Abstract
As a common kind of food, pepper is well known for its special effects on the physiological state of human individuals. Capsaicin, the main component of pepper, is speculated to be linked with intestinal microorganisms on account of their direct contact. Herein, we first utilized mouse models and 16S rRNA high-throughput sequencing to compare the differences in intestinal flora between mouse groups with and without capsaicin treatment by gavage. The mice in the two groups showed significantly distinct performance in terms of body weight, leukocyte count, fecal humidity, and constituent ratios of intestinal bacteria, such as Faecalibacterium, Akkermansia, Roseburia, Helicobacter, and Bacteroides species. In particular, the Faecalibacterium abundance was the most highly variable among the 5 bacterial genera. Based on statistical analysis and comparison, the variation tendency of body weight, leukocyte count, and fecal humidity was closely related to the bacteria. In conclusion, capsaicin could affect the physiological state of mice by changing the constitution of the intestinal flora.
Introduction
As the intestinal flora plays a key role in the process of human immunity, nutrition, and metabolism, the intestinal flora is considered an “organ” equivalent in the human body and has attracted much attention in recent years. The human intestine provides a good habitat for microorganisms, harboring more than 1000 kinds of symbiotic microorganisms. The number of microorganisms in the adult intestine is not only much larger than that of microorganisms on the body surface but also 10 times the number of human cells.1 Many studies have verified that the intestinal flora is closely related to the health of the host, which affects the metabolic phenotype2−4 as well as the immunoregulation.5,6 Diet stands out among all the factors contributing to the adjustment of the intestinal microbial structure. The intestinal microecology was directly and dynamically influenced by different dietary species and quantities.7 The metabolites generated from undigested food by intestinal microorganisms own a potential impact on human health, which acts as a key bridge between diet and human health.8
The balance of the intestinal flora is closely related to human health. Lu et al.9,10 and Qin et al.11 found intestinal flora imbalance in H7N9 avian influenza patients. Qin et al.12 used metagenomic technology and found that type II diabetes has symptoms of moderate intestinal microecological imbalance, decreased abundance of butyric acid bacteria and increased abundance of opportunistic pathogens. It is also noted that 23 strains may serve as biomarkers to distinguish between type II diabetes patients and healthy people. Delzenne et al.13 found that intestinal barrier function damage is one of the causes of obesity and type II diabetes.14 In 2009, the Zhao Liping Laboratory studied the effects of a high-fat diet and a normal diet on intestinal microorganisms and metabolism in normal mice and gene knockout mice. After sequencing the DNA of the intestinal flora by a high-throughput sequencing technique, it was found that the phylogenetic relationship of the intestinal flora community was basically consistent with that reflected by full-length 16S rRNA gene analysis.15,16 A study in 2014 by Duca et al.17 found that obese mice had a higher proportion of thick-walled bacteria/Bacteroides species than obesity-resistant mice under the same diet and that there were three unique “fat bacteria”, Oscillibacter and Clostridium clusters XIVa and IV, in obese mice. When transplanted into sterile mice, the intestinal flora of obese mice was able to successfully replicate the obesity phenotypes. In 2016, Zhu et al.18 found that intestinal microorganisms directly promote platelet hypersensitivity by producing trimethylamine N-oxide, which increases the risk of thrombosis. In addition, intestinal microorganisms are also associated with common human diseases, such as liver disease,19−21 intestinal diseases,22,23 and nervous system diseases.24,25
Pepper is more and more popular in our daily diet in these years. Following different cultures and climates, people in different regions possess different eating habits for pepper. It is observed that pepper consumption frequently worsens the physiological states of human individuals, such as sore throat, mouth ulcer, constipation, acne, and so on, which were summarized as “getting inflamed”. Nevertheless, relevant research studies and mechanisms have not yet been clearly illuminated. In view of the facts that the close relationship among intestinal flora, human health, and pepper, it is of significance to explore the series of influence brought by pepper consumption.
In this study, we first used the mouse intestine to simulate the human intestine to study the effects of dietary habits associated with pepper on the intestinal flora. Through 16S rRNA high-throughput sequencing, we compared the differences in intestinal flora between mouse groups with and without pepper consumption. The mice in the two groups showed significantly distinct performance in terms of body weight, leukocyte count, fecal humidity, and constituent ratios of intestinal bacteria. Furthermore, the variation tendency of body weight, leukocyte count, and fecal humidity were closely related to the intestinal flora. In conclusion, capsaicin could affect the physiological state of mice by changing the constitution of the intestinal flora. Our study not only provided insight into the relationship between pepper and the intestinal flora but also provided a significant foundation for research on the effects of a variety of foods on human health.
Results and Discussion
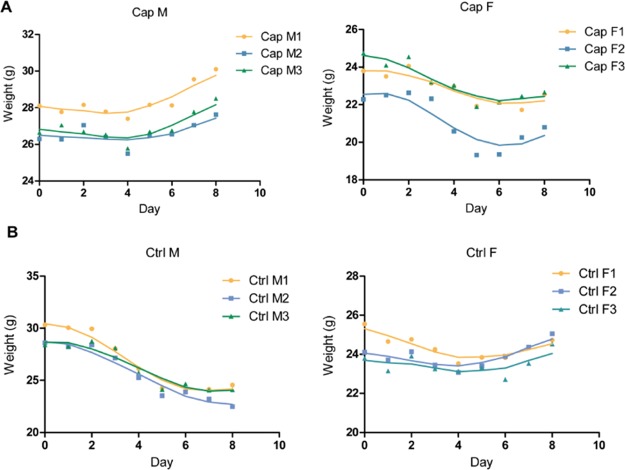
Effects of capsaicin on mice body weight. As shown in Figure 1, after gastric perfusion, the average body weight of Cap M raised 9.6% from day 4, while those in the control group declined 18.3% after day 2. Compared to male mice, the body weight of female mice changed slightly. For female mice, the body weight of Cap F slightly decreased, but Ctrl F did not change significantly after gavage.
Figure 1.
Body weight changes of mice. (A) Capsaicin-treated mice were continuously given gastric perfusion of capsaicin solution for one week, and their body weight was measured daily. (B) Control mice were given the same volume of soybean oil for one week and weighed daily.
Effects of capsaicin on blood leukocyte content in mice. From the variation tendency on day 2 in Figure 2, the number of blood leukocytes among all the 4 groups of mice decreased consistently than day 0. For the male mice, the leukocyte counts of the capsaicin-treated ones began to increase to a maximum after day 2, and the controlled ones increased after day 4 and decreased after day 6. For the female mice, the leukocyte numbers in the two groups fell between day 0 and day 2 and increased between day 2 and day 8. O’Keefe et al.23 reported that gastric perfusion of capsaicin could enhance short-term hematopoietic function. Therefore, gastric perfusion of capsaicin might be the cause of the upward trend in the leukocyte content from day 2.
Figure 2.
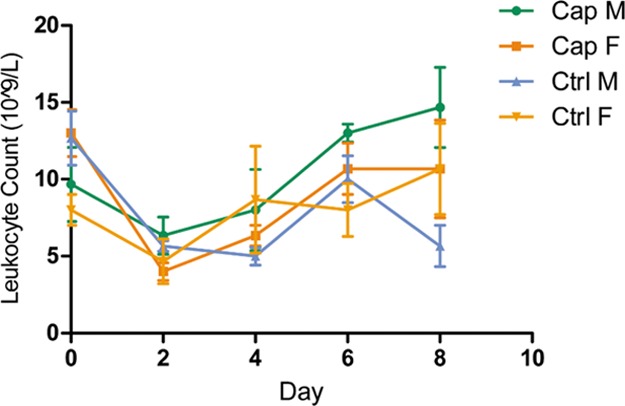
Blood leukocyte count changes of mice. Blood leukocytes on days 0, 2, 4, 6, and 8 in each mouse were counted, and the average of each group along with the error bars is plotted on the graph.
Effects of capsaicin on fecal water content in mice. In regard to male mice, the fecal moisture content of Cap M increased from 62.4 to 62.9%, while Ctrl M declined from 63.9 to 51.1% after 2 days (Figure 3). As for female mice, the fecal moisture content of Cap F fluctuated in a range from 54.2 to 67.4%. Besides, the moisture content range of Ctrl F was relatively small, ranging from 55.0 to 65.0%.
Figure 3.
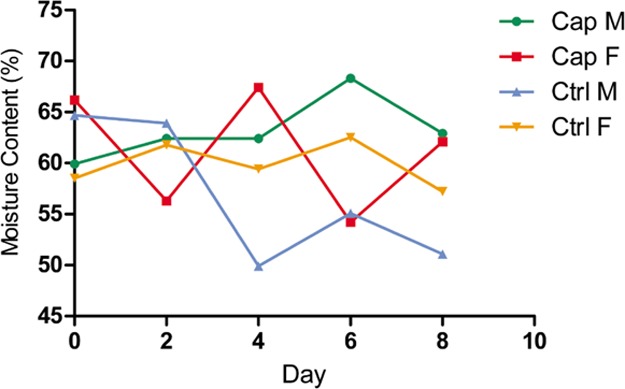
Content change of water in mouse feces. The fecal moisture content on days 0, 2, 4, 6, and 8 of each group of mice was calculated and plotted to create a line graph.
A preliminary speculation observed from Cap M is that capsaicin may stimulate water intake, while soybean oil may inhibit the desire to drink in the control group. Consequently, the moisture content in the capsaicin group was higher than that in the control group. There exists the possibility that capsaicin may have practical influence on the original intestinal microflora of the mice. The demands for water consumption after the flora variation differ as well, resulting in a noteworthy difference in the fecal moisture content. The fecal moisture content affects the physical properties of the feces, such as softness and dryness. It is recognized that dry and hard feces easily lead to rectum and anus damage because of friction, which would cause a series of health problems further, such as constipation, hemorrhoids, and even intestinal cancer.
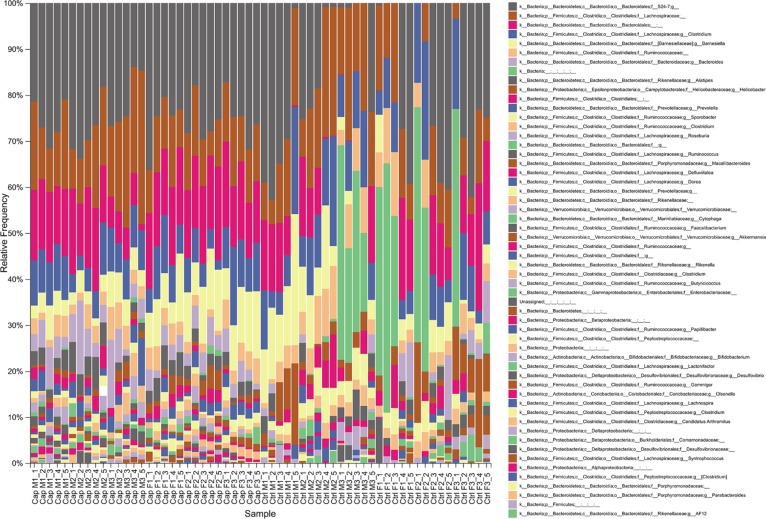
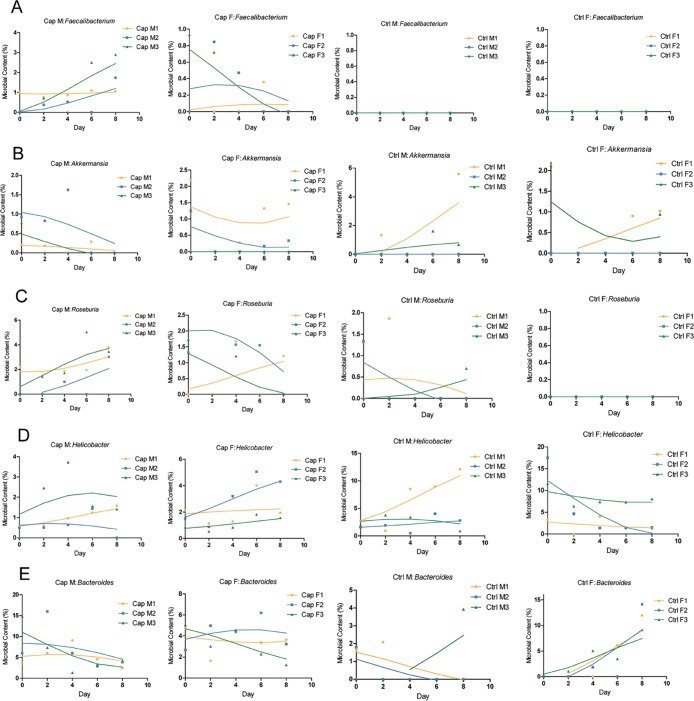
Effects of capsaicin on the intestinal flora in mice. Figure 4 reflected the relative abundance of the intestinal flora constituents of each mouse at the genus level basing on high-throughput sequencing. The composition of intestinal microbes in each mouse experiment was visually depicted. From the fecal metagenome analysis of the capsaicin-treated ones, it was concluded that several kinds of bacteria, such as Faecalibacterium and Akkermansia species, owned obvious variation trends at the genus level. It is worth noting that, Faecalibacterium and Akkermansia are reported as immune regulation and body weight control related factors to human health.26−32
Figure 4.
Intestinal flora sequencing results (genus level). Mice feces were collected during the experiment, and the relative frequency of the intestinal flora components was subsequently obtained by high-throughput sequencing. The relative frequency of intestinal flora constituents at the genus level in mice fecal samples collected on different dates is shown.
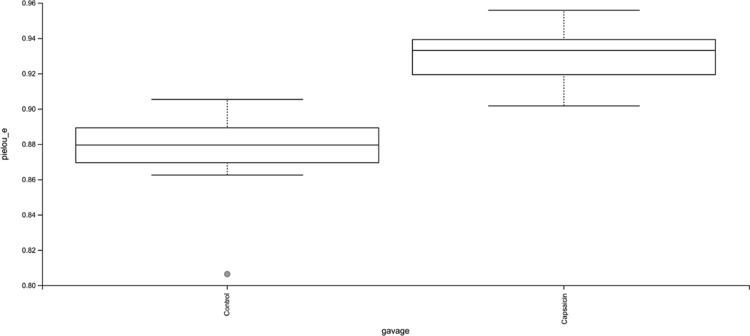
According to the analysis of the intestinal flora uniformity (Figure 5), the evenness of the capsaicin group was significantly higher than that of the control group (P < 0.001). It can be concluded that capsaicin affects the number of intestinal flora constituents in mice.
Figure 5.
Effects of capsaicin on the evenness of the intestinal microbiota in mice. Box plot showed the difference in Pielou’s evenness index of the mouse intestinal flora between the capsaicin group and the control group.
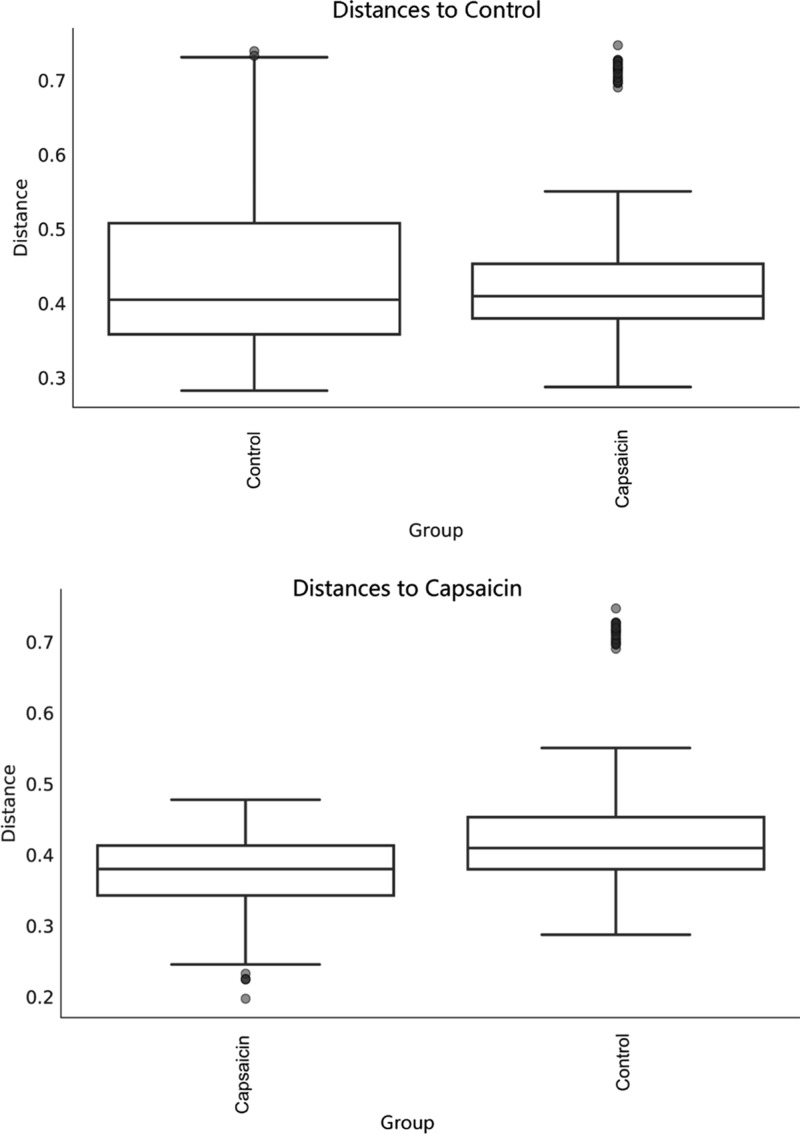
According to Figure 6, the intestinal microflora difference among the mice in the capsaicin group was significantly smaller than that in the control group (P = 0.002). In combination with Figure 5, administration of capsaicin via gavage changed the structure of the intestinal flora of mice and made it more evenly distributed.
Figure 6.
Differences in the intestinal microflora between mice in each group based on unweighted UniFrac distance.
Faecalibacterium prausnitzii, the only known species of Faecalibacterium, is a Gram-positive bacterium and is one of the most highly enriched and most important symbiotic bacteria in the human intestinal microbiome.33,34 This species boosts the immune system26 and is associated with Crohn’s disease, obesity, asthma, and major depressive disorder.27,28,35,36 As shown in Figure 7A, Faecalibacterium appeared in the capsaicin group but not the control group, suggesting that capsaicin was an important factor for Faecalibacterium existence in the intestinal flora of the capsaicin group mice. It can be seen from Figure 7A that in the capsaicin group, the changes in abundance of Faecalibacterium in the intestines of male and female mice were different. The Faecalibacterium content in the male mice increased gradually with gastric perfusion of the capsaicin solution, while that in the female mice showed a tendency to increase first and then disappear. The abovementioned changes reminded us that the effects of capsaicin on the intestinal Faecalibacterium content in mice was sex sensitive.
Figure 7.
Changes in intestinal flora composition. (A–E) Faecalibacterium, Akkermansia, Roseburia, Helicobacter, and Bacteroides were screened according to the sequencing results, and the curve was fitted according to their relative levels in the intestinal flora of each mouse.
Figures 1A and 7A indicated that the changing trend of mice body weight in the capsaicin group was consistent with the content of the intestinal Faecalibacterium. It is a remarkable fact that Faecalibacterium is obesity related.27,28 Hence, it could be speculated that capsaicin might play a particular role in the body weight of male mice. Moreover, the Faecalibacterium content increased with gastric perfusion of capsaicin, which further led to body weight gain in male mice. Basing on above findings, it is worth conducting additional research studies focusing on the relationship between Faecalibacterium and capsaicin with larger samples. In the near future, it is also significant and necessary to examine the body weight variation tendency with Faecalibacterium among human volunteers in response to spicy diet.
Akkermansia muciniphila (A. muciniphila) is currently the only known species in the Akkermansia genus and exists in the human intestine. The results of the International Cooperative Human Twins study showed that decreased A. muciniphila abundance was related to increasing risk of diabetes mellitus and obesity.29 The Akkermansia content of the intestines of mice in the capsaicin group reduced, when the body weight showed an opposite trend (Figure 7B). The Akkermansia content of Cap F decreased first and then increased, and the body weight of these mice exhibited a downward tendency. The flora content of the mice in the control group showed an upward trend, while that of the female mice showed no obvious trend (Figure 7B). It can be concluded that the effect of capsaicin on the Akkermansia content of the intestine in mice is sex sensitive. Based on Figures 1 and 7B, it could be concluded that the Akkermansia content of the intestinal flora and body weight may be inversely proportional to each other. In addition, the changing trend of the Akkermansia content in the intestines of Cap M was similar to that of the blood leukocyte count (Figure 2), which decreased first and then increased. Caesar suggested that the Akkermansia genus could reduce inflammation in the body.37 After gastric perfusion for 2 days, the leukocyte count increased, and the Akkermansia content decreased. After 8 days, the leukocyte count and Akkermansia content both exhibited an upward trend. To sum up, capsaicin might cause inflammation in the body along with an increase in the leukocyte count in mice by affecting the abundance of Akkermansia bacteria in the intestine.
Roseburia species are Gram-positive anaerobic bacteria that produce butyrate and inhabit the human colon. Increasing abundance of Roseburia species is associated with body weight loss and reduced glucose intolerance in mice.30 Capsaicin enhanced the Roseburia content in the intestines of the mice and decreased the intestinal Roseburia content in female mice, indicating that the effect of capsaicin on the Roseburia content in the intestines of the mice was also sex sensitive (Figure 7C). According to Figures 1 and 7C, the Roseburia content of the intestine in mice might be inversely proportional to the mice body weight. In conclusion, capsaicin may have an effect on mice body weight by altering the amount of Roseburia in mice.
Helicobacter pylori is the most widely known species of Helicobacter, which is Gram-negative and infects up to 50% of the population. Some strains of these bacteria are pathogenic to humans because they are closely related to peptic ulcers, chronic gastritis, duodenal inflammation, and gastric cancer.38,39Figure 7D revealed that the Helicobacter content in the capsaicin group was lower than that in the control group, and it was concluded that capsaicin might reduce the Helicobacter content in the intestines of mice to a certain extent. Because Helicobacter has a certain pathogenicity, it is believed that capsaicin may reduce the probability of disease caused by Helicobacter in the body by inhibiting the activity of H. pylori.
Bacteroides is a genus of Gram-negative, obligate anaerobic bacteria that play an important role in the processing of complex molecules into simpler molecules in the host intestine. Previous literature suggested that members of Bacteroides could affect thin or obese phenotypes in humans.31 According to Figure 7E, the Bacteroides content in the intestines of Cap M decreased when compared with that of Ctrl M, while that of the female mice exhibited relatively little change. It could be considered that there was a sex-based difference in the effect of capsaicin on the Bacteroides content. The mice body weight variation of the capsaicin group was different between male and female. Therefore, it was believed that Bacteroides content might be associated with mice body weight.
In this study, we came to the conclusion finally that capsaicin could affect the composition and content of the intestinal flora in mice. There were inconsistencies in the change trends of the intestinal flora between male and female mice, which implied gender sensitivity. Additionally, the changes in the intestinal flora of mice caused by capsaicin might be involved in altering the body weight of mice. After gastric perfusion of capsaicin solution, initially absent Faecalibacterium, recognized as obesity-related bacterium, was detected in the intestines of male and female mice. Furthermore, the body weight of the male mice as well as the content of Faecalibacterium in their intestines increased consistently.
Materials and Methods
Animals
Male and female ICR clean-grade mice, aged 3 weeks were purchased from Wu’s Laboratory Animal (Fuzhou, China), fed with basic feed, and provided free access to drinking water in the test environment for one week to adapt. The Animal Care and Use Committee of the College of Biological Science and Engineering, FuZhou University, approved the experimental protocols in this study.
Pharmacy
To prepare the capsaicin solution, synthesized capsaicin (97% capsaicin, provided by Sinopharm, China) was dissolved in soybean oil (edible grade) to form 0.8 mg/mL capsaicin solution. To prepare the leukocyte diluent, 0.1 mL of 25% glutaraldehyde was added to 100 mL of 12% glacial acetic acid and mixed evenly.40
Animal Treatment
The capsaicin-administered mice were assigned into the control group and capsaicin group (fed with capsaicin-soybean oil solution). In the capsaicin group, 3 male mice were termed Cap M while 3 female mice were termed Cap F. In the control group, 3 male mice were termed Ctrl M and 3 female mice were termed Ctrl F. The capsaicin group was treated with gastric perfusion (capsaicin of 8 mg/kg/d mice body weight) from 16:00 to 17:00 for one week. The control group was fed with the corresponding volume of soybean oil solution at the same time. The body weight data were collected daily, and the blood leukocyte content, fecal water content, and intestinal flora composition of the mice were measured at days 0, 2, 4, 6, and 8.
DNA Isolation, PCR, and 16S rRNA Gene Analysis
DNA extraction of mouse feces was accomplished by using a fecal DNA extraction kit (Weiyin, China), followed by using the bacterial 16S rRNA V3–V4 primers (upstream primer: 5′ CCTAYGGGRBGCAAG 3 ′, downstream primer: 5′ GGACTACNNGGGTATCTAAT 3 ′) to amplify the DNA template. PCR amplification was conducted in a total volume of 10 μL containing 5 μL of 2× Taq PCR Mix (CWbio, China), 0.4 μL of each primer, 0.5 μL of DNA template, and 3.7 μL of ddH2O. PCRs were performed under the following conditions: initial denaturation at 94 °C for 3 min; 30 cycles of 94 °C for 30 s, 53 °C for 30 s, and 72 °C for 30 s; and a final extension at 72 °C for 3 min.
The Vazyme Universal DNA Library Prep Kit for ILLUMINAV2 was used to prepare libraries of the PCR amplification products for next-generation sequencing. The paired-end 250-bp mode of an Illumina HiSeq 2500 sequencer was used. Then, the sequencing results were analyzed using the QIIME2 software package.41
Determination of Immune Indicators
Twenty microliters of blood was collected from each mouse using the tail tip-cutting method and then mixed with 0.38 mL of leukocyte diluent. A small amount of suspension was dripped into the counting pool of a hemocytometer, and then, the number of leukocytes was counted via microscopy.
Determination of Water Content in Feces
A tube of feces was collected from each mouse cage at 9:00 every morning. The net weight of each tube was controlled in the range of 180–210 mg, which was recorded as the wet weight. After weighing, tubes with opened lips were placed in an oven and dried at 80 °C for 24 h. Then, the weight was measured again and recorded as dry weight.
Author Contributions
⊥ F.W., X.H., and Y.C. contributed equally to this work.
This work was financially supported by the National Natural Science Foundation of China (31301537), Young Teachers Education Research Project, Department of Education, Fujian (JAT170080), and the Project of Student Research Training Program of Fuzhou University (24173).
The authors declare no competing financial interest.
Notes
All the sequencing raw data were uploaded to the NCBI-SRA database under the accession number of PRJNA573302.
Notes
The Animal Care and Use Committee of the College of Biological Science and Engineering, Fuzhou University, approved the experimental protocols in this study.
References
- Backhed F.; Ley R. E.; Sonnenburg J. L.; Peterson D. A.; Gordon J. I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Li M.; Wang B.; Zhang M.; Rantalainen M.; Wang S.; Zhou H.; Zhang Y.; Shen J.; Pang X.; Zhang M.; Wei H.; Chen Y.; Lu H.; Zuo J.; Su M.; Qiu Y.; Jia W.; Xiao C.; Smith L. M.; Yang S.; Holmes E.; Tang H.; Zhao G.; Nicholson J. K.; Li L.; Zhao L. Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 2117–2122. 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y.; Wang Q.; Chang R.; Zhou X.; Xu C. Intestinal Barrier Function-Non-alcoholic Fatty Liver Disease Interactions and Possible Role of Gut Microbiota. J. Agric. Food Chem. 2019, 67, 2754–2762. 10.1021/acs.jafc.9b00080. [DOI] [PubMed] [Google Scholar]
- Goodman A. L.; McNulty N. P.; Zhao Y.; Leip D.; Mitra R. D.; Lozupone C. A.; Knight R.; Gordon J. I. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 2009, 6, 279–289. 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. K.; Mazmanian S. K. Has the Microbiota Played a Critical Role in the Evolution of the Adaptive Immune System?. Science 2010, 330, 1768–1773. 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosato T.; Tomida K.; Otani H. Effect ofLactobacillus pentosusONRIC b0240 on Intestinal IgA Production in Mice Fed Differing Levels of Protein. J. Agric. Food Chem. 2011, 59, 2646–2651. 10.1021/jf104240d. [DOI] [PubMed] [Google Scholar]
- Guo W.-L.; Pan Y.-Y.; Li L.; Li T.-T.; Liu B.; Lv X.-C. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018, 9, 3419–3431. 10.1039/c8fo00836a. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Lv Y.; Zhang D.; Liu H.; Dong L.; Ming T.; Su X. In Vivo Effects of Salbutamol Residues on Blood Lipid, Lung Structure, Gene Expression, and Gut Microorganism Composition. ACS Omega 2019, 4, 20644. 10.1021/acsomega.9b02701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.; Zhang C.; Qian G.; Hu X.; Zhang H.; Chen C.; Liang W.; Gao H.; Yang Y.; Li L. An analysis of microbiota-targeted therapies in patients with avian influenza virus subtype H7N9 infection. BMC Infect. Dis. 2014, 14, 359. 10.1186/1471-2334-14-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Yang Y.; Wu Z.; Weng P. The Modulatory Effect of Anthocyanins from Purple Sweet Potato on Human Intestinal Microbiota in Vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. 10.1021/acs.jafc.6b00586. [DOI] [PubMed] [Google Scholar]
- Qin N.; Zheng B. W.; Yao J.; Guo L. H.; Zuo J.; Wu L. J.; Zhou J. W.; Liu L.; Guo J.; Ni S. J.; Li A.; Zhu Y. X.; Liang W. F.; Xiao Y. H.; Ehrlich S. D.; Li L. J. Influence of H7N9 virus infection and associated treatment on human gut microbiota. Sci. Rep. 2015, 5, 14771. 10.1038/srep14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J.; Li Y.; Cai Z.; Li S.; Zhu J.; Zhang F.; Liang S.; Zhang W.; Guan Y.; Shen D.; Peng Y.; Zhang D.; Jie Z.; Wu W.; Qin Y.; Xue W.; Li J.; Han L.; Lu D.; Wu P.; Dai Y.; Sun X.; Li Z.; Tang A.; Zhong S.; Li X.; Chen W.; Xu R.; Wang M.; Feng Q.; Gong M.; Yu J.; Zhang Y.; Zhang M.; Hansen T.; Sanchez G.; Raes J.; Falony G.; Okuda S.; Almeida M.; LeChatelier E.; Renault P.; Pons N.; Batto J.-M.; Zhang Z.; Chen H.; Yang R.; Zheng W.; Li S.; Yang H.; Wang J.; Ehrlich S. D.; Nielsen R.; Pedersen O.; Kristiansen K.; Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Delzenne N. M.; Cani P. D.; Everard A.; Neyrinck A. M.; Bindels L. B. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia 2015, 58, 2206–2217. 10.1007/s00125-015-3712-7. [DOI] [PubMed] [Google Scholar]
- Lasalle M.; Hoguet V.; Hennuyer N.; Leroux F.; Piveteau C.; Belloy L.; Lestavel S.; Vallez E.; Dorchies E.; Duplan I.; Sevin E.; Culot M.; Gosselet F.; Boulahjar R.; Herledan A.; Staels B.; Deprez B.; Tailleux A.; Charton J. Topical Intestinal Aminoimidazole Agonists of G-Protein-Coupled Bile Acid Receptor 1 Promote Glucagon Like Peptide-1 Secretion and Improve Glucose Tolerance. J. Med. Chem. 2017, 60, 4185–4211. 10.1021/acs.jmedchem.6b01873. [DOI] [PubMed] [Google Scholar]
- Ye L.; Yan Y.; Chen Q.; Zhao L.; Ling N.; Pang G., Application of High-throughput Sequencing Technology in Studying Matagenomics of Intestinal Microbiota. J. Chin. Inst. Food Sci. Technol. 2016. [Google Scholar]
- Yu X.; Jiang W.; Shi Y.; Ye H.; Lin J. Applications of sequencing technology in clinical microbial infection. J. Cell Mol. Med. 2019, 23, 7143–7150. 10.1111/jcmm.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca F. A.; Sakar Y.; Lepage P.; Devime F.; Langelier B.; Doré J.; Covasa M. Replication of Obesity and Associated Signaling Pathways Through Transfer of Microbiota From Obese-Prone Rats. Diabetes 2014, 63, 1624–1636. 10.2337/db13-1526. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Gregory J. C.; Org E.; Buffa J. A.; Gupta N.; Wang Z.; Li L.; Fu X.; Wu Y.; Mehrabian M.; Sartor R. B.; McIntyre T. M.; Silverstein R. L.; Tang W. H. W.; DiDonato J. A.; Brown J. M.; Lusis A. J.; Hazen S. L. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L.-X.; Fang D.-Q.; Shi D.; Chen D.-Y.; Yan R.; Zhu Y.-X.; Chen Y.-F.; Shao L.; Guo F.-F.; Wu W.-R.; Li A.; Shi H.-Y.; Jiang X.-W.; Jiang H.-Y.; Xiao Y.-H.; Zheng S.-S.; Li L.-J. Alterations and correlations of the gut microbiome, metabolism and immunity in patients with primary biliary cirrhosis. Environ. Microbiol. 2016, 18, 2272–2286. 10.1111/1462-2920.13401. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Guo J.; Qian G.; Fang D.; Shi D.; Guo L.; Li L. Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J. Gastroenterol. Hepatol. 2015, 30, 1429–1437. 10.1111/jgh.12932. [DOI] [PubMed] [Google Scholar]
- Ma H.; Zhang B.; Hu Y.; Wang J.; Liu J.; Qin R.; Lv S.; Wang S. Correlation Analysis of Intestinal Redox State with the Gut Microbiota Reveals the Positive Intervention of Tea Polyphenols on Hyperlipidemia in High Fat Diet Fed Mice. J. Agric. Food Chem. 2019, 67, 7325–7335. 10.1021/acs.jafc.9b02211. [DOI] [PubMed] [Google Scholar]
- Verma R.; Verma A. K.; Ahuja V.; Paul J. Real-Time Analysis of Mucosal Flora in Patients with Inflammatory Bowel Disease in India. J. Clin. Microbiol. 2010, 48, 4279–4282. 10.1128/jcm.01360-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe S. J. D.; Li J. V.; Lahti L.; Ou J. H.; Carbonero F.; Mohammed K.; Posma J. M.; Kinross J.; Wahl E.; Ruder E.; Vipperla K.; Naidoo V.; Mtshali L.; Tims S.; Puylaert P. G. B.; DeLany J.; Krasinskas A.; Benefiel A. C.; Kaseb H. O.; Newton K.; Nicholson J. K.; de Vos W. M.; Gaskins H. R.; Zoetendal E. G. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 6342. 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.; Wang T.; Jin F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 2016, 59, 1006–1023. 10.1007/s11427-016-5083-9. [DOI] [PubMed] [Google Scholar]
- Hsiao E. Y.; McBride S. W.; Hsien S.; Sharon G.; Hyde E. R.; McCue T.; Codelli J. A.; Chow J.; Reisman S. E.; Petrosino J. F.; Patterson P. H.; Mazmanian S. K. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel S.; Martín R.; Rossi O.; Bermúdez-Humarán L.; Chatel J.; Sokol H.; Thomas M.; Wells J.; Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Balamurugan R.; George G.; Kabeerdoss J.; Hepsiba J.; Chandragunasekaran A. M. S.; Ramakrishna B. S. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br. J. Nutr. 2010, 103, 335–338. 10.1017/s0007114509992182. [DOI] [PubMed] [Google Scholar]
- Furet J.-P.; Ling-Chun K.; Julien T.; Christine P.; Arnaud B.; Jean-Luc B.; Denis M.; Gérard C.; JoëL D.; Corneliu H. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M.; Lim M. Y.; Yun H. S.; Tickle T. L.; Sung J.; Song Y. M.; Lee K.; Franzosa E. A.; Morgan X. C.; Gevers D.; Lander E. S.; Xavier R. J.; Birren B. W.; Ko G.; Huttenhower C. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 2016, 8, 17. 10.1186/s13073-016-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. K.; Tremaroli V.; Clemmensen C.; Kovatcheva-Datchary P.; Myronovych A.; Karns R.; Wilson-Pérez H. E.; Sandoval D. A.; Kohli R.; Bäckhed F. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014, 509, 183. 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura V. K.; Faith J. J.; Rey F. E.; Cheng J. Y.; Duncan A. E.; Kau A. L.; Griffin N. W.; Lombard V.; Henrissat B.; Bain J. R.; Muehlbauer M. J.; Ilkayeva O.; Semenkovich C. F.; Funai K.; Hayashi D. K.; Lyle B. J.; Martini M. C.; Ursell L. K.; Clemente J. C.; Van Treuren W.; Walters W. A.; Knight R.; Newgard C. B.; Heath A. C.; Gordon J. I. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfeng W.; Lin L.; Chenggong Y. The preventive effect of Faecalibacterium prausnitzii combined with Kuikeling on colitis of rats. J. Nanjing Med. Univ. Nat. Sci. 2016, 36, 1079–1084. 10.7655/NYDXBNS20160911. [DOI] [Google Scholar]
- Khan M. T.; Duncan S. H.; Stams A. J. M.; van Dijl J. M.; Flint H. J.; Harmsen H. J. M. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J. 2012, 6, 1578–1585. 10.1038/ismej.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passalacqua L. F. M.; Jimenez R. M.; Fong J. Y.; Lupták A. Allosteric Modulation of the Faecalibacterium prausnitzii Hepatitis Delta Virus-like Ribozyme by Glucosamine 6-Phosphate: The Substrate of the Adjacent Gene Product. Biochemistry 2017, 56, 6006–6014. 10.1021/acs.biochem.7b00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H.; Pigneur B.; Watterlot L.; Lakhdari O.; Bermudez-Humaran L. G.; Gratadoux J.-J.; Blugeon S.; Bridonneau C.; Furet J.-P.; Corthier G.; Grangette C.; Vasquez N.; Pochart P.; Trugnan G.; Thomas G.; Blottiere H. M.; Dore J.; Marteau P.; Seksik P.; Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 16731–16736. 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.; Ling Z.; Zhang Y.; Mao H.; Ma Z.; Yin Y.; Wang W.; Tang W.; Tan Z.; Shi J. fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Caesar R.; Tremaroli V.; Kovatcheva-Datchary P.; Cani P. D.; Bäckhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell D. S. Helicobacter pylori: Molecular Genetics and Cellular Biology. Edited by Yoshio Yamaoka. Norfolk (United Kingdom): Caister Academic Press. $310.00. ix + 261 p.; ill.; index. ISBN: 978-1-904455-31-8. 2008. Q. Rev. Biol. 2010, 85, 110. 10.1086/650262. [DOI] [Google Scholar]
- Joshi S.; Fedoseyenko D.; Mahanta N.; Ducati R. G.; Feng M.; Schramm V. L.; Begley T. P. Antibacterial Strategy against H. pylori: Inhibition of the Radical SAM Enzyme MqnE in Menaquinone Biosynthesis. ACS Med. Chem. Lett. 2019, 10, 363–366. 10.1021/acsmedchemlett.8b00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiazeng L.; Hongli W.; Zhongchao H.. Blood experiment (in Chinese); SHANGHAI SCIENTIFIC & TECHNICAL PUBLISHERS: ShangHai, 1997; p 696. [Google Scholar]
- Bolyen E.; Rideout J. R.; Dillon M. R.; Bokulich N. A.; Abnet C.; Al-Ghalith G. A.; Alexander H.; Alm E. J.; Arumugam M.; Asnicar F.; Bai Y.; Bisanz J. E.; Bittinger K.; Brejnrod A.; Brislawn C. J.; Brown C. T.; Callahan B. J.; Caraballo-Rodríguez A. M.; Chase J.; Cope E.; Da Silva R.; Dorrestein P. C.; Douglas G. M.; Durall D. M.; Duvallet C.; Edwardson C. F.; Ernst M.; Estaki M.; Fouquier J.; Gauglitz J. M.; Gibson D. L.; Gonzalez A.; Gorlick K.; Guo J.; Hillmann B.; Holmes S.; Holste H.; Huttenhower C.; Huttley G.; Janssen S.; Jarmusch A. K.; Jiang L.; Kaehler B.; Kang K. B.; Keefe C. R.; Keim P.; Kelley S. T.; Knights D.; Koester I.; Kosciolek T.; Kreps J.; Langille M. G. I.; Lee J.; Ley R.; Liu Y.-X.; Loftfield E.; Lozupone C.; Maher M.; Marotz C.; Martin B. D.; McDonald D.; McIver L. J.; Melnik A. V.; Metcalf J. L.; Morgan S. C.; Morton J.; Naimey A. T.; Navas-Molina J. A.; Nothias L. F.; Orchanian S. B.; Pearson T.; Peoples S. L.; Petras D.; Preuss M. L.; Pruesse E.; Rasmussen L. B.; Rivers A.; Robeson I. I. M. S.; Rosenthal P.; Segata N.; Shaffer M.; Shiffer A.; Sinha R.; Song S. J.; Spear J. R.; Swafford A. D.; Thompson L. R.; Torres P. J.; Trinh P.; Tripathi A.; Turnbaugh P. J.; Ul-Hasan S.; van der Hooft J. J. J.; Vargas F.; Vázquez-Baeza Y.; Vogtmann E.; von Hippel M.; Walters W.; Wan Y.; Wang M.; Warren J.; Weber K. C.; Williamson C. H. D.; Willis A. D.; Xu Z. Z.; Zaneveld J. R.; Zhang Y.; Zhu Q.; Knight R.; Caporaso J. G. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Prepr. 2018, 6, e27295v2. [DOI] [PMC free article] [PubMed] [Google Scholar]