Short abstract
Infection-induced chronic pain is an under-studied pain condition. One example is apical periodontitis, which evokes considerable mechanical allodynia that persists after treatment in 7% to 12% of patients. Available analgesics often provide incomplete relief. However, a preclinical model to study pain mechanisms associated with apical periodontitis is not available. Here, we report a mouse model of apical periodontitis to facilitate studies determining mechanisms mediating persistent infection-induced pain. Mice were anesthetized and the left first molar was exposed to the oral environment for six weeks. Bone resorption, as an indicator of apical periodontitis, was quantified using microcomputed tomography. Mechanical allodynia was determined using extraoral von-Frey filaments in both male and female mice. The expression of c-fos in the medullary dorsal horn was assessed using immunohistochemistry. Mice with apical periodontitis developed significant mechanical allodynia by day 7 that was maintained for 42 days. Mechanical thresholds were significantly lower in females compared to males. Administration of ibuprofen, morphine, or MK-801 reversed mechanical allodynia. Finally, apical periodontitis triggered an upregulation of c-fos in the medullary dorsal horn. Collectively, this model simulates signs of clinical pain experienced by patients with apical periodontitis, detects sex differences in allodynia, and permits the study of peripheral and central trigeminal pain mechanisms.
Keywords: Apical periodontitis, pain model, central sensitization, orofacial pain, dental pain, mechanical allodynia
Introduction
Chronic pain from a microbial etiology is a common, but understudied clinical condition that is distinct in its pathophysiology, as it is comprised of both neuropathic and inflammatory pain mechanisms.1 Apical periodontitis is typically caused by microbial infection of the dental pulp.2–4 Radiographically, it is characterized by bone resorption around the inflamed/infected tooth and clinically by exquisite mechanical allonia. Approximately 60%–85% of patients with apical periodontitis report mechanical allodynia at the inflamed tooth, and 90% of these patients experience referred pain at a distant intra- or extraoral site.5 It is important to note that apical periodontitis differs from periodontal disease with the latter being asymptomatic despite having a microbial etiology as well.6 The Global Burden of Disease study reported that tooth pain was ranked fifth out of 64 conditions of acute sequelae around the world, having occurred in more than 200 million cases in 2013.7 A National Practice-Based Research Network study suggests that the duration of pretreatment pain significantly increases the risk of developing chronic pain by 19%.8 Moreover, about 25% of patients with unrelenting postoperative dental pain appear to develop “neuropathic-like” pain9 of which infection-induced nerve injury is a plausible cause. Pain due to apical periodontitis is therefore a highly prevalent type of infection-induced pain that relies heavily on the physical elimination of a bacterial etiology by way of root canal treatment procedures. However, despite the elimination of etiology, persistent endodontic pain that lasts greater than six months ensues in 7%–12% of patients.8,10,11 Interestingly, antimicrobial drugs provide little-to-no relief12–16 in patients with post-endodontic pain suggestive of neuronal activation in a condition that is primed by a bacterial etiology but that persists due to nociceptive changes that are unaffected by microbial targeting. This is a significant public health problem as nearly 15 million root canal procedures are performed yearly and the chronic use of currently available pharmacotherapies have known serious adverse effects (www.cdc.gov/opioids/index.html).17 Therefore, a preclinical model of infection-induced odontogenic pain may offer utility in understanding the mechanisms of nociceptive changes observed in patients with chronic odontogenic pain. Importantly, similar to other chronic pain conditions, sex-related differences have been reported in these patients.18 The aim of this study was therefore to develop a model of persistent odontogenic pain that (1) models the symptoms of odontogenic pain patients, (2) mimics observed sex-related differences in odontogenic pain, and (3) provides insight into mechanisms mediating infection-induced chronic pain conditions.
Methods
Animals
The animal protocol was approved by the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee and conforms to International Association for the Study of Pain guidelines. Six- to eight-week-old adult inbred Balb/c male and female mice (Charles River, Wilmington, MA) were used in the experiments. Animals were housed for at least five days prior to the start of any experiments.
Drugs
Ibuprofen was purchased from Sigma-Aldrich (St. Louis, MO). The drug was diluted in 100% DMSO and administered at a 200 mg/kg dose19,20 in 2.5% Tween made in saline. Morphine was purchased from Henry Schein (Melville, NY) and (5S,10R)-(+)- 5-Methyl-10,11-dihydro-5H-dibenzo [a, d]cyclohepten-5, 10-imine hydrogen maleate (MK-801) was purchased from Tocris (Minneapolis, MN). Both drugs were dissolved in saline. Morphine was administered at a 30 mg/kg dose21–24 and MK-801 was injected at a 0.3 mg/kg dose.25,26 All drugs were administered intraperitoneally at a 200 µl volume.
In vivo mouse model of apical periodontitis
This in vivo model is based on previously published studies.27–30 All animals were anesthetized by intraperitoneal (i.p.) injection of ketamine (75 mg/kg)/dexmedetomidine (1 mg/kg). Animals in the apical periodontitis group were then placed on a custom jaw retraction apparatus, and pulpal exposure of the maxillary left first molar was performed using a sterile size #1/4 surgical length round bur rotated by a low-speed handpiece to the depth of the diameter of the bur with the aid of a surgical microscope (Zeiss, Ontario, CA). Pulp exposure was verified by inserting an #8 K-file (DENTSPLY Maillefer, Ballaigues, Switzerland) into pulp canals (Figure 1(a)). Animals in the control group were anesthetized and placed on the jaw retraction apparatus but did not undergo pulp exposures. Anesthesia was reversed by intraperitoneal injection of Antisedan™ (Zoetis, Florham Park, NJ) (1 mg/kg). Teeth were left open to the oral environment for up to six weeks (42 days). The body weight of animals in both groups was measured weekly after pulp exposure.
Figure 1.
Images demonstrating pulp exposures and site of mechanical allodynia. (a) Representative image demonstrating pulp exposure of mouse maxillary left first molar tooth. (b) Schematic representing area of von Frey testing for mechanical allodynia.
In vivo mouse model of apical periodontitis-induced mechanical allodynia
All behavior tests were done by observers blinded to treatment allocation. Withdrawal thresholds to mechanical forces were determined with von Frey filaments as previously described.31,32 Briefly, mice were lightly restrained by their tail and allowed to stand on a mesh platform. This habituation took no more than 2–3 min. von Frey filaments were then applied to their left vibrissal pad border and cheek (Figure 1(b)) to collect baseline mechanical thresholds. Following pulp exposures, all animals were tested for development of mechanical allodynia once a week for six weeks (days 1, 7, 14, 21, 28, 35, and 42). For all behavioral testing, animals were subjected to a sequential series of calibrated von Frey filaments (bending forces of 0.008–6 g). Head withdrawal, face swipe, and grimace were considered as a positive response. Each force was applied six times at intervals of 3–5 s. In addition to mechanical allodynia, we also observed animals for spontaneous nociceptive behaviors (i.e., grooming and scratching after pulp exposures or application of von Frey filaments).
For experiments evaluating ibuprofen, morphine, and MK-801 analgesia, drugs and their respective vehicles were injected i.p. 60 min prior to behavioral testing on day 21.
Microcomputed tomography
Maxillae were harvested and placed in phosphate buffer (PB) and transferred to air-filled vials to be scanned in a microcomputed tomography (µCT) system (Bruker Skyscan1172, Kontich, Belgium) with the following settings: 60 kV, 167 uA, 0.5 mm aluminum filter, 0.7° rotation step, four frames averaging 2000 × 1336 charge-coupled device (CCD), 680 msec exposure, and 10 µm voxel size. Left- and right-sided maxillae were oriented orthogonally with respect to the buccal/lingual view. Regions of interest (ROIs) were hand contoured to include all areas of the M1 tooth socket. ROIs started on the buccal side when the root space is encapsulated by bone and continued to the lingual side where the root space is encapsulated by bone. The total hand contoured volume of interest (VOI) was determined to be the total volume (TV). By subtracting the bone volume (BV) from the TV, the void volume (VV) was calculated. In order to maximize the lesion void volume, the ROI includes both bone and molar. Percent VV was calculated by dividing VV by TV (VV/TV).
Immunohistochemistry
At day 21, mice were deeply anesthetized with an intraperitoneal injection of ketamine (75 mg/kg)/dexmedtomidine (1 mg/kg) and transcardially perfused with 100 ml of 0.9% saline followed by 200 ml of fixative consisting of 4% paraformaldehyde in 0.1 M PB. The medullary dorsal horn and trigeminal ganglia (TG) were removed, postfixed for 20 min, rinsed in PB, and placed in cold 0.1 M PB with 30% sucrose overnight. Tissues were embedded in Neg-50 (Richard Allan, Kalamazoo, MI) and sectioned in the horizontal plane at 30 µm with a cryostat. Sections were placed onto Superfrost Plus™ slides (Fisher Scientific, Waltham, MA), dried, and stored at 20°C until stained. Immunostaining was performed as described previously.33 Briefly, tissue sections were permeabilized and blocked for nonspecific protein binding with blocking solution consisting of 4% normal goat serum (Sigma-Aldrich), 2% bovine gamma-globulin (Sigma-Aldrich), and 0.3% Triton X-100 (Fisher Scientific) in phosphate-buffered saline (PBS) for 90 min prior to incubation with primary antibodies in blocking solution for 16 h. Table 1 lists the antibodies, dilutions, and sources used in this study. Sections were rinsed with PBS, incubated in secondary antibody in blocking solution for 90 min, rinsed in PBS and H2O, dried, and coverslipped with Vectashield™. Secondary antibodies were purchased from Molecular Probes, Eugene, OR. All immunostaining procedures were performed at room temperature. Sections were evaluated and images obtained with a Nikon Eclipse 90i microscope equipped with a C1si laser scanning confocal imaging system. Images were acquired in z-stack at 20× and 40× objective and identical laser gain settings. Images were taken using fixed acquisition parameters across all groups and were unaltered from that initially taken.
Table 1.
Antibody List for Immunohistochmistry.
| Primary antibody | Dilution | Company | Secondary antibody |
|---|---|---|---|
| c-fos (sc-8047) | 1:50 | Santa Cruz Biotechnology, Inc. (Dallas, TX) | Goat anti-mouse 1:200 Alexa 568 |
| CGRP (C8198) | 1:300 | Sigma-Aldrich (St. Louis, MO) | Goat anti-rabbit 1:200 Alexa 488 |
| NeuN | 1:300 | Abcam (Cambridge, UK) | Goat anti-rabbit Alexa 488 |
CGRP: calcitonin gene-related peptide.
Data analysis
The µCT experiments were conducted with n = 3 maxillae/group and data were analyzed using two-way analysis of variance (ANOVA) with Sidak’s multiple comparison test. All data were analyzed using GraphPad (San Diego, CA) Prism software version 7.0. All behavior experiments were conducted with n = 6–10 animals/group, and the resulting stimulus–response curve was plotted and analyzed via nonlinear regression analysis. EF50 values (50% response rate) were calculated and plotted (mean ± standard error of the mean). Data were analyzed using two-way ANOVA with Sidak’s multiple comparison test.
Results
The µCT analyses were conducted to verify the induction of apical periodontitis as measured by periradicular bone loss. The results demonstrate large regions of bone destruction around the apices of the maxillary left first molars as observed in the coronal and axial views (Figure 2(a) and (b)). Quantification of void volume demonstrated a significantly larger void volume on the exposed molar (left side) compared to the untreated (right side) in the apical periodontitis group (p < 0.0001), with no left–right differences observed in the control group (Figure 2(c)). Both groups exhibited similar increases in body weights during the experiment (data not shown).
Figure 2.
Effect of pulp exposures on induction of apical periodontitis using µCT analysis. (a) Representative left (L) and right (R) coronal scans of control animals (upper panel) and apical periodontitis animals (lower panel; arrow). (b) Representative left (L) and right (R) axial scans of control animals (left side) and apical periodontitis animals (right side; arrow). (c) Quantification of % void volume between left and right side of control and apical periodontitis animals. Statistical analysis was performed using two-way ANOVA with Sidak’s multiple comparison test (N = 3 maxillae/group; error bars = standard error of the mean; ****p < 0.001 compared to right side). Note: Data generated using male mice.
For assessment of mechanical allodynia, baseline mechanical thresholds were collected prior to pulp exposure (Figure 3(b)). Thereafter, mechanical allodynia was measured by applying von Frey filaments with increasing forces to the left vibrissal pad and cheek in both the control (Figure 3(c)) and apical periodontitis groups. Male mice with apical periodontitis displayed significant mechanical allodynia by day 7 that was maintained for at least 42 days (Figure 3(a)). A cyclical pattern was observed with lowest values seen at days 7, 21, and 35. However, these were not different statistically. By day 21, the EF50 values for the apical periodontitis group was reduced by ∼40% as compared to the control group (Figure 3(a); apical periodontitis: 0.18 g ± 0.048 vs. control 0.47 ± 0.021 g; p < 0.005). Mechanical thresholds on the contralateral side at day 21 exhibited no difference between the control and apical periodontitis groups (data not shown).
Figure 3.
Effect of pulp exposures on development of mechanical allodynia in male mice. (a) EF50 values comparing control and apical periodontitis animals on days 1, 7, 14, 21, 28, 35, and 42 after pulp exposures to the left maxillary left first molar (*BL = Baseline). (b–d) Stimulus-response curves comparing control (c) and apical periodontitis (d) animals on days 1, 7, 14, 21, 28, 35, and 42 after pulp exposures to the left maxillary left first molar. Statistical analysis was performed using two-way ANOVA with Sidak’s multiple comparison test (N = 10 animals/group; error bars = standard error of the mean; ****p < 0.001 compared to control group at all time points).
We performed similar experiments as above using female mice. Our data demonstrate a similar overall pattern compared to male animals, with mechanical allodynia present at day 7 and continuing to at least day 42 (Figure 4(a)). Once again, a cyclical pattern was observed from day 21 through 42. However, values were not different statistically. Female mice with apical periodontitis demonstrated significant mechanical allodynia with an approximate 34% reduction in mechanical thresholds in the apical periodontitis group as compared to the control group (Figure 4(a); apical periodontitis: 0.10 ± 0.035 g vs. control: 0.29 ± 0.036 g).
Figure 4.
Effect of pulp exposures on development of mechanical allodynia in female mice. (a) EF50 values comparing control and apical periodontitis animals on days 1, 7, 14, 21, 28, 35, and 42 after pulp exposures to the left maxillary left first molar (*BL = Baseline). (b–d) Stimulus-response curves comparing control (c) and apical periodontitis (d) animals on days 1, 7, 14, 21, 28, 35, and 42 after pulp exposures to the left maxillary left first molar. Statistical analysis was performed using two-way ANOVA with Sidak’s multiple comparison test (N = 6 animals/group; error bars = standard error of the mean; ****p < 0.001 compared to control group at all time points).
While the overall temporal patterns were similar, sex differences in the actual mechanical threshold values were observed (Figure 5). Female mice displayed significantly lower baselines thresholds (Figure 5; males: 0.47 g vs. females: 0.29 g) compared to males. This finding was also observed at day 21 between the two apical periodontitis groups (Figure 5; males: 0.18 g vs. females: 0.10 g).
Figure 5.
Comparison of mechanical allodynia between male and female mice. Mean EF50 values between male and female mice at BL and day 21 post-pulp exposure. Statistical analysis was performed using two-way ANOVA with Sidak’s multiple comparison test (N = 6–10 animals/group; error bars = standard error of the mean; ****p < 0.001 within group, **p < 0.01 compared to control group at all time points).
Upon establishing that the induction of apical periodontitis produces extraoral mechanical allodynia in mice, we conducted interventional experiments to evaluate the effect of various known analgesics on providing analgesia. Nonsteroidal anti-inflammatory drugs (NSAIDs) are first line in the pharmacological management of pain from apical periodontitis. The results demonstrate that ibuprofen 200 mg/kg significantly (p < 0.05) reversed mechanical allodynia compared to vehicle control (Figure 6(a)). Similarly, morphine administration reversed the mechanical allodynia (Figure 6(b); p < 0.05) without demonstration of any motor deficits. Patients often report pain from apical periodontitis that is referred to extraoral sites, likely due to central sensitization.5 To evaluate if this model of persistent allodynia due to apical periodontitis produces central sensitization, we administered MK-801, a noncompetitive antagonist of the N-Methyl-D-aspartate (NMDA) receptor. The injection of MK-801 significantly reversed mechanical allodynia in mice with apical periodontitis (Figure 6(c); p < 0.01). Importantly, these three drugs all reversed mechanical allodynia in mice with apical periodontitis, but not in the control animals.
Figure 6.
Effect of known analgesics on reversal of apical periodontitis-induced mechanical allodynia. (a–c) Mean EF50 values at BL and day 21 post-pulp exposure with and without i.p. injection of ibuprofen (a), morphine (b), and MK-801 (c). Statistical analysis was performed using two-way ANOVA with Sidak’s multiple comparison test (N = 4 animals/group; error bars = standard error of the mean; ****p < 0.001 within group, **p < 0.01, p < 0.05 compared to respective control group at all time points). Note: Data generated using male mice.
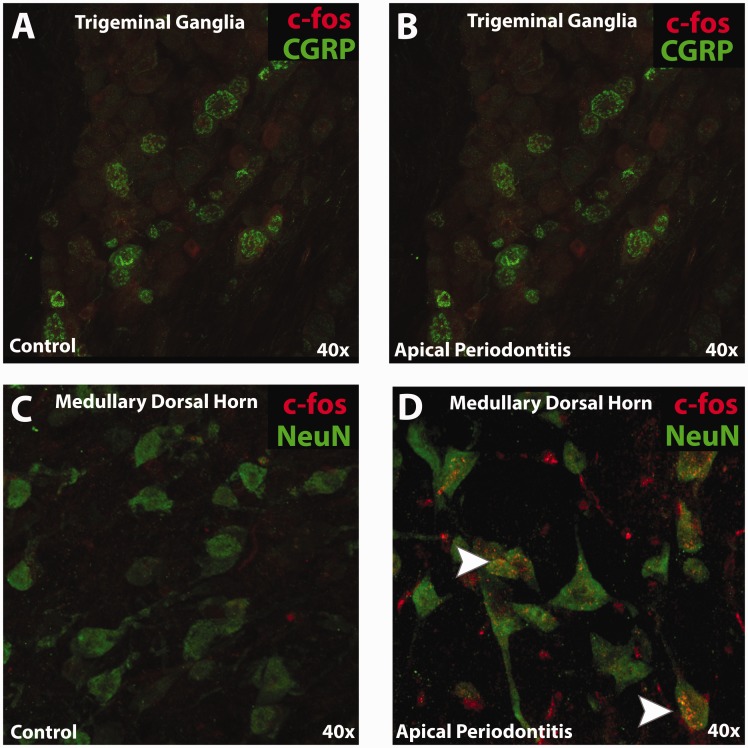
Upregulation of c-fos is one of the several factors that are often associated with central sensitization34 and provides an anatomical marker of neuronal activation. Here, we evaluated the expression of c-fos in the medullary dorsal horn at day 21. The results demonstrate a robust c-fos labeling in animals with apical periodontitis, while minimal-to-no labeling was observed in control animals (Figure 7(b)). Moreover, this expression of c-fos was restricted to regions of lamina I and II of the medullary dorsal horn. Furthermore, co-localization studies with NeuN demonstrate that c-fos was upregulated in cell bodies of neurons only in animals with apical periodontitis (Figure 7(b)). We also evaluated the expression of c-fos in the TG and observed no expression of this marker with calcitonin gene-related peptide (CGRP)-positive neurons (Figure 7(a)).
Figure 7.
Immunohistochemistry of c-fos in trigeminal ganglia and medullar dorsal horn. (a and b) Representative immunohistochemical images evaluating expression of c-fos and CGRP in the V2 region of the trigeminal ganglia between control and apical periodontitis animals. (c and d) Representative immunohistochemical images evaluating expression of c-fos and NeuN in lamina I and II of the left medullary dorsal horn between control and apical periodontitis animals. Arrows in (d) indicates co-localization of c-fos with NeuN. Note: Data generated using male mice.
Discussion
The pathogenesis of apical periodontitis has been extensively studied including regulation of the peripheral nociceptor. Owing to a polymicrobial etiology, toll-like receptor-(TLR)35,36 and non-TLR37–39 mechanisms have been proposed. The role of bacterial endotoxins such as lipoteichoic acid (LTA) and lipopolysaccharide (LPS) has been long known to activate and sensitize trigeminal sensory neurons.35,36 Other non-TLR mechanisms suggest that bacterium-induced pain is not dependent on tissue edema or immune cell activation but on the concentration of the bacterial load.37–39 Additionally, phenotypic changes such as nerve sprouting have been demonstrated.40,41 However, infection-induced signaling leading to central sensitization and persistent pain has not been evaluated. This is primarily due to lack of an appropriate preclinical model that closely mimics the initiation, progression, and maintenance of persistent endodontic pain.
The present study aims at developing a disease-specific persistent pain model to study mechanisms of bacteria-induced chronic pain conditions. Here, we adapted a well-established and validated method for the induction of apical periodontitis27–29 by creating standardized pulp exposures in the maxillary left first molar of mice that were exposed to the oral microbiome for six weeks. The results from µCT studies demonstrated significant bone loss in the periapical region of the maxillary left first molar tooth at the end of six weeks and corroborates previous findings.29 Moreover, mice that underwent molar surgeries demonstrated significant mechanical allodynia at an extraoral site as early as day 1 post-pulp exposures, and this effect persisted for six weeks. Additionally, this effect was reversible by known analgesics demonstrating involvement of the nociceptive pathways. Data from clinical studies indicate that ∼90% of patients with pain on mastication (i.e., mechanical allodynia) also experience pain at a site other than the inflamed tooth such as adjacent teeth, tooth from the opposing arch, and/or extraoral pain (74%) proximal to the inflamed tooth.5 Instead of focusing on pain on mastication in mice, we chose to test extraoral sites, which are feasible, reproducible, and a clinically simulative model that may reflect central sensitization. Therefore, our model simulates this clinical symptom for measurement of pain in animals with apical periodontitis.
In order to determine the involvement of central pain pathways in our model, we utilized two different approaches. The behavioral assay demonstrates reversal of mechanical allodynia using MK-801, an NMDA receptor antagonist (Figure 6(c)). The immunohistochemical analysis demonstrates the selective expression of c-fos, an immediate-early gene, and a transcriptional factor in lamina I and II of the medullary dorsal horn in injured animals (Figure 7(d) and (e)). The induction of c-fos specifically after activation of primary afferent sensory neurons such as Aδ and unmyelinated C fibers and not upon activation of non-noxious Aβ, Aα and unmyelinated low threshold C fibers42 makes it a useful marker to study changes post-injury and post-drug treatment in an animal model. As previously reported,42 we did not observe expression of c-fos in the TG in either groups. Collectively, the findings of mechanical allodynia at distant site, combined with MK-801 reversal and the c-fos upregulation, all support the hypothesis that this model evokes neuronal plasticity in the trigeminal medullary dorsal horn.
A critical feature of central sensitization is the recruitment of non-nociceptive fibers and the conversion of nociceptive-specific second-order neurons into wide dynamic range neurons in the continued transmission of pain in the setting of chronic pain states. As seen from Figure 3(a), baseline mechanical thresholds at 0.47 g force caused face withdrawal in control animals, while a force as small as 0.18 g elicited face withdrawal in apical periodontitis animals. Furthermore, Figures 3(d) and 4(d) demonstrate significant leftward shift at both, innocuous and noxious forces. These findings implicate the development of both, mechanical allodynia and hyperalgesia in animals with apical periodontitis. Future studies using transgenic models can aid in the dissection of neuronal subtypes and their functional regulation in mediating apical periodontitis-induced mechanical hypersensitivity.
Numerous studies provide evidence for greater prevalence of chronic pain in females.43 Sex-related differences are a consistent finding in dental pain as well.44,45 Clinical studies evaluating sex differences in pain from apical periodontitis demonstrate that mechanical allodynia thresholds from an inflamed tooth differ greatly between male and female participants.18,46 We attempted to reproduce this clinical finding in our model. Baseline mechanical thresholds were significantly lower in females than males and corroborate findings from clinical studies.18,46 This finding was also consistent at 21 days after pulp exposure. Taken together, these results for the first time provide a model depicting sexual dimorphism at both basal and postoperative stages of a chronic pain condition seen routinely in endodontic patients.
Conclusions
Collectively, currently available animal models that mimic infection-induced chronic pain utilize spontaneous, immune-mediated, or a single pathogen-mediated infectious models.47–52 These, however, do not account for the clinical polymicrobial infection that exists in the development and maintenance of persistent odontogenic pain. The present model was designed to mimic the clinical conditions of apical periodontitis and offers the potential for mechanistic and therapeutic studies using this combined model of inflammatory and neuropathic pain.
Author Note
Obadah Austah is also affiliated with Department of Endodontics, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by start-up funds by University of Texas Health Science Centre San Antonio.
ORCID iD
Nikita B Ruparel https://orcid.org/0000-0001-9583-0856
References
- 1.Luo S, Perry GM, Levinson SR, Henry MA. Nav1.7 expression is increased in painful human dental pulp. Mol Pain 2008; 4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graunaite I, Lodiene G, Maciulskiene V. Pathogenesis of apical periodontitis: a literature review. J Oral Maxillofac Res 2012; 2: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akamine A, Hashiguchi I, Toriya Y, Maeda K. Immunohistochemical examination on the localization of macrophages and plasma cells in induced rat periapical lesions. Endod Dent Traumatol 1994; 10: 121–128. [DOI] [PubMed] [Google Scholar]
- 4.Okiji T, Kawashima N, Kosaka T, Kobayashi C, Suda H. Distribution of Ia antigen-expressing nonlymphoid cells in various stages of induced periapical lesions in rat molars. J Endod 1994; 20: 27–31. [DOI] [PubMed] [Google Scholar]
- 5.Falace DA, Reid K, Rayens MK. The influence of deep (odontogenic) pain intensity, quality, and duration on the incidence and characteristics of referred orofacial pain. J Orofac Pain 1996; 10: 232–239. [PubMed] [Google Scholar]
- 6.Gaurilcikaite E, Renton T, Grant AD. The paradox of painless periodontal disease. Oral Dis 2017; 23: 451–463. [DOI] [PubMed] [Google Scholar]
- 7.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nixdorf DR, Law AS, Lindquist K, Reams GJ, Cole E, Kanter K, Nguyen RH, Harris DR, National Dental PBRN Collaborative Group. Frequency, impact, and predictors of persistent pain after root canal treatment: a national dental PBRN study. Pain 2016; 157: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark GT. Persistent orodental pain, atypical odontalgia, and phantom tooth pain: when are they neuropathic disorders? J Calif Dent Assoc 2006; 34: 599–609. [PubMed] [Google Scholar]
- 10.Polycarpou N, Ng YL, Canavan D, Moles DR, Gulabivala K. Prevalence of persistent pain after endodontic treatment and factors affecting its occurrence in cases with complete radiographic healing. Int Endod J 2005; 38: 169–178. [DOI] [PubMed] [Google Scholar]
- 11.Nixdorf DR, Moana-Filho EJ, Law AS, McGuire LA, Hodges JS, John MT. Frequency of persistent tooth pain after root canal therapy: a systematic review and meta-analysis. J Endod 2010; 36: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry M, Reader A, Beck M. Effect of penicillin on postoperative endodontic pain and swelling in symptomatic necrotic teeth. J Endod 2001; 27: 117–123. [DOI] [PubMed] [Google Scholar]
- 13.Walton RE, Chiappinelli J. Prophylactic penicillin: effect on posttreatment symptoms following root canal treatment of asymptomatic periapical pathosis. J Endod 1993; 19: 466–470. [DOI] [PubMed] [Google Scholar]
- 14.Pickenpaugh L, Reader A, Beck M, Meyers WJ, Peterson LJ. Effect of prophylactic amoxicillin on endodontic flare-up in asymptomatic, necrotic teeth. J Endod 2001; 27: 53–56. [DOI] [PubMed] [Google Scholar]
- 15.Abbott AA, Koren LZ, Morse DR, Sinai IH, Doo RS, Furst ML. A prospective randomized trial on efficacy of antibiotic prophylaxis in asymptomatic teeth with pulpal necrosis and associated periapical pathosis. Oral Surg Oral Med Oral Pathol 1988; 66: 722–733. [DOI] [PubMed] [Google Scholar]
- 16. AAE Position Statement: AAE guidance on the use of systemic antibiotics in endodontics. J Endod 2017; 43: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 17.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013; 382: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan AA, Owatz CB, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. Measurement of mechanical allodynia and local anesthetic efficacy in patients with irreversible pulpitis and acute periradicular periodontitis. J Endod 2007; 33: 796–799. [DOI] [PubMed] [Google Scholar]
- 19.Jones CK, Peters SC, Shannon HE. Synergistic interactions between the dual serotonergic, noradrenergic reuptake inhibitor duloxetine and the non-steroidal anti-inflammatory drug ibuprofen in inflammatory pain in rodents. Eur J Pain 2007; 11: 208–215. [DOI] [PubMed] [Google Scholar]
- 20.Jones CK, Peters SC, Shannon HE. Efficacy of duloxetine, a potent and balanced serotonergic and noradrenergic reuptake inhibitor, in inflammatory and acute pain models in rodents. J Pharmacol Exp Ther 2005; 312: 726–732. [DOI] [PubMed] [Google Scholar]
- 21.Luger NM, Mach DB, Sevcik MA, Mantyh PW. Bone cancer pain: from model to mechanism to therapy. J Pain Symptom Manage 2005; 29: S32–S46. [DOI] [PubMed] [Google Scholar]
- 22.Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci USA 2003; 100: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansikka H, Zhao C, Sheth RN, Sora I, Uhl G, Raja SN. Nerve injury induces a tonic bilateral mu-opioid receptor-mediated inhibitory effect on mechanical allodynia in mice. Anesthesiology 2004; 100: 912–921. [DOI] [PubMed] [Google Scholar]
- 24.King T, Vardanyan A, Majuta L, Melemedjian O, Nagle R, Cress AE, Vanderah TW, Lai J, Porreca F. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain 2007; 132: 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liaw WJ, Zhu XG, Yaster M, Johns RA, Gauda EB, Tao YX. Distinct expression of synaptic NR2A and NR2B in the central nervous system and impaired morphine tolerance and physical dependence in mice deficient in postsynaptic density-93 protein. Mol Pain 2008; 4: 45–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Kohno T, Amaya F, Brenner GJ, Ito N, Allchorne A, Ji RR, Woolf CJ. Bradykinin produces pain hypersensitivity by potentiating spinal cord glutamatergic synaptic transmission. J Neurosci 2005; 25: 7986–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song M, Alshaikh A, Kim T, Kim S, Dang M, Mehrazarin S, Shin KH, Kang M, Park NH, Kim RH. Preexisting periapical inflammatory condition exacerbates tooth extraction-induced bisphosphonate-related osteonecrosis of the jaw lesions in mice. J Endod 2016; 42: 1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paula-Silva FW, Petean IB, da Silva LA, Faccioli LH. Dual role of 5-lipoxygenase in osteoclastogenesis in bacterial-induced apical periodontitis. J Endod 2016; 42: 447–454. [DOI] [PubMed] [Google Scholar]
- 29.Austah ON, Ruparel NB, Henry MA, Fajardo RJ, Schmitz JE, Diogenes A. Capsaicin-sensitive innervation modulates the development of apical periodontitis. J Endod 2016; 42: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 30.AlShwaimi E, Purcell P, Kawai T, Sasaki H, Oukka M, Campos-Neto A, Stashenko P. Regulatory T cells in mouse periapical lesions. J Endod 2009; 35: 1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci 2008; 28: 10482–10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chodroff L, Bendele M, Valenzuela V, Henry M, Ruparel S. BDNF Signaling contributes to oral cancer pain in a preclinical orthotopic rodent model. Molecular Pain 2016; 12: 1744806916666841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry MA, Freking AR, Johnson LR, Levinson SR. Increased sodium channel immunofluorescence at myelinated and demyelinated sites following an inflammatory and partial axotomy lesion of the rat infraorbital nerve. Pain 2006; 124: 222–233. [DOI] [PubMed] [Google Scholar]
- 34.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 2011; 90: 759–764. [DOI] [PubMed] [Google Scholar]
- 36.Ferraz CC, Henry MA, Hargreaves KM, Diogenes A. Lipopolysaccharide from Porphyromonas gingivalis sensitizes capsaicin-sensitive nociceptors. J Endod 2011; 37: 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu IM, Pinho-Ribeiro FA, Woolf CJ. Pain and infection: pathogen detection by nociceptors. Pain 2016; 157: 1192–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Pena C, Talavera A, Kichko T, Navia B, Sanchez A, Senaris R, Reeh P, Perez-Garcia MT, Lopez-Lopez JR, Voets T, Belmonte C, Talavera K, Viana F. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun 2014; 5: 3125–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013; 501: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byers MR, Narhi MV. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med 1999; 10: 4–39. [DOI] [PubMed] [Google Scholar]
- 41.Byers MR, Suzuki H, Maeda T. Dental neuroplasticity, neuro-pulpal interactions, and nerve regeneration. Microsc Res Tech 2003; 60: 503–515. [DOI] [PubMed] [Google Scholar]
- 42.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 1987; 328: 632–634. [DOI] [PubMed] [Google Scholar]
- 43.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013; 111: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Estrela C, Guedes OA, Silva JA, Leles CR, Estrela CR, Pecora JD. Diagnostic and clinical factors associated with pulpal and periapical pain. Braz Dent J 2011; 22: 306–311. [DOI] [PubMed] [Google Scholar]
- 45.Nusstein JM, Beck M. Comparison of preoperative pain and medication use in emergency patients presenting with irreversible pulpitis or teeth with necrotic pulps. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 96: 207–214. [DOI] [PubMed] [Google Scholar]
- 46.Khan AA, McCreary B, Owatz CB, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. The development of a diagnostic instrument for the measurement of mechanical allodynia. J Endod 2007; 33: 663–666. [DOI] [PubMed] [Google Scholar]
- 47.Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice AS. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain 2007; 130: 2688–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleetwood-Walker SM, Quinn JP, Wallace C, Blackburn-Munro G, Kelly BG, Fiskerstrand CE, Nash AA, Dalziel RG. Behavioural changes in the rat following infection with varicella-zoster virus. J Gen Virol 1999; 80: 2433–2436. [DOI] [PubMed] [Google Scholar]
- 49.Takasaki I, Andoh T, Shiraki K, Kuraishi Y. Allodynia and hyperalgesia induced by herpes simplex virus type-1 infection in mice. Pain 2000; 86: 95–101. [DOI] [PubMed] [Google Scholar]
- 50.Lynch JL, Gallus NJ, Ericson ME, Beitz AJ. Analysis of nociception, sex and peripheral nerve innervation in the TMEV animal model of multiple sclerosis. Pain 2008; 136: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nickel JC, Olson ME, Costerton JW. Rat model of experimental bacterial prostatitis. Infection 1991; 19: S126–S130. [DOI] [PubMed] [Google Scholar]
- 52.Dolan JC, Lam DK, Achdjian SH, Schmidt BL. The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J Neurosci Methods 2010; 187: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]