Abstract
Background
Although 18F- FDG PET/CT is validated in baseline workup of esophageal cancer to detect distant metastases, it remains underused in assessing local staging and biology of the primary tumor. This study aimed to evaluate the association between 18F- FDG PET/CT-derived parameters of esophageal cancer, and its clinico-pathological features and prognosis.
Methods
All patients (n = 86) with esophageal adenocarcinoma or squamous cell cancer operated between 2005 and 2014 were analyzed. Linear regression was used to identify clinico-pathologic features of esophageal cancer associated with the tumor’s maximal Standardized Uptake Value (SUVmax), Total Lesion Glycolysis (TLG) and Metabolic Tumor Volume (MTV). ROC curve analysis was performed to precise the optimal cutoff of each variable associated with a locally advanced (cT3/4) status, long-term survival and recurrence. Kaplan Meier curves and Cox regression were used for survival analyses.
Results
High baseline SUVmax was associated with cT3/4 status and middle-third tumor location, TLG with a cT3/4 and cN+ status, whereas MTV only with active smoking. A cT3/4 status was significantly predicted by a SUVmax > 8.25 g/mL (p < 0.001), TLG > 41.7 (p < 0.001) and MTV > 10.70 cm3 (p < 0.01) whereas a SUVmax > 12.7 g/mL was associated with an early tumor recurrence and a poor disease-free survival (median 13 versus 56 months, p = 0.030), particularly in squamous cell cancer.
Conclusions
Baseline 18F- FDG PET/CT has a high predictive value of preoperative cT stage, as its parameters SUVmax, TLG and MTV can predict a locally advanced tumor with high accuracy. A SUVmax > 12.7 g/mL may herald early tumor recurrence and poor disease-free survival.
Keywords: Esophageal cancer, TNM stage, Nuclear imaging, Metabolic imaging, 18FDG PET/CT
Background
Esophageal cancer is associated with aggressive lymphatic spread, resulting often in locally advanced or metastatic disease upon diagnosis [1]. Metabolic imaging with 18F- Fluorodeoxyglucose Positron Emission Tomography/Computerized Tomography (18F- FDG -PET/CT) has been integrated into the preoperative workup of esophageal cancer for the detection of distant suspicions lesions [1–3], interval metastases [4] or assessment of response to neoadjuvant treatment [5]. Esophageal cancer workup should use three-modality staging with Computerized Tomography (CT), endoscopic ultrasound (EUS) and 18F- FDG PET/CT [2, 3], as failure to identify locally advanced tumors (cT3/4 or N+) may lead to omission of neoadjuvant treatment before surgery, compromising patient survival [2, 6]. To this day, 18F- FDG PET/CT is not primarily used for local staging of the primary tumor due to its poor spatial resolution. However, Malik et al. recently demonstrated a significant predictive value of Metabolic Tumor Volume (MTV) in differentiating early-stage (cT1/2) from locally advanced (cT3/4) lesions [7]. Obtaining accurate cTN staging information through 18F- FDG PET/CT may be of prime importance particularly in cases where EUS is unavailable or if the tumor is obstructive (up to 19% of patients) [7]. Furthermore, although the predictive value of 18F- FDG PET/CT derived parameters maximal standardized Uptake Value (SUVmax), Total Lesion Glycolysis (TLG) and MTV on long-term survival of esophageal cancer has been extensively reported in the literature [8], the absence of universally accepted thresholds and the paucity of data for each histological type limit the predictive value for the individual patient.
The aim of our study was to assess the clinico-pathological correlations and staging value of 18F- FDG PET/CT derived parameters SUVmax, TLG and MTV, as well as their predictive value in patient survival and tumor recurrence.
Methods
All patients operated for esophageal adenocarcinoma or squamous cell cancer, with curative intent, from 2005 to 2014 in our tertiary referral center and a baseline 18F- FDG PET/CT in the preoperative workup were included in this study. Demographic, clinical and histological data were retrieved from our prospectively maintained database.
In all patients, routine preoperative staging was performed by esophagogastroduodenoscopy, EUS and thoraco-abdominal CT scan. Since 2005 18F- FDG PET/CT was integrated in the baseline preoperative workup, according to current recommendations [2]. TNM stage was defined according to the 7th TNM classification [9]. Neoadjuvant treatment was administered for locally advanced lesions (cT3/4 and/or N+), with 5FU-platin or carboplatin-paclitaxel based chemotherapy and external beam radiation of 41–54 Gy. R0 resection was defined as the presence of tumor within 1 mm of resection margins. Postoperative follow-up included a thoraco-abdominal CT scan every 4 months for the first two postoperative years and further workup in cases of suspected recurrence [10]. Early recurrence was defined as any documented recurrence in the first 12 postoperative months. Follow-up data were last updated in November 2018, to assure a minimum follow-up of 4 years for all patients.
Baseline 18F- FDG PET/CT and derived parameters
Since the beginning of this study, we introduced our own PET/quality control program used in several national and international PET studies [11] until our center participated to the quality control program by the European Association of Nuclear Medicine (EANM)- EARL as PET/CT Center of Excellence in October 2011, for which we have been accredited each year so far. For 18F- FDG PET/CT, patients fasted for at least 6 h before and blood glucose was measured before administration of the radiotracer and required to be < 8.5 mmol/L, otherwise the scan was rescheduled. Each patient received 5.5 MBq/kg until 08/2011 or 3.5 MBq/kg thereafter of 18F- FDG intravenously and remained in a calm and warm area for 1 h post injection. Thereafter, the patient was asked to void and subsequently was placed in the scanner. Images were acquired on PET/CT scanner (Discovery LS until 08/2011 and thereafter Discovery D690 TOF; GE Healthcare, Waukesha, WI) with scatter and point-spread function recovery corrections. The CT scan (140 kV, 80 mA pith 1.5 until 08/2011 and thereafter 120 keV, 80–200 AutomA/SmartmA, pitch 1.375) was used for attenuation correction. The CT scan was followed by a PET over the same body region (3–5 min/bed position acquired in 2-D mode until 08/2011 thereafter 1 min 30 s–2 min/bed position acquired in 3-D mode). Images were reconstructed using OSEM (2 iterations, 26 subsets) with a 5.4-mm FWHM post-filter and 3.91-mm FWHM loop filter until 08/2011 and thereafter using OSEM (3 iterations, 16 subsets) with 5-mm postfilter and PSF and TOF recovery corrections. Two nuclear medicine physicians (AP, JOP) closely reviewed the images using for analysis an Advantage Workstation (version 4.6, GE Healthcare, Waukesha, WI) using PET VCAR software to compute SUVmean, SUVmax, TLG and MTV.
SUVmax was defined as the point of maximal radiotracer uptake value within the delineated tumor volume (g/mL). MTV represents the metabolically active volume of the main tumor (cm3), whereas TLG was computed as the product of MTV multiplied by the tumor’s SUVmean. In order to define the contouring margin of primary tumor, a volume of interest around the tumor was drawn carefully to incorporate the target lesion in transaxial, sagittal and coronal planes. For tumor delineation we used a 42% threshold, as it is the most commonly used in the literature [7, 12].
Statistical analysis
Linear regression was performed to assess correlation between several clinicopathological variables and baseline SUVmax, TLG and MTV. For each PET/CT derived parameter, a Receiver Operating Characteristic (ROC) curve analysis was performed to assess whether an optimal cutoff could be associated with locally advanced lesions (cT3/4), overall survival and early tumor recurrence. Overall and disease-free survival were analyzed with the Kaplan-Meier method and log-rank test as well as and a Cox regression analysis. Based on previously published differences in FDG uptake between adenocarcinoma and squamous cell cancer [3, 13], subgroup analyses were separately carried out for each histological type. Co-variates with a p-value< 0.2 on a univariate level were entered to a backward elimination process, allowing to build the final multivariate model with the lowest Akaike Information Criterion (AIC) value. Significance level was set at p < 0.05 and all tests were two-sided. Statistical analysis was performed with RStudio (version 3.2.3, RStudio Team 2015, Boston, USA) and SPSS (version 23.0, Chicago, USA).
Results
From the 141 patients operated in the study period, 89 had a baseline 18F- FDG PET/CT in their workup (63%). Three of them were excluded from analysis because of histology other than adenocarcinoma or squamous cell; thus, the current series consists of 86 patients. Baseline patient characteristics are outlined in Table 1. Adenocarcinoma represented 53% and squamous cell histology 47% of all lesions; 94% of all tumors were FDG-avid in 18F- FDG PET/CT.
Table 1.
Baseline demographics and preoperative workup of all patients
| Variable | N = 86 |
|---|---|
| Median age, years [range] | 63 [38–82] |
| Male Gender (%) | 66 (77) |
| Active smoking (%) | 38 (44) |
| ASA class (%) | |
| 1–2 | 61 (71) |
| 3–4 | 25 (29) |
| Tumor location (%) | |
| Upper third | 3 (4) |
| Middle third | 27 (31) |
| Distal third | 29 (34) |
| Gastroesophageal junction | 27 (31) |
| Clinical T stage (cT) | |
| cT1–2 | 21 (24) |
| cT3–4 | 63 (73) |
| Missing data | 2 (2) |
| Clinical N stage (cN) | |
| cN0 | 33 (38) |
| cN+ | 49 (57) |
| Missing data | 4 (5) |
| Tumor histology | |
| Adenocarcinoma | 46 (53) |
| Squamous cell carcinoma | 40 (47) |
| Preoperative workup | |
| CT | 86 (100) |
| EUS | 78 (91) |
| EUS non-obstructive lesion | 73 (85) |
| 18F- FDG PET/CT | 86 (100) |
| 18F- FDG PET/CT -avid lesion | 81 (94) |
| Neoadjuvant treatment | 71 (82) |
| Operative approach | |
| Transthoracic (Lewis) | 65 (76) |
| Three-field (McKeown) | 19 (22) |
| Transhiatal | 2 (2) |
ASA American Society of Anesthesiology, CT Computerized tomography, EUS Endoscopic ultrasound, 18F- FDG PET/CT 18F-Fluorodeoxyglucose Positron Emission Tomography CT
Baseline 18F- FDG PET/CT -derived parameters and initial tumor staging
SUVmax (maximal standardized uptake value)
Median baseline SUVmax was 12.1 g/mL (range 2.8–48.0) for all tumors. Middle third tumor location, advanced cT and cN stage as well as squamous cell histology were associated with higher SUVmax values on a univariate level, however only tumor location and cT stage remained significant on multiple regression (Table 2). cT3/4 tumors had an expected SUVmax 6.61 higher than a cT1–2 lesion (β coefficient 6.61, 95%CI 2.40, 10.81, p = 0.002), and middle third tumors an expected SUVmax 7.01 higher than GEJ lesions (β coefficient 7.01, 95%CI 0.71–13.32, p = 0.029). The multivariable model presented a good fit to the data (R2 = 0.2804, F-statistic 4.676 on 6 and 72 DF, p < 0.0001).
Table 2.
Linear regression analysis for baseline SUVmax
| Variable | Unadjusted β coefficient | 95%CI | P-value | Adjusted β coefficient | 95%CI | P-value |
|---|---|---|---|---|---|---|
| cT stage | ||||||
| cT1–2 | Ref | Ref | ||||
| cT3–4 | 7.76 | 3.64,11.87 | < 0.001 | 6.61 | 2.40,10.81 | 0.002 |
| cN stage | ||||||
| cN0 | Ref | Ref | ||||
| cN+ | 4.12 | 0.29,7.95 | 0.038 | 3.28 | −0.49,7.05 | 0.087 |
| Tumor location | ||||||
| GEJ | Ref | Ref | ||||
| Distal third | 1.41 | −2.99,5.80 | 0.53 | 1.59 | −3.10,6.29 | 0.50 |
| Middle third | 6.27 | 1.80,10.75 | 0.007 | 7.01 | 0.71,13.32 | 0.029 |
| Superior third | −1.42 | −11.26,8.42 | 0.78 | −1.47 | −12.05,9.11 | 0.78 |
| Histology | ||||||
| Adenocarcinoma | Ref | |||||
| Squamous cell | 3.84 | 0.23,7.45 | 0.040 | −0.630 | −5.84,4.58 | 0.81 |
SUVmax Maximal standardized uptake value, GEJ Gastroesophageal junction, 95%CI 95% confidence intervals, Ref Reference category (β coefficient = 0)
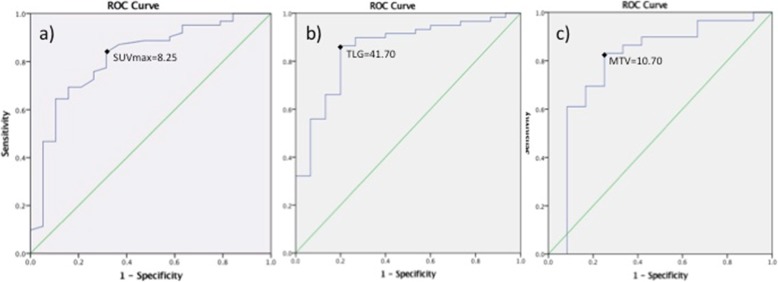
Baseline SUVmax presented a good prognostic value of a cT3/4 status in ROC curve analysis (Fig. 1a). A SUVmax > 8.25 g/mL predicted a cT3/4 lesion with a sensitivity of 84% and a specificity of 68%. Overall accuracy as indicated by the area under the curve (AUC) was 82% (AUC = 0.816, 95%CI = 0.704–0.928, p < 0.001).
Fig. 1.
ROC curve analyses for the predictive value of 18FDG-PET/CT derived parameters in relation to a cT3/4 status. All three parameters predicted significantly cT3–4 status of the primary tumor. a SUVmax > 8.25 g/mL (sensitivity 83.9%, specificity 68.4%, p < 0.001), b TLG > 41.7 g (sensitivity 86.4%, specificity 80%, p < 0.001), c MTV > 10.7 cm3 (sensitivity 83.1%, specificity 75%, p = 0.01)
TLG (Total lesion glycolysis)
Median TLG for all tumors was 122.1 (range 1–1179). Simple linear regression revealed higher TLG values for cT3/4 and cN+ tumors, and both co-variates remained significant in multivariate analysis; expected TLG for cT3/4 tumors was 162.9 higher than cT1/2 tumors (β coefficient 162.95, 95% CI 31.39–294.51, p = 0.016), and cN+ had a TLG 145.83 higher than cN0 lesions (β coefficient 145.83, 95% 34.47–256.19, p = 0.010). (Table 3) The model presented a good fit to the data (R2 = 0.1852, F-statistic 7.841 on 2 and 69DF, p < 0.001).
Table 3.
Linear regression analysis for baseline TLG
| Variable | Unadjusted β coefficient | 95%CI | P-value | Adjusted β coefficient | 95%CI | P-value |
|---|---|---|---|---|---|---|
| cT stage | ||||||
| cT1–2 | Ref | Ref | ||||
| cT3–4 | 198.42 | 61.42,335.43 | 0.006 | 162.95 | 31.39,294.51 | 0.016 |
| cN stage | ||||||
| cN0 | Ref | Ref | ||||
| cN+ | 168.77 | 58.05,279.48 | 0.004 | 145.83 | 35.47,256.19 | 0.010 |
TLG Total lesion glycolysis, 95%CI 95% confidence intervals, Ref Reference category (β coefficient = 0)
ROC curve analysis identified a TLG > 41.7 g as the optimal cutoff to detect a cT3/4 lesion, with a sensitivity of 86%, a specificity of 80% and an overall accuracy of 85% (AUC 0.852, 95%CI 0.744–0.960, p < 0.001) (Fig. 1b).
MTV (metabolic tumor volume)
Median MTV for all FDG-avid tumors was 22.7 cm3 (range 1–519). Univariate analysis identified only active smoking being associated with higher baseline MTV (β coefficient 32.81, 95%CI 4.99–70.62, p = 0.093) and thus, no multivariable analysis was possible for this parameter.
In ROC curve analysis a baseline MTV > 10.70 cm3 was identified as the optimal cutoff to predict cT3/4 status (sensitivity 83.1%; specificity 75%, AUC 0.799, 95%CI 0.640–0.959, p = 0.01) (Fig. 1c).
Prognostic value of 18F- FDG PET/CT -derived parameters for recurrence and patient survival
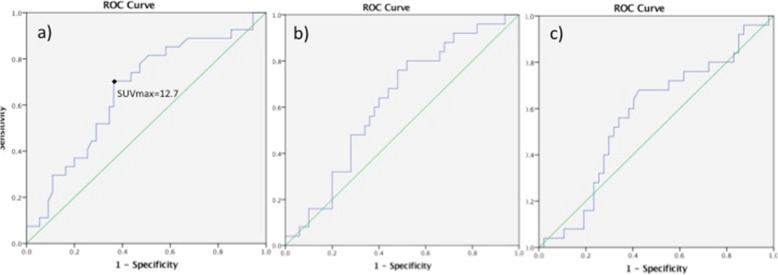
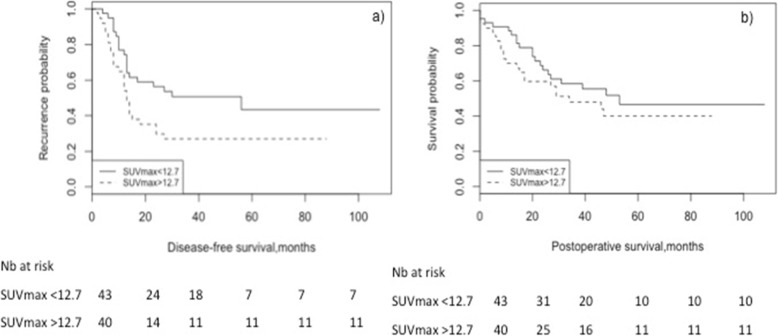
Among the three parameters studied, SUVmax at baseline was the only one with a significant predictive value for early tumor recurrence, within the 1st postoperative year (Fig. 2). A SUVmax > 12.7 g/mL predicted early recurrence with 70.4% sensitivity and 64.6% specificity (AUC 0.660, 95% CI 0.535–0.785, p = 0.019) (Fig. 2a). Patients with a SUVmax ≤ 12.7 g/mL at baseline had a median disease-free survival (DFS) of 56 months (95%CI 7.7–104), versus 13 (95%CI 10.4–15.7) for those with a SUVmax > 12.7 g/mL (p = 0.030) (Fig. 3). Cox regression confirmed SUVmax > 12.7 g/mL as an independent predictor of DFS (HR 2.54, 95%CI 1.26, 5.09, p = 0.009), along with preoperative active smoking and pT3/4 status (Table 4).
Fig. 2.
ROC curve analyses for 18FDG-PET/CT derived parameters as predictors of early tumor recurrence. a A SUVmax > 12.7 g/mL was identified as the optimal threshold for early recurrence, with a sensitivity of 70.4%, specificity 63.6% (AUC 0.660, p = 0.019). No optimal cutoff was defined for b TLG (AUC 0.624, 95% CI 0.495–0.753, p = 0.081) or c MTV (AUC 0.570, 95%CI 0.431–0.709, p = 0.332)
Fig. 3.
Baseline SUVmax as a predictor of disease-free and overall postoperative survival. a SUVmax > 12.7 g/mL was a significant predictor of poor disease-free survival (median DFS 13 versus 56 months, p = 0.030), b but not of overall survival
Table 4.
Cox regression analysis for disease-free survival (DFS)
| Variable | Unadjusted HR | 95%CI | P-value | Adjusted HR | 95%CI | P-value |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Active smoking | 2.22 | 1.23,4.01 | 0.008 | 2.22 | 1.15,4.27 | 0.017 |
| SUVmax> 12.7 g/mL | 2.10 | 1.16,3.81 | 0.014 | 2.54 | 1.26,5.09 | 0.009 |
| pT stage | ||||||
| pT0 | Ref | Ref | ||||
| pT1 | 0.30 | 0.06,1.45 | 0.135 | 0.59 | 0.11,3.04 | 0.532 |
| pT2 | 1.32 | 0.49,3.57 | 0.577 | 1.55 | 0.56,4.28 | 0.393 |
| pT3 | 2.78 | 1.18,6.55 | 0.019 | 3.31 | 1.37,8.00 | 0.008 |
| pT4 | 11.93 | 2.25,63.29 | 0.004 | 7.24 | 1.34,39.22 | 0.022 |
| Resection margins | ||||||
| R0 | Ref | |||||
| R+ | 2.47 | 1.04,5.89 | 0.041 | 2.09 | 0.77,5.68 | 0.147 |
| Adenocarcinoma | ||||||
| Active smoking | 2.34 | 1.04–5.28 | 0.039 | 2.54 | 1.05–6.14 | 0.039 |
| SUVmax> 12.7 g/mL | 1.87 | 0.85–4.11 | 0.119 | 1.54 | 0.65–3.62 | 0.323 |
| pN stage | ||||||
| pN0 | Ref | Ref | ||||
| pN1 | 5.76 | 2.06–16.09 | < 0.001 | 4.04 | 1.29–12.58 | 0.016 |
| pN2 | 5.24 | 1.78–15.49 | 0.003 | 3.88 | 1.25–12.02 | 0.019 |
| pN3 | 9.31 | 1.82–47.49 | 0.007 | 8.67 | 1.50–50.07 | 0.016 |
| Resection margins | ||||||
| R0 | Ref | |||||
| R+ | 4.37 | 1.44–13.25 | 0.009 | 2.78 | 0.78–9.94 | 0.115 |
| Squamous cell cancer | ||||||
| cT stage | ||||||
| cT1–2 | Ref | Ref | ||||
| cT3–4 | 2.74 | 0.92–8.10 | 0.069 | 0.96 | 0.19–4.78 | 0.961 |
| SUVmax> 12.7 g/mL | 1.82 | 0.76–4.36 | 0.176 | 5.06 | 1.44–17.71 | 0.011 |
| pT stage | ||||||
| pT0 | Ref | Ref | ||||
| pT1 | 0.84 | 0.16–4.33 | 0.833 | 1.23 | 0.21–7.17 | 0.816 |
| pT2 | 1.95 | 0.56–6.76 | 0.290 | 5.67 | 1.34–24.01 | 0.018 |
| pT3 | 5.97 | 1.95–18.29 | 0.002 | 5.05 | 1.10–23.16 | 0.037 |
| pT4 | 11.75 | 1.17–118.29 | 0.037 | 10.67 | 0.93–122.39 | 0.057 |
| pN stage | ||||||
| pN0 | Ref | Ref | ||||
| pN1 | 0.22 | 0.03–1.67 | 0.144 | 0.09 | 0.01–0.92 | 0.042 |
| pN2 | 4.51 | 1.42–14.31 | 0.010 | 4.71 | 1.00–22.14 | 0.049 |
| pN3 | 4.39 | 0.95–20.32 | 0.058 | 6.72 | 0.90–49.88 | 0.062 |
HR Hazard ratio, 95%CI 95% confidence intervals, Ref Reference category, SUVmax Maximal standardized uptake value
When the two histological subtypes were analyzed separately, there was no significant association of SUVmax with DFS for adenocarcinoma. For squamous cell carcinoma, a baseline SUVmax > 12.7 g/mL along with pT and pN stage independently predicted worse DFS (Table 4). The distinct metabolic profile of the two histological types is illustrated in Fig. 4.
Fig. 4.
Axial and coronal 18−FDG PET/CT fusion images of two distinct esophageal malignancies with different metabolic profiles. Illustration of the distinct metabolic profile a cT1N0 adenocarcinoma of the gastroesophageal junction (Panels a and b, SUVmax = 5.5, TLG = 5) versus a cT3N+ squamous cell middle third lesion (Panels c and d, SUVmax = 12.8, TLG = 311.5). The white arrows show the primary tumor hypercaptation in each image
No association was found between baseline SUVmax and overall survival (OS), neither on the Kaplan-Meier (Fig. 3) nor the Cox regression analysis. In the latter, only active smoking (HR 2.30, 95% CI 1.15–4.62, p = 0.019) and pT4 stage (HR 21.42, 95%CI 5.00–91.72, p < 0.0001) independently predicted OS. None of the variables remained independent predictors of OS in adenocarcinoma or squamous cell subtypes.
Median follow-up of all patients, calculated with the reverse Kaplan-Meier method, was 50 months (95%CI 45.33–54.66).
Discussion
In this study, higher baseline SUVmax of esophageal cancer was significantly related to a middle-third tumor location and a cT3/4 stage, whereas higher TLG was related to cT3/4 and cN+ stage. Baseline SUVmax > 8.25 g/mL, TLG > 41.7 and MTV > 10.7 cm3 were associated with cT/4 stage, whereas SUVmax ≥ 12.7 g/mL predicted early recurrence and poor disease-free survival.
Currently, 18F- FDG PET/CT is mostly used for the detection of distant metastases as it can identify suspicious lesions as small as 1 cm [2, 14, 15]. Walker et al. reported 18F- FDG PET/CT -detected distant lesions precluding curative treatment in 21% of patients [15], even though the specificity of an FDG ‘hot spot’ remains low [16]. Until recently, limited spatial resolution of PET for esophageal wall layers and adjacent structures had restrained this modality as a detector of distant metastases [15]. Recent data, however, reinforce the role of 18F- FDG PET/CT in better defining cTNM stage and the tumor’s biology, the latter being FDG-avid in 84–92% of cases especially if it infiltrates the submucosa [7, 15, 17].
It is generally admitted that all 18F- FDG PET/CT-derived parameters are higher in advanced and aggressive tumors, however no universally accepted cutoffs exist to this day, limiting their cTNM staging value in the individual patient. Malik et al. suggested SUVmax > 4.1 (sensitivity 85%, and specificity 48%) and MTV > 23.4 cm3 (sensitivity 64%, specificity 67%) as optimal thresholds to distinguish cT1/2 from cT3/4 lesions [7]. In our study, SUVmax and TLG presented a higher predictive value than MTV in preoperative staging; ROC curve analysis yielded as significant cutoff values to predict preoperative cT3/4 status with high accuracy a SUVmax > 8.25 g/mL, TLG > 41.7 and MTV > 10.70 cm3. This discrepancy may be explained by the predominance of adenocarcinoma in the Malik study (75% of patients, versus 53% in the present study); adenocarcinoma has being described as less FDG-avid with lower SUVmax values compared to squamous cell cancer, probably in relation to increased expression of the HK-II biomarker [3, 13]. However, as in our study tumor histology was not independently associated with SUVmax (Table 2), no separate cutoffs of SUVmax were justified for each type.
One might argue that EUS is sufficient to identify locally advanced lesions (cT3/4 or N+) and thus to direct the patient to neoadjuvant treatment before surgery. However, previous data from our institution suggest a rather low rate of accurate usT (51%) and usN (72%) staging, with the highest rates of understaging among active smokers [18]. Indeed, a three-modality workup strategy (18F- FDG PET/CT, CT and EUS) offers the highest probability (84%) to correctly select patients for surgery, a fortiori when 18F- FDG PET/CT is the first exam performed [3]. Complementary use of these exams could improve staging accuracy, directing patients with locally advanced lesions to neoadjuvant treatment and avoiding its unnecessary toxicity for early-stage tumors.
Although several studies have reported poor long-term prognosis associated with high baseline SUVmax its prognostic value for the individual patient remains limited, as great variability is seen in the suggested cutoffs (ranging from 3 to 9 g/mL) [16, 19–22]. This variability might be linked to patient-related factors (e.g. cardiac output, tumor histology) but also to the PET/CT configuration and interobserver variability. To overcome these limitations, tumor-to-blood Standard Uptake Ratio (SUR) has been recently published as a promising predictor of survival [23, 24]. Hofheinz et al. [23] suggest the superiority of baseline SUR over SUVmax to predict overall survival in squamous cell cancer patients treated with definitive chemoradiation, whereas Bütof et al. [24] propose the restaging SUR value along with baseline MTV as a reliable survival predictor. In a recent meta-analysis, Han et al. reinforce the prognostic value of MTV and TLG for overall survival, although no specific cutoff value is suggested and both histological types are jointly taken into account [8]. In the present study none of the 18F- FDG PET/CT derived parameters demonstrated significant association with overall survival.
The added value of our study lies in the identification of SUVmax > 12.7 g/mL as an independent predictor of early tumor recurrence, within the 1st postoperative year. This result is of major clinical importance, as it might help early identification of patients with resectable esophageal cancer, who may not benefit from surgical resection as their risk of short-term recurrence is significantly increased. Indeed, we observed a significantly shorter DFS for squamous cell tumors with a baseline SUVmax > 12.7 g/mL; as definitive chemoradiation is a valid treatment option for this histological type, the above threshold may provide valuable prognostic information for the individual patient and guide accordingly therapeutic management. Schreurs et al. previously reported worse DFS for patients with baseline SUVmax > 3.67 g/mL, although no correlation with overall survival was found in that study either [3]. Markers of aggressive biology (GLUT-1, p53, Ki-67, HK-II) were studied in relation to SUVmax, although no clear immunohistochemical profile was found for high-FDG uptake tumors compared to the others [3]. Thus, even though it is generally admitted that high baseline SUVmax may herald tumor aggressiveness and early recurrence, the underlying mechanism remains poorly understood. Our team previously reported active smoking as an independent predictor of early recurrence after esophagectomy [25], which is being confirmed in the present analysis along with a baseline SUVmax > 12.7 g/mL, and may express pathologic DNA-methylation patterns and tumor proliferation genes [26].
This study has some limitations that need to be addressed. Retrospective analysis has an inherent drawback in data completeness, even though our institutional database is maintained prospectively, with a stringent follow-up of all patients. The use of two different PET/CT scanners over the years might have introduce some bias in SUVmax measurements especially in the smaller-volume lesions, because of a better resolution recovery in the newer PET/CT scanner (from September 2011 and thereafter). However, both scanners measured similar SUV for lesions above 1.2 cm diameter (about 4 cm3). Since the mean MTV in our series was well above this threshold (48 cm3 ± 83 cm3), it can be estimated that this scanner change had little effect on the results. Moreover, we compared the SUVmax across both scanners in lesions with a MTV ≥30 cm3 (where SUVmax would be similar with both scanners) vs. lesions < 30 cm3, where the new scanner could measure SUVmax better. No differences were observed across scanners in the large lesions (MTV ≥ 30 cm3: SUVmax 20.6 ± 7.8 g/mL for the new scanner vs. 17.3 ± 12.0 g/mL for the previous one, p = 0.36). This was also the case for the smaller lesions (MTV < 30 cm3: SUVmax 9.9 ± 4.9 g/mL for the new scanner vs. 11.9 ± 4.4 g/mL for the previous one, p = 0.17). Results of this comparative analysis between the two CT scans are provided as supplementary material, and enhance our belief that the scanner change could not influence significantly our results.
Although there was practically no heterogeneity in 18F- FDG PET/CT results over the years, inaccuracies in the preoperative workup may have occurred for both T and N staging especially with EUS, confounding associations with 18F- FDG PET/CT parameters. As mentioned above, histological type and patient-related factors may influence 18F- FDG PET/CT-derived parameters and limit the use of universally accepted cutoffs. In the present study, due to the small number of patients per histological type our subgroup analyses can be considered as exploratory, needing validation in larger cohorts. Prospective validation of the suggested cutoffs should take into account the specificities of histological types studied.
Conclusions
18F- FDG PET/CT derived parameters SUVmax > 8.25 g/mL, TLG > 41.7 g and MTV > 10.70 cm3 were significantly associated with locally advanced cT3/4 stage and a baseline SUVmax > 12.7 g/mL with early tumor recurrence and poor disease-free survival, particularly for squamous cell cancer. These findings add to the existing knowledge of 18F- FDG PET/CT’s value in esophageal cancer management and reinforce its predictive value in terms of tumor recurrence and survival. A prospective study is currently running in our institution to correlate these values with high-resolution CT and MR imaging characteristics of the primary tumor.
Supplementary information
Additional file 1. Comparison of the SUVmax values for large (MTV>=30cm3) and small (MTV<30cm3) tumors, for the two CT scanners used in the study.
Acknowledgements
The authors would like to thank Dr. Penelope St-Amour for English language editing.
Abbreviations
- 18F- FDG PET/CT
18F- Fluorodeoxyglucose Positron Emission Tomography/Computerized Tomography
- 5-FU
5 Fluoro-Uracil
- 95%CI
95% Confidence Intervals
- AIC
Akaike Information Criterion
- ASA
American Society of Anesthesiologists
- AUC
Area under the curve
- CT
Computerized Tomography
- DFS
Disease-free survival
- EANM
European Association of Nuclear Medicine
- EARL
EANM Research Limited
- EUS
Endoscopic ultrasonography
- GEJ
Gastroesophageal junction
- HR
Hazard ratio
- MTV
Metabolic Tumor Volume
- OS
Overall survival
- ROC
Receiver Operator Characteristic
- SUR
Standard Uptake Ratio
- SUVmax
Maximal Standardized Uptake Value
- TLG
Total Lesion Glycolysis
Authors’ contributions
SM and AP study design, data collection, PET/CT interpretation, statistical analysis and drafting of the manuscript. MW, PA and ND drafting and clinical review of the manuscript. JP study design, PET/CT interpretation, drafting manuscript and critical review of the manuscript. MS study design, statistical analysis, drafting and critical review of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The data that support the findings of this study are available from authors upon request and with permission of the Lausanne University Ethics Committee.
Ethics approval and consent to participate
The study was approved by the Lausanne University Institutional Review Board. On the basis of the general research contentment form, the need for individual consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Styliani Mantziari and Anastasia Pomoni contributed equally to this work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12880-019-0401-x.
References
- 1.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2016;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 2.Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v50–v57. doi: 10.1093/annonc/mdw329. [DOI] [PubMed] [Google Scholar]
- 3.Schreurs LMA, Janssens ACJW, Groen H, et al. Value of EUS in determining curative resectability in reference to CT and FDG-PET: the optimal sequence in preoperative staging of esophageal cancer? Ann Surg Oncol. 2016;23:1021–1028. doi: 10.1245/s10434-011-1738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroese TE, Goense L, van Hillegersberg R, et al. Detection of distant interval metastases after neoadjuvant therapy for esophageal cancer with 18F-FDG PET(/CT): a systematic review and meta-analysis. Dis Esophagus. 2018;31(12). 10.1093/dote/doy055. [DOI] [PubMed]
- 5.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8(9):797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 6.Pasquali S, Yim G, Vohra RS, et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectable esophageal carcinoma: a network meta-analysis. Ann Surg. 2017;265(3):481–491. doi: 10.1097/SLA.0000000000001905. [DOI] [PubMed] [Google Scholar]
- 7.Malik V, Johnston C, O’Toole D, et al. Metabolic tumor volume provides complementary prognostic information to EUS staging in esophageal and junctional cancer. Dis Esophagus. 2017;30(3):1–8. doi: 10.1111/dote.12505. [DOI] [PubMed] [Google Scholar]
- 8.Han S, Kim YJ, Woo S, et al. Prognostic value of volumetric parameters of pretreatment 18F-FDG PET/CT in esophageal cancer: a systematic review and meta-analysis. Clin Nucl Med. 2018;43(12):887–894. doi: 10.1097/RLU.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Gospodarowicz MK, Wittekind C. Oesophagus including oesophagogastric junction malignant tumors. In: TNM UICC classification of malignant tumours. 7th ed: Wiley-Blackwell Editions; 2011. p. 66–72.
- 10.Allemann P, Mantziari S, Wagner D, et al. Curative treatment for esophageal cancer. Results of a multidisciplinary consensus; traitement curatif du cancer de l’oesophage: consensus multidisciplinaire multicentrique. Rev Med Suisse. 2016;12(523):1165–1169. [PubMed] [Google Scholar]
- 11.Gnesin S, Deshayes E, Camus F, et al. Quantification and monitoring of PET/CT data in multicentre trials: the Swiss SAKK 56/07 trial experience. Médecine Nucléaire. 2017;41:259–266. doi: 10.1016/j.mednuc.2017.06.004. [DOI] [Google Scholar]
- 12.Xu W, Yu S, Ma Y, et al. No Effect of different segmentation algorithms on metabolic tumor volume measured on 18F-FDG PET/CT of cervical primary squamous cell carcinoma. Nucl Med Commun. 2017;38(3):259–265. doi: 10.1097/MNM.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imperiale A, Cimarelli S, Brigand C, et al. Does the association of 18F-FDG uptake intensity and lesion topography reveal histological phenotype and tumor differentiation in esophageal cancer? Hell J Nucl Med. 2011;14(3):239–242. [PubMed] [Google Scholar]
- 14.van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98(3):547–557. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker A, Spier B, Perlman S, et al. Integrated PET/CT fusion imaging and endoscopic ultrasound in the pre-operative staging and evaluation of esophageal cancer. Mol Imaging Biol. 2011;13(1):166–171. doi: 10.1007/s11307-010-0306-0. [DOI] [PubMed] [Google Scholar]
- 16.Malik V, Johnston C, Donohoe C, et al. (18)F-FDG PET-detected synchronous primary neoplasms in the staging of esophageal cancer: incidence, cost, and impact on management. Clin Nucl Med. 2012;37(12):1152–1158. doi: 10.1097/RLU.0b013e31827083ba. [DOI] [PubMed] [Google Scholar]
- 17.Kato H, Nakajima M, Sohda M, et al. The clinical application of 18F-fluorodeoxyglucose positron emission tomography to predict survival in patients with operable esophageal cancer. Cancer. 2009;115(14):3196–3203. doi: 10.1002/cncr.24399. [DOI] [PubMed] [Google Scholar]
- 18.Winiker M, Mantziari S, Figueiredo SG, et al. Accuracy of preoperative staging for a priori resectable esophageal cancer. Dis Esophagus. 2018;31(1):1–6. doi: 10.1093/dote/dox113. [DOI] [PubMed] [Google Scholar]
- 19.Omloo JMT, Sloof GW, Boellaard R, et al. Importance of fluorodeoxyglucose-positron emission tomography (FDG-PET) and endoscopic ultrasonography parameters in predicting survival following surgery for esophageal cancer. Endoscopy. 2008;40(6):464–471. doi: 10.1055/s-2008-1077302. [DOI] [PubMed] [Google Scholar]
- 20.Cerfolio RJ, Bryant AS. Maximum standardized uptake values on positron emission tomography of esophageal cancer predicts stage, tumor biology, and survival. Ann Thorac Surg. 2006;82(2):391–395. doi: 10.1016/j.athoracsur.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 21.Rizk N, Downey RJ, Akhurst T, et al. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. Ann Thorac Surg. 2006;81(3):1076–1081. doi: 10.1016/j.athoracsur.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 22.Hatt M, Visvikis D, Albarghach NM, et al. Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumour delineation methodology. Eur J Nucl Med Mol Imaging. 2011;38(7):1191–1202. doi: 10.1007/s00259-011-1755-7. [DOI] [PubMed] [Google Scholar]
- 23.Hofheinz F, Li Y, Steffen IG, et al. Confirmation of the prognostic value of pretherapeutic tumor SUR and MTV in patients with esophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2019;46(7):1485–1494. doi: 10.1007/s00259-019-04307-6. [DOI] [PubMed] [Google Scholar]
- 24.Bütof Rebecca, Hofheinz Frank, Zöphel Klaus, Schmollack Julia, Jentsch Christina, Zschaeck Sebastian, Kotzerke Jörg, van den Hoff Jörg, Baumann Michael. Prognostic Value of Standardized Uptake Ratio in Patients with Trimodality Treatment of Locally Advanced Esophageal Carcinoma. Journal of Nuclear Medicine. 2018;60(2):192–198. doi: 10.2967/jnumed.117.207670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantziari S, Allemann P, Winiker M, Demartines N, Schäfer M. Locoregional tumor extension and preoperative smoking are significant risk factors for early recurrence after esophagectomy for cancer. World J Surg. 2018;42(7):2209–2217. doi: 10.1007/s00268-017-4422-8. [DOI] [PubMed] [Google Scholar]
- 26.Kaz AM, Grady WM, Stachler MD, Bass AJ. Genetic and epigenetic alterations in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am. 2015;44(2):473–489. doi: 10.1016/j.gtc.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Comparison of the SUVmax values for large (MTV>=30cm3) and small (MTV<30cm3) tumors, for the two CT scanners used in the study.
Data Availability Statement
The data that support the findings of this study are available from authors upon request and with permission of the Lausanne University Ethics Committee.