Abstract
Introduction:
In 2014, we implemented a geriatric hip fracture patient care pathway at our institution which was designed to improve outcomes and decrease time to surgery.
Materials and Methods:
We analyzed retrospective data from 463 patients, aged greater than 50, who had surgical treatment for a closed hip fracture due to a low-energy injury between 2013 and 2016 at an academic institution. Objective outcome measures included time to surgery, mortality rate, and total hospital length of stay. Our primary goal was to decrease the time to surgery for definitive fracture fixation to within 24 hours of admission to the hospital for patients who were medically fit for surgery.
Results:
We implemented a multidisciplinary, collaborative approach to address the needs of this specific patient population. Prior to implementing the pathway in 2013, our baseline time to surgery within 24 hours was 74.67%. After implementation, we had incremental yearly increases in the percentage of patients operated on within 24 hours, 82.31% in 2014 (P = .10) and 84.14% in 2015 (P = .04). During the study period, our overall time to surgery was reduced by 27% with an initial average of 20.22 hours in 2013, decreasing to 15.33 hours in 2014, and 14.63 hours in 2015. Our mortality rate at 1 year was 16% in 2013, 17% in 2014, and 15% in 2015.
Conclusion:
With implementation of the pathway, we were able to expedite surgical care for our patients and demonstrate a 10% improvement in the percentage of patients able to have surgery within 24 hours over a 3-year period. Our mortality and hospital length of stay, however, remained the same. Through this collaborative process and system standardization, we believe we have significantly improved not only direct patient care but their overall hospital experience. We continue to make improvements in our pathway.
Keywords: geriatric hip fracture, time to surgery, length of stay, mortality, elderly
Introduction
The number of patients with fragility fractures of the proximal femur is steadily increasing.1 The World Health Organization estimates that by 2050 a total of 6 million hip fractures will occur worldwide every year.2 As many as 70% of those who suffer a fragility fracture will be admitted to skilled nursing care following fracture repair, and 10% will remain in nursing homes for a year or longer.3-5 The 1-year mortality rate after fixation for a fragility fracture varies but is commonly cited to be as high as 30% in patients undergoing surgical fixation.6 Thus, these fractures are a sentinel event that often results in permanent functional impairment, immobility, institutionalization, and even death.5,6
Timely surgical care decreases patient discomfort and morbidity and helps facilitate expedient rehabilitation for ambulation, which in turn helps the patient to return to their baseline function quickly and safely.6-9 Perioperative care of these patients has received substantial attention in recent years in order to improve primary outcomes such as mortality and hospital length of stay.2-10
Prior to surgery, patients are bedridden and in considerable pain. Many have posited that unnecessary delays in surgical repair result in prolonged immobilization, which negatively affects postoperative outcome due to the increased risk of venous thromboembolism, urinary tract infections, atelectasis, and pressure ulcer development.10-13
We developed a collaborative, multidisciplinary approach to facilitate hip fracture care for our patients. The Departments of Orthopaedics and Rehabilitation, Medicine, and Anesthesiology collectively decided that hip fracture patients, if medically stable, should not have to wait any longer than 24 hours for their hip surgery. We felt that it was our responsibility to decrease the time that these patients wait to have surgery in order to both decrease their pain and accelerate their rehabilitation. The purpose of this article is to share our experiences developing this multidisciplinary approach and provide insight into the results of our efforts.
Materials and Methods
Between July 1, 2013, and June 30, 2016, we tracked the outcomes of patients with low-energy closed hip fractures admitted to our institution. In July 2014, we implemented a multidisciplinary geriatric hip fracture protocol to improve patient outcomes and decrease time to surgery (TTS). On August 6, 2015, our institutional review board granted expedited approval for this retrospective review study (STUDY00003092). All patients age greater than 50 diagnosed with an isolated proximal femur fracture from a low-energy mechanism that went on to have surgical fixation or arthroplasty were followed. Patients were initially evaluated in the emergency department (ED) at our institution prior to admission. Patients were excluded if they presented as a trauma activation or if they sustained a proximal femur fracture from a high-energy mechanism. Time to surgery was reported as the length of time from admission to the hospital (placement of admission order) to the time the patient entered the operating room. This would account for the time that the patient stays in the ED until a bed is available. We recorded hospital length of stay, discharge disposition, age, gender, international normalized ratio (INR), readmissions within 30 days of discharge, whether there was a preoperative echocardiogram (echo) completed, whether patients were anticoagulated, and overall mortality rates at 1 year post-surgery.
Our protocol for managing patients with hip fractures is as follows: When a patient suspected of having a fragility hip fracture is seen in the ED, an initial X-ray of the injured hip is obtained. Once the fracture diagnosis is confirmed, the orthopedic service is consulted for further treatment recommendations. The orthopedic service then evaluates the patient and consults the medicine service to provide recommendations for medical optimization and cardiac risk assessment.14 The medicine attending then determines, based on medical comorbidities, if the patient should be admitted to the orthopedic service with medical comanagement or admitted directly to the medicine service. Examples of medical comorbidities that would deem a patient appropriate for admission to the medicine service include any active condition that would delay the patient from going to the operating room such as congestive heart failure (CHF), uncontrolled arrhythmias, and chronic obstructive pulmonary disease (Figure 1).
Figure 1.
Hip fracture protocol.
A preoperative anesthesia evaluation is performed for risk assessment and recommendations for optimization including ordering nonroutine tests (ie, echo) and the need to obtain any pertinent outside medical records. When the patient is scheduled for surgery, the chief scheduling anesthesiologist (CSA) is contacted by a third-year orthopedic resident. If the admission is during normal work hours, this communication happens immediately upon admission. For admissions after hours, the orthopedic resident calls the CSA the next day to arrange a surgery time.
If an echo is ordered during daytime hours, it is completed by an echo technician. This is facilitated by communicating directly with the head echo technologist to facilitate expedient completion of the test. If the echo is ordered during evening hours, the cardiology fellow on call is contacted to facilitate completion of the test (as a first case the following morning or as soon as possible). We did not specifically track how many were actual first case starts. The echo would then be completed and read by the cardiology fellow (Figure 1).
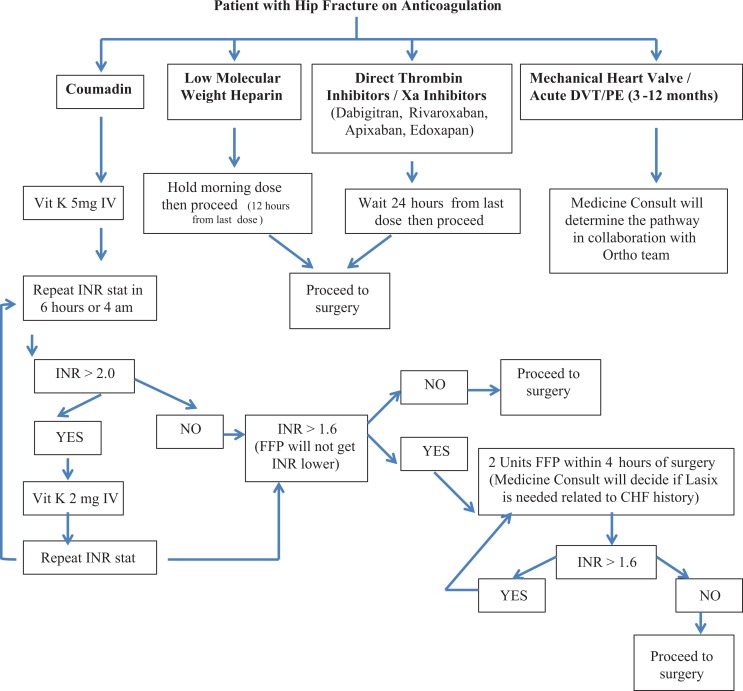
If a patient is determined to have a supratherapeutic INR preoperatively, the hip fracture anticoagulation pathway is followed (Figure 2). If the patient was on warfarin preoperatively, 5 mg of intravenous (IV) vitamin K is administered in the ED with a repeat INR ordered 6 hours later. If the repeat INR is greater than 2, an additional dose of 2 mg of vitamin K is administered IV and an INR repeated. If the repeat INR is 1.6 or less, we proceed with surgery. If it is greater than 1.6, 2 units of fresh frozen plasma (FFP) are given and the INR repeated. Fresh frozen plasma is administered until the INR is less than 1.6. While there are reports of vitamin K taking up to 36 hours to lower INR, the medicine consultants felt that INR is significantly lowered within a 12-hour window of administration of IV formulations. Using FFP as first line for reduction of INR could be problematic in patients who have heart failure, where a colloid fluid load may trigger chronic heart failure exacerbation. Thrombotic risks are also lower with IV vitamin K.15
Figure 2.
Hip fracture anticoagulation pathway. Note: Heart failure or creatinine clearance <30, then Medicine Consult Service will determine pathway for therapeutic or prophylactic dosing after surgery in collaboration with the ortho team. A creatinine clearance <30 is important—sometimes you need to use therapeutic lovenox to bridge after surgery which has a creatinine threshold.
Furosemide is administered as needed to prevent fluid overload. If the patient was previously taking low-molecular-weight heparin (LMWH), surgery is delayed for 12 hours after the last dose of LMWH. If the patient was taking a direct thrombin inhibitor, surgery is delayed for 24 hours from the last dose. If the patient had a mechanical heart valve, an acute deep vein thrombosis, or a pulmonary embolus within the last year, a tailored anticoagulation regimen specific to the patient’s comorbidities and bleeding risk was implemented.
Results
A total of 463 patients were included in the study. There were 162 males (34.9%) and 301 females (65.1%). Average age at the time of fracture was 79.8 years of age (51-99). Forty-two percent of patients sustained intertrochanteric hip fractures, 48% femoral neck fractures, and 9% subtrochanteric hip fractures. Three percent of our patients sustained fragility fractures of the contralateral hip within the study period.
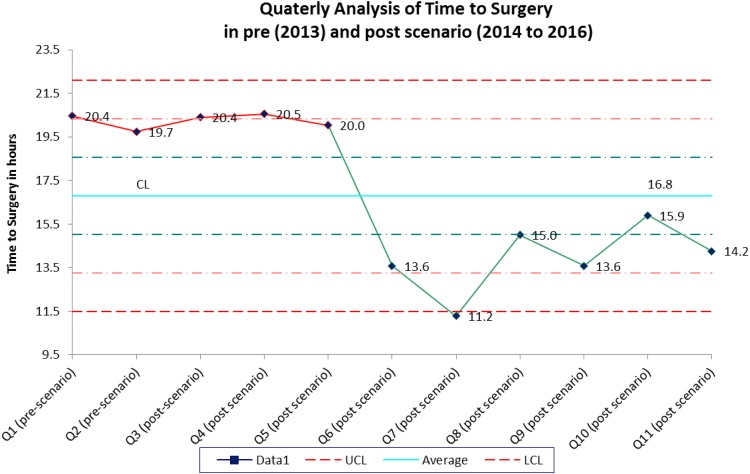
Prior to implementation of our fragility fracture pathway in 2013, the average TTS was 20.22 hours (1.5-119.7). After initiating the pathway, it significantly decreased to 15.33 (1.4-109.8) hours in 2014 (P = .01) and 14.63 (1.4-54.5) hours in 2015 (P = .00). Our baseline percent of patients who made it to the operating room within 24 hours was 74.67% in 2013, 82.31% in 2014 (P = .10), and 84.14% in 2015 (P = .04). We also did a quarterly analysis of TTS in pre- (2013) and postimplementation (2014-2016) (Figure 3). In the third post-quarter following implementation of the hip fracture pathway (Q5), we started to see sustained improvement in timeliness of care for our patients. Length of stay was 5.49 days (1.2-21.3) in 2013 prior to implementation, 5.91 days (2.6-31.5) in 2014 (P = .30), and 6.31 days (1.3-28.8) in 2015 (P = .04).
Figure 3.
Time to surgery. We calculated average time to surgery in each quarter in pre (2013) and post (2014-2016) scenario. CL means a center line representing the median of the data, UCL means upper control limit, and LCL means lower control limit.
“Reason(s) for delay” to surgery were recorded to improve our pathway. The most common reasons for delay in getting patients to the operating room within 24 hours were INR reversal and medical optimization. The INR reversal was the cause of delay in 33% of patients in 2013, 31% in 2014, and 30% in 2015. Preoperative medical optimization was the reason for delay greater than 24 hours in 33% of cases in 2013, 34% of cases in 2014, and 9% in 2015. In 2013, 1 patient needed further evaluation due to a new colon cancer diagnosis, 1 patient was being treated for pneumonia, 2 were being treated for a urinary tract infection, and 3 patients required optimization due to liver failure. In 2014, 4 patients were delayed for optimization of CHF exacerbations. Other causes for delays (each in one person) in 2014 included the need for medical optimization of arrhythmias, gastrointestinal bleeding, chronic kidney disease, hypothermia, pneumonia, and acute altered mental status. In 2015, 4 patients were delayed due to an acute gastrointestinal bleed, 2 for CHF exacerbations, and 1 each for pneumonia and a new diagnosis of chronic myelogenous leukemia. The need to obtain an echo was also an important factor in getting patients to the operating room in a timely manner. In 2013, 25% of patients were delayed greater than 24 hours due to the need for an echo. In 2014, it decreased to 7%, but increased to 26% in 2015 (Table 1).
Table 1.
Demographic Data of Patients With a Hip Fracture at the Time of Presentation.
| All Patients | 2013 | 2014 | 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | <24 hours | >24 hours | Total | <24 hours | >24 hours | Total | <24 hours | >24 hours | ||
| Number of patients (%) | 463 | 154 | 115 (74.67) | 39 | 164 | 135 (82.31) | 29 | 145 | 122 (84.14) | 23 |
| Number of males | 162 | 50 | 36 | 14 | 57 | 46 | 11 | 55 | 42 | 13 |
| Number of females | 301 | 104 | 79 | 25 | 107 | 89 | 18 | 90 | 80 | 10 |
| Average age in years | 79.79 | 78.71 | 79.71 | 74.86 | 79.05 | 78.70 | 81.06 | 81.14 | 81.47 | 79.43 |
| Average time to surgery in hours (range) | 16.74 | 20.22 (1.5-119.7) | 12.13 | 44.08 | 15.33 (1.4-109.8) | 10.06 | 39.83 | 14.63 (1.4-54.5) | 11.38 | 31.86 |
| Number of patients on anticoagulation | 82 | 25 | 10 | 14 | 26 | 17 | 9 | 32 | 23 | 9 |
| Average INR | 1.29 | 1.26 | 1.12 | 1.69 | 1.26 | 1.22 | 1.46 | 1.34 | 1.28 | 1.64 |
| Average hospital length of stay in days (range) | 5.90 | 5.49 (1.2- 21.2) | 4.97 | 7.01 | 5.91 (2.6-31.5) | 5.45 | 8.06 | 6.31 (1.3-28.8) | 6.19 | 6.96 |
| Number of patients readmitted within 30 days | 60 | 20 | 12 | 8 | 16 | 11 | 5 | 24 | 19 | 5 |
| Mortality within 30 days | 22 | 6 | 6 | 0 | 7 | 5 | 2 | 9 | 7 | 2 |
| Mortality within 1 year | 75 | 25 | 14 | 11 | 28 | 20 | 8 | 22 | 20 | 2 |
Abbreviation: INR, internal normalized ratio.
Our 1-year mortality rate pre-and postpathway implementation was 16% in 2013, 17% in 2014, and 15% in 2015. After discharge, the majority of our patients went to either a rehabilitation or skilled nursing facility (SNF). In 2013, 41% of our patients were discharged to an SNF and 44% to a rehabilitation facility. In 2014, 32% to an SNF and 46% to a rehabilitation facility, and in 2015, 40% to an SNF and 46% to a rehabilitation facility.
Discussion
There has been a wealth of research on multidisciplinary geriatric fracture care, the first article published on the topic by Devas in 1967.16 It is well known that there is an increased risk of morbidity, mortality, and cost associated with fragility fractures in the elderly population.3,17 Our hip fracture pathway was a collaborative initiative that involved the Departments of Orthopaedics and Rehabilitation, Internal Medicine, and Anesthesiology. Our goal was to safely perform surgery within 24 hours of the written hospital admission order. Once agreed upon, our pathway was then codified in a formal written document. It sets expectations and enhanced communication between multiple specialties. Prior to implementing the pathway, the baseline percentage of patients who went to the operating room within 24 hours of admission was 74%. After implementation, we improved this to 84% over the following 2 years. We also showed sustained improved timeliness of care in the third post-quarter of implementation of the program (Figure 3). One could likely anticipate 3 months to see effective culture change.
To accomplish this degree of improvement, we tracked the timeliness of care for each patient with a fragility hip fracture during their hospitalization to better understand our system processes and identify areas for improvement. We recognized that some patients were waiting unnecessarily for hip fracture surgery which contributed to a more prolonged pain experience by the patient. Our aim was, therefore, to increase the number of hip fracture patients who went to the operating room safely within 24 hours of their admission to the hospital. Enhanced communication between the Departments of Orthopaedics and Rehabilitation, Medicine, and Anesthesiology was critical. Schedule planning at 6:15 am between Orthopaedics and Anesthesiology facilitated surgery scheduling as early as possible during the day. Prior to implementing this pathway, a patient would wait to have their surgery until the end of the list of operative cases for the day. Once a time for an available operating room was determined by the anesthesia team, the orthopedic team then was responsible to arrange for a surgeon to perform the case. This required a significant amount of collaboration among the orthopedic surgeon team, and oftentimes it was our orthopedic trauma surgeons or joint replacement surgeons who would perform the surgery. Surgeon availability as a reason for delay in surgery was 10% in 2013, 28% in 2014, and 35% in 2015 (Table 2). Delay in 2013 was lower because the practice at that time was to perform the cases at the end of the day. When we moved to performing the cases during the day, surgeon availability became more of a challenge which resulted in delayed surgery in one-third of the cases. Implementing the pathway was, nonetheless, very effective at reducing the TTS by 27% for all patients over 3 years and increased the percentage of patients able to have surgery within 24 hours by 10%. Improved communication between the orthopedic anesthesiology faculty regarding operating room and surgeon availability was considered the main reason for this improvement. Given the unanticipated nature of these cases, we felt that this would be difficult to improve upon but we continue to look for ways to improve our hip fracture pathway. The Department of Internal Medicine played an important role in the perioperative optimization of our patients. Their recommendations are now communicated directly to the anesthesiology attending on call. Prior to this, communication between Medicine and Anesthesiology was inconsistent. The key to collaboration and communication to facilitate hip fracture care was the result of a commitment to the goal by all 3 departments.
Table 2.
Reasons for Time to Surgery Greater than 24 Hours.
| Reasons for Time to Surgery >24 hours | 2013 | 2014 | 2015 | |||
|---|---|---|---|---|---|---|
| Patients | Percentage | Patients | Percentage | Patients | Percentage | |
| INR reversal | 13 | 33 | 9 | 31 | 7 | 30 |
| Need for echocardiogram | 9 | 23 | 2 | 7 | 6 | 26 |
| Medical optimization | 13 | 33 | 10 | 34 | 2 | 9 |
| OR/surgeon availability | 4 | 10 | 8 | 28 | 8 | 35 |
Abbreviation: INR, international normalized ratio; OR, operating room.
The requirement for echo prior to surgery created delays in getting patients to surgery. In our institution, echos are not performed after-hours unless they are an emergency. These studies are typically requested by either Medicine or Anesthesiology for preoperative risk assessment, optimization, and perioperative management. The majority of delays in obtaining this study were due to the fact that, unless emergent, echos were not available after-hours due to the nature of staffing at our hospital. Implementation of a standardized multidisciplinary approach to obtaining an echo was initiated to improve time to obtaining an echo (Figure 1). Our data demonstrate that after the first year of implementing the pathway to obtain an echo, the incidence of a delay in TTS of greater than 24 hours due to the need for an echo decreased from 25% in 2013 to 7% and 2014. Unfortunately, in 2015, this delay increased back to baseline at 26%. When we examined this more closely, we determined it was most likely related to the fact that a new group of cardiology fellows started the program and were not well versed in the echo protocol for hip fracture patients. As a result, new cardiology fellows receive this information as part of their on-boarding orientation prior to starting fellowship.
The INR reversal also caused a significant delay in approximately one-third of our patients. Prior to implementing the pathway, there was considerable variability in managing INR reversal. To make the practice more consistent, we added a standardized protocol for INR reversal to our pathway. This was created in conjunction with our Internal Medicine colleagues. Special attention was paid to specific situations such as volume overload with the use of FFP in patients with heart failure (Figure 2). The incidence of delay for INR reversal, however, remained relatively unchanged at 33% in 2013, 31% in 2014, and 30% of in 2015. Despite the lack of improvement in delays specifically related to INR reversal, we feel that this pathway has had an impact in that it created a standardized process and less confusion.
The second year of pathway implementation demonstrated similar results to the pilot year both in terms of postoperative mortality and hospital length of stay. We did not see any effective change in these 2 metrics following initiation of our program. We are currently in the process of optimizing postsurgical care and outpatient placement. This is a very complicated process requiring multiple points of intervention. There is a growing body of literature supporting the value of a geriatric specialist including a reduction in hospital morbidity and mortality while also reducing the cost of care.18,19 At our institution, we are currently investigating the potential role of a geriatric specialist within our system. This could aid in patient management and care coordination with a goal to improve the transition from hospital discharge to home or a secondary care facility. Currently, at our institution, discharge disposition is managed by a team of social workers and care coordinators. As anyone who works in this environment is aware, there are many complexities to this process. In the United Kingdom, a National Hip Fracture Database was developed to assist in the creation of best practice guidelines and improved care through future research endeavors.20,21 Other countries have also followed in the creation of orthogeriatric models of care.20-25 In the United States, Zuckerman et al showed interdisciplinary care for the complex medical management of patients with hip fractures with reduced postoperative complications, intensive care transfers, and discharges to nursing homes (Table 3).26
Table 3.
Disposition.
| Discharge Disposition | 2013 | 2014 | 2015 | |||
|---|---|---|---|---|---|---|
| Patients | Percentage | Patients | Percentage | Patients | Percentage | |
| Transfer to skilled nursing facility | 63 | 41 | 52 | 32 | 59 | 41 |
| Transfer to rehabilitation facility | 67 | 44 | 75 | 46 | 66 | 46 |
| Discharge to other institution | 3 | 2 | 5 | 3 | 1 | 1 |
| Discharge to federal facility | 2 | 1 | 3 | 2 | 1 | 1 |
| Hospice | 1 | 1 | 2 | 1 | 1 | 1 |
| Died in hospital | 4 | 3 | 2 | 1 | 4 | 3 |
| Discharge to home | 14 | 9 | 25 | 15 | 13 | 9 |
| Total | 154 | 100 | 164 | 100 | 145 | 100 |
Our research has limitations. First, this was a retrospective review of a health-care intervention that we implemented to create a more efficient pathway for patients with hip fractures to get to the operating room for definitive fracture treatment. As such, we did not have preoperative data or other preintervention data to assess the specific patient perspective which could have provided us with very useful information. We feel, however, that reporting our experience and lessons learned will prove to be helpful to other organizations that may want to develop a more coordinated process for patients with hip fractures to safely and efficiently obtain surgical care.
Second, while mortality is a commonly reported outcome in the literature when researching geriatric hip fractures, the scope of our project is too small to truly reflect effect on mortality. Lastly, our work was done at a large academic institution at a single center. Some of the difficulties we encountered may not be generalizable to other practice types.
In summary, we describe a multidisciplinary approach to facilitate care for our hip fracture patient population, which showed improved timeliness of surgical care (Table 4). Prior to our intervention, we appreciated that delays in surgery created a situation where our patients had to lay in bed, in pain, for long periods of time. We wanted to improve the timeliness of surgical care for these individuals. Our pathway improved the overall proportion of patients getting to the operating room within 24 hours. We did this through a collaborative, multidisciplinary approach that involved multiple care teams whose goal was to assess and optimize each patient in a timely and systematic way which we feel improved their hospital experience and quality of care.
Table 4.
Lessons Learned.
|
Abbreviation: INR, international normalized ratio.
Footnotes
Authors’ Note: All authors meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals including the following: (1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alex Burton, MD  https://orcid.org/0000-0003-0136-5354
https://orcid.org/0000-0003-0136-5354
References
- 1. Cummings SR, Rubin SM, Black D. The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen. Clin Orthop Relat Res. 1990;252:163–166. [PubMed] [Google Scholar]
- 2. World Health Organization. Prevention and Management of Osteoporosis. Geneva, Switzerland: World Health Organization (WHO); 2014:EB11413. [Google Scholar]
- 3. Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51(3):364–370. jgs51110 [pii]. [DOI] [PubMed] [Google Scholar]
- 4. Marottoli RA, Berkman LF, Cooney LM., Jr Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861–866. [DOI] [PubMed] [Google Scholar]
- 5. Steiner JF, Kramer AM, Eilertsen TB, Kowalsky JC. Development and validation of a clinical prediction rule for prolonged nursing home residence after hip fracture. J Am Geriatr Soc. 1997;45(12):1510–1514. [DOI] [PubMed] [Google Scholar]
- 6. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334:1519–1525. doi:10.1056/NEJM199606063342307. [DOI] [PubMed] [Google Scholar]
- 7. Hamlet WP, Lieberman JR, Freedman EL, Dorey FJ, Fletcher A, Johnson EE. Influence of health status and the timing of surgery on mortality in hip fracture patients. Am J Orthop (Belle Mead NJ). 1997;26(9):621–627. [PubMed] [Google Scholar]
- 8. Villar RN, Allen SM, Barnes SJ. Hip fractures in healthy patients: operative delay versus prognosis. Br Med J (Clin Res Ed). 1986;293(6556):1203–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bredahl C, Nyholm B, Hindsholm KB, Mortensen JS, Olesen AS. Mortality after hip fracture: results of operation within 12 h of admission. Injury. 1992;23(2):83–86. [DOI] [PubMed] [Google Scholar]
- 10. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738–1743. doi:10.1001/jama.291.14.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Versluysen M. How elderly patients with femoral fracture develop pressure sores in hospital. Br Med J (Clin Res Ed). 1986;292(6531):1311–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hefley FG, Jr, Nelson CL, Puskarich-May CL. Effect of delayed admission to the hospital on the preoperative prevalence of deep-vein thrombosis associated with fractures about the hip. J Bone Joint Surg Am. 1996;78(4):581–583. [DOI] [PubMed] [Google Scholar]
- 13. Hedstrom M, Grondal L, Ahl T. Urinary tract infection in patients with hip fractures. Injury. 1999;30(5):341–343. [DOI] [PubMed] [Google Scholar]
- 14. Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850. doi:10.1056/NEJM197710202971601. [DOI] [PubMed] [Google Scholar]
- 15. Narick C, Triulzi DJ, Yazer MH. Transfusion-associated circulatory overload after plasma transfusion. Transfusion. 2012;52(1):160–165. [DOI] [PubMed] [Google Scholar]
- 16. Irvine RE, Devas MB. The geriatric orthopaedic unit. J bone Joint Surg (Br). 1967;49B:186–187. [Google Scholar]
- 17. Hasserius R, Karlsson MK, Jonsson B, Redlund-Johnell I, Johnell O. Long-term morbidity and mortality after a clinically diagnosed vertebral fracture in the elderly—a 12- and 22-year follow-up of 257 patients. Calcif Tissue Int. 2005;76:235–242. doi:10.1007/s00223-004-2222-2. [DOI] [PubMed] [Google Scholar]
- 18. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20:172–178; discussion 9-80 doi:10.1097/01.bot.0000202220.88855.16. [DOI] [PubMed] [Google Scholar]
- 19. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4:10–15. doi:10.1177/2151458513495238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffenberg R, James OFW, Brocklehurst JC, et al. Fractured neck of femur: prevention and management. Summary and recommendations of a report of the Royal College of Physicians. J R Coll Physicians, Lond. 1989;23(1):8–14. [PMC free article] [PubMed] [Google Scholar]
- 21. Duthie RB. Orthopaedic Services: Waiting Time for Outpatient Appointments and In-Patient Treatment: Report of a Working Part to the Secretary of State for Social Services. London: HM Stationary Office; 1981. [Google Scholar]
- 22. Lefroy RB. Treatment of patients with fractured neck of the femur in a combined unit. Med J Aust. 1980;2(12):669–670. [DOI] [PubMed] [Google Scholar]
- 23. Lundstrom M, Edlund A, Lundstrom G, Gustafson Y. Reorganization of nursing and medical care to reduce the incidence of postoperative delirium and improve rehabilitation outcome in elderly patients treated for femoral neck fractures. Scand J Caring Sci. 1999;13(3):193–200. [PubMed] [Google Scholar]
- 24. Huusko TM, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Randomised, clinically controlled trial of intensive geriatric rehabilitation in patients with hip fracture: subgroup analysis of patients with dementia. BMJ. 2000;321:1107–1111. doi:10.1136/bmj.321.7269.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adunsky A, Arad M, Levi R, Blankstein A, Zeilig G, Mizrachi E. Five-year experience with the ‘Sheba’ model of comprehensive orthogeriatric care for elderly hip fracture patients. Disabil Rehabil. 2005;27:1123–1127. doi:10.1080/09638280500056030. [DOI] [PubMed] [Google Scholar]
- 26. Zuckerman JD, Sakales SR, Fabian DR, Frankel VH. Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program. Clin Orthop Relat Res. 1992:274:213–225. [PubMed] [Google Scholar]