Abstract
This study aims to measure the plasma levels of rivaroxaban and apixaban among Asian patients with atrial fibrillation and compare the results with expected drug levels from clinical studies. A total of 73 patients taking rivaroxaban and 105 patients taking apixaban were enrolled. Peak and trough levels were measured using ultra‐high performance liquid chromatography with tandem mass spectrometry. The percentage of those with drug levels within the expected range reported in clinical studies was significantly higher in the apixaban group than in the rivaroxaban group, both for trough (84.8% vs. 64.4%; P = 0.002) and peak levels (76.9% vs. 33.8%; P < 0.001). After adjusting for age, sex, kidney function, appropriate dose, and adherence, patients taking rivaroxaban were still less likely to have peak and trough levels within the expected drug levels. Our real‐world data suggests that Asian patients taking rivaroxaban are more likely to have out‐of‐expected drug levels than those taking apixaban.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THIS TOPIC?
☑ The exposure of nonvitamin K antagonist oral anticoagulants (NOACs) can change by disease status. One‐size‐fits‐all dosing does not necessarily ensure an identical treatment effect among patients. Measuring the drug level remains the most arbitrary method to evaluate the pharmacological effect in emergent circumstances.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We recruited Asian patients taking rivaroxaban or apixaban therapy. The NOAC peak and trough levels were measured and compared with expected drug levels from previous studies to investigate the potential risk of extreme drug levels.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Patients taking rivaroxaban were more likely than those taking apixaban to have drug levels lower than the expected value reported in previous clinical studies. Furthermore, the low rivaroxaban trough level was associated with inappropriately ordered rivaroxaban dosage, and a high apixaban peak level was associated with female sex.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Assessment of rivaroxaban and apixaban levels provides important information on the quality of anticoagulation therapy. Adjusting NOAC dosage against the labeled recommendation should be discouraged.
Nonvitamin K antagonist oral anticoagulant (NOAC) therapy has replaced warfarin as the first‐line treatment for preventing stroke and systemic thromboembolism in patients with atrial fibrillation (AF).1, 2, 3 Unlike warfarin, NOAC targets a specific coagulation factor. All NOAC agents claim to have linear and predictable pharmacokinetic and pharmacodynamic properties, have low drug−drug interaction, and do not require routine laboratory monitoring.4 Nevertheless, the exposure for NOACs is still affected by such patient characteristics as sex, age, body weight, ethnicity, renal function, and comedications.4, 5, 6 Clinical studies have elucidated the exposure relationship responses. The Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY) and the Effective Anticoagulation with Factor Xa Next‐Generation in Atrial Fibrillation‐Thrombolysis in Myocardial Infarction 48 (ENGAGE AF‐TIMI 48) studies have shown an association between low plasma drug levels and increased risk of ischemic stroke.7, 8 Therefore, the current one‐size‐fits‐all dosing approach does not yield ideal outcomes for all patients. Measuring NOAC levels remains the most arbitrary method by which to guide critical decisions in cases of occult thrombosis or major bleeding.4
Rivaroxaban and apixaban are the two most frequently prescribed factor Xa inhibitors.4 The most remarkable difference between them is the frequency of drug administration (i.e., once daily for rivaroxaban and twice daily for apixaban).4 The labeled dosage for rivaroxaban in Japan is unique (i.e., 15 mg daily rather than 20 mg) based on a pharmacokinetic model‐based study.5 In Taiwan, most physicians follow the dosage recommendations for Japan, considering the similar ethnic characteristics, such as low body weight.9 Currently, rivaroxaban and apixaban both lack exposure and response analysis data. One recently published study from Germany investigated the correlation between antifactor Xa activity (AXA) in rivaroxaban or apixaban users and the severity of ischemic stroke at the time of admission.10 The results showed that low NOAC levels were associated with higher stroke severity (as represented by higher initial National Institutes of Health Stroke Scale scores), persistence in neurological deficits, and cerebral infarction on brain image.10
A knowledge gap exists between the NOAC level reported in clinical studies and real‐world usage, especially in Asians. In pharmacokinetic studies, Asian ethnicity leads to increased rivaroxaban and apixaban exposure, whether or not the elevation was clinically relevant.6, 11 Our main purpose was to recruit Taiwanese patients with AF taking rivaroxaban or apixaban, analyze their plasma NOAC levels, compare our results to the reported expected drug levels from previous studies, and identify potential risk factors that drive inappropriately high or low drug levels.
Results
Baseline patient characteristics and adherence
A total of 198 patients were enrolled into the present study. After excluding 9 patients with no AF diagnosis and 11 patients who failed to provide ≥ 1 blood sample, 178 patients had NOAC level monitoring, including 73 patients taking rivaroxaban (15 mg once daily, 34 patients; 10 mg once daily, 39 patients) and 105 patients taking apixaban (5 mg twice daily, 44 patients; 2.5 mg twice daily, 61 patients). The flowchart for participant enrollment is shown in Figure 1 . The demographic characteristics of the rivaroxaban and apixaban groups are displayed in Table 1 . Patients in the apixaban group had worse serum creatinine (SCr) levels (1.2 ± 0.5 vs. 1.0 ± 0.3 mg/dL; P = 0.020) but similar creatinine clearance (CrCL; 50.0 ± 20.0 vs. 52.4 ± 19.5 mL/minutes; P = 0.438), and many more had been ordered an inappropriately adjusted dose compared with the rivaroxaban group (37.5% vs. 22.5%; P = 0.046). Among patients ordered an inappropriate NOAC dose, most received an unnecessarily adjusted low dose, especially in the apixaban group (rivaroxaban 62.5% vs. apixaban 94.9%; P = 0.002). The proportion of patients with normal alanine transaminase levels was similar between the two groups, and none of the patients enrolled in this study had bilirubin levels exceed twofold higher than the upper limit.
Figure 1.
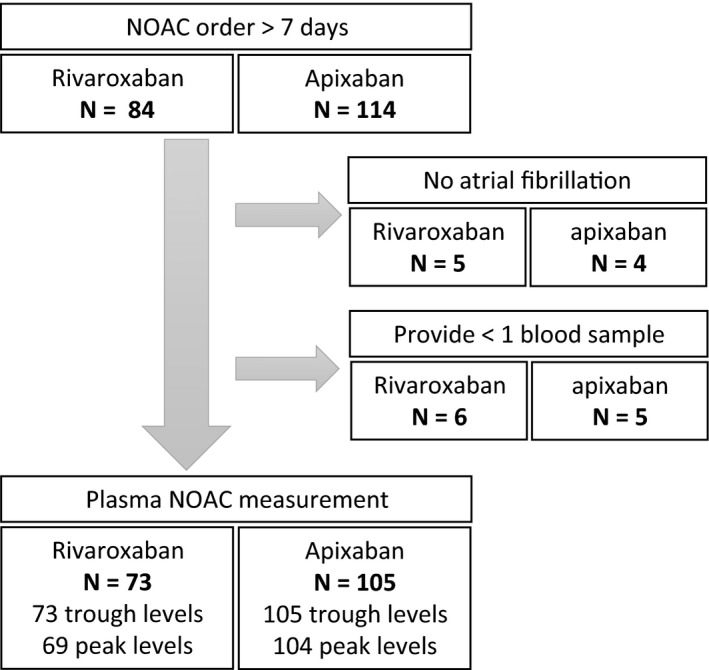
The enrollment of this present study. NOAC, nonvitamin K antagonist oral anticoagulant.
Table 1.
Baseline characteristics between rivaroxaban and apixaban
| Rivaroxaban (N = 73) | Apixaban (N = 105) | P value | |
|---|---|---|---|
| Age, year | 74.9 ± 7.5 | 77.3 ± 9.1 | 0.067 |
| Male | 37 (50.7) | 60 (57.1) | 0.445 |
| Body weight, kg | 61.8 ± 11.6 | 64.9 ± 10.3 | 0.061 |
| CRE, mg/dL | 1.0 ± 0.3 | 1.2 ± 0.5 | 0.020* |
| CrCL, mL/minutes | 52.4 ± 19.5 | 50.0 ± 20.0 | 0.438 |
| ALT < 35 U/La | 62 (93.9) | 77 (87.5) | 0.272 |
| CHA2DS2VAScb | 3.9 ± 1.5 | 4.3 ± 1.4 | 0.070 |
| HAS‐BLEDc | 2.2 ± 0.8 | 2.5 ± 0.8 | 0.075 |
| Comorbidities | |||
| IS or TIA | 29 (39.7) | 52 (49.5) | 0.223 |
| CHF | 15 (20.5) | 18 (17.1) | 0.564 |
| Hypertension | 55 (75.3) | 84 (80.0) | 0.468 |
| Diabetes | 20 (27.4) | 26 (24.8) | 0.730 |
| MI or PAOD | 5 (6.8) | 16 (15.2) | 0.102 |
| Malignancy | 10 (13.7) | 17 (16.2) | 0.678 |
| Bleeding history | 10 (13.7) | 20 (19.0) | 0.418 |
| ICH | 1 (1.4) | 6 (5.7) | 0.243 |
| GI bleeding | 5 (6.8) | 7 (6.7) | 1.000 |
| Other bleeding | 6 (8.2) | 8 (7.6) | 1.000 |
| NOAC levels | |||
| Fasted status while monitoring | 28 (38.4) | 34 (32.4) | 0.428 |
| Trough, ng/mL | 39.2 ± 60.8 | 105.9 ± 57.2 | Not applicable |
| Lower than ranged | 23 (31.5) | 11 (10.5) | 0.001* |
| Within ranged | 47 (64.4) | 89 (84.8) | 0.002* |
| Higher than ranged | 3 (4.1) | 5 (4.8) | 1.000 |
| Peak, ng/mLe | 186.4 ± 100.8 | 221.3 ± 116.5 | Not applicable |
| Lower than ranged | 38 (55.9) | 2 (1.9) | < 0.001* |
| Within ranged | 23 (33.8) | 80 (76.9) | < 0.001* |
| Higher than ranged | 7 (10.3) | 22 (21.2) | 0.094 |
| NOAC use | |||
| Good adherencef | 62 (84.9) | 78 (74.3) | 0.097 |
| Low‐dose regimeng | 39 (53.4) | 61 (58.1) | 0.169 |
| Appropriate doseh | 55 (77.5) | 65 (62.5) | 0.046* |
| Lower than recommended | 10 | 37 | 0.002* |
| Concurrent medications | |||
| Amiodarone | 16 (21.9) | 33 (31.4) | 0.176 |
| Dronedarone | 4 (5.5) | 2 (1.9) | 0.229 |
| NSAID | 1 (1.4) | 1 (1.0) | 1.000 |
| Aspirin | 1 (1.4) | 1 (1.0) | 1.000 |
| Clopidogrel | 0 (0) | 3 (2.9) | 0.270 |
Data are expressed as mean ± SD or number (percentage). For characteristics significantly different between two groups, the P‐value is marked by label "*."
ALT, alanine transaminase; CHF, congestive heart failure; CrCL, creatinine clearance (estimated by Cockcorfot‐Gault Formula); CRE, serum creatinine; GI, gastrointestinal; ICH, intracranial hemorrhage; IS, ischemic stroke; MI, myocardial infarction; NOAC, nonvitamin K antagonist oral anticoagulants; NSAID, nonsteroidal anti‐inflammatory drugs; PAOD, peripheral arterial vascular disease; TIA, transient ischemic attack; U/L, upper limit.
aALT data was missing in 7 patients in the rivaroxaban group and 17 patients in the apixaban group. bCHA2DS2VASc score: To evaluate the risk for ischemic stroke among patients with atrial fibrillation. Higher score indicates higher risk of ischemic stroke. One point was assigned to congestive heart failure, hypertension, age 65–74 years, diabetes, female sex, or vascular disease, and two points were assigned to age ≥ 75 years and previous stroke or transient ischemic attack history. cHASBLED score: To evaluate the risk for bleeding. Higher score indicates higher risk. One point is assigned to hypertension, abnormal liver function, abnormal renal function, stroke history, bleeding history, labile international normalized ratio (INR) during warfarin therapy, age over 65 years, antiplatelet agent, nonsteroidal anti‐inflammatory drug, or ethanol use. The item labile INR was not calculated in the present study. dThe data was compared with the expected rivaroxaban level reported in pharmacokinetic studies, and the expected apixaban level showed in the product monograph. eFive patients in the rivaroxaban group and one patient in the apixaban group did not monitor the peak levels. fGood adherence was defined as no self‐reported missed NOAC dose in the past 1 week, during NOAC treatment, and no reasons other than forgetting to take the NOAC dose. This was evaluated by providing participants a three‐item questionnaire. gLow‐dose regimen was defined as 10 mg o.d. for rivaroxaban and 2.5 mg b.i.d. for apixaban. hAppropriate dose was defined as ordering the NOAC according to the product labeling, including correct dose and frequency per indication, and appropriately adjusted renal dose. iConcurrent medications: None of our patients concomitantly used verapamil, azole antifungal agents, protease inhibitors (P‐glycoprotein (P‐gp) inhibitors), and rifampin, enzyme‐inducing antiepileptic drugs, such as phenytoin and phenobarbital (P‐gp inducers).
In terms of medication adherence, 62 patients (84.9%) in the rivaroxaban group reported good adherence, compared with 78 patients (74.3%) in the apixaban group (P = 0.097). The baseline characteristics of the high and low‐dose groups of those taking rivaroxaban and apixaban are displayed in Tables S1 and S2 . Patients in the low‐dose group were older and had worse renal function. For rivaroxaban, patients in the low‐dose group were also thinner.
Plasma rivaroxaban levels
Patients in the rivaroxaban group contributed 73 trough and 69 peak levels. Most of the trough levels were measured at 20–26 hours from the last rivaroxaban dose (62 patients; 84.9%), and most of the peak levels were measured at 1−4 hours after rivaroxaban ingestion (67 patients; 97.1%). More specifically, 78.3% of peak levels were measured at 2 hours ± 5 minutes. At the time of peak level measurement, 28 patients (40.6%) were in fasted status (15 mg daily, 18 patients; 10 mg daily, 10 patients). Six patients (8.2%) had undetectable trough levels. The 5th and 95th percentiles for trough and peak levels were 7.3–123.4 ng/mL and 70.2–390.0 ng/mL, compared with 12–137 ng/mL and 178–343 ng/mL, respectively, reported in clinical pharmacokinetic studies.4, 12 Figure 2 a shows the distribution of rivaroxaban levels according to the appropriateness of ordered dose, and Figure 2 c shows the distribution of rivaroxaban levels according to CrCL. The drug levels did not differ significantly among patients with CrCL ≥ or < 50 mL/minutes, both for the trough and peak levels. Forty‐seven rivaroxaban levels fell within the expected range (64.4%) for trough and 23 (33.8%) for peak levels. Most out‐of‐range rivaroxaban levels were lower than the expected range (23 trough levels; 31.5%; and 38 peak levels; 55.9%). The trough and peak levels were highly correlated (Spearman's correlation coefficient: 0.46; P < 0.001). The rivaroxaban levels were similar regardless of the fasted status at the time of measurement, either for the trough or peak levels (fasted vs. nonfasted, trough level, 34.6 ± 65.5 and 36.8 ± 55.7 ng/mL, P = 0.881; peak level, 170.3 ± 105.1 and 197.6 ± 97.5 ng/mL, P = 0.275).
Figure 2.
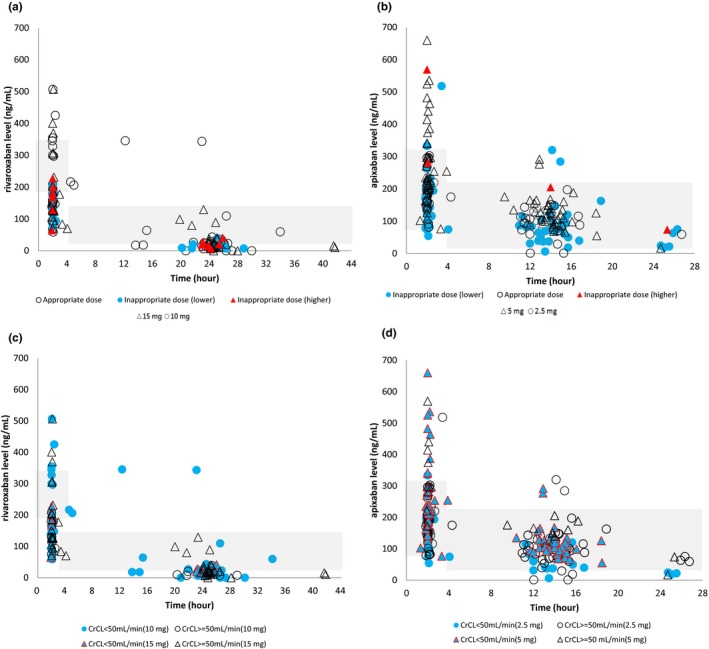
The distribution of rivaroxaban levels (a, c) and apixaban levels (b, d) according to appropriate or inappropriately ordered dose and creatinine clearance (CrCL). Mark shape: circle, rivaroxaban 10 mg daily a, c, and apixaban 2.5 mg twice daily b, d; triangle, rivaroaban 15 mg daily a, c and apixaban 5 mg twice daily b, d. a to b, Mark fill color, white, appropriately ordered drug dose; red, the ordered dose was higher than the labeled recommendation; and blue, the ordered dose was lower than the labeled recommendation. c to d, Mark fill color, white, CrCL ≥ 50 mL/minutes, and blue, CrCL < 50 mL/minutes. Shadows indicated the expected drug level reported in clinical studies.
Plasma apixaban levels
Patients in the apixaban group contributed 105 trough and 104 peak levels. Most of the trough levels were measured at 10–14 hours from the last apixaban dose (70 patients; 66.7%), and all of the peak levels were measured at 1−4 hours after apixaban ingestion. More specifically, 69.2% of peak levels were measured at 2 hours ± 5 minutes. At the time of peak‐level measurement, 34 patients (32.7%) were in fasted status (5 mg twice daily, 12 patients; 2.5 mg twice daily, 22 patients). Three patients (2.9%) had undetectable trough levels. The 5th and 95th percentiles for trough and peak levels for apixaban 5 mg twice daily were 66.5–203.8 and 110.6‐534.5 ng/mL, compared with 41–230 and 91–321 ng/mL, respectively. The 5th and 95th percentiles for trough and peak levels for apixaban 2.5 mg twice daily were 18.3–162.6 and 77.9–296.7 ng/mL, compared with 34–162 and 69–221 ng/mL, respectively, reported in clinical studies.4, 12 Figure 2 b displays the distribution of apixaban levels according to the appropriateness of ordered dose, and Figure 2 d shows the distribution of apixaban levels according to CrCL. The drug levels did not differ significantly among patients with CrCL ≥ or < 50 mL/minutes, both for the trough and peak levels. Eighty‐nine apixaban levels (84.8%) fell within the expected range for trough and 80 for peak levels (76.9%). Most out‐of‐range trough apixaban levels were lower than the expected range (11 trough levels; 10.5%). By contrast, most out‐of‐range peak apixaban levels were higher than the expected range (22 peak levels; 21.2%). The correlation between trough and peak levels was significant (Spearman's correlation coefficient: 0.64; P < 0.001). The apixaban levels were similar regardless of the fasted status at the time of measurement, either for the trough or peak levels (fasted vs. nonfasted, trough level, 106.6 ± 51.9 and 101.1 ± 62.4 ng/mL, P = 0.655; peak level, 221.7 ± 113.3 and 221.1 ± 118.7 ng/mL, P = 0.978).
Factors that caused drug levels to fall out of expected ranges
Compared with the apixaban group, the rivaroxaban group was significantly less likely to have drug levels within the expected range, either for the trough (P = 0.002) or the peak levels (P < 0.001). After adjusting for age, sex, SCr, appropriately ordered or inappropriately ordered NOAC dose, and good or suboptimal self‐reported adherence, patients in the rivaroxaban group remained significantly less likely to have trough and peak levels within the expected drug levels (odds ratio (OR) for trough 0.279, 95% confidence interval (CI) = 0.13–0.62, P = 0.002; for peak, OR = 0.172, 95% CI = 0.08–0.35, P < 0.001).
We further conducted multivariate regression to analyze factors driving the lower or higher‐than‐range drug levels. For rivaroxaban, lower‐than‐range trough levels were significantly associated with inappropriately ordered dosage (OR = 4.24; 95% CI = 1.02–17.68; P = 0.047). For apixaban, higher‐than‐range peak levels were associated with female sex (OR = 3.46; 95% CI = 1.19–10.07; P = 0.023). Details of the multivariate regression analysis are displayed in Tables 2 and 3 .
Table 2.
Multivariate regression for rivaroxaban levels
| Covariate | Rivaroxaban (trough) | Rivaroxaban (peak) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Multivariate regression for lower‐than‐range rivaroxaban level | ||||||
| Age, year | 1.03 | (0.96−1.12) | 0.426 | 0.96 | (0.90−1.04) | 0.325 |
| Male sex | 2.50 | (0.71−8.83) | 0.156 | 1.76 | (0.55−5.66) | 0.343 |
| CrCL, mL/minutes | 1.01 | (0.97−1.04) | 0.771 | 0.97 | (0.94−1.00) | 0.055 |
| Appropriate dosea | 4.24 | (1.02−17.68) | 0.047 * | 2.61 | (0.61−11.13) | 0.196 |
| Good adherencea | 1.35 | (0.33−5.52) | 0.676 | 0.28 | (0.06−1.35) | 0.112 |
| Covariate | Rivaroxaban (trough)b | Rivaroxaban (peak) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Multivariate regression for higher‐than‐range rivaroxaban level | ||||||
| Age, year | 0.92 | (0.71−1.18) | 0.493 | 0.94 | (0.85−1.05) | 0.258 |
| Male sex | 0.00 | (0.00) | 0.998 | 0.59 | (0.12−2.83) | 0.506 |
| CrCL, mL/minutes | 1.05 | (0.92−1.19) | 0.476 | 0.99 | (0.95−1.03) | 0.553 |
| Appropriate dosea | 0.00 | (0.00) | 1.000 | 0.00 | (0) | 0.998 |
| Good adherencea | 0.00 | (0.00) | 1.000 | 0.74 | (0.07−7.42) | 0.797 |
Bold value indicated covariate which significantly affected the drug levels.
CI, confidence interval; CrCL, creatinine clearance; OR, odds ratio.
Definition for appropriate dose and good adherence were mentioned in Table 1.
There were only two patients who had trough rivaroxaban levels higher than the expected range.
Table 3.
Multivariate regression for apixaban levels
| Covariate | Apixaban (trough) | Apixaban (peak)b | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Multivariate regression for lower‐than‐range apixaban level | ||||||
| Age, year | 1.05 | (0.96−1.14) | 0.313 | 0.84 | (0.64−1.10) | 0.210 |
| Male sex | 0.57 | (0.13−2.48) | 0.457 | 1.60 | (0.08−34.11) | 0.762 |
| CrCL, mL/minutes | 1.01 | (0.97−1.05) | 0.739 | 0.93 | (0.84−1.03) | 0.163 |
| Appropriate dosea | 1.83 | (0.43−7.76) | 0.416 | 1.27 | (0.04−41.89) | 0.895 |
| Good adherencea | 2.26 | (0.58−8.86) | 0.240 | 4.19 | (0.22−81.27) | 0.344 |
| Covariate | Apixaban (trough) | Apixaban (peak) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Multivariate regression for higher‐than‐range apixaban level | ||||||
| Age, year | 1.01 | (0.87−1.16) | 0.950 | 1.06 | (0.99−1.14) | 0.080 |
| Male sex | 0.63 | (0.08−4.96) | 0.660 | 3.46 | (1.19−10.07) | 0.023 * |
| CrCL, mL/minutes | 1.04 | (0.97−1.12) | 0.260 | 1.02 | (0.99−1.05) | 0.224 |
| Appropriate dosea | 0.50 | (0.06−4.35) | 0.529 | 1.08 | (0.35−3.31) | 0.890 |
| Good adherencea | 6.34 | (0.84−47.63) | 0.073 | 0.82 | (0.25−2.70) | 0.744 |
Bold value indicated covariate which significantly affected the drug levels.
CI, confidence interval; CrCL, creatinine clearance; OR, odds ratio.
Definition for appropriate dose and good adherence were mentioned in Table 1.
There was only two patients who had levels lower than the expected range.
Discussion
Our present study had several main findings. First, patients in the apixaban group tended to be ordered an inappropriately reduced dose. Second, patients in the rivaroxaban group were more likely to have a low drug level. Third, low rivaroxaban trough levels were linked to the inappropriately reduced dose and high apixaban peak levels were connected to female sex.
The possible causes for inappropriately reduced apixaban dose can be multifocal. On the one hand, Asian physicians tend to overadjust anticoagulant doses in order to avoid drug‐related bleeding.13, 14 On the other hand, the unique dose‐reduction criteria are confusing.4 Around 60% of our study participants who were ordered half‐dose apixaban carried less than two fragile characteristics for dose reduction. Interestingly, despite the large proportion of patients with an inadequately low apixaban dose, most apixaban levels were still within the expected range, whether for the peak or the trough levels. In a population pharmacokinetic study, the apixaban exposure increased by 17.7% for Japanese and 4.5% for other Asians.6 Although the change in exposure did not seem clinically relevant, differences in patient characteristics between clinical settings and trials may lead to a more prominent effect in drug exposure. Similarly, the effect of female sex on apixaban exposure was also considered to be clinically irrelevant in clinical studies.6 However, our multiple regression analysis identified sex as an important factor for high apixaban peak level. Collectively, whether an Asian‐specific dosing regimen or female‐specific dose adjustment recommendation is necessary requires more investigation.
In contrast, around 75% of patients in the rivaroxaban group received the appropriate dose as per the Japanese package insert,15 but only 30% of the peak levels were within the expected range. The results for trough levels were similar. If we propose a level of > 30 ng/mL as effective for anticoagulant activity,16, 17 a standard reported in some expert opinion, only 27% of the trough levels fulfilled this criteria. If we use the threshold for severe stroke reported in the German study, only 14% reached the cutoff point.10 A study in Singapore reported results similar to ours: under identical dosing regimens, drug levels for Singaporean patients was lower than 50% of that measured in white patients, for both the trough and peak levels.18 However, one Canadian study with a design similar to ours showed that most out‐of‐range rivaroxaban levels exceeded the higher end of the range (around 40%).19
Several causes can lead to low rivaroxaban levels, as observed in our study. First is the unique low‐dose regimen. Originally, the 15 mg daily regimen was selected according to a model‐based pharmacokinetic study, which showed similar rivaroxaban exposure for Japanese patients taking 15 mg daily and white patients taking 20 mg daily.5 However, the pharmacokinetic data showed that ethnic Chinese patients, unlike Japanese patients, did not have a more prominent increase in rivaroxaban exposure compared with white patients.11 From our real‐world study, whether the low‐dose regimen is appropriate for all Asian populations warrants reconsideration.
The second cause is the unique dosing regimen. The recommendation of once‐daily use was derived from the pharmacodynamic study, which showed an identical anti‐Xa response curve between once‐daily rivaroxaban and enoxaparin in patients with venous thromboembolism.20 Actually, no dose‐selection study has been performed in patients with AF.5 The once‐daily dosage frequency did improve adherence in patients with chronic cardiovascular disease.21 However, the effect of a delayed or missed dose could be more prominent than in twice‐daily dosing regimens,22 especially for rivaroxaban with its relatively short half‐life. In addition, the half‐life of rivaroxaban seemed even shorter in Chinese patients than in white patients (7.57 vs. 9.07 hours).23 In our study, six patients had undetectable trough levels. All had good self‐reported drug adherence and properly ordered rivaroxaban dose, but had a few hours of delay from the planned time for trough monitoring. Even such a short delay in rivaroxaban administration can have an obvious effect on drug levels.
The last cause is the inappropriately reduced rivaroxaban dose. Although fewer rivaroxaban patients had an unnecessarily reduced dose than apixaban patients, more than half still received a lower‐than‐recommended dose. As mentioned before, rivaroxaban exposure did not increase in Chinese patients.11 Because the half‐life was shorter than in white patients23; inappropriately reducing rivaroxaban dosage led to low drug levels. Our results in multiple regression analyses were in line with the observation: low rivaroxaban trough level was associated with inappropriately reduced dose.
Both rivaroxaban and apixaban are substrates for p‐glycoprotein (P‐gp) and breast cancer resistant gene protein (BCRP). These proteins are drug efflux membrane transporters. Concurrently, the use of medications that inhibit P‐gp or BCRP may lead to increase in NOAC levels.24 In our study, the proportion of patients concomitantly taking amiodarone/dronedarone (potent P‐gp inhibitors) was similar in the rivaroxaban and apixaban groups. On the other hand, polymorphisms over genes encoding P‐gp or BRCP may also influence NOAC levels.25 Investigating the pharmacogenetics of rivaroxaban and apixaban should be a future direction.
Monitoring NOAC levels is important for several reasons. The first is to measure drug adherence. One study with a similar design to ours reported that adherence significantly affected dabigatran levels.26 As the half‐life for NOAC is short, the effect of a missed dose can be more prominent than in warfarin.4 The second is to ensure the quality of NOAC therapy. In patients who concurrently take drugs having interactions with NOAC, with increased bleeding risks, and who were ordered an off‐label reduced NOAC dose, plasma drug‐level assessment helps physicians understand the current status of NOAC therapy. The third reason is to guide proper management in emergent circumstances.4 Despite the prominent reduction in intracranial hemorrhage risk for NOAC compared with warfarin, occult thrombosis and critical bleeding were still reported in postmarketing use.10, 26 Data from clinical studies showed that inappropriate trough levels affect effectiveness, just as inappropriate peak levels affect safety.7, 8 Similar findings were recently reported from Germany, which showed a correlation of low rivaroxaban and apixaban levels with a more severe risk of stroke.10 These reports suggest the potential benefits of routine drug monitoring. This pilot study helped facilities develop a standard process for monitoring NOAC levels and our data clearly demonstrate that a certain percentage of patients receiving NOACs may still fail to have drug levels within the expected therapeutic range, even with proper prescription and good compliance.
We acknowledge some limitations to our study. First, we directly measured plasma NOAC levels rather than using the AXA assays. In low drug levels, an absence of AXA on the assay may happen and excludes clinically relevant concentrations. To quantitatively measure plasma drug level, using highly sensitive ultra‐high performance liquid chromatography with tandem mass spectrometry system remained the gold standard and should be primarily selected.4, 27 Moreover, the clinical outcomes were not incorporated in this study. We were not able to answer whether the relatively low rivaroxaban levels observed in the present study was of clinical significance. A larger scale study with a longer follow‐up period is necessary to draw the optimal therapeutic range for rivaroxaban and apixaban in terms of effectiveness and safety. Second, medication adherence was recorded by a self‐reported questionnaire, which may overexaggerate the actual level of adherence. However, we use a three‐item questionnaire modified from the Morisky Medication Adherence Questionnaire to ensure the quality of adherence measurement. In addition, to avoid misclassification, patients who failed to answer all of the questions on the questionnaire were deemed to be poor adherent. To better validate adherence, a more ideal method would be to physically check the pillbox, medication blister pack, and refill record.
In conclusion, compared with the data from clinical studies, our real‐world study suggests that Asian patients taking rivaroxaban are more likely to have out‐of‐expected drug levels compared with those taking apixaban.
Methods
Study design
This is a prospective study conducted from January 2016 through December 2018 in the inpatient and outpatient departments of National Taiwan University Hospital (NTUH), a tertiary medical center with 9,000 outpatient appointments per day and 2,500 inpatient beds. Patients aged > 20 years, diagnosed with AF, and taking rivaroxaban or apixaban for > 7 days fulfilled the inclusion criteria. Patients who were pregnant, breastfeeding, refused to provide informed consent, or failed to comply with at least one blood sample collection were excluded from the study. The study protocol was approved by the Institutional Ethics Committee of NTUH and subjects provided written informed consent to participate.
Plasma rivaroxaban and apixaban level measurements
For each patient, two blood samples (each 5 mL) were collected via venous puncture. The trough level was collected right before the next NOAC dose. After collecting the trough level, the patient was asked to take NOAC and then remain at NTUH for at least 2 hours so we could collect the peak level. The peak level was measured at 1–4 hours after drug administration. The blood samples were collected in tubes containing K2EDTA anticoagulant (BD vacutainer; Becton, Dickinson, Franklin Lakes, NJ). All participants were also asked to answer a three‐item questionnaire regarding their drug adherence. Self‐reported good adherence was defined as no missed NOAC doses within the past week, never forgetting to take NOAC during treatment, and not missing taking NOAC for reasons other than forgetting. Patients with any unanswered questions on the questionnaire were deemed to have poor adherence.
The blood samples were centrifuged at 3,000 relative centrifugal force at 4°C for 15 minutes to obtain the plasma samples, then stored at −80°C before analysis. Each plasma sample (each 100 μL) was deproteinized by adding 400 μL of 100% methanol and homogenized at 1,000 rpm for 2 minutes in a Geno/Grinder 2010 (SPEX SamplePrep, Metuchen, NJ), centrifuged at 15,000 rpm for 5 minutes, then the supernatant was dried in a SpeedVac system overnight, and the sample reconstituted in 200 μL of methanol. The reconstituted sample was then filtered for injection into a ultra‐high performance liquid chromatography with tandem mass spectrometry system (0.22‐μm PP membrane filters; RC‐4, Sartorius, Göttingen, Germany). Instrumentation included an Agilent 1290 UHPLC system coupled with an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA) and an HSS T3 C18 (100*2.1 mm, 1.8 μm) column (Waters). The mobile phase consisted of 0.1% formic acid with 10 mM ammonium acetate (solvent A) and 0.1% formic acid with 10 mM ammonium acetate in 90% acetonitrile solution (solvent B). The gradient elution program was linear from 15% to 48% B for 0–3 minutes; linear from 48% to 95% B for 3–3.5 minutes; then held at 95% B for 1.5 minutes. The flow rate was set at 0.3 mL/minutes, and the injection volume was 3 μL. The JetStream electrospray ionizer (Agilent Technologies) was used as the ion source. The mass spectroscopy parameters were set as follows: a 350°C drying gas temperature, a 10 L/minutes drying gas flow rate, a 45 psi nebulizer pressure, a 350°C sheath gas temperature, an 11 L/minutes sheath gas flow rate, a 3,500 V capillary voltage, and a 500 V nozzle voltage. Mass spectroscopy acquisition was executed in multiple reaction monitoring mode and the mass transitions were 460.2 → 443.1 and 460.2 → 199 for apixaban, 464.2 → 203.1 for 13d3 apixaban, 436.1 → 144.9 and 436.1 → 72.9 for rivaroxaban, and 442.1 → 144.9 for 13C6 rivaroxaban.
Clinical data acquisition
For each participant, we also collected demographic characteristics, medical history, laboratory tests, concomitant medications, and NOAC prescription details through the electronic medical records. We recorded each participant's age, sex, weight, height, results of renal function tests and liver function tests, comorbidities (including ischemic stroke, transient ischemic attack, pulmonary embolism, deep vein thrombosis, congestive heart failure, hypertension, diabetes, peripheral artery occlusion disease, and myocardial infarction), concurrent medications, including antiplatelet agents, nonsteroidal anti‐inflammatory drugs (regular use only), amiodarone, dronedarone, verapamil, and enzyme‐inducing antiepileptic drugs, and the details of NOAC orders, including dose, frequency, order initiation/end date, and the antithrombotic drug prescribed before and after NOAC use.
We calculated CrCL using the Cockroft−Gault method. The risk of thromboembolism was evaluated using the CHA2DS2‐VASc score,28 and the risk of bleeding was evaluated using the HAS‐BLED score.29 The item “labile INR” was excluded from the HAS‐BLED score because most of our patients had not used warfarin before NOAC. We also evaluated whether the patient received the correct NOAC dose according to indication and renal function, categorizing them into appropriate or inappropriate dose groups. For rivaroxaban, the standard dose to prevent thromboembolism in patients with AF was defined as 15 mg daily as per Japanese labeling.30 Patients with CrCL of 15–50 mL/minutes should have their dose adjusted to 10 mg daily.30 Patients with CrCL < 15 mL/minutes or those undergoing dialysis, according to the labeling in our country should not take rivaroxaban; hence, they were assigned to the inappropriate dose group. For apixaban, the standard dose was defined as 5 mg twice daily.4 Patients with two or more of the following characteristics should have their dose adjusted to 2.5 mg twice daily: age ≥ 80 years, SCr ≥ 1.5 mg/dL or body weight ≤ 60 kg.4 Patients with CrCL < 25 mL/minutes or those undergoing dialysis should not use apixaban according to the labeling in our country, and these patients were assigned to the inappropriate dose group.
Statistical analysis
Descriptive analysis was used to obtain the average, SD, 5th and 95th percentiles. The difference between the rivaroxaban and the apixaban group was tested using the Student's t‐test or the χ2 test, as appropriate. The NOAC level in our present study was compared with the expected drug level reported in clinical studies.4 All measured drug levels were then categorized as “within or above the range” or “lower than the range.” Univariate analysis was performed first, followed by multivariate regression to investigate the factors driving drug levels to be lower than the expected range. Due to the relatively small sample size, we also performed bootstrapping analysis on top of the multivariate regression to ensure the reliability of the observations in the present study. All analyses were performed using IBM SPSS Statistics for Windows, version 21.0 (IBM, Armonk, NY). Any P < 0.05 was considered statistically significant.
Funding
This project was partially funded by a research grant from the Ministry of Science and Technology in Taiwan (107‐2314‐B‐002‐068‐MY3). This research was part of the National Science and Technology program.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
S.Y.L., C.H.K., S.J.Y., L.K.T., Y.B.L., C.F.H., S.C.T., and J.S.J. wrote the manuscript. S.Y.L., S.C.T., and J.S.J. designed the research, performed the research, and analyzed the data.
Supporting information
Table S1. Comparison between 2 rivaroxaban groups. Table S2. Comparisons between 2 apixaban groups.
Acknowledgments
We acknowledge the support from the Ministry of Science and Technology, Department of Neurology, and Department of Pharmacy in National Taiwan University Hospital.
References
- 1. Chiang, C.E. et al 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J. Arrhythm. 33, 345–367 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. January, C.T. et al 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 74, 104–132 (2019). [DOI] [PubMed] [Google Scholar]
- 3. Kirchhof, P. et al 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 37, 2893–2962 (2016). [DOI] [PubMed] [Google Scholar]
- 4. Steffel, J. et al The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 39, 1330–1393 (2018). [DOI] [PubMed] [Google Scholar]
- 5. Tanigawa, T. et al Model‐based dose selection for phase III rivaroxaban study in Japanese patients with non‐valvular atrial fibrillation. Drug Metab. Pharmacokinet. 28, 59–70 (2013). [DOI] [PubMed] [Google Scholar]
- 6. Cirincione, B. et al Population pharmacokinetics of apixaban in subjects with nonvalvular atrial fibrillation. CPT Pharmacometrics Syst. Pharmacol. 7, 728–738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chao, T.F. et al Clinical outcomes, edoxaban concentration, and anti‐factor Xa activity of Asian patients with atrial fibrillation compared with non‐Asians in the ENGAGE AF‐TIMI 48 trial. Eur. Heart J. 40, 1518–1527 (2019). [DOI] [PubMed] [Google Scholar]
- 8. Reilly, P.A. et al The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY Trial (Randomized Evaluation of Long‐Term Anticoagulation Therapy). J. Am. Coll. Cardiol. 63, 321–328 (2014). [DOI] [PubMed] [Google Scholar]
- 9. Huang, H.Y. , Lin, S.Y. , Cheng, S.H. & Wang, C.C. Effectiveness and safety of different rivaroxaban dosage regimens in patients with non‐valvular atrial fibrillation: a nationwide, population‐based cohort study. Sci. Rep. 8, 3451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macha, K. et al Cerebral ischemia in patients on direct oral anticoagulants. Stroke 50, 873–879 (2019). [DOI] [PubMed] [Google Scholar]
- 11. Mueck, W. , Stampfuss, J. , Kubitza, D. & Becka, M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin. Pharmacokinet. 53, 1–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byon, W. , Garonzik, S. , Boyd, R.A. & Frost, C.E. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin. Pharmacokinet. 10.1007/s40262-019-00775-z. [e‐pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan, Y.H. et al Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in Asians with nonvalvular atrial fibrillation. J. Am. Heart Assoc. 7, pii:e008150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin, Y.C. et al Effectiveness and safety of standard‐ and low‐dose rivaroxaban in Asians with atrial fibrillation. J. Am. Coll. Cardiol. 72, 477–485 (2018). [DOI] [PubMed] [Google Scholar]
- 15. Hori, M. et al Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J‐ROCKET AF study. Circulation J. 76, 2104–2111 (2012). [DOI] [PubMed] [Google Scholar]
- 16. Steiner, T. , Weitz, J.I. & Veltkamp, R. Anticoagulant‐associated intracranial hemorrhage in the era of reversal agents. Stroke 48, 1432–1437 (2017). [DOI] [PubMed] [Google Scholar]
- 17. Pernod, G. et al Management of major bleeding complications and emergency surgery in patients on long‐term treatment with direct oral anticoagulants, thrombin or factor‐Xa inhibitors. Proposals of the Working Group on Perioperative Haemostasis (GIHP) – March 2013. Ann. Fr. Anesth. Reanim. 32, 691–700 (2013). [DOI] [PubMed] [Google Scholar]
- 18. Ng Tsai, H.O. et al Comparison of rivaroxaban concentrations between Asians and Caucasians and their correlation with PT/INR. J. Thromb. Thrombolysis 46, 541–548 (2018). [DOI] [PubMed] [Google Scholar]
- 19. Gulilat, M. et al Interpatient variation in rivaroxaban and apixaban plasma concentrations in routine care. Canadian J. Cardiol. 33, 1036–1043 (2017). [DOI] [PubMed] [Google Scholar]
- 20. Kubitza, D. , Berkowitz, S.D. & Misselwitz, F. Evidence‐based development and rationale for once‐daily rivaroxaban dosing regimens across multiple indications. Clin. Appl. Thromb. Hemost. 22, 412–422 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weeda, E.R. , Coleman, C.I. , McHorney, C.A. , Crivera, C. , Schein, J.R. & Sobieraj, D.M. Impact of once‐ or twice‐daily dosing frequency on adherence to chronic cardiovascular disease medications: a meta‐regression analysis. Int. J. Cardiol. 216, 104–109 (2016). [DOI] [PubMed] [Google Scholar]
- 22. Vrijens, B. & Heidbuchel, H. Non‐vitamin K antagonist oral anticoagulants: considerations on once‐ vs. twice‐daily regimens and their potential impact on medication adherence. Europace 17, 514–523 (2015). [DOI] [PubMed] [Google Scholar]
- 23. Zhao, X. et al Safety, pharmacokinetics and pharmacodynamics of single/multiple doses of the oral, direct factor Xa inhibitor rivaroxaban in healthy Chinese subjects. Br. J. Clin. Pharmacol. 68, 77–88 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vranckx, P. , Valgimigli, M. & Heidbuchel, H. The significance of drug–drug and drug–food interactions of oral anticoagulation. Arrhythmia Electrophysiol. Rev. 7, 55–61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanuri, S.H. & Kreutz, R.P. Pharmacogenomics of novel direct oral anticoagulants: newly identified genes and genetic variants. J. Personal. Med. 9, pii: E7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin, S.Y. et al Factors affecting serum concentration of dabigatran in Asian patients with non‐valvular atrial fibrillation. J. Formosan Med. Assoc. 118, 1154–1160 (2019). [DOI] [PubMed] [Google Scholar]
- 27. Gosselin, R.C. , et al International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb. Haemost. 118, 437–450 (2018). [DOI] [PubMed] [Google Scholar]
- 28. Lip, G.Y. , Nieuwlaat, R. , Pisters, R. , Lane, D.A. & Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest 137, 263–272 (2010). [DOI] [PubMed] [Google Scholar]
- 29. Pisters, R. , Lane, D.A. , Nieuwlaat, R. , de Vos, C.B. , Crijns, H.J. & Lip, G.Y. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 138, 1093–1100 (2010). [DOI] [PubMed] [Google Scholar]
- 30. Hori, M. et al Safety and efficacy of adjusted dose of rivaroxaban in Japanese patients with non‐valvular atrial fibrillation: subanalysis of J‐ROCKET AF for patients with moderate renal impairment. Circ. J. 77, 632–638 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison between 2 rivaroxaban groups. Table S2. Comparisons between 2 apixaban groups.


