Abstract
Background
Patients with Congenital heart disease (CHD) require repetitive imaging of the pulmonary vasculature throughout their life. In this study, we compared a novel Compressed SENSE accelerated (factor 9) electrocardiogram (ECG)- and respiratory-triggered 3D modified Relaxation-Enhanced Angiography without Contrast and Triggering (modified REACT-non-contrast-enhanced magnetic resonance angiography (modified REACT-non-CE-MRA)) with standard non-ECG-triggered time-resolved 4D CE-MRA for imaging of the pulmonary arteries and veins in patients with CHD.
Methods
This retrospective analysis of 25 patients (June 2018–April 2019) with known or suspected CHD was independently conducted by two radiologists executing measurements on modified REACT-non-CE-MRA and 4D CE-MRA on seven dedicated points (inner edge): Main pulmonary artery (MPA), right and left pulmonary artery, right superior and inferior pulmonary vein, left superior (LSPV) and inferior pulmonary vein. Image quality for arteries and veins was evaluated on a four-point scale in consensus.
Results
Twenty-three of the 25 included patients presented a CHD. There was a high interobserver agreement for both methods of imaging at the pulmonary arteries (ICC ≥ 0.96); at the pulmonary veins, modified REACT-non-CE-MRA showed a slightly higher agreement, pronounced at LSPV (ICC 0.946 vs. 0.895). Measurements in 4D CE-MRA showed higher diameter values compared to modified REACT-non-CE-MRA, at the pulmonary arteries reaching significant difference (e.g. MPA: mean 0.408 mm, p = 0.002). Modified REACT-non-CE-MRA (average acquisition time 07:01 ± 02:44 min) showed significant better image quality than 4D CE-MRA at the pulmonary arteries (3.84 vs. 3.32, p < 0.001) and veins (3.32 vs. 2.72, p = 0.015).
Conclusions
Compressed SENSE accelerated (factor 9) ECG- and respiratory-triggered 3D modified REACT-non-CE-MRA allows for reliable and fast imaging of the pulmonary arteries and veins with higher image quality and slightly higher interobserver agreement than 4D CE-MRA without contrast agent and associated disadvantages. Therefore, it represents a clinically suitable technique for patients requiring repetitive imaging of the pulmonary vasculature, e.g. patients with CHD.
Keywords: Magnetic resonance angiography, 4D CE-MRA, Contrast agent, Congenital heart disease, Pulmonary vasculature
Background
With an incidence of 6–8/1000 at birth, congenital heart disease (CHD) comprises a wide range of different manifestations regarding the cardiovascular system potentially leading to death if left untreated. CHD has shown a serious improvement of survival over the past decades due to advancement in surgical techniques and early diagnosis, mostly owing to the widely use of fetal echocardiography [1–3]. Echocardiography represents the primary imaging modality of choice as it allows for fast, accurate and non-invasive imaging of cardiac function and vessel morphology [3–5]. However, it suffers from limitations such as user dependency and limited field of view (FOV) in growing patients [6, 7].
Given the radiation dose as well as the use of iodinated contrast agent in computed tomography (CT)-angiography (CTA) and digital subtraction angiography (DSA) with the invasiveness of the latter, cardiovascular magnetic resonance (CMR) has been established as the non-invasive imaging of choice to evaluate the different vascular territories of the thorax in patients with CHD and has to be regarded as the gold standard [6, 8–10]. Besides 4D flow CMR, contrast-enhanced MR-angiography (CE-MRA) has proven to sufficiently detect vascular abnormalities and has shown technical progress over the past decades with the development of time-resolved 4D CE-MRA [8, 11–13]. However, the accurate application of CE-MRA and 4D CE-MRA is technically demanding and shows further limitations such as nephrogenic systemic fibrosis (NSF) [14] and long term retention of gadolinium.
Therefore, many non-CE-MRA techniques have been developed in the past, including sequences based on turbo spin echo (TSE), spoiled gradient echo sequences, steady-state free precession (SSFP), and balanced SSFP (bSSFP) with SSFP and bSSFP being the most widely used for the assessment of the pulmonary vasculature in patients with CHD as well as other diseases affecting the pulmonary vessels, e.g. pulmonary hypertension (PH) [15–19]. Recently, a novel 3D Relaxation-Enhanced Angiography without Contrast and Triggering (REACT) sequence, a combination of non-volume-selective short tau inversion recovery (STIR) pulse, a T2 preparation (T2 prep) pulse, and dual gradient echo Dixon (mDIXON XD), was introduced. It combines the benefits of SSFP with robust fat and background suppression [20].
The purpose of this study was to investigate the feasibility of a novel electrocardiogram (ECG)- and navigator-triggered 3D non-CE-MRA based on a modified REACT approach (modified REACT-non-CE-MRA) for the imaging of the pulmonary arteries and veins in patients with CHD and to compare measurement values and image quality to standard 4D CE-MRA.
Methods
Patient population
Patients were retrospectively selected from our internal database of 26 consecutive patients over a ten-month study period (June 2018–April 2019) receiving a dedicated clinical protocol regarding known or suspected CHD including both, 4D CE-MRA and modified REACT-non-CE-MRA. Insufficient contrast in 4D CE-MRA led to exclusion of patients. There were no exclusions regarding pathologies or operative treatment. Due to the retrospective design of the study, the local ethics committee waived written informed consent requirement in the patient cohort.
Image acquisition
All scans were performed on a clinical whole body 1.5 T CMR system (Philips Ingenia, Philips Healthcare, Best, The Netherlands) equipped with a dedicated 28-channel coil for cardiac imaging. The protocol comprised a non-CE-MRA using a modified REACT approach, a 4D CE-MRA, and 2D bSSFP breath hold cine imaging in standard orientations (4-chamber, 2-chamber, 3-chamber, short axis, transversal, left ventricular outflow tract, right ventricular outflow tract (RVOT)) as well as phase contrast velocity measurements of the main pulmonary artery (MPA) and the ascending aorta.
For 4D CE-MRA, a 3D spoiled gradient echo sequence was used. Gadobutrol (Gadovist, Bayer HealthCare Pharmaceuticals, Berlin, Germany; 0.1 ml/kg body weight) was injected at a flow-rate of 2 ml/second into an antecubital vein. Patients were asked to perform a breath hold during the acquisition. To allow for high spatio-temporal resolution, the acquisition was combined with parallel imaging using SENSitivity Encoding (SENSE) and a keyhole technique where 20% of the central k-space was acquired in each dynamic (4D Track, Philips Healthcare). The keyhole data was then combined with outer k-space data from a reference scan during image reconstruction.
For non-CE-MRA, imaging was based on a modified flow-independent REACT sequence. 3D magnetization-prepared mDIXON XD (Philips Healthcare) was combined with a 30 ms T2 prep sequence. Since it was verifiable that background suppression of mDIXON XD in combination with T2 prep was sufficient for cardiovascular applications, no STIR preparation was applied, contrary to the original REACT sequence as introduced by Yoneyama et al. [20]. To compensate for cardiac and respiratory motion, ECG-triggering (end-diastolic) and respiratory navigator-triggering were added to the originally proposed REACT sequence, therefore being referred to as “modified” REACT-non-CE-MRA throughout the manuscript. Data were acquired in the coronal plane. For acceleration of image acquisition, Compressed SENSE (Philips Healthcare), a combination of compressed sensing and parallel imaging using SENSE, was used [21, 22]. For data acquisition, a variable density incoherent sampling pattern with high-density sampling in the center and continuously increased undersampling towards the k-space periphery was employed. Image reconstruction was based on an iterative L1 norm minimization, assuring data consistency and image sparsity in the wavelet domain. Additionally, the reconstruction was regularized by coil sensitivity distribution and SENSE parallel imaging. Reconstruction was done online on the standard hardware as provided by the manufacturer of the CMR system. An acceleration factor of 9 was used, resulting in a nominal scan time of 02:11 min.
Detailed imaging parameters are given in Table 1.
Table 1.
Imaging parameters of modified REACT-non-CE-MRA and 4D CE-MRA. FOV = field of view. TR = repetition time. TE = echo time
| REACT-non-CE-MRA | 4D CE-MRA | |
|---|---|---|
| Acquisition matrix | 235 × 299 × 100 | 268 × 268 × 25 |
| Resolution [mm] | 1.7 × 1.7 × 1.7 | 1.5 × 1.5 × 4 |
| FOV [mm] | 400 × 508 × 170 | 400 × 400 × 100 |
| Flip Angle [deg] | 10 | 30 |
| TR/TE1/TE2 [ms] | 6.3/1.8/4 | 2.8/1.05 |
| T2 preperation [ms] | 30 | – |
| k-space lines per heartbeat | 35 | – |
| Acceleration factor | Compressed SENSE 9 | SENSE 3 |
| Temporal resolution | – | 1 s |
| Nominal scan time [min] | 02:11 | ~ 0:24 |
| Subtraction | – | CE – native |
Measurement
Anonymized images of 4D CE-MRA and modified REACT-non-CE-MRA were presented in random order to two radiologists (L.P., A.W.), each with at least two years of experience in cardiovascular imaging, who independently conducted the measurement on seven distinct measurement points:
MPA (2 cm distal of the pulmonary valve as correlated by RVOT/transversal cine if necessary).
Right pulmonary artery (RPA, 1 cm distal of the bifurcation).
Left pulmonary artery (LPA, 1 cm distal of the bifurcation).
Right superior pulmonary vein (RSPV, 1 cm proximal of the ostium).
Right inferior pulmonary vein (RIPV, 1 cm proximal of the ostium).
Left superior pulmonary vein (LSPV, 1 cm proximal of the ostium).
Left inferior pulmonary vein (LIPV, 1 cm proximal of the ostium).
For each point, the measurement (inner diameter approach) was conducted on source images using the manual Multiplanar-Reconstruction-(MPR) tool in IMPAX EE (Agfa Healthcare N.V., Mortsel, Belgium) in manual perpendicular alignment. Maximum intensity projection images were not used since they lead to an apparent reduction in vessel diameter resulting in an underestimation [23]. Measurement points were excluded when they could not be assessed due to susceptibility or severe pulsation artifacts.
Image quality evaluation
Image quality was evaluated by both observers in consensus on a four-point scale regarding sharpness, presence of pulsation artifacts at the levels of measurement, and anatomic delineation. Quality was rated on a Likert scale of 1 to 4: 1 non-diagnostic, 2 poor image quality with substantial blurring impairing diagnostic confidence, 3 intermediate image quality with mild blurring, and 4 good image quality without any blurring and resulting high diagnostic confidence.
Statistical analysis
Data are shown as mean ± standard deviation (SD), unless noted otherwise. Statistical significance was set at p < 0.05. For each point of measurement, the average diameter of two tangential measurements was used for analysis. To evaluate interobserver reliability, intraclass correlation coefficients (ICCs) were calculated. Bland-Altman analysis was conducted to assess differences regarding measurement values of the pulmonary arteries and veins using 4D CE-MRA and modified REACT-non-CE-MRA. A paired t-test was used to evaluate the significance of differences between pulmonary artery and vein measurements on both modalities. For evaluation of differences regarding image quality, the Wilcoxon matched pair test was applied. Statistical analysis and graph creation were performed using JMP (Version 14.1.0, SAS Institute, Cary, North Carolina, USA).
Results
Study population and baseline characteristics
Of the 26 patients, one patient was excluded due to insufficient contrast in 4D CE-MRA, resulting in a study population of 25 patients (39 ± 20 years, body mass index 23.8 ± 5.2; 15 male subjects). Twenty-three patients presented a CHD, the most frequent being an atrial septal defect (n = 7), followed by ventricular septal defect (n = 5), transposition of the great arteries (n = 3), tetralogy of Fallot (TOF, n = 2) and pulmonary atresia (n = 2). 10 patients had surgery for CHD prior to the examination.
Imaging
All included imaging studies were executed without any complications. Modified REACT-non-CE-MRA showed an average total acquisition time of 7:01 ± 2:44 min (depending on the patient’s breathing frequency as well as heart rate), 4D CE-MRA of 2:14 ± 1:01 min.
Interobserver agreement of modified REACT-non-CE-MRA and 4D CE-MRA
At pulmonary arteries, modified REACT-non-CE-MRA and 4D CE-MRA showed comparable ICCs between 0.95 and 0.99. At pulmonary veins, modified REACT-non-CE-MRA achieved a higher agreement than 4D CE-MRA with the highest difference at LSPV (0.95 versus 0.90). Detailed results are given in Table 2.
Table 2.
Interobserver correlation coefficients of both methods of imaging and the dedicated measurement points with values > 0.8 indicating excellent correlation
| MPA | RPA | LPA | RSPV | RIPV | LSPV | LIPV | |
|---|---|---|---|---|---|---|---|
| Modified REACT-non-CE-MRA | 0.9904 | 0.9866 | 0.9792 | 0.935 | 0.959 | 0.9457 | 0.9595 |
| 4D CE-MRA | 0.9778 | 0.9834 | 0.9594 | 0.9101 | 0.9478 | 0.895 | 0.9347 |
MPA = main pulmonary artery. RPA = right pulmonary artery. LPA = left pulmonary artery. RSPV = right superior pulmonary vein. RIPV = right inferior pulmonary vein. LSPV = left superior pulmonary vein. LIPV = left inferior pulmonary vein
Detailed comparison between 4D CE-MRA and modified REACT-non-CE-MRA
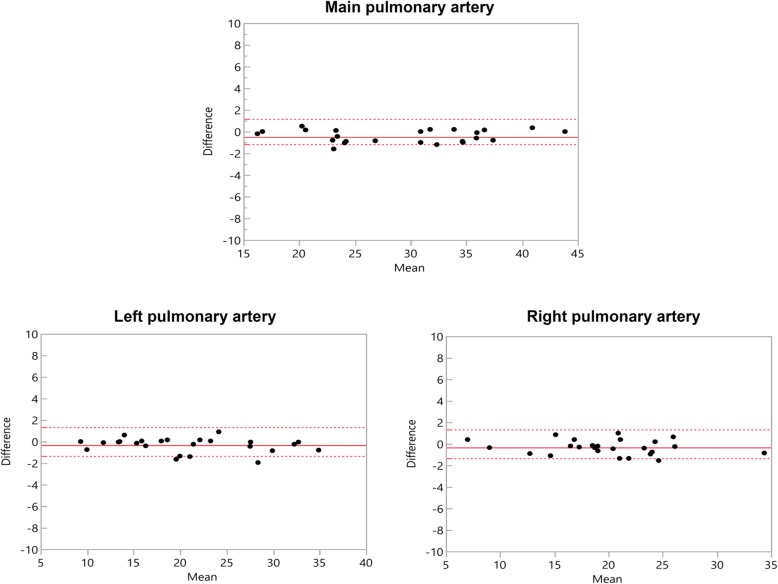
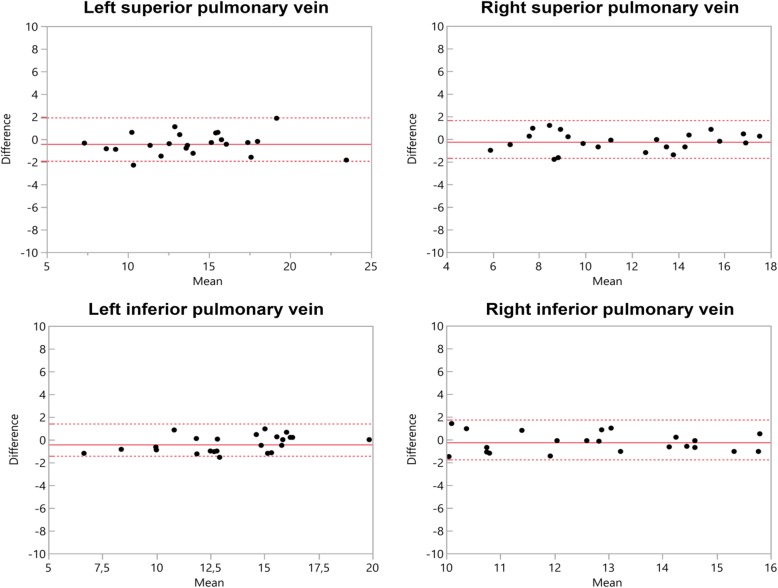
4D CE-MRA showed greater diameters at all points of measurement with significant differences at MPA (p = 0.002), RPA (p = 0.019), LPA (p = 0.026) (Table 3). At the pulmonary veins, no significant difference was noted with RSPV yielding the highest difference (0.396 mm) (Table 3). Bland-Altman comparisons of the differences regarding measurement values of the pulmonary arteries and veins assessed by 4D CE-MRA and modified REACT-non-CE-MRA with corresponding 95% confidence intervals are given in Figs. 1 and 2. Due to impaired image quality, measurement was not possible at the LSPV in one patient and at the LIPV in three patients using 4D CE-MRA. In modified REACT-non-CE-MRA, all measurements were conducted sufficiently.
Table 3.
Average measurement diameters and differences as well as the results of the paired t-test between differences of both methods of imaging at the dedicated measurement points, bold indicating statistical significance (p < 0.05)
| MPA | RPA | LPA | RSPV | RIPV | LSPV | LIPV | |
|---|---|---|---|---|---|---|---|
| Modified REACT non-CE MRA, mean, diameter, mm, SD | 29.0 ± 7.5 | 20.7 ± 7.1 | 19.7 ± 5.6 | 13.8 ± 3.7 | 13.3 ± 3.1 | 11.5 ± 3.4 | 12.7 ± 2.0 |
| 4D CE-MRA, mean, diameter, mm, SD | 29.4 ± 7.5 | 21.0 ± 7.3 | 20.0 ± 5.7 | 14.2 ± 3.7 | 13.7 ± 3.0 | 11.8 ± 3.5 | 13.0 ± 2.0 |
| Differences, mean, mm | 0.4 | 0.3 | 0.3 | 0.4 | 0.4 | 0.2 | 0.3 |
| 95% confidence interval, cm | 0.2 to 0.7 | 0.1 to 0.6 | 0.0 to 0.6 | 0.0 to 0.9 | 0.1 to 0.7 | −0.1 to 0.6 | − 0.1 to 0.7 |
| p value | 0.002 | 0.019 | 0.026 | 0.0638 | 0.0199 | 0.192 | 0.173 |
MPA = main pulmonary artery. RPA = right pulmonary artery. LPA = left pulmonary artery. RSPV = right superior pulmonary vein. RIPV = right inferior pulmonary vein. LSPV = left superior pulmonary vein. LIPV = left inferior pulmonary vein
Fig. 1.
Bland–Altman comparison of the measured diameters of the pulmonary arteries assessed by modified REACT-non-CE-MRA and 4D CE-MRA. The middle line indicates the mean bias of the diameter measurements whereas the dotted lines represent the 95% confidence interval. Values are given in mm
Fig. 2.
Bland–Altman comparison of the measured diameters of the pulmonary veins assessed by modified REACT-non-CE-MRA and 4D CE-MRA. The middle line indicates the mean bias of the diameter measurements whereas the dotted lines represent the 95% confidence interval. Values are given in mm
Comparison of image quality between 4D CE-MRA and modified REACT-non-CE-MRA
Modified REACT-non-CE-MRA showed average image quality scores of 3.8 for pulmonary arteries and 3.3 for veins compared to values of 3.3 for arteries (p < 0.001) and 2.7 for veins (p = 0.015) in 4D CE-MRA.
Figures 3, 4, 5 and 6 give exemplary comparisons of modified REACT-non-CE-MRA and 4D CE-MRA.
Fig. 3.

Multiplanar reformatted image of the main pulmonary artery (arrowheads) in an 11-year-old patient with pulmonary atresia after implantation of a Contegra conduit and multiple angioplasties of both pulmonary arteries. The pulmonary arteries can be clearly delineated in modified REACT-non-CE-MRA (left) compared to the blurred appearance in 4D CE-MRA (right)
Fig. 4.

CMR-imaging in a 56-year-old patient with sinus venosus atrial septal defect and suspected associated anomalous pulmonary venous return. Modified REACT-non-CE-MRA (left) clearly depicts connection of the right superior pulmonary vein with the superior vena cava (arrowheads) whereas 4D CE-MRA (right) shows pulsation artefacts hampering diagnosis
Fig. 5.

Multiplanar reformatted image of the main pulmonary artery (arrowheads) in a 39-year-old patient with situs inversus totalis and dextrocardia with transposition of the great artery and arterial switch operation (left: modified REACT-non-CE-MRA, right: 4D CE-MRA). Modified REACT-non-CE-MRA shows superior delineation of the vessel wall compared to 4D CE-MRA due to pulsation artifacts
Fig. 6.

Multiplanar reformatted image of the left pulmonary veins (arrowheads; left: modified REACT-non-CE-MRA, right: 4D CE-MRA) in a 71-year-old patient with patent ductus arteriosus. Modified REACT-non-CE-MRA shows improved delineation of the vessel wall compared to 4D CE-MRA
Discussion
In our study, we retrospectively compared a novel Compressed SENSE accelerated navigator- and ECG-triggered 3D modified REACT-non-CE-MRA with standard non-ECG-triggered 4D CE-MRA for the imaging of the pulmonary vessels in patients with CHD. The major findings of this study are the following: 1. 4D CE-MRA showed greater diameters for the pulmonary vessels in comparison to modified REACT-non-CE-MRA, at the arteries with significant difference. 2. Modified REACT-non-CE-MRA offers a significantly higher image quality of the pulmonary vasculature and a slightly higher interobserver agreement at the pulmonary veins than 4D CE-MRA.
In line with previous works, which compared non-ECG-triggered CE-MRA with ECG-triggered non-CE-MRA (SSFP, end-diastolic) for the imaging of pulmonary arteries and veins, 4D CE-MRA showed higher measurement values of the pulmonary vessels compared to modified REACT-non-CE-MRA, at the arteries with a significant difference [16, 24]. These differences are mainly due to pulsation and breathing artifacts in 4D CE-MRA and the resulting hampered vessel delineation. These pulsation artifacts are pronounced in patients with CHD such as TOF who show highly pulsatile circulations of the pulmonary arteries [23]. Furthermore, the motion and the changing size of the pulmonary veins and arteries throughout the cardiac cycle have to be considered when comparing the non-triggered acquisition of 4D CE-MRA with the ECG-triggered (end-diastolic) acquisition of modified REACT-non-CE-MRA, hence resulting in physiological differences which lead to greater diameters in 4D CE-MRA [16, 23–26]. Furthermore, the above-mentioned factors also implicate the slightly higher interobserver agreement of modified REACT-non-CE-MRA at the pulmonary veins. In line with above-mentioned studies, which have shown that other ECG-triggered and respiratory-gated non-CE-MRA sequences such as 3D SSFP outperform untriggered CE-MRA in terms of image quality, modified REACT-non-CE-MRA with respective triggering provided significant higher image quality scores of the pulmonary arteries and veins than 4D CE-MRA [15, 16, 18].
Patients with CHD require repetitive imaging of the pulmonary vasculature throughout their life and CMR has to be regarded as the gold standard of imaging with CE-MRA being used to evaluate the different vascular territories of the thorax and to detect its pathologies [6, 8–10]. However, the accurate execution of CE-MRA and 4D CE-MRA is technically demanding, as image quality depends on good coordination among the contrast injection, exact timing of data acquisition and patient cooperation for breath holding, subsequently limiting its use in incompliant patients [9, 16]. Recently, 4D CE-MRA has been developed, which offers the possibility to acquire a series of volume angiograms in quick succession therefore simplifying the timing of acquisition in relation to the passage of the contrast bolus [9]. Nevertheless, the majority of time-resolved MRA uses some form of data sharing across time, making them sensitive to respiratory motion artifacts; therefore the majority is performed during a breath-hold. New techniques of free-breathing time-resolved MRAs are technically feasible, but show decreased signal- and contrast-to-noise ratios [9].
Furthermore, the use of gadolinium contrast poses a drawback potentially leading to NSF in end-stage renal disease, anaphylactic reactions or extravasation. Contrast agents show high costs and require an intravenous access, potentially limiting its use in the clinical routine [14, 27–30]. Given the growing literature on long-term gadolinium deposition within the brain and the uncertainty regarding its long-term effects, repetitive application of gadolinium contrast, especially in children, should be executed cautiously [31–33].
Consequently, many non-CE-MRA methods have been developed over the past decades with SSFP and bSSFP being routinely used for thoracoabdominal vessels. 3D bSSFP/SSFP show advantages such as high signal-to-noise ratios, high blood-to-tissue contrast due to its bright-blood signal and flow independence [34–37]. However, they are highly sensitive to off-resonance effects caused by B0 heterogeneities in the main magnetic field and disruptions of the steady state due to highly pulsatile flow or motion and show high background signals [15, 38, 39]. Therefore, image quality can be impaired by signal loss, banding artifacts and insufficient fat suppression. These effects are pronounced in higher magnetic fields such as 3 T and in large FOVs. When applied in large FOVs, a long acquisition time is required, consequently limiting its use in clinical routine [15, 37].
Recently, a Compressed SENSE accelerated 3D REACT-non-CE-MRA was introduced, which overcomes these limitations by the following: On the one hand, mDIXON XD combines the above-mentioned benefits of SSFP with reduced sensitivity to inhomogeneities in the magnetic field [20, 40]. mDIXON XD provides robust suppression of fat and background and allows for separation of water and fat, consequently leading to insensitivity of REACT-non-CE-MRA to inhomogeneities in the magnetic field, even in large FOVs [41]. Therefore, it provides high-resolution scans in large FOVs and allows the application in higher magnetic fields such as 3 T, where inhomogeneities are expected to be higher. On the other hand, with the advent of new acceleration techniques such as compressed sensing, shorter acquisition times beyond current parallel imaging techniques are possible, especially when combining the advantages of both techniques [22, 42]. In this work, Compressed SENSE was employed, allowing for image-acquisition acceleration factors currently not achievable by compressed sensing or parallel imaging alone. Consequently, this directly addresses shortcomings of non-CE-MRA techniques as identified in previous work [15, 36]. The Compressed SENSE technique was fully integrated on the clinical system, resulting in reconstruction times below one minute and an overall scan time of 7:01 ± 2:44 min, lower than 3D SSFP for the same kind of investigation and FOV (10:01 ± 4.5 min) [15]. REACT-non-CE-MRA was combined with respiratory navigator-triggering and ECG-triggering to compensate for respiratory and cardiac motion, therefore being referred to as “modified” REACT-non-CE-MRA in this study. Given its flow-independency, REACT-non-CE-MRA can in principle also be used without triggering, making it a versatile alternative to CE-MRA, especially for patients who are unable to perform a breath-hold, e.g. children.
Modified REACT-non-CE-MRA only delivers a stationary depiction of the vessels and does not include dynamic flow conditions as 4D CE-MRA [8]. Given the fact that modified REACT-non-CE-MRA enables a simultaneous display of arterial and venous vessels of the thorax unlike 4D CE-MRA, resulting images might appear overloaded. However, since the vessels are displayed sharply and in good quality, a sufficient differentiation is possible.
Clinical applications
Besides the depiction of the pulmonary vasculature in CHD, modified REACT-non-CE-MRA may offer an alternative to CTA or other MRA techniques for patients with PH or suspected pulmonary embolism as well as for imaging of the aorta in patients with connective tissue disease. Further, its use in other vascular territories, e.g. the extracranial arteries, might pose an additional clinical application and may warrant future investigation.
Limitations
The size and the heterogeneity of the study population with its wide range of different CHDs and postoperative alterations may be regarded as a drawback of this study.
We did not compare modified REACT-non-CE-MRA to DSA and regarded untriggered breath-hold 4D CE-MRA as the reference standard. Furthermore, no comparison to other ECG-triggered and respiratory-gated non-CE-MRA sequences such as 3D SSFP was conducted in this study, which may nurture future investigations.
Additionally, the lower resolution of 4D CE-MRA consequently leads to an inferior image quality than modified REACT-non-CE-MRA and offers lower image quality than standard CE-MRA [8]. The comparison of the ECG- and navigator-triggered modified REACT-non-CE-MRA with an untriggered breath-hold first-pass CE-MRA (4D CE-MRA) may represent a limitation of this work given the fact that ECG-gating as well as navigator-gating with slow-infusion of contrast media have proven to increase the image quality of CE-MRA [43–47]. However, ECG-gating with respect to contrast bolus arrival proves to be challenging in daily clinical routine.
Conclusions
Compressed SENSE accelerated (factor 9) ECG- and respiratory navigator-triggered 3D modified REACT-non-CE-MRA allows for robust and reliable imaging of the pulmonary vasculature in CHD with a higher image quality and a slightly higher interobserver agreement than 4D CE-MRA without the need of gadolinium contrast. Given its short acquisition time, it represents a clinically applicable alternative for patients with CHD in need of repetitive imaging of the pulmonary vessels.
Acknowledgements
Not applicable.
Abbreviations
- bSSFP
Balanced SSFP
- CE-MRA
Contrast-enhanced MR-angiography
- CHD
Congenital heart disease
- CMR
Cardiovascular magnetic resonance
- CT
Computed tomography
- CTA
CT-angiography
- DSA
Digital subtraction angiography
- ECG
Electrocardiogram
- FOV
Field of view
- ICC
Intraclass correlation coefficients
- LIPV
Left inferior pulmonary vein
- LPA
Left pulmonary artery
- LSPV
Left superior pulmonary vein
- mDIXON XD
Dual gradient echo Dixon
- MPA
Main pulmonary artery
- MPR
Multiplanar reconstruction
- NSF
Nephrogenic systemic fibrosis
- PH
Pulmonary hypertension
- REACT
Relaxation-Enhanced Angiography without Contrast and Triggering
- RIPV
Right inferior pulmonary vein
- RPA
Right pulmonary artery
- RSPV
Right superior pulmonary vein
- RVOT
Right ventricular outflow tract
- SD
Standard deviation
- SENSE
SENSitivity encoding
- SSFP
Steady-state free precession
- STIR
Short tau inversion recovery
- T2 prep
T2 preparation
- TOF
Tetralogy of Fallot
- TSE
Turbo spin echo
Authors’ contributions
Conceptualization: LP, JD, AW, JG, KL, DM, CN, TH, AB, KW. Methodology: LP, JD, AW, KW, SL, DM, CN, TH, AB. Formal analysis and investigation: LP, SL, AW, KW, JD, CN. Writing - original draft preparation: LP, KW, AW, JG, KL. Writing - review and editing: JD, DM, CN, TH, AB, JG, KL, KW. Resources: JD, CN, DM, KW, TH, AB. Supervision: DM, JD, CN, KW, TH, AB. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to data protection but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The local ethics committee approved this study. Due to the retrospective design of the study, the local ethic committee waived written informed consent requirement in the patient cohort.
Ethics comittee: Ethikkommission, Medizinische Fakultät der Universität zu Köln.
Committee’s reference number: 19–1217.
Consent for publication
Regarding the images attached to the manuscript, written informed consent for publication was obtained from the patients.
Competing interests
The following authors of this manuscript declare relationships with the following companies:
Kilian Weiss - employee of Philips Healthcare.
David Maintz - speakers’ bureau Philips Healthcare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/21/2020
The original publication of this article [1], contained graphical errors in Figs. 1 and 2. This does not impact the display of the mean differences of the Bland-Altman plots. The updated figures (Fig. 1 and Fig. 2) are published in this correction article.
Contributor Information
Lenhard Pennig, Email: Lenhard.pennig@uk-koeln.de.
Anton Wagner, Email: Anton.wagner@uk-koeln.de.
Kilian Weiss, Email: Kilian.weiss@uk-koeln.de.
Simon Lennartz, Email: Simon.lennartz@uk-koeln.de.
Jan-Peter Grunz, Email: Grunz_j@ukw.de.
David Maintz, Email: David.maintz@uk-koeln.de.
Kai Roman Laukamp, Email: Kai.laukamp@uk-koeln.de.
Tilman Hickethier, Email: Tilman.hickethier@uk-koeln.de.
Claas Philip Naehle, Email: Claas.naehle@uk-koeln.de.
Alexander Christian Bunck, Email: Alexander.bunck@uk-koeln.de.
Jonas Doerner, Email: Jonas.doerner@uk-koeln.de.
References
- 1.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115(2):163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 3.Mcleod G, Shum K, Gupta T, Chakravorty S, Kachur S, Bienvenu L, et al. Echocardiography in congenital heart disease. Prog Cardiovasc Dis. 2018;61(5–6):468–475. doi: 10.1016/j.pcad.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Gutgesell HP, Huhta JC, Latson LA, Huffines D, McNamara DG. Accuracy of two-dimensional echocardiography in the diagnosis of congenital heart disease. Am J Cardiol. 1985;55(5):514–518. doi: 10.1016/0002-9149(85)90237-1. [DOI] [PubMed] [Google Scholar]
- 5.Popescu BA, Andrade MJ, Badano LP, Fox KF, Flachskampf FA, Lancellotti P, et al. European Association of Echocardiography recommendations for training, competence, and quality improvement in echocardiography. Eur J Echocardiogr. 2009;10(8):893–905. doi: 10.1093/ejechocard/jep151. [DOI] [PubMed] [Google Scholar]
- 6.Ntsinjana HN, Hughes ML, Taylor AM. The role of cardiovascular magnetic resonance in pediatric congenital heart disease. J Cardiovasc Magn Reson. 2011;13:51. doi: 10.1186/1532-429X-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgartner H, Bonhoeffer P, De Groot NMS, de Haan F, Deanfield JE, Galie N, et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31(23):2915–2957. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 8.Vogt FM, Theysohn JM, Michna D, Hunold P, Neudorf U, Kinner S, et al. Contrast-enhanced time-resolved 4D MRA of congenital heart and vessel anomalies: image quality and diagnostic value compared with 3D MRA. Eur Radiol. 2013;23(9):2392–2404. doi: 10.1007/s00330-013-2845-7. [DOI] [PubMed] [Google Scholar]
- 9.Steeden JA, Pandya B, Tann O, Muthurangu V. Free breathing contrast-enhanced time-resolved magnetic resonance angiography in pediatric and adult congenital heart disease. J Cardiovasc Magn Reson. 2015;17:38. doi: 10.1186/s12968-015-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Stelt F, Siegerink SN, Krings GJ, et al. Three-dimensional rotational angiography in pediatric patients with congenital heart disease: a literature review. Pediatr Cardiol. 2019;40(2):257–264. doi: 10.1007/s00246-019-02052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnamurthy R, Bahouth SM, Muthupillai R. 4D contrast-enhanced MR angiography with the keyhole technique in children: technique and clinical applications. RadioGraphics. 2016;36(2):523–537. doi: 10.1148/rg.2016150106. [DOI] [PubMed] [Google Scholar]
- 12.Grist TM, Mistretta CA, Strother CM, Turski PA. Time-resolved angiography: past, present, and future. J Magn Reson Imaging. 2012;36(6):1273–1286. doi: 10.1002/jmri.23646. [DOI] [PubMed] [Google Scholar]
- 13.Zhong L, Schrauben EM, Garcia J, Uribe S, Grieve SM, Elbaz MSM, et al. Intracardiac 4D flow MRI in congenital heart disease: recommendations on behalf of the ISMRM flow & motion study group. J Magn Reson Imaging. 2019;50(3):677–681. doi: 10.1002/jmri.26858. [DOI] [PubMed] [Google Scholar]
- 14.Perazella MA. Advanced kidney disease, gadolinium and nephrogenic systemic fibrosis: the perfect storm. Curr Opin Nephrol Hypertens. 2009;18(6):519–525. doi: 10.1097/MNH.0b013e3283309660. [DOI] [PubMed] [Google Scholar]
- 15.François CJ, Tuite D, Deshpande V, Jerecic R, Weale P, Carr JC. Pulmonary vein imaging with unenhanced three-dimensional balanced steady-state free precession MR angiography: initial clinical evaluation. Radiology. 2009;250(3):932–939. doi: 10.1148/radiol.2502072137. [DOI] [PubMed] [Google Scholar]
- 16.Krishnam MS, Tomasian A, Malik S, Singhal A, Sassani A, Laub G, et al. Three-dimensional imaging of pulmonary veins by a novel steady-state free-precession magnetic resonance angiography technique without the use of intravenous contrast agent: initial experience. Investig Radiol. 2009;44(8):447–453. doi: 10.1097/RLI.0b013e3181a7c6cb. [DOI] [PubMed] [Google Scholar]
- 17.Edelman RR, Silvers RI, Thakrar KH, Metzl MD, Nazari J, Giri S, et al. Nonenhanced MR angiography of the pulmonary arteries using single-shot radial quiescent-interval slice-selective (QISS): a technical feasibility study. J Cardiovasc Magn Reson. 2017;19(1):48. doi: 10.1186/s12968-017-0365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnam MS, Tomasian A, Deshpande V, Tran L, Laub G, Finn JP, et al. Noncontrast 3D steady-state free-precession magnetic resonance angiography of the whole chest using nonselective radiofrequency excitation over a large field of view: comparison with single-phase 3D contrast-enhanced magnetic resonance angiography. Investig Radiol. 2008;43(6):411–420. doi: 10.1097/RLI.0b013e3181690179. [DOI] [PubMed] [Google Scholar]
- 19.Johns CS, Swift AJ, Hughes PJC, Ohno Y, Schiebler M, Wild JM. Pulmonary MR angiography and perfusion imaging—a review of methods and applications. Eur J Radiol. 2017;86:361–370. doi: 10.1016/j.ejrad.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Yoneyama M, Zhang S, Hu HH, Chong LR, Bardo D, Miller JH, et al. Free-breathing non-contrast-enhanced flow-independent MR angiography using magnetization-prepared 3D non-balanced dual-echo Dixon method: a feasibility study at 3 tesla. Magn Reson Imaging. 2019;63:137–146. doi: 10.1016/j.mri.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 22.Lustig M, Donoho D, Sparse PJM, MRI The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 23.Shariat M, Schantz D, Yoo SJ, Wintersperger BJ, Seed M, Alnafisi B, et al. Pulmonary artery pulsatility and effect on vessel diameter assessment in magnetic resonance imaging. Eur J Radiol. 2014;83(2):378–383. doi: 10.1016/j.ejrad.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Syed MA, Peters DC, Rashid H, Arai AE. Pulmonary vein imaging: comparison of 3D magnetic resonance angiography with 2D cine MRI for characterizing anatomy and size. J Cardiovasc Magn Reson. 2005;7(2):355–360. doi: 10.1081/jcmr-200053458. [DOI] [PubMed] [Google Scholar]
- 25.Hauser TH, Yeon SB, Kissinger KV, Josephson ME, Manning WJ. Variation in pulmonary vein size during the cardiac cycle: Implications for non-electrocardiogram-gated imaging. Am Heart J. 2006;152(5):974.e1–974.e6. doi: 10.1016/j.ahj.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Burman ED, Keegan J, Kilner PJ. Pulmonary artery diameters, cross sectional areas and area changes measured by cine cardiovascular magnetic resonance in healthy volunteers. J Cardiovasc Magn Reson. 2016;18:12. doi: 10.1186/s12968-016-0230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. Am J Roentgenol. 2016;207(2):229–233. doi: 10.2214/AJR.15.15842. [DOI] [PubMed] [Google Scholar]
- 28.Von Knobelsdorff-Brenkenhoff F, Bublak A, El-Mahmoud S, Wassmuth R, Opitz C, Schulz-Menger J. Single-Centre survey of the application of cardiovascular magnetic resonance in clinical routine. Eur Heart J Cardiovasc Imaging. 2013;14(1):62–68. doi: 10.1093/ehjci/jes125. [DOI] [PubMed] [Google Scholar]
- 29.Jung J-W, Kang H-R, Kim M-H, Lee W, Min KU, Han MH, et al. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology. 2012;264(2):414–422. doi: 10.1148/radiol.12112025. [DOI] [PubMed] [Google Scholar]
- 30.Heshmatzadeh Behzadi A, Farooq Z, Newhouse JH, Prince MR. MRI and CT contrast media extravasation. Med.(Baltimore) 2018;97(9):e0055. doi: 10.1097/MD.0000000000010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanda T, Matsuda M, Oba H, Toyoda K, Furui S. Gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;277(3):924–925. doi: 10.1148/radiol.2015150697. [DOI] [PubMed] [Google Scholar]
- 32.Gulani V, Calamante F, Shellock FG, Kanal E. Reeder SB; International Society for Magnetic Resonance in medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16(7):564–570. doi: 10.1016/S1474-4422(17)30158-8. [DOI] [PubMed] [Google Scholar]
- 33.Olchowy C, Cebulski K, Łasecki M, Chaber R, Olchowy A, Kałwak K, et al. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity-a systematic review. PLoS One. 2017;12(2):e0171704. doi: 10.1371/journal.pone.0171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bannas P, Groth M, Rybczynski M, Sheikhzadeh S, von Kodolitsch Y, Graessner J, et al. Assessment of aortic root dimensions in patients with suspected Marfan syndrome: Intraindividual comparison of contrast-enhanced and non-contrast magnetic resonance angiography with echocardiography. Int J Cardiol. 2013;167(1):190–196. doi: 10.1016/j.ijcard.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 35.Potthast S, Mitsumori L, Stanescu LA, Richardson ML, Branch K, Dubinsky TJ, et al. Measuring aortic diameter with different MR techniques: comparison of three-dimensional (3D) navigated steady-state free-precession (SSFP), 3D contrast-enhanced magnetic resonance angiography (CE-MRA), 2D T2 black blood, and 2D cine SSFP. J Magn Reson Imaging. 2010;31(1):177–184. doi: 10.1002/jmri.22016. [DOI] [PubMed] [Google Scholar]
- 36.Von Knobelsdorff-Brenkenhoff F, Gruettner H, Trauzeddel RF, Greiser A, Schulz-Menger J. Comparison of native high-resolution 3D and contrast-enhanced MR angiography for assessing the thoracic aorta. Eur Heart J Cardiovasc Imaging. 2014;15(6):651–658. doi: 10.1093/ehjci/jet263. [DOI] [PubMed] [Google Scholar]
- 37.Morita Satoru, Masukawa Ai, Suzuki Kazufumi, Hirata Masami, Kojima Shinya, Ueno Eiko. Unenhanced MR Angiography: Techniques and Clinical Applications in Patients with Chronic Kidney Disease. RadioGraphics. 2011;31(2):E13–E33. doi: 10.1148/rg.312105075. [DOI] [PubMed] [Google Scholar]
- 38.Bangerter NK, Cukur T, Hargreaves BA, Hu BS, Brittain JH, Park D. Three-dimensional fluid-suppressed T2-prep flow-independent peripheral angiography using balanced SSFP. Magn Reson Imaging. 2011;29(8):1119–1124. doi: 10.1016/j.mri.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Çukur T, Lee JH, Bangerter NK, Hargreaves BA, Nishimura DG. Non-contrast-enhanced flow-independent peripheral MR angiography with balanced SSFP. Magn Reson Med. 2009;61(6):1533–1539. doi: 10.1002/mrm.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eggers H, Brendel B, Duijndam A, Herigault G. Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med. 2011;65(1):96–107. doi: 10.1002/mrm.22578. [DOI] [PubMed] [Google Scholar]
- 41.Leiner T, Habets J, Versluis B, Geerts L, Alberts E, Blanken N, et al. Subtractionless first-pass single contrast medium dose peripheral MR angiography using two-point Dixon fat suppression. Eur Radiol. 2013;23(8):2228–2235. doi: 10.1007/s00330-013-2833-y. [DOI] [PubMed] [Google Scholar]
- 42.Liang D, Liu B, Wang J, Ying L. Accelerating SENSE using compressed sensing. Magn Reson Med. 2009;62(6):1574–1584. doi: 10.1002/mrm.22161. [DOI] [PubMed] [Google Scholar]
- 43.Dabir D, Naehle CP, Clauberg R, Gieseke J, Schild HH, Thomas D. High-resolution motion compensated MRA in patients with congenital heart disease using extracellular contrast agent at 3 tesla. J Cardiovasc Magn Reson. 2012;14:75. doi: 10.1186/1532-429X-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tandon A, James L, Henningsson M, Botnar RM, Potersnak A, Greil GF, et al. A clinical combined gadobutrol bolus and slow infusion protocol enabling angiography, inversion recovery whole heart, and late gadolinium enhancement imaging in a single study. J Cardiovasc Magn Reson. 2016;18(1):66. doi: 10.1186/s12968-016-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen KL, Han F, Zhou Z, Brunengraber DZ, Ayad I, Levi DS, et al. 4D MUSIC CMR: value-based imaging of neonates and infants with congenital heart disease. J Cardiovasc Magn Reson. 2017;19(1):40. doi: 10.1186/s12968-017-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groves EM, Bireley W, Dill K, Carroll TJ, Carr JC. Quantitative analysis of ECG-gated high-resolution contrast-enhanced MR angiography of the thoracic aorta. Am J Roentgenol. 2007;188(2):522–528. doi: 10.2214/AJR.05.1467. [DOI] [PubMed] [Google Scholar]
- 47.Galizia MS, Febbo JA, Popescu AR, Bi X, Collins J, Markl M, et al. Steady state imaging of the thoracic vasculature using inversion recovery FLASH and SSFP with a blood pool contrast agent. J Cardiovasc Magn Reson. 2012;14(Suppl 1):51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to data protection but are available from the corresponding author on reasonable request.