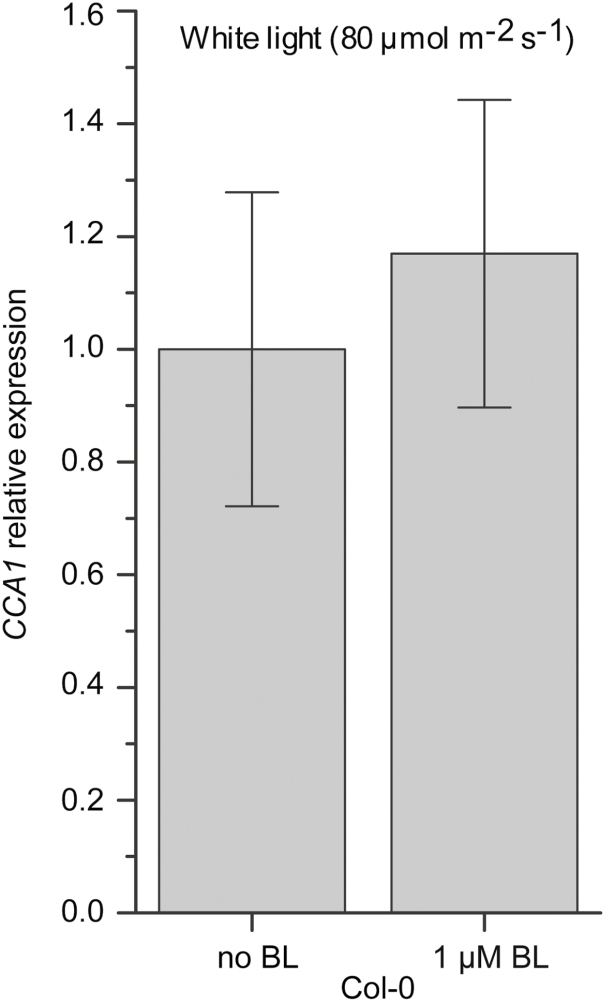
In addition to being a repressor of the BR-inactivating genes BAS1 and SOB7, ATAF2 is transcriptionally suppressed by exogenous BL, which forms a feedback regulatory loop (Peng et al., 2015). This led to the hypothesis that CCA1 may also be feedback regulated by exogenous BL. When treated with BL, CCA1 transcript accumulation in Col-0 did not show any significant change (Fig. 6). The result indicated that unlike ATAF2, CCA1 is not subject to BR-mediated transcriptional feedback regulation.
Fig. 6.
CCA1 is not feedback regulated by BRs. CCA1 transcript accumulations in Col-0 did not show any significant change when treated with 1 μM BL. Four-day-old seedlings grown at 25 °C in 80 μmol m−2 s−1 continuous white light were used for RNA extraction. Each qRT–PCR value is the mean of results from three biological replicates×three technical replicates (n=9). Error bars denote the SE.
CCA1 interacts with ATAF2 at both the DNA–protein and protein–protein levels
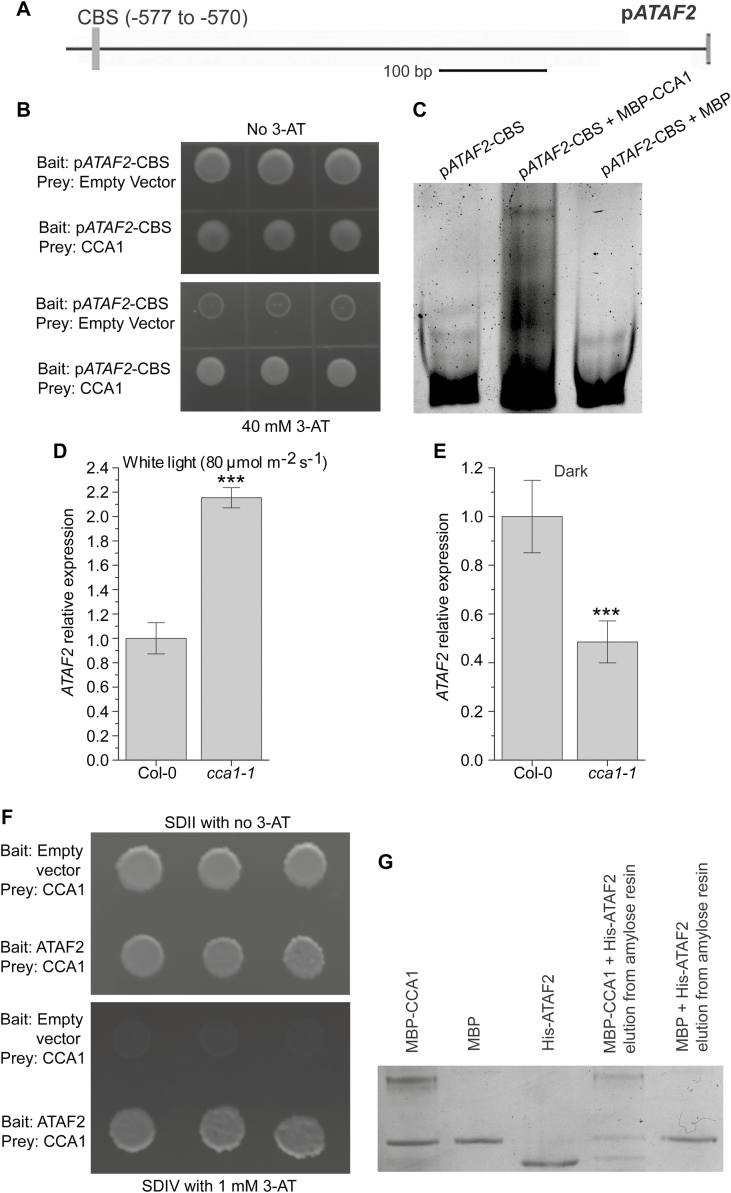
Since CCA1 and ATAF2 share the same binding sites (the EE and CBS) (Peng et al., 2015; Fig. 1), and both act as repressors of the BR-inactivating genes BAS1 and SOB7 (Peng et al., 2015; Figs 2–4), we tested whether these two TFs genetically or physically interact. Like BAS1 and SOB7, the ATAF2 promoter also contains a CBS motif (−577 to −570; Fig. 7A), which is a potential binding target for CCA1. A 63 bp ATAF2 promoter fragment, pATAF2-CBS (−598 to −536; Supplementary Table S1), was used as the bait in a targeted Y1H assay to test its interaction with CCA1. CBS is the only predicted TF-binding site harbored by pATAF2-CBS. The Y1H result demonstrates that CCA1 can bind to the promoter of ATAF2 (Fig. 7B). Their interaction was further confirmed by EMSA (Fig. 7C). Compared with Col-0, cca1-1 seedlings showed significantly higher ATAF2 transcript accumulation when grown in continuous white light (Fig. 7D), whereas the opposite trend was observed in dark-grown seedlings (Fig. 7E). The results above reveal that CCA1 can act as either a repressor or an activator of ATAF2 expression depending on the light conditions. On the other hand, no significant changes of CCA1 expression in ataf2-2 seedlings were observed in either continuous light (Supplementary Fig. S3A) or dark conditions (Supplementary Fig. S3B), which indicates that ATAF2 is not a transcriptional regulator of CCA1. Furthermore, CCA1 physically interacts with ATAF2 in a targeted Y2H assay (Fig. 7F). In a pull-down assay, MBP–CCA1 and His-ATAF2 can be eluted together from the amylose resin, whereas the resin only retained MBP from the mixture of MBP and His-ATAF2 (Fig. 7G). The pull-down result further confirmed physical interaction between CCA1 and ATAF2.
Fig. 7.
CCA1 interacts with ATAF2 at both DNA–protein and protein–protein levels. (A) The ATAF2 promoter contains a CBS motif. (B) CCA1 binds pATAF2-CBS in a targeted Y1H assay. Their interaction was further confirmed by EMSA (C). Compared with Col-0, cca1-1 seedlings showed significantly higher ATAF2 transcript accumulation when grown in continuous white light (D), whereas the opposite trend was observed in dark-grown seedlings (E). (F) CCA1 physically interacts with ATAF2 in a targeted Y2H assay. (G) Physical interaction between CCA1 and ATAF2 was further confirmed by a pull-down assay. MBP–CCA1 and His-ATAF2 can be eluted together from the amylose resin whereas the resin only retained MBP from the mixture of MBP and His-ATAF2 (G). For Y1H assay, the pATAF2-CBS bait was integrated into the mutant HIS3 locus of the yeast strain YM4271. The bait-integrated yeast clone with the lowest self-activation was transformed with the CCA1 prey construct and empty prey vector (negative control). The interaction between CCA1 and pATAF2-CBS was tested by yeast tolerance to 3-AT. For Y2H assay, the CCA1 prey construct was used to transform yeast strain A. After testing for self-activation, the resulting clone was used for transformation of the ATAF2 bait construct and the empty bait vector (negative control). The CCA1–ATAF2 interaction was tested by yeast tolerance to 3-AT and the ability to grown on SDIV medium. All yeast clones were grown at 28 °C for 3–4 d. Three independent clones are shown for each Y1H or Y2H sample. For EMSA, MBP–CCA1 was incubated with pATAF2-CBS and separated by non-denaturing PAGE. The DNA probe and protein–DNA complex were stained by SYBR Green. MBP was used as a negative control. For the pull-down assay, MBP–CCA1 and His-ATAF2 were mixed and incubated overnight at 4 °C with end over end mixing, and then loaded onto the amylose resin. After washing away unbound proteins, the bound proteins were eluted using elution buffer containing 10 mM maltose. As the negative control, the mixture of MBP and His-ATAF2 went through identical procedures. The elution samples were analyzed by SDS–PAGE and stained using Coomassie brilliant blue R-250. Four-day-old seedlings grown at 25 °C in 80 μmol m−2 s−1 continuous white light were used for RNA extraction. Each qRT–PCR value is the mean of results from three biological replicates×three technical replicates (n=9). Error bars denote the SE. Two-tailed Student’s t-test was used to determine the significance of differences. ***P<0.001.
ATAF2, BAS1, and SOB7 are all subject to circadian regulation
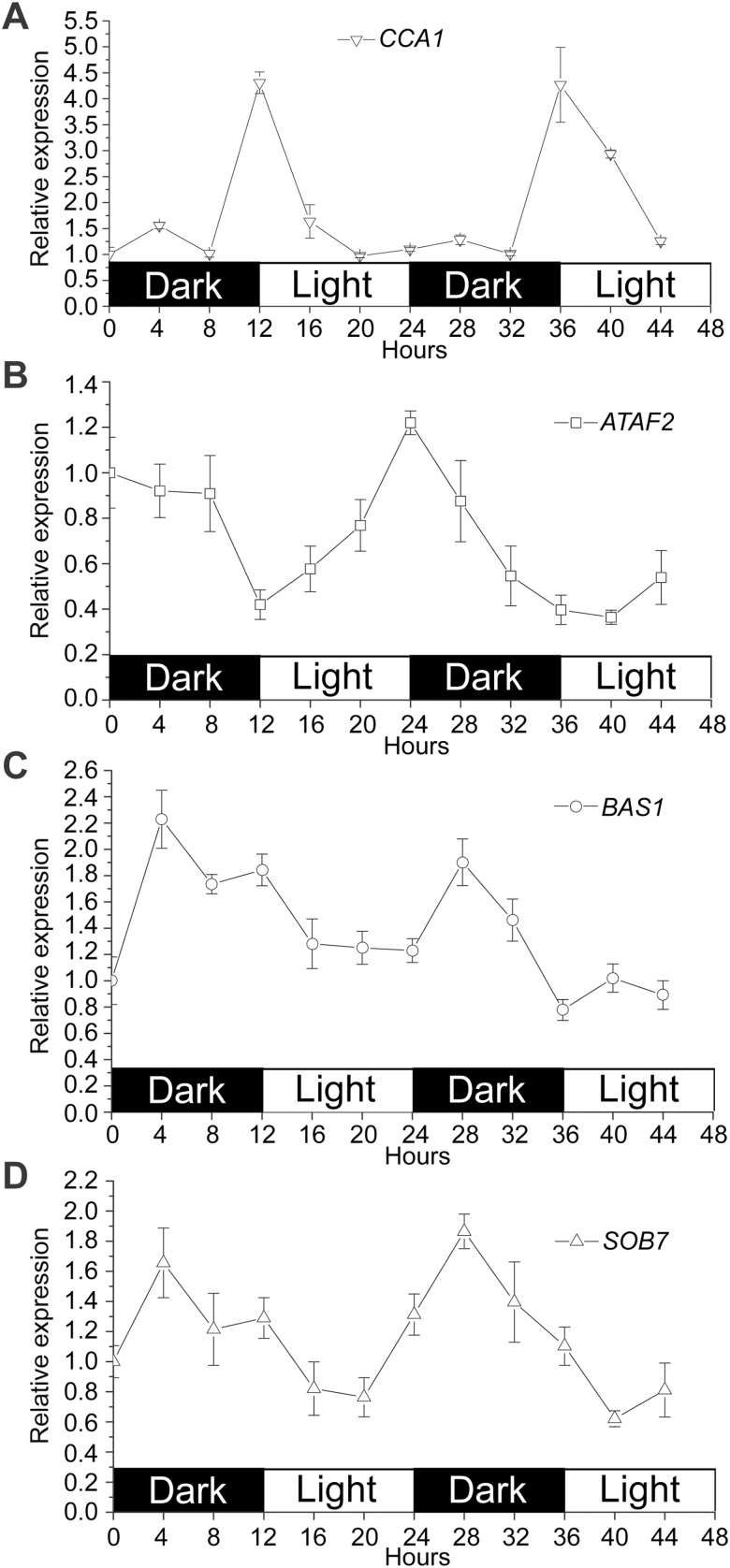
Since CCA1 acts as a core regulator for the circadian clock (Wang and Tobin, 1998), we tested whether ATAF2, BAS1, and SOB7 expression are circadian regulated in wild-type (Col-0) Arabidopsis seedlings (Fig. 8). After seedlings were grown in a 12 h/12 h light/dark cycle for 7 d, gene expression was monitored at 4 h intervals for 2 d under the same photoperiod setting. CCA1 expression was used as a reference (Fig. 8A). Consistent with previous observations (Wang and Tobin, 1998), CCA1 expression was largely stable, with the exception of peak levels occurring at dawn (Fig. 8A). ATAF2, BAS1, and SOB7 were all circadian regulated with distinct expression patterns (Fig. 8B–D). Transcript accumulation of ATAF2 kept decreasing in the dark period and began to increase after transiting to light (Fig. 8B). In contrast, both BAS1 and SOB7 showed higher expression levels in the dark than under light, and their transcript accumulation peaks appeared after entering the dark period for 4 h (Fig. 8C, D).
Fig. 8.
ATAF2, BAS1, and SOB7 are all circadian regulated with distinct expression patterns. (A) As a reference, CCA1 expression was largely stable, with the exception of peak levels occurring at dawn. (B) Transcript accumulation of ATAF2 kept decreasing in the dark period and began to increase after transiting to light. In contrast, both BAS1 (C) and SOB7 (D) showed higher expression levels in the dark than under light, and their transcript accumulation peaks appeared after entering the dark period for 4 h. After Col-0 seedlings were grown in a 12 h/12 h light/dark cycle for 7 d, gene expression was monitored at 4 h intervals for 2 d under the same photoperiod setting. Each qRT–PCR value is the mean of results from three biological replicates×three technical replicates (n=9). Error bars denote the SE.
Discussion
BAS1, SOB7, and multiple other BR-inactivating genes contribute to BR homeostasis
BR inactivation can be achieved via multiple approaches in Arabidopsis, including hydroxylation (Neff et al., 1999; Turk et al., 2003), glycosylation (Poppenberger et al., 2005; Husar et al., 2011), acylation (Roh et al., 2012; M. Wang et al., 2012; K. Schneider et al., 2012; Choi et al., 2013; W. Zhu et al., 2013; Zhang and Xu, 2018), and other unknown or unconfirmed mechanisms (Nakamura et al., 2005; Takahashi et al., 2005; Turk et al., 2005; Marsolais et al., 2007; Yuan et al., 2007; Thornton et al., 2010; Sandhu and Neff, 2013). At least 10 BR-inactivating genes have been identified in Arabidopsis including; P450 hydroxylases, glycosyltransferases, acyltransferases, sulfotransferases, and a reductase. The redundancy of BR-inactivating pathways is consistent with the fact that BRs act in local tissues at extremely low endogenous concentrations (He et al., 2005; Kim et al., 2006; Symons et al., 2008). The role of catabolism in maintaining BR homeostasis appears to be as critical as the biosynthesis and signaling pathways, since tissue-specific BR levels can be fine-tuned by multiple inactivating enzymes and their upstream TF regulatory cascades. For example, LOB negatively regulates BR accumulation by activating BAS1 expression at organ boundaries (Bell et al., 2012). As two transcriptional repressors of BAS1 and/or SOB7, ATAF2 and ARF7 integrate BR inactivation with auxin biosynthesis and signaling, seedling photomorphogenesis, disease resistance, and stress tolerance (Peng et al., 2015; Youn et al., 2016).
CCA1 is a direct repressor of both BAS1 and SOB7
The existence of EE and CBS motifs in BAS1 and SOB7 promoters (Peng et al., 2015; Fig. 1A) indicates that these two genes may be included in the regulatory network of the core circadian clock protein CCA1. The genomic approach of ChIP-Seq did not identify BAS1 or SOB7 as a target of CCA1 (Nagel et al., 2015; Kamioka et al., 2016). However, our focused analysis demonstrated that CCA1 is a direct repressor of both BAS1 and SOB7 (Figs 1–4). Since P450s play critical roles in the metabolism of diverse secondary compounds, they have been used as reporters for different nodes in the circadian clock network (Pan et al., 2009). Therefore, it is not surprising that both BAS1 and SOB7 are subject to the transcriptional regulation of CCA1. The lack of BAS1 and SOB7 in the genomic characterization of CCA1 targets can be explained by the inherent bias of the ChIP-Seq approach in enriching highly expressed loci (Teytelman et al., 2013). The cause of this bias may be that DNA from actively transcribed regions tends to be more exposed to binding proteins and antibodies due to nucleosome depletion (Teytelman et al., 2013). Since both BAS1 and SOB7 have extremely low expression levels that are restricted to specific tissues (Neff et al., 1999; Turk et al., 2003, 2005; Sandhu et al., 2012; Peng et al., 2015), the two genes are more likely to be filtered out than other loci in the ChIP-Seq assay.
CCA1 regulates multiple BR signaling and metabolic genes
There have been established associations between CCA1 and BRs. Two TF-encoding genes involved in BR signaling, ATBS1 (ACTIVATION-TAGGED BRI1 SUPPRESSOR 1)-INTERACTING FACTOR 1 (AIF1; H. Wang et al., 2009) and MYB-LIKE 2 (MYBL2; Ye et al., 2012), have been identified by both Nagel et al. (2015) and Kamioka et al. (2016) as direct targets of CCA1. CCA1 also binds to the promoter of the BR biosynthetic gene DWF4 and activates its expression (Zheng et al., 2018). This report, together with our finding that CCA1 directly suppresses the BR-inactivating genes BAS1 and SOB7 (Figs 1–4), suggests that CCA1 is an overall positive regulator of BR accumulation.
CCA1 is selective in binding EE and CBS elements
Although EEs and CBSs are confirmed binding motifs for CCA1, CCA1 does not associate with all of the EEs or CBSs in the Arabidopsis genome (Kamioka et al., 2016). This binding may require appropriate sequence context within the broader regulatory region (Kamioka et al., 2016). CCA1 also prefers to bind EEs relative to CBSs (Nagel et al., 2015; Kamioka et al., 2016). Consistent with these findings, CCA1 did not bind pBAS1-CBS1, but did interact with pBAS1-EE in our study (Fig. 1B, D, E, G). In contrast, ATAF2 is able to bind both pBAS1-EE and pBAS1-CBS1 (Peng et al., 2015).
CCA1 and ATAF2 have overlapping and distinct patterns in suppressing BAS1 and SOB7
Disruption of either ATAF2 (Peng et al., 2015) or CCA1 (Fig. 3A–T) led to the expansion of BAS1 and SOB7 expression to additional tissues, but the suppressing patterns of CCA1 and ATAF2 are not identical. Compared with CCA1, ATAF2 disruption caused an enhanced expansion of BAS1 and SOB7 in seedlings and flowers (Fig. 3A–P). These tissue-specific pattern differences may reflect the distinct expression patterns of CCA1 and ATAF2 (Fig. 3Q, S).
About a quarter of the T1 pBAS1:BAS1-GUS/cca1-1 (Fig. 2A) and pSOB7:SOB7-GUS/cca1-1 (Fig. 2B) transformants showed the BR-dwarf phenotype. Similar BR-dwarfs were previously observed in pBAS1:BAS1-GUS/ataf2-2 and pSOB7:SOB7-GUS/ataf2-2 transformants (Peng et al., 2015). However, there is no visible dwarfism in cca1-1, ataf2-2, or the cca1-1 ataf2-2 double mutant. Since GUS translational fusions can increases protein stability in Arabidopsis (Chae et al., 2012; Spartz et al., 2012), BAS1–GUS and SOB7–GUS may be more likely to confer BR-dwarfing than their native forms in the cca1-1 and/or ataf2-2 mutant backgrounds. There are also other factors that may contribute to the BR-dwarf phenotype observed in pBAS1:BAS1-GUS and SOB7:SOB7-GUS transgenic plants in the cca1-1 and/or ataf2-2 mutant background. There is an additional BAS1 or SOB7 copy from the original Arabidopsis genome in each transgenic plant, which can result in higher BAS1 or SOB7 expression. Depending on the insert location of the transgene, adjacent enhancer elements may increase gene expression. Some of the remote suppressing cis-regulatory elements may not be included in the pBAS1:BAS1-GUS and pSOB7:SOB7-GUS constructs. An increase of BAS1 or SOB7 expression caused by all these factors may not be sufficient to induce the BR-dwarf phenotype in transgenic plants in the Col-0 background but can lead to dwarfism in the cca1-1 and/or ataf2-2 mutant backgrounds. In contrast, none of the four factors mentioned above exists in the original cca1-1, ataf2-2, or cca1-1 ataf2-2 genetic backgrounds.
CCA1 and ATAF2 additively suppress SOB7 expression in white light
In both light- and dark-grown seedlings, CCA1 and ATAF2 suppress BAS1 expression without an additive effect (Fig. 4A, B). In contrast, CCA1 and ATAF2 additively suppress SOB7 expression in seedlings grown in continuous white light (Fig. 4C). However, suppression of SOB7 expression by CCA1 and ATAF2 is not additive in darkness (Fig. 4D). This light-dependent collaborative suppression of SOB7 helps to explain the observation that cca1-1 ataf2-2 seedlings only show greater insensitivity to exogenous BL treatments than either of the single mutants when grown in white light but not in darkness (Fig. 5A–F). Although BL is not likely to be a preferred substrate for SOB7 (Thornton, et al., 2010), increased expression of SOB7 can still reduce the overall endogenous levels of BRs. It is important to note that the differential regulatory patterns on BAS1 and SOB7 expression are probably influenced by the binding of CCA1 and ATAF2 (Peng et al., 2015; Fig. 1), light- and tissue-specific regulation of CCA1 and ATAF2 expression (Wang and Tobin, 1998; Peng et al., 2015; Fig. 8), and CCA1–ATAF2 interactions at both the DNA–protein and protein–protein levels (Fig. 7). Though we have shown that CCA1 and ATAF2 physically interact via targeted Y2H analysis and the pull-down assay (Fig. 7F, G), attempts to test their in planta interaction via bimolecular fluorescence complementation did not generate positive results. Thus, CCA1–ATAF2 physical interactions in planta may be transient, tissue specific, or require post-translational modification of either protein. In the dark, cca1-1 ataf2-2 showed slightly but significantly lower BAS1 transcript accumulation than either single mutant (Fig. 4B). It is possible that CCA1 and ATAF2 forms a heterodimer to suppress BAS1 expression in the dark. Disruption of either protein abolishes the suppression effect, but the protein left can still bind to the BAS1 promoter to prevent potential binding of other TFs on the same site. When both CCA1 and ATAF2 are disrupted, they lose the transcriptional suppression effect as well as the ability of DNA to bind to the BAS1 promoter. The binding of additional repressors may lead to decreased BAS1 expression in cca1-1 ataf2-2 when compared with either single mutant.
Light and BRs have complex effects on CCA1’s role in BR homeostasis
As part of a feedback regulation loop, ATAF2 expression can be suppressed by external BL treatments (Peng et al., 2015). Additionally, microarray data showed that three other members of the ATAF subfamily, ATAF1 (ANAC002), ANAC102, and ANAC032, are also transcriptionally down-regulated by BL (Kleinow et al., 2009). In contrast, CCA1 is not feedback regulated by BL in our study (Fig. 6). Since CCA1 is a core regulator for the circadian clock, it is not surprising that BRs do not have a significant impact on its expression. With the treatment of 1000 nM BL, cca1-1 and Col-0 showed similar BL response phenotypes when grown under darkness or a 12 h/12 h light/dark photoperiod, but cca1-1 was still more insensitive to BL than Col-0 under continuous light (Fig. 5A–F). This observation indicates that high concentrations of BRs together with darkness may attenuate CCA1’s function in maintaining BR homeostasis, which is consistent with the primary role of CCA1 in circadian regulation and the previous observation that CCA1 is transcriptionally induced by light (Wang and Tobin, 1998).
The circadian oscillation pattern of ATAF2 is different from that of BAS1 and SOB7
BAS1 and SOB7 have a similar circadian oscillation pattern that shows higher expression in the dark, whereas ATAF2’s oscillation cycle is largely opposite, with expression decreasing in the dark and increasing in the light period (Fig. 8B–D). This observation is consistent with our previous finding that ATAF2 is a repressor for BAS1 and SOB7 expression (Peng et al., 2015). The expression of CCA1 itself is also subject to circadian oscillation, with peak levels occurring at dawn (Fig. 8A). With the exception of the dawn period, CCA1 expression levels are relatively low and stable (Fig. 8A). The circadian oscillation pattern of CCA1 is largely consistent with our observation that CCA1 suppresses ATAF2 expression in the light but the effect switches to promotion in darkness (Fig. 7C, D). The comparison of oscillation patterns between CCA1 and BAS1/SOB7 (Fig. 8A, C, D) also largely supports our observation that CCA1 is a repressor of BAS1 and SOB7 expression (Figs 2–4). Peak CCA1 expression levels appear when plants enter the light photoperiod (Fig. 8A). In contrast, both BAS1 and SOB7 have peak expression levels after entering the dark photoperiod for 4 h, and they both have generally lower transcript accumulations during the light photoperiod (Fig. 8C, D). Although both CCA1 and ATAF2 are repressors of BAS1 and SOB7, the circadian oscillation pattern of ATAF2 is different from that of CCA1 (Fig. 8A, B). CCA1 expression can be immediately induced by light, but the effect of light switches to suppression after 1 h (Wang and Tobin, 1998). In contrast, light consistently promotes ATAF2 expression during the whole light photoperiod (Fig. 8B). Unlike CCA1 showing higher accumulation only during the dawn period (Fig. 8A), ATAF2 has opposite expression patterns with similar patterns in light and dark photoperiods (Fig. 8B). Despite the overall consistency between the circadian oscillation patterns and the suppression/activation relationships of CCA1, ATAF2, BAS1, and SOB7, there are still discrepancies during some time periods. These discrepancies can be explained by the fact that the circadian clock is regulated by multiple players besides CCA1, such as CCA1’s closely related partner LATE ELONGATED HYPOCOTYL (LHY; Schaffer et al., 1998).
Light regulates ATAF2 expression via either the circadian or the photomorphogenic pathway
In seedlings grown under a 12 h light and 12 h dark photoperiod, ATAF2 expression gradually drops in the dark and increases steadily after the transition to light, with transcript accumulation levels peaking at the beginning of the evening and being the lowest around dawn (Fig. 8B). On the other hand, we previously found that ATAF2 has higher transcript accumulation in dark-grown etiolated seedlings than in seedlings grown under continuous white light, and that the expression of ATAF2 in white light is fluence rate dependent (Peng et al., 2015). ATAF2 expression can also be suppressed when etiolated seedlings are transferred to white light (Peng et al., 2015). These results indicate that ATAF2 is transcriptionally regulated by light via either the circadian or the photomorphogenic pathway. When seedlings are grown under a light/dark circadian photoperiod, ATAF2 expression is induced during the light period. In contrast, ATAF2 has higher transcript accumulation in seedlings undergoing skotomorphogenesis than in photomorphogenic seedlings.
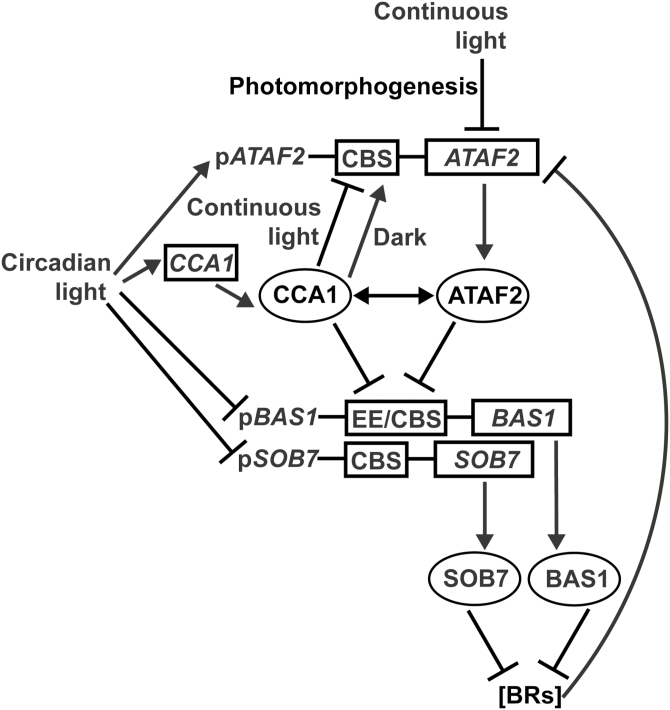
Current model
We summarized the roles of CCA1 and ATAF2 in regulating BR inactivation and how circadian and photomorphogenic pathways are incorporated (Fig. 9). Both CCA1 and ATAF2 suppress the expression of the BR-inactivating genes BAS1 and SOB7 via direct binding to their promoters. However, the role of CCA1 and ATAF2 with regard to BR inactivation is dynamic with respect to the light environment. Both BAS1 and SOB7 are circadian regulated, with higher expression in the dark period. Transcriptionally induced by light, CCA1 plays a role in the oscillation of BAS1 and SOB7. While ATAF2 expression is feedback suppressed by BRs, CCA1 is not subject to this transcriptional regulation. CCA1 suppresses ATAF2 expression in seedlings grown under light but switches to an activator for ATAF2 in etiolated seedlings. In addition, CCA1 may also physically interact with ATAF2 at the protein level. It is important to point out, however, that this model only focuses on the components characterized in this study. Clearly, other CCA1- and ATAF2-interacting proteins, as well as additional regulatory TFs, are likely to have an impact on the overall regulation of the BR-inactivating genes BAS1 and SOB7.
Fig. 9.
Model for the roles of CCA1 and ATAF2 in regulating BR inactivation and the incorporation of circadian and photomorphogenic pathways. Both CCA1 and ATAF2 suppress the expression of BR-inactivating genes BAS1 and SOB7 via direct binding to their promoters. BRs promote Arabidopsis hypocotyl growth under light. BAS1 and SOB7 inhibit hypocotyl elongation by catabolizing BRs. Both BAS1 and SOB7 are circadian regulated with higher expression in the dark period. Transcriptionally induced by light, CCA1 plays a role in the oscillation of BAS1 and SOB7. While ATAF2 expression is feedback suppressed by BRs, CCA1 is not subject to such transcriptional regulation. CCA1 suppresses ATAF2 expression in seedlings grown under light but switches to an activator for ATAF2 in etiolated seedlings. CCA1 can also physically interact with ATAF2 at the protein level. Light induces ATAF2 expression in seedlings undergoing a circadian photoperiod but acts as a repressor when seedlings transit from skotomorphogenesis to photomorphogenesis.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Sequence of the ATAF2 promoter fragment used for targeted Y1H.
Fig. S1. qRT–PCR assays on CCA1 and ATAF2 transcript accumulations demonstrate that cca1-1, ataf2-2, and cca1-1 ataf2-2 are all gene knockout mutants.
Fig. S2. CCA1 modulates the tissue-specific expression patterns of BAS1 and SOB7 in leaves and siliques.
Fig. S3. Compared with Col-0, ataf2-2 seedlings did not show significant changes of CCA1 expression in either continuous light or darkness.
Acknowledgements
This research was supported by the United States National Science Foundation project #1656265 (to MMN) and by the USDA National Institute of Food and Agriculture, Hatch Umbrella Project #1015621 (to MMN). The authors would like to thank Dr James N. Culver (University of Maryland) for providing the pATAF2::GUS construct. We also thank Dr Gaganjot Sidhu and Shahbaz Ahmad in the Neff Lab for their critical review and comments on this manuscript. The authors have no conflict of interest to declare.
Glossary
Abbreviations:
- 3-AT
3-aminotriazole
- BL
brassinolide
- BR
brassinosteroid
- CBS
CCA1-binding site
- P450
cytochrome P450
- EE
Evening Element
- TF
transcription factor
- Y1H
yeast one-hybrid
- Y2H
yeast two-hybrid
References
- Arvidsson S, Kwasniewski M, Riaño-Pachón DM, Mueller-Roeber B. 2008. QuantPrime—a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinformatics 9, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Min YK, Nagata N, Yamagishi K, Takatsuto S, Fujioka S, Murofushi N, Yamaguchi I, Yoshida S. 2000. Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiology 123, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir Y, Jaillais Y, Epple P, Balsemão-Pires E, Dangl JL, Chory J. 2012. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proceedings of the National Academy of Sciences, USA 109, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EM, Lin WC, Husbands AY, Yu L, Jaganatha V, Jablonska B, Mangeon A, Neff MM, Girke T, Springer PS. 2012. Arabidopsis lateral organ boundaries negatively regulates brassinosteroid accumulation to limit growth in organ boundaries. Proceedings of the National Academy of Sciences, USA 109, 21146–21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Isaacs CG, Reeves PH, Maloney GS, Muday GK, Nagpal P, Reed JW. 2012. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. The Plant Journal 71, 684–697. [DOI] [PubMed] [Google Scholar]
- Choi S, Cho YH, Kim K, Matsui M, Son SH, Kim SK, Fujioka S, Hwang I. 2013. BAT1, a putative acyltransferase, modulates brassinosteroid levels in Arabidopsis. The Plant Journal 73, 380–391. [DOI] [PubMed] [Google Scholar]
- Delessert C, Kazan K, Wilson IW, Van Der Straeten D, Manners J, Dennis ES, Dolferus R. 2005. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis. The Plant Journal 43, 745–757. [DOI] [PubMed] [Google Scholar]
- Deplancke B, Dupuy D, Vidal M, Walhout AJ. 2004. A gateway-compatible yeast one-hybrid system. Genome Research 14, 2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplancke B, Vermeirssen V, Arda HE, Martinez NJ, Walhout AJ. 2006. Gateway-compatible yeast one-hybrid screens. Cold Spring Harbor Protocols doi: 10.1101/pdb.prot4590. [DOI] [PubMed] [Google Scholar]
- Favero DS, Jacques CN, Iwase A, Le KN, Zhao J, Sugimoto K, Neff MM. 2016. SUPPRESSOR OF PHYTOCHROME B4-#3 represses genes associated with auxin signaling to modulate hypocotyl growth. Plant Physiology 171, 2701–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero DS, Le KN, Neff MM. 2017. Brassinosteroid signaling converges with SUPPRESSOR OF PHYTOCHROME B4-#3 to influence the expression of SMALL AUXIN UP RNA genes and hypocotyl growth. The Plant Journal 89, 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Oda A, Yoshida R, et al. 2008. Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. The Plant Cell 20, 2960–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Tobin EM. 1999. Loss of the circadian clock-associated protein 1 in Arabidopsis results in altered clock-regulated gene expression. Proceedings of the National Academy of Sciences, USA 96, 4176–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Fujioka S, Blancaflor EB, Miao S, Gou X, Li J. 2010. TCP1 modulates brassinosteroid biosynthesis by regulating the expression of the key biosynthetic gene DWARF4 in Arabidopsis thaliana. The Plant Cell 22, 1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL, Kay SA. 2005. Positive and negative factors confer phase-specific circadian regulation of transcription in Arabidopsis. The Plant Cell 17, 1926–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang ZY. 2005. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307, 1634–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SU, Lee SB, Kim HH, Paek KH. 2012. ATAF2, a NAC transcription factor, binds to the promoter and regulates NIT2 gene expression involved in auxin biosynthesis. Molecules and Cells 34, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husar S, Berthiller F, Fujioka S, et al. 2011. Overexpression of the UGT73C6 alters brassinosteroid glucoside formation in Arabidopsis thaliana. BMC Plant Biology 11, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamioka M, Takao S, Suzuki T, Taki K, Higashiyama T, Kinoshita T, Nakamichi N. 2016. Direct repression of evening genes by CIRCADIAN CLOCK-ASSOCIATED1 in the Arabidopsis circadian clock. The Plant Cell 28, 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HB, Kwon M, Ryu H, Fujioka S, Takatsuto S, Yoshida S, An CS, Lee I, Hwang I, Choe S. 2006. The regulation of DWARF4 expression is likely a critical mechanism in maintaining the homeostasis of bioactive brassinosteroids in Arabidopsis. Plant Physiology 140, 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinow T, Himbert S, Krenz B, Jeske H, Koncz C. 2009. NAC domain transcription factor ATAF1 interacts with SNF1-related kinases and silencing of its subfamily causes severe developmental defects in Arabidopsis. Plant Science 177, 360–370. [Google Scholar]
- Li G, Siddiqui H, Teng Y, et al. 2011. Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis. Nature Cell Biology 13, 616–622. [DOI] [PubMed] [Google Scholar]
- Marsolais F, Boyd J, Paredes Y, Schinas AM, Garcia M, Elzein S, Varin L. 2007. Molecular and biochemical characterization of two brassinosteroid sulfotransferases from Arabidopsis, AtST4a (At2g14920) and AtST1 (At2g03760). Planta 225, 1233–1244. [DOI] [PubMed] [Google Scholar]
- Michael TP, McClung CR. 2002. Phase-specific circadian clock regulatory elements in Arabidopsis. Plant Physiology 130, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahage ISP, Sakamoto S, Nagano M, Ishikawa T, Kawai-Yamada M, Mitsuda N, Yamaguchi M. 2018. An NAC domain transcription factor ATAF2 acts as transcriptional activator or repressor dependent on promoter context. Plant Biotechnology 35, 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel DH, Doherty CJ, Pruneda-Paz JL, Schmitz RJ, Ecker JR, Kay SA. 2015. Genome-wide identification of CCA1 targets uncovers an expanded clock network in Arabidopsis. Proceedings of the National Academy of Sciences, USA 112, E4802–E4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Satoh T, Tanaka S, Mochizuki N, Yokota T, Nagatani A. 2005. Activation of the cytochrome P450 gene, CYP72C1, reduces the levels of active brassinosteroids in vivo. Journal of Experimental Botany 56, 833–840. [DOI] [PubMed] [Google Scholar]
- Neff MM, Nguyen SM, Malancharuvil EJ, et al. 1999. BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proceedings of the National Academy of Sciences, USA 96, 15316–15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T, Chen J, Yin Y. 2017. Cross-talk of brassinosteroid signaling in controlling growth and stress responses. The Biochemical Journal 474, 2641–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Michael TP, Hudson ME, Kay SA, Chory J, Schuler MA. 2009. Cytochrome P450 monooxygenases as reporters for circadian-regulated pathways. Plant Physiology 150, 858–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Zhao J, Neff MM. 2015. ATAF2 integrates Arabidopsis brassinosteroid inactivation and seedling photomorphogenesis. Development 142, 4129–4138. [DOI] [PubMed] [Google Scholar]
- Poppenberger B, Fujioka S, Soeno K, et al. 2005. The UGT73C5 of Arabidopsis thaliana glucosylates brassinosteroids. Proceedings of the National Academy of Sciences, USA 102, 15253–15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenberger B, Rozhon W, Khan M, Husar S, Adam G, Luschnig C, Fujioka S, Sieberer T. 2011. CESTA, a positive regulator of brassinosteroid biosynthesis. The EMBO Journal 30, 1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneda-Paz JL, Breton G, Para A, Kay SA. 2009. A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science 323, 1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh H, Jeong CW, Fujioka S, Kim YK, Lee S, Ahn JH, Choi YD, Lee JS. 2012. Genetic evidence for the reduction of brassinosteroid levels by a BAHD acyltransferase-like protein in Arabidopsis. Plant Physiology 159, 696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu KS, Hagely K, Neff MM. 2012. Genetic interactions between brassinosteroid-inactivating P450s and photomorphogenic photoreceptors in Arabidopsis thaliana. G3 2, 1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu KS, Neff MM. 2013. The Arabidopsis gene ATST4a in not a typical brassinosteroid catabolic gene. Plant Signaling & Behavior 8, doi: 10.4161/psb.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Peto C, Chory J. 2007. The epidermis both drives and restricts plant shoot growth. Nature 446, 199–202. [DOI] [PubMed] [Google Scholar]
- Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carré IA, Coupland G. 1998. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93, 1219–1229. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Breuer C, Kawamura A, et al. 2012. Arabidopsis PIZZA has the capacity to acylate brassinosteroids. PLoS One 7, e46805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnejat-Bushehri S, Tarkowska D, Sakuraba Y, Balazadeh S. 2016. Arabidopsis NAC transcription factor JUB1 regulates GA/BR metabolism and signalling. Nature Plants 2, 16013. [DOI] [PubMed] [Google Scholar]
- Spartz AK, Lee SH, Wenger JP, Gonzalez N, Itoh H, Inzé D, Peer WA, Murphy AS, Overvoorde PJ, Gray WM. 2012. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. The Plant Journal 70, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons GM, Reid JB. 2004. Brassinosteroids do not undergo long-distance transport in pea. Implications for the regulation of endogenous brassinosteroid levels. Plant Physiology 135, 2196–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons GM, Ross JJ, Jager CE, Reid JB. 2008. Brassinosteroid transport. Journal of Experimental Botany 59, 17–24. [DOI] [PubMed] [Google Scholar]
- Takasaki H, Maruyama K, Takahashi F, Fujita M, Yoshida T, Nakashima K, Myouga F, Toyooka K, Yamaguchi-Shinozaki K, Shinozaki K. 2015. SNAC-As, stress-responsive NAC transcription factors, mediate ABA-inducible leaf senescence. The Plant Journal 84, 1114–1123. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nakazawa M, Shibata K, Yokota T, Ishikawa A, Suzuki K, Kawashima M, Ichikawa T, Shimada H, Matsui M. 2005. shk1-D, a dwarf Arabidopsis mutant caused by activation of the CYP72C1 gene, has altered brassinosteroid levels. The Plant Journal 42, 13–22. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Asami T, Yoshida S, Nakamura Y, Matsuo T, Okamoto S. 2005. Brassinosteroid homeostasis in Arabidopsis is ensured by feedback expressions of multiple genes involved in its metabolism. Plant Physiology 138, 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teytelman L, Thurtle DM, Rine J, van Oudenaarden A. 2013. Highly expressed loci are vulnerable to misleading ChIP localization of multiple unrelated proteins. Proceedings of the National Academy of Sciences, USA 110, 18602–18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LE, Rupasinghe SG, Peng H, Schuler MA, Neff MM. 2010. Arabidopsis CYP72C1 is an atypical cytochrome P450 that inactivates brassinosteroids. Plant Molecular Biology 74, 167–181. [DOI] [PubMed] [Google Scholar]
- Thornton LE, Peng H, Neff MM. 2011. Rice CYP734A cytochrome P450s inactivate brassinosteroids in Arabidopsis. Planta 234, 1151–1162. [DOI] [PubMed] [Google Scholar]
- Turk EM, Fujioka S, Seto H, Shimada Y, Takatsuto S, Yoshida S, Denzel MA, Torres QI, Neff MM. 2003. CYP72B1 inactivates brassinosteroid hormones: an intersection between photomorphogenesis and plant steroid signal transduction. Plant Physiology 133, 1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk EM, Fujioka S, Seto H, et al. 2005. BAS1 and SOB7 act redundantly to modulate Arabidopsis photomorphogenesis via unique brassinosteroid inactivation mechanisms. The Plant Journal 42, 23–34. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu Y, Fujioka S, Asami T, Li J, Li J. 2009. Regulation of Arabidopsis brassinosteroid signaling by atypical basic helix–loop–helix proteins. The Plant Cell 21, 3781–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liu X, Wang R, Li W, Rodermel S, Yu F. 2012. Overexpression of a putative Arabidopsis BAHD acyltransferase causes dwarfism that can be rescued by brassinosteroid. Journal of Experimental Botany 63, 5787–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Culver JN. 2012. DNA binding specificity of ATAF2, a NAC domain transcription factor targeted for degradation by Tobacco mosaic virus. BMC Plant Biology 12, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Goregaoker SP, Culver JN. 2009. Interaction of the Tobacco mosaic virus replicase protein with a NAC domain transcription factor is associated with the suppression of systemic host defenses. Journal of Virology 83, 9720–9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Kenigsbuch D, Sun L, Harel E, Ong MS, Tobin EM. 1997. A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. The Plant Cell 9, 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Tobin EM. 1998. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93, 1207–1217. [DOI] [PubMed] [Google Scholar]
- Wei Z, Yuan T, Tarkowská D, Kim J, Nam HG, Novák O, He K, Gou X, Li J. 2017. Brassinosteroid biosynthesis is modulated via a transcription factor cascade of COG1, PIF4, and PIF5. Plant Physiology 174, 1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakir E, Hilman D, Kron I, Hassidim M, Melamed-Book N, Green RM. 2009. Posttranslational regulation of CIRCADIAN CLOCK ASSOCIATED1 in the circadian oscillator of Arabidopsis. Plant Physiology 150, 844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Li L, Guo H, Yin Y. 2012. MYBL2 is a substrate of GSK3-like kinase BIN2 and acts as a corepressor of BES1 in brassinosteroid signaling pathway in Arabidopsis. Proceedings of the National Academy of Sciences, USA 109, 20142–20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JH, Kim MK, Kim EJ, Son SH, Lee JE, Jang MS, Kim TW, Kim SK. 2016. ARF7 increases the endogenous contents of castasterone through suppression of BAS1 expression in Arabidopsis thaliana. Phytochemistry 122, 34–44. [DOI] [PubMed] [Google Scholar]
- Yuan T, Fujioka S, Takatsuto S, Matsumoto S, Gou X, He K, Russell SD, Li J. 2007. BEN1, a gene encoding a dihydroflavonol 4-reductase (DFR)-like protein, regulates the levels of brassinosteroids in Arabidopsis thaliana. The Plant Journal 51, 220–233. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu L. 2018. Arabidopsis BRASSINOSTEROID INACTIVATOR2 is a typical BAHD acyltransferase involved in brassinosteroid homeostasis. Journal of Experimental Botany 69, 1925–1941. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li J. 2012. Regulation of brassinosteroid biosynthesis and inactivation. Journal of Integrative Plant Biology 54, 746–759. [DOI] [PubMed] [Google Scholar]
- Zhao J, Favero DS, Peng H, Neff MM. 2013. Arabidopsis thaliana AHL family modulates hypocotyl growth redundantly by interacting with each other via the PPC/DUF296 domain. Proceedings of the National Academy of Sciences, USA 110, E4688–E4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zhang F, Wang S, Su Y, Ji X, Jiang P, Chen R, Hou S, Ding Y. 2018. MLK1 and MLK2 coordinate RGA and CCA1 activity to regulate hypocotyl elongation in Arabidopsis thaliana. The Plant Cell 30, 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JY, Sae-Seaw J, Wang ZY. 2013. Brassinosteroid signalling. Development 140, 1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Wang H, Fujioka S, Zhou T, Tian H, Tian W, Wang X. 2013. Homeostasis of brassinosteroids regulated by DRL1, a putative acyltransferase in Arabidopsis. Molecular Plant 6, 546–558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.