Introduction
Diabetic neuropathies are the most prevalent chronic complications of diabetes. This heterogeneous group of conditions affects different parts of the nervous system and presents with diverse clinical manifestations. The early recognition and appropriate management of neuropathy in the patient with diabetes is important for a number of reasons:
Diabetic neuropathy is a diagnosis of exclusion. Nondiabetic neuropathies may be present in patients with diabetes and may be treatable by specific measures.
A number of treatment options exist for symptomatic diabetic neuropathy.
Up to 50% of diabetic peripheral neuropathies may be asymptomatic. If not recognized and if preventive foot care is not implemented, patients are at risk for injuries to their insensate feet.
Recognition and treatment of autonomic neuropathy may improve symptoms, reduce sequelae, and improve quality of life.
Among the various forms of diabetic neuropathy, distal symmetric polyneuropathy (DSPN) and diabetic autonomic neuropathies, particularly cardiovascular autonomic neuropathy (CAN), are by far the most studied (1–4). There are several atypical forms of diabetic neuropathy as well (1–4). Patients with prediabetes may also develop neuropathies that are similar to diabetic neuropathies (5–10). Table 1 provides a comprehensive classification scheme for the diabetic neuropathies.
Table 1.
Classification for diabetic neuropathies
| Diabetic neuropathies |
|---|
| A. Diffuse neuropathy |
| DSPN |
| • Primarily small-fiber neuropathy |
| • Primarily large-fiber neuropathy |
| • Mixed small- and large-fiber neuropathy (most common) |
| Autonomic |
| Cardiovascular |
| • Reduced HRV |
| • Resting tachycardia |
| • Orthostatic hypotension |
| • Sudden death (malignant arrhythmia) |
| Gastrointestinal |
| • Diabetic gastroparesis (gastropathy) |
| • Diabetic enteropathy (diarrhea) |
| • Colonic hypomotility (constipation) |
| Urogenital |
| • Diabetic cystopathy (neurogenic bladder) |
| • Erectile dysfunction |
| • Female sexual dysfunction |
| Sudomotor dysfunction |
| • Distal hypohydrosis/anhidrosis, |
| • Gustatory sweating |
| Hypoglycemia unawareness |
| Abnormal pupillary function |
| B. Mononeuropathy (mononeuritis multiplex) (atypical forms) |
| Isolated cranial or peripheral nerve (e.g., CN III, ulnar, median, femoral, peroneal) |
| Mononeuritis multiplex (if confluent may resemble polyneuropathy) |
| C. Radiculopathy or polyradiculopathy (atypical forms) |
| Radiculoplexus neuropathy (a.k.a. lumbosacral polyradiculopathy, proximal motor amyotrophy) |
| Thoracic radiculopathy |
| Nondiabetic neuropathies common in diabetes |
| Pressure palsies |
| Chronic inflammatory demyelinating polyneuropathy |
| Radiculoplexus neuropathy |
| Acute painful small-fiber neuropathies (treatment-induced) |
Due to a lack of treatments that target the underlying nerve damage, prevention is the key component of diabetes care. Screening for symptoms and signs of diabetic neuropathy is also critical in clinical practice, as it may detect the earliest stages of neuropathy, enabling early intervention. Although screening for rarer atypical forms of diabetic neuropathy may be warranted, DSPN and autonomic neuropathy are the most common forms encountered in practice. The strongest available evidence regarding treatment pertains to these forms.
This Position Statement is based on several recent technical reviews, to which the reader is referred for detailed discussion and relevant references to the literature (3,4,11–16).
PREVENTION
Prevention of diabetic neuropathies focuses on glucose control and lifestyle modifications. Available evidence pertains only to DSPN and CAN, and most of the large trials that have evaluated the effect of glucose control on the risk of complications have included DSPN and CAN as secondary outcomes or as post hoc analyses rather than as primary outcomes. In addition, in some of these trials, the outcome measures used to evaluate neuropathy may have limited ability to detect a benefit, if present.
Recommendations
Optimize glucose control as early as possible to prevent or delay the development of distal symmetric polyneuropathy and cardiovascular autonomic neuropathy in people with type 1 diabetes. A
Optimize glucose control to prevent or slow the progression of distal symmetric polyneuropathy in people with type 2 diabetes. B
Consider a multifactorial approach targeting glycemia among other risk factors to prevent cardiovascular autonomic neuropathy in people with type 2 diabetes. C
Glucose Control
Enhanced glucose control in people with type 1 diabetes dramatically reduces the incidence of DSPN (78% relative risk reduction) (17–19). In contrast, enhanced glucose control in people with type 2 diabetes reduces the risk of developing DSPN modestly (5%–9% relative risk reduction) (20,21). In a small trial of Japanese patients with early type 2 diabetes, intensive insulin treatment was associated with improvement in selected DSPN measures (22), and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial reported a modest but significant DSPN risk reduction with the glycemia intervention in individuals with type 2 diabetes after 5 years of follow-up (21). Yet, no effects are observed in other large trials (20,23–25). This discrepancy highlights the differences between type 1 and type 2 diabetes and emphasizes the point that many people with type 2 diabetes develop DSPN despite adequate glucose control (20,25). The presence of multiple comorbidities, polypharmacy, hypoglycemia, and weight gain might have attenuated the effects of glucose control in these trials and contributed to inconsistent findings (25). Specific glucose-lowering strategies may also contribute to the discrepancy. For example, participants, particularly men, in the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI 2D) study treated with insulin sensitizers had a lower incidence of DSPN over 4 years than those treated with insulin/sulfonylurea (26). This outcome may be a result of less weight gain and less hypoglycemia (26). Last, the fact that many patients have had asymptomatic hyperglycemia for many years prior to the diagnosis of type 2 diabetes may also explain the limited benefit in these patients.
Similar to the findings in DSPN, the most robust evidence for CAN prevention was reported in type 1 diabetes. Intensive glucose control designed to achieve near-normal glycemia reduced the risk of incident CAN during the Diabetes Control and Complications Trial (DCCT) by 45% and by 31% in its follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC) study (27). The highly reproducible and sensitive testing protocol, the robust definitions used for CAN, and the large sample size in DCCT/EDIC enhance the validity of the results and support the rationale for implementing and maintaining tight glucose control as early as possible in the course of type 1 diabetes. In contrast, glycemic control in type 2 diabetes has not consistently lowered the risk of CAN (25). However, a multifactorial intervention, including a lifestyle component, targeting glucose and cardiovascular disease risk factors reduced the risk of CAN by 60% in people with type 2 diabetes (28).
Lifestyle Modifications
The best models to date regarding parameters for an evidence-based, intensive lifestyle intervention come from the Diabetes Prevention Program (DPP) (29), the Steno-2 Study (28), the Italian supervised treadmill study (30), and the University of Utah type 2 diabetes study (31). The latter study recently reported nerve fiber regeneration in patients with type 2 diabetes engaged in an exercise program compared with loss of nerve fibers in those who only followed standard of care. Overall, such an approach focuses on either exercise alone (supervised aerobic and/or resistance training) (30,31) or combined dietary modification and exercise. There is no consensus regarding dietary regimens, and although the DPP used a low-calorie, low-fat diet, others have championed a Mediterranean diet that is moderately lower in carbohydrate (45%) and higher in fat (35%–40%), with less than 10% of saturated fat.
Although the DPP (32) and the Impaired Glucose Tolerance Neuropathy (IGTN) study (33) reported benefits of lifestyle interventions on measures of CAN and DSPN, respectively, these trials did not include subjects with established diabetes. In addition, in the DPP, indices of CAN improved with the lifestyle intervention and did not change in the other arms (32).
DSPN
Most common among diabetic neuropathies is chronic DSPN, accounting for about 75% of the diabetic neuropathies (1,3). A simple definition of DSPN for clinical practice is the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes.
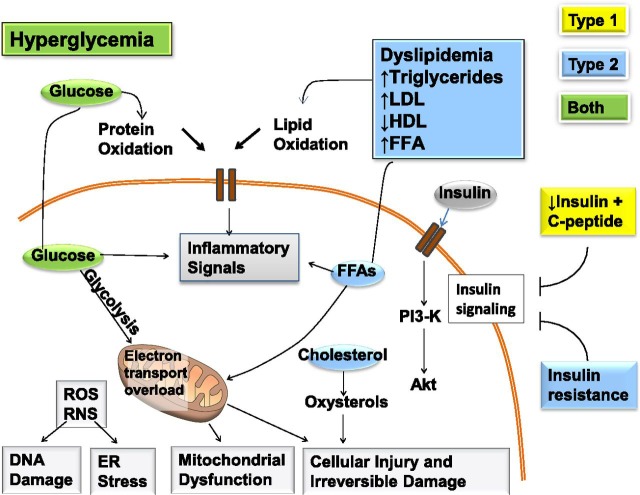
Experimental studies suggest a multifactorial pathogenesis of DSPN (Fig. 1), but the causes remain unknown (34–37). A prevailing view of the pathogenesis is that oxidative and inflammatory stress may, in the context of metabolic dysfunction, damage nerve cells (34–37).
Figure 1.
Mechanisms of diabetic neuropathy. Factors linked to type 1 diabetes (yellow), type 2 diabetes (blue), and both (green) cause DNA damage, endoplasmic reticulum stress, mitochondrial dysfunction, cellular injury, and irreversible damage. The relative importance of the pathways in this network will vary with cell type, disease profile, and time. ER, endoplasmic reticulum; FFA, free fatty acids; PI3-K, phosphatidylinositol-3 kinase; RNS, reactive nitrogen species; ROS, reactive oxygen species. Adapted and reprinted from Callaghan et al. (20), with permission from Elsevier.
Estimates of the incidence and prevalence of DSPN vary greatly (25,38–40), but evidence from several large observational cohorts (41,42) and the DCCT/EDIC (27,43) suggests that DSPN occurs in at least 20% of people with type 1 diabetes after 20 years of disease duration. DSPN may be present in at least 10%–15% of newly diagnosed patients with type 2 diabetes (44,45), with rates increasing to 50% after 10 years of disease duration (25,26). Rates in youth with type 1 and type 2 diabetes approach those observed in adult populations (46). DSPN has been associated with glycemia (14,33–35), height (47) (perhaps as a proxy for nerve length), smoking (48), blood pressure, weight, and lipid measures (49,50).
There is emerging evidence that DSPN, especially the painful small-fiber neuropathy subtype, may be present in 10%–30% of subjects with impaired glucose tolerance, also known as prediabetes (5–10) or metabolic syndrome (51).
DSPN is the most important cause of foot ulceration, and it is also a prerequisite in the development of Charcot neuroarthropathy (CN) (52). The reader is referred to several other reviews that cover this topic (52,53). Foot ulceration and CN are both recognized as late complications of DSPN (52,54). These late complications drive amputation risk and economic costs of diabetic neuropathy and are also predictors of mortality.
DSPN is also a major contributor to falls and fractures (55–57), through more advanced small- and large-fiber dysfunction, with loss of sensory, proprioception, temperature discrimination, and pain, all ultimately leading to unsteadiness, recurrent minor injuries, and an increased risk of falls. These recurrent minor injuries may further contribute to the pathogenesis of CN (58).
Screening and Diagnosis
Recommendations
All patients should be assessed for distal symmetric polyneuropathy starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes and at least annually thereafter. B
Consider screening patients with prediabetes who have symptoms of peripheral neuropathy. B
Assessment should include a careful history and either temperature or pinprick sensation (small-fiber function) and vibration sensation using a 128-Hz tuning fork (large-fiber function). All patients should have an annual 10-g monofilament testing to assess for feet at risk for ulceration and amputation. B
Electrophysiological testing or referral to a neurologist is rarely needed for screening, except in situations where the clinical features are atypical, the diagnosis is unclear, or a different etiology is suspected. Atypical features include motor greater than sensory neuropathy, rapid onset, or asymmetrical presentation. B
Patients with type 1 diabetes for 5 or more years and all patients with type 2 diabetes should be assessed annually for DSPN using medical history and simple clinical tests. Up to 50% of patients may experience symptoms of DSPN (Table 2), whereas the rest are asymptomatic. Patients may not volunteer symptoms but on inquiry may reveal that they are experiencing numbness or other positive symptoms of DSPN.
Table 2.
Symptoms and signs of DSPN
| Large myelinated nerve fibers | Small myelinated nerve fibers | |
|---|---|---|
| Function |
Pressure, balance |
Nociception, protective sensation |
| Symptoms§ |
Numbness, tingling, poor balance |
Pain: burning, electric shocks, stabbing |
| Examination (clinically diagnostic)** | Ankle reflexes: reduced/absent Vibration perception: reduced/absent 10-g monofilament: reduced/absent Proprioception: reduced/absent | Thermal (cold/hot) discrimination: reduced/absent** Pinprick sensation: reduced/absent** |
§To document the presence of symptoms for diagnosis;
**Documented in symmetrical, distal to proximal pattern.
Symptoms vary according to the class of sensory fibers involved. The most common early symptoms are induced by the involvement of small fibers and include pain and dysesthesias (unpleasant sensations of burning) (1,4,59,60). Neuropathic pain may be the first symptom that prompts patients to seek medical care and is present in up to 25% of individuals with DSPN (61–63). Characteristically, the pain is burning, lancinating, tingling, or shooting (electric shock–like); occurs with paresthesias; presents in varying combinations; and is typically worse at night. Neuropathic pain may be accompanied by an exaggerated response to painful stimuli (hyperalgesia) and pain evoked by contact, e.g., with socks, shoes, and bedclothes (allodynia). Neuropathic pain can lead to interference with daily activities, disability, psychosocial impairment, and reduced health-related quality of life (64–66). The direct and indirect economic burden associated with neuropathic pain is substantial (67–69).
The involvement of large fibers may cause numbness, tingling without pain, and loss of protective sensation. Loss of protective sensation indicates the presence of DSPN and is a risk factor for diabetic foot ulceration. Patients can also initially present with an insensate, numb foot due to the loss of large fibers. Patients frequently state that their feet feel like they are wrapped in wool or they are walking on thick socks. It is the loss of the “gift of pain” that permits patients with plantar neuropathic ulcers to walk on the lesions, inducing chronicity, frequently complicated by infection (70).
The following clinical tests may be used to assess small- and large-fiber function distal to proximal (Table 2):
Small-fiber function: pinprick and temperature sensation
Large-fiber function: vibration perception, proprioception, 10-g monofilament, and ankle reflexes
A 128-Hz tuning fork can be used for the assessment of vibration perception. Assessment of light-touch perception using a 10-g monofilament should include evaluation on the dorsal aspect of the great toe bilaterally as previously validated by Perkins et al. (71). The 10-g monofilament is a useful clinical tool mainly for detecting more advanced neuropathy and identifying patients at increased risk of ulceration and amputation (72).
Assessments should follow the typical DSPN pattern, starting distally (the dorsal aspect of the hallux) on both sides and move proximally until a sensory threshold is identified (72). Combining at least two examinations will increase the sensitivity and specificity of detecting DSPN, as demonstrated in several cohorts of patients with type 1 and type 2 diabetes including children and adolescents (26,46,73–79).
The diagnosis of DSPN is principally a clinical one (Table 2). A combination of typical symptomatology and symmetrical distal sensory loss or typical signs in the absence of symptoms in a patient with diabetes is highly suggestive of DSPN and may not require additional evaluation or referral. As up to half of the patients may be asymptomatic, a diagnosis may only be made on examination or, in some cases, when the patient presents with a painless foot ulcer.
Clinicians should note that the 10-g monofilament test included for the annual DSPN screening and diagnosis is different than the diagnosis of the “high-risk foot” for ulceration, a late DSPN complication that requires that four sites (first, third, and fifth metatarsal heads and plantar surface of distal hallux) be tested on each foot (80).
Consider excluding neuropathy with causes other than diabetes (Table 3) by undertaking a family and medication history and performing relevant investigations (e.g., serum B12, folic acid, thyroid function, complete blood count, metabolic panel, and a serum protein immunoelectrophoresis) (81).
Table 3.
Differential diagnosis of diabetic neuropathies
| Metabolic disease |
| Thyroid disease (common) |
| Renal disease |
| Systemic disease |
| Systemic vasculitis |
| Nonsystemic vasculitis |
| Paraproteinemia (common) |
| Amyloidosis |
| Infectious |
| HIV |
| Hepatitis B |
| Lyme |
| Inflammatory |
| Chronic inflammatory demyelinating polyradiculoneuropathy |
| Nutritional |
| B12* |
| Postgastroplasty |
| Pyridoxine |
| Thiamine |
| Tocopherol |
| Industrial agents, drugs, and metals |
| Industrial agents |
| Acrylamide |
| Organophosphorous agents |
| Drugs |
| Alcohol |
| Amiodarone |
| Colchicine |
| Dapsone |
| Vinka alkaloids |
| Platinum |
| Taxol |
| Metals |
| Arsenic |
| Mercury |
| Hereditary |
| Hereditary motor, sensory, and autonomic neuropathies |
*B12 deficiency is more commonly associated with malabsorption rather than nutritional deficiency.
Electrophysiological testing or referral to a neurologist is rarely needed for diagnosis, except in situations where the clinical features are atypical, the diagnosis is unclear, or a different etiology is suspected (2,38,40,80). Atypical features that warrant referral include motor greater than sensory neuropathy, asymmetry of symptoms and signs, and rapid progression.
Foot Complications
The simple yet comprehensive clinical exam is principally designed to identify those at risk for the late complications who need education on preventative foot self-care and regular podiatric foot care. Recently an even simpler foot exam, the “3-minute diabetic foot exam,” has been proposed (82). This is intended not only for physicians but also for other health care professionals who may only have 15 min for the entire diabetes annual review; it requires no equipment and provides simple advice on education on preventative foot self-care.
Management
Recommendations
Tight glucose control targeting near-normal glycemia in patients with type 1 diabetes dramatically reduces the incidence of distal symmetric polyneuropathy and is recommended for distal symmetric polyneuropathy prevention in type 1 diabetes. A
In patients with type 2 diabetes with more advanced disease and multiple risk factors and comorbidities, intensive glucose control alone is modestly effective in preventing distal symmetric polyneuropathy and patient-centered goals should be targeted. B
Lifestyle interventions are recommended for distal symmetric polyneuropathy prevention in patients with prediabetes/metabolic syndrome and type 2 diabetes. B
Prevention
Please refer to Prevention on page 136.
Pathogenetic Therapies
Despite the recent major advances in elucidating the pathogenesis of diabetic neuropathy, there remains a lack of treatment options that effectively target the natural history of DSPN (83) or reverse DSPN once established. Several pathogenetic pharmacotherapies have been investigated (36), but evidence from randomized clinical trials is very limited (81,83,84). Advances in DSPN disease modification need to be confirmed with further robust evidence from clinical trials, together with a better understanding of the mechanisms of action of promising treatments (83).
Pain Management
Recommendations
Consider either pregabalin or duloxetine as the initial approach in the symptomatic treatment for neuropathic pain in diabetes. A
Gabapentin may also be used as an effective initial approach, taking into account patients’ socioeconomic status, comorbidities, and potential drug interactions. B
Although not approved by the U.S. Food and Drug Administration, tricyclic antidepressants are also effective for neuropathic pain in diabetes but should be used with caution given the higher risk of serious side effects. B
Given the high risks of addiction and other complications, the use of opioids, including tapentadol or tramadol, is not recommended as first- or second-line agents for treating the pain associated with DSPN. E
No compelling evidence exists in support of glycemic control or lifestyle management as therapies for neuropathic pain in diabetes or prediabetes (33,85), which leaves only pharmaceutical interventions.
At present, pregabalin and duloxetine have received regulatory approval for the treatment of neuropathic pain in diabetes by the U.S. Food and Drug Administration (FDA), Health Canada, and the European Medicines Agency. The opioid, tapentadol, has regulatory approval in the U.S. and Canada, but the evidence of its use is weaker (15).
A large evidence base supports pharmacological treatment of neuropathic pain in diabetic neuropathy using other agents of different classes, as documented by several recent guidelines and systematic reviews (15,16,20,86,87). It is important to mention that only a few trials that targeted pain in peripheral neuropathic pain were carried out in DSPN alone. However, the results of studies performed on peripheral nondiabetic neuropathic pain or mixed neuropathic pain may be applicable to patients with neuropathic pain due to DSPN.
Although there are broad general agreements among the recommendations, there are some inconsistencies that are, in part, a consequence of whether the guidelines are specific for painful DSPN or whether they address neuropathic pain due to all causes (15,16,20,86,87).
Below we summarize the available evidence on the most effective agents for DSPN pain starting with the currently approved drugs and continuing with the other agents based on mechanism of action and strength of evidence. Evidence levels are assigned based on the strength of the published clinical evidence for the efficacy and safety of the agents for the treatment of DSPN pain, which should be considered in clinical decision making. However, a certain degree of publication bias should be considered, given that many negative trials may not have been published (15).
Additional information on dose titration, adverse effects, number needed to treat, and safety is presented in Table 4.
Table 4.
| Drug class | Agent | Dose |
NNT range 30–50% improvement** | Common adverse events | Major adverse events | |
|---|---|---|---|---|---|---|
| Initial | Effective | |||||
| Anticonvulsants |
||||||
| Pregabalin* (15,86,88–94) |
25–75 mg, 1–3×/day |
300–600 mg/day |
3.3–8.3 |
• Somnolence |
• Angioedema |
|
| • Dizziness |
• Hepatotoxicity |
|||||
| • Peripheral edema |
• Rhabdomyolysis |
|||||
| • Headache |
• Suicidal thoughts and behavior |
|||||
| • Ataxia |
• Seizures after rapid discontinuation |
|||||
| • Fatigue |
• Thrombocytopenia |
|||||
| • Xerostomia |
||||||
| • Weight gain |
||||||
| Gabapentin (15,86,96,105–111) |
100–300 mg, 1–3×/day |
900–3,600 mg/day |
3.3–7.2 |
• Somnolence |
• Stevens-Johnson syndrome |
|
| • Dizziness |
• Suicidal thoughts and behavior |
|||||
| • Ataxia |
• Seizures after rapid discontinuation |
|||||
| • Fatigue |
||||||
| Antidepressants |
||||||
| Serotonin-norepinephrine reuptake inhibitors |
Duloxetine* (15,86,94,96,98–101) |
20–30 mg/day |
60–120 mg/day |
3.8–11 |
• Nausea |
• Stevens-Johnson syndrome |
| • Somnolence |
• Hepatotoxicity |
|||||
| • Dizziness |
• Hypertensive crisis |
|||||
| • Constipation |
• Gastrointestinal hemorrhage |
|||||
| • Dyspepsia |
• Delirium |
|||||
| • Diarrhea |
• Myocardial infarction |
|||||
| • Xerostomia |
• Cardiac arrhythmias |
|||||
| • Anorexia |
• Glaucoma |
|||||
| • Headache |
• Suicidal thoughts and behavior |
|||||
| • Diaphoresis |
• Shift to mania in patients with bipolar disorder |
|||||
| • Insomnia |
• Seizures |
|||||
| • Fatigue |
• Severe hyponatremia |
|||||
| • Decreased libido |
• Fragility bone fractures |
|||||
| • Serotonin syndrome |
||||||
| • Neuroleptic malignant syndrome |
||||||
| Venlafaxine (15,16,20,86,87,126,127) |
37.5 mg/day |
75–225 mg/day |
5.2–8.4 |
• Nausea |
• Same as duloxetine |
|
| • Somnolence |
||||||
| • Dizziness |
||||||
| • Constipation |
||||||
| • Dyspepsia |
||||||
| • Diarrhea |
||||||
| • Xerostomia |
||||||
| • Anorexia |
||||||
| • Headache |
||||||
| • Diaphoresis |
||||||
| • Insomnia |
||||||
| • Fatigue |
||||||
| • Decreased libido |
||||||
| Tricyclic antidepressants |
Amitriptyline (16,110,112–116) |
10–25 mg/day |
25–100 mg/day |
2.1–4.2 |
• Xerostomia |
• Delirium |
| • Somnolence |
• Cardiac arrhythmias |
|||||
| • Fatigue |
• Conduction abnormalities |
|||||
| • Headache |
• Myocardial infarction |
|||||
| • Dizziness |
• Heart failure exacerbation |
|||||
| • Insomnia |
• Stroke |
|||||
| • Orthostatic hypotension |
• Seizures |
|||||
| • Anorexia |
• Hepatotoxicity |
|||||
| • Nausea |
• Bone marrow suppression |
|||||
| • Urinary retention |
• Suicidal thoughts and behavior |
|||||
| • Constipation |
• Shift to mania in bipolar disorder |
|||||
| • Blurred vision |
• Neuroleptic malignant syndrome |
|||||
| • Accommodation |
• Serotonin syndrome |
|||||
| • Disturbance |
• Severe hyponatremia |
|||||
| • Mydriasis |
• Fragility bone fractures |
|||||
| • Weight gain |
||||||
| Desipramine (113,118–121,122) |
• Same as above |
• Same as above |
||||
| Nortriptyline (15,16,86,87,113,114,120,121,123) |
• Same as above |
• Same as above |
||||
| Opioids |
||||||
| Tramadol (15,16,86,87,109,130) |
50 mg, 1–2×/day |
210 mg/day |
3.1–6.4 |
• Somnolence |
• Confusion |
|
| • Nausea |
• Seizures |
|||||
| • Vomiting |
• Cardiac arrhythmias |
|||||
| • Constipation |
• Hypertension |
|||||
| • Light-headedness |
• Hypersensitivity reactions |
|||||
| • Dizziness |
• Stevens-Johnson syndrome |
|||||
| • Headache |
||||||
| Tapentadol* (103,104,135) |
Immediate release:50–100 mg, 4–6×/day |
Immediate-release: day 1: 700 mg; after day 1, 60 mg/day |
N/A |
• Somnolence |
• Respiratory depression |
|
| • Nausea |
• Serotonin syndrome |
|||||
| Extended release:50 mg, 2×/day | Extended release:50 mg, 2×/day | • Vomiting |
• Seizures |
|||
| • Constipation |
• Hypertension |
|||||
| • Dizziness | • Neonatal opioid withdrawal syndrome | |||||
NNT, number needed to treat. *FDA approved.
**FDA considers 30–50% improvement to be significant.
Approved Medications
Pregabalin and duloxetine have received regulatory approval for the treatment of neuropathic pain in diabetes in the U.S., Europe, and Canada.
Pregabalin, a calcium channel α2-δ subunit ligand, is an effective treatment for neuropathic pain associated with DSPN. It is the most extensively studied drug by far in DSPN, with the majority of studies being positive regarding the proportion of responders with at least 30%–50% improvement in pain (15,86,88–94). There is also some evidence suggesting a dose response, with a weaker effect with 300 vs. 600 mg/day (88). However, not all trials with pregabalin have been positive (15,86,95,96), especially when treating advanced refractory patients (93). Pregabalin, in contrast to gabapentin (see below), has a linear, dose-proportional absorption in the therapeutic dose range (150–600 mg/day) (88). In addition, pregabalin has a more rapid onset of action and more limited dosage range that requires minimal titration. Adverse effects may be more severe in older patients (97) and may be attenuated by lower starting doses and more gradual titration.
Duloxetine is a selective norepinephrine and serotonin reuptake inhibitor. Doses of 60 and 120 mg/day showed efficacy in the treatment of pain associated with DSPN in multicenter randomized trials, although some of these had a rather high drop-out rate (15,86,94,96,98–101). Duloxetine was also suggested to induce improvement in neuropathy-related quality of life (100). In longer-term studies, a small increase in A1C was reported in people with diabetes treated with duloxetine compared with placebo (102). Adverse events may again be more severe in older people but may be attenuated with lower doses and progressive titrations of duloxetine.
Tapentadol extended release is a novel centrally acting opioid analgesic that exerts its analgesic effects through both μ-opioid receptor agonism and noradrenaline reuptake inhibition. Extended-release tapentadol was approved by the FDA for the treatment of neuropathic pain associated with diabetes based on data from two multicenter randomized withdrawal, placebo-controlled phase 3 trials (103,104). However, both used an enriched design and therefore are not generalizable, and a recent systematic review and meta-analysis by the International Association for the Study of Pain Special Interest Group on Neuropathic Pain (NeuPSIG) found the evidence of the effectiveness of tapentadol in reducing neuropathic pain inconclusive (15). Therefore, given the high risk for addiction and safety concerns compared with the relatively modest pain reduction, the use of tapentadol extended release is not recommended as first- or second-line treatment.
Anticonvulsants
Gabapentin, like pregabalin, also binds the calcium channel α2-δ subunit and has shown efficacy in a number of clinical trials for treating the pain associated with DSPN (15,86,96,105–111). However, not all painful DSPN studies, some of which are unpublished, have been positive (15,107).
Given its pharmacokinetic profile, gabapentin requires gradual titration and doses up to 1,800–3,600 mg are generally needed to be clinically effective (96,105–107). Adverse effects may be more severe in older patients (97).
Monoamine Reuptake Inhibitors
The monoamine reuptake inhibitors—tricyclic antidepressants, selective serotonin reuptake inhibitors, and norepinephrine and serotonin reuptake inhibitors—increase synaptic monoamine levels and directly influence the activity of the descending neurons.
Amitriptyline, although not FDA approved, is the most used of the tricyclic agents. Many previous guidelines recommend the medication as a first-line treatment based on few randomized, blinded, placebo-controlled clinical trials that reported significant improvement in neuropathic pain (110,112–116). The effectiveness appeared unrelated to the antidepressant effect (112). A recent Cochrane Review questioned the quality of evidence on amitriptyline by raising concerns for bias given the small sample size in most and concluded that in fact there is no clear evidence for a beneficial effect for amitriptyline on DSPN pain, especially when balanced against spectrum of side effects (117). However, there was no good evidence of a lack of effect either (117).
The secondary amines, nortriptyline and desipramine, have a less troublesome side effect profile than the tertiary amines, amitriptyline and imipramine, although fewer randomized controlled trials were performed with these agents, and the potential for bias was high given the small size (113,118–123). The use of these agents is preferable, particularly in older and side effect–prone patients (113,118–121).
Several studies have suggested that there is an increased risk of myocardial ischemia and arrhythmogenesis associated with tricyclic agents (124,125). Because of concerns of possible cardiotoxicity, tricyclic antidepressants should be used with caution in patients with known or suspected cardiac disease.
Venlafaxine, a selective norepinephrine and serotonin reuptake inhibitor, in doses between 150 and 225 mg/day has shown some effectiveness in the treatment of painful DSPN (126,127). Both venlafaxine and duloxetine (see above) inhibit the reuptake of serotonin and norepinephrine without the muscarinic, histaminic, and adrenergic side effects that accompany the use of the tricyclic agents (98–100,102). However, the level of evidence for pain reduction associated with DSPN is higher with duloxetine (see above). Venlafaxine may lower the seizure threshold, and gradual tapering is recommended to avoid the emergence of adverse events upon discontinuation (126,127).
Opioid and Atypical Opioid Analgesics
Tramadol is a centrally acting analgesic with pain relief mediated by a weak μ-opioid receptor agonist activity and inhibition of norepinephrine and serotonin reuptake (128,129). It is an effective agent in the treatment of painful diabetic peripheral neuropathy compared with placebo as demonstrated by two large multicenter trials (129,130), and it appears to have long-term effects (131). Although tramadol has a lower potential for abuse compared with other opioids, given these safety concerns, it is not recommended for use as first- or second-line agent.
Controlled-release oxycodone improved pain scores in two single-center trials in patients with painful diabetic neuropathy, one of which had a small sample size (132,133). It may provide additional analgesia for patients on α2-δ ligand treatment (134). As with all opioids, it is not recommended for use as first-, second-, or third-line agent.
Warnings on All Opioids
Despite the demonstrated effectiveness of opioids in the treatment of neuropathic pain (15,132,134,135), there is a high risk of addiction, abuse, sedation, and other complications and psychosocial issues even with short-term opioid use. For these reasons, opioids are not recommended in the treatment of painful DSPN before failure of other agents that do not have these associated concerns (136–138).
Although add-on therapy with strong opioids may be required in some patients who do not respond to all other combinations, referral to specialized pain clinics is recommended in these cases to avoid risks.
Additional Considerations for Pain Management
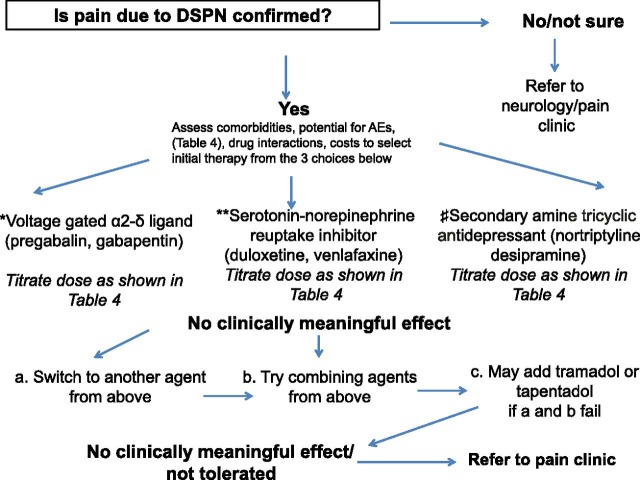
Combination therapy, including combinations with opioids, may provide effective treatment for diabetic neuropathic pain at lower doses (94,139). A detailed approach for pain management is amply covered in other literature (15,109), and a simple algorithm for clinical practice use is shown in Fig. 2.
Figure 2.
Algorithm for management of the patient with pain because of DSPN. AE, adverse events.*Pregabalin is FDA approved for painful DSPN, whereas gabapentin is not. Pharmacokinetic profile, spectrum of AEs, drug interactions, comorbidities, and costs to be considered in selecting the agent of choice. **Duloxetine is FDA approved for painful DSPN, whereas venlafaxine is not. Pharmacokinetic profile, spectrum of AEs, drug interactions, comorbidities, and costs to be considered in selecting the agent of choice. #None is FDA approved for painful DSPN. Spectrum of AEs, drug interactions, and comorbidities need be considered if selecting these agents.
Treatment of Foot Complications
Detailed treatment of foot ulceration and CN is beyond the scope of this statement, and the reader is referred to a relevant review (54). Effective off-loading that prevents patients with plantar neuropathic ulcers to walk on the lesions is the key to successful management (52,54). Off-loading, usually with casting, and careful follow-up and repeated investigations are also key components for the management of CN (52,54). Ongoing education and regular podiatry follow-up can reduce the incidence of foot complications in those found to be at “high risk.” Early intervention for foot lesions and CN or suspected CN can slow or reverse progression.
Fall Prevention
Recommendation
Tests assessing gait and balance may be considered in people with distal symmetric polyneuropathy to evaluate the risk of falls. E
DSPN may also compromise balance in daily activities (58). For instance, progressive loss of proprioception (diminished sensation) and later weakness, superimposed on age-related functional impairments, lead to imbalance and unsteadiness in gait, with increased likelihood of a fall (55,58). A decline in cognitive function, polypharmacy, and neuropathic pain may further contribute. In addition, treatment of neuropathic pain often requires dosages and drug combinations that may further increase the fall risk due to cognitive impairment, drowsiness, dizziness, blurred vision, and gait disturbances (97,109). Older patients are the most susceptible (97,109). Therefore, tests assessing gait and balance may be considered in clinical practice to evaluate risk of falls in patients at risk (55,58).
Psychosocial Factors
Recommendations
Consider treatment with duloxetine, pregabalin, and gabapentin to improve quality of life in patients with neuropathic pain. C
Assess the effects of distal symmetric polyneuropathy on quality of life to improve adherence and response to neuropathic pain treatment. E
Assessing the effects of DSPN on a patient’s quality of life is emerging as a component of patient care and may play an important part in the adherence and the response to therapies in patients with neuropathic pain (140). Some studies report an improvement in quality of life in people with painful DSPN treated with duloxetine (100), pregabalin (141), and gabapentin (106,142). A longitudinal study has shown that DSPN is a risk factor for depression and the strongest symptom associated with depression was unsteadiness. Pain with DSPN may also give rise to symptoms of anxiety (143). Two research tools that can be used to assess quality of life that are neuropathy specific are the Neuro-QoL (Quality of Life in Neurological Disorders) (144) and QOL-DN (Norfolk Quality of Life-Diabetic Neuropathy) instruments (145).
Diabetic Autonomic Neuropathies
Autonomic neuropathies affect the autonomic neurons (parasympathetic, sympathetic, or both) and are associated with a variety of site-specific symptoms. The symptoms and signs of autonomic dysfunction should be elicited carefully during the medical history and physical examination. Major clinical manifestations of diabetic autonomic neuropathy include hypoglycemia unawareness, resting tachycardia, orthostatic hypotension, gastroparesis, constipation, diarrhea, fecal incontinence, erectile dysfunction, neurogenic bladder, and sudomotor dysfunction with either increased or decreased sweating.
Although CAN is the most studied and clinically relevant of the diabetic autonomic neuropathies, gastrointestinal, genitourinary, and sudomotor dysfunction should be considered in the optimal care of patients with diabetes.
CAN
Although CAN prevalence is very low in newly diagnosed patients with type 1 diabetes (146), CAN prevalence increases substantially with diabetes duration (13,25), and prevalence rates of at least 30% were observed in the DCCT/EDIC cohort after 20 years of diabetes duration (27,147). In type 2 diabetes, the prevalence of CAN also increases with diabetes duration and may be present in up to 60% of patients with type 2 diabetes after 15 years (13,148,149). CAN may affect youth, especially young women and those with elevated A1C levels, with prevalence rates of at least 20% reported in youth with type 1 or type 2 diabetes (150). In addition, CAN is present in patients with impaired glucose tolerance, insulin resistance, or metabolic syndrome (10,32,151).
A timely diagnosis of CAN may have important clinical implications, as CAN is an independent risk factor for cardiovascular mortality, arrhythmia, silent ischemia, any major cardiovascular event, and myocardial dysfunction (152–157). Data from two large cardiovascular outcomes trials that included 31,531 patients with stable heart disease and/or diabetes followed for a median of 5 years reported that heart rate, an indirect measure of CAN, analyzed as either categorical (baseline heart rate >70 vs. ≤70 bpm) or across heart rate quintile, was independently associated with significant increases in cardiovascular disease (CVD) events and all-cause death (158). CAN may also be associated with hemodynamic instability or cardiorespiratory arrest (159). CAN was the strongest risk factor for mortality in a large cohort of patients with type 1 diabetes participating in the EURODIAB Prospective Cohort Study (160), and a meta-analysis of several trials reported higher mortality risk with worse measures of CAN (152).
Conclusive evidence that supports CAN as an independent predictor of mortality was confirmed in more than 8,000 participants with type 2 diabetes in the ACCORD trial (154). Hazard ratios for all-cause and CVD mortality in those with CAN were as high as 2.14 after adjusting for all traditional CVD risk factors and many other risk factors, including use of various classes of medication use (154).
It was also suggested that intensification of glucose and blood pressure management may increase the risk of a cardiovascular event in people with signs of CAN (161–165). Similarly, emerging evidence demonstrates an association between CAN and glucose variability, especially in the hypoglycemic range (150,164).
In addition, CAN independently predicts the progression of diabetic nephropathy and chronic kidney disease in diabetes (13,166–168).
Screening and Diagnosis
Recommendations
Symptoms and signs of autonomic neuropathy should be assessed in patients with microvascular and neuropathic complications. E
In the presence of symptoms or signs of cardiovascular autonomic neuropathy, tests excluding other comorbidities or drug effects/interactions that could mimic cardiovascular autonomic neuropathy should be performed. E
Consider assessing symptoms and signs of cardiovascular autonomic neuropathy in patients with hypoglycemia unawareness. C
The most common symptoms of CAN occur upon standing and include light-headedness, weakness, palpitations, faintness, and syncope (13,169,170) (Table 5). The patient should be asked about these symptoms when a medical history is taken in the office, although the correlation of symptoms with overall autonomic deficits is weak (149,171). However, these symptoms may occur quite late in the disease course (25,27,147,149). It may be appropriate to screen patients with hypoglycemia unawareness, as this may be associated with CAN (81).
Table 5.
Symptoms and signs associated with diabetic autonomic neuropathy
| CAN | Gastrointestinal | Urogenital | Sudomotor |
|---|---|---|---|
| Resting tachycardia | Gastroparesis (Gastropathy) | Bladder dysfunction | Dry skin |
Abnormal blood pressure regulation
|
|
|
|
| Orthostatic hypotension (all with standing) | Esophageal dysfunction | Male sexual dysfunction | |
|
|
|
|
| Orthostatic tachycardia or bradycardia and chronotropic incompetence (all with standing) | Diabetic diarrhea | Female sexual dysfunction | |
|
|
|
|
| Exercise intolerance | Constipation | ||
|
In its early stages, CAN may be completely asymptomatic and detected only by decreased heart rate variability (HRV) with deep breathing (13,169,170). Testing HRV may be done in the office by either 1) taking an electrocardiogram recording as a patient begins to rise from a seated position or 2) taking an electrocardiogram recording during 1–2 min of deep breathing with calculation of HRV (11,81,170).
In more advanced cases, patients may present with resting tachycardia (>100 bpm) and exercise intolerance (13,170). Advanced disease may also be associated with orthostatic hypotension (a fall in systolic or diastolic blood pressure by >20 mmHg or >10 mmHg, respectively, upon standing without an appropriate increase in heart rate) (172). Orthostatic hypotension is usually easy to document in the office. In most cases of CAN, there is no compensatory increase in the heart rate, despite hypotension (173).
The diagnosis includes documentation of symptoms (Table 5) and signs of CAN, which include impaired HRV, higher resting heart rate, and presence of orthostatic hypotension. In a symptomatic patient presenting with resting tachycardia, with a history of poor glucose control, or when the diagnosis of CAN is likely, clinicians may not need to perform additional tests given costs and burden.
Exclusion of other comorbidities or drug effects/interactions that may present with the symptoms or signs of CAN and that mimic CAN may be needed (81,174) (Table 6). In addition, polypharmacy may also directly or indirectly impact CAN.
Table 6.
Diagnostic algorithm for CAN
| Symptoms | Signs/diagnostic tests | Differential workup | |
|---|---|---|---|
| Resting tachycardia |
Palpitations |
Clinical exam: resting heart rate >100 bpm |
• Anemia |
| Could be asymptomatic |
• Hypothyroidism |
||
| • Fever |
|||
| • CVD (atrial fibrillation, flutter, other) |
|||
| • Dehydration |
|||
| • Adrenal insufficiency |
|||
| • Medications |
|||
| • Sympathomimetic agents (asthma) |
|||
| • Over-the-counter cold agents containing ephedrine or pseudoephedrine |
|||
| • Dietary supplements (e.g., ephedra alkaloids) |
|||
| • Smoking, alcohol, caffeine |
|||
| • Recreational drugs (cocaine, amphetamines, methamphetamine, mephedrone) |
|||
| Orthostatic hypotension |
Light-headedness |
Clinical exam: a reduction of >20 mmHg in the systolic blood pressure or >10 mmHg in diastolic blood pressure |
• Adrenal insufficiency |
| Weakness |
• Intravascular volume depletion |
||
| Faintness |
• Blood loss/acute anemia |
||
| Visual impairment |
• Dehydration |
||
| Syncope |
• Pregnancy/postpartum |
||
| • CVD |
|||
| • Arrhythmias |
|||
| • Heart failure |
|||
| • Myocarditis |
|||
| • Pericarditis |
|||
| • Valvular heart disease |
|||
| • Alcohol |
|||
| • Medication |
|||
| • Antiadrenergics |
|||
| • Antianginals |
|||
| • Antiarrhythmics |
|||
| • Anticholinergics |
|||
| • Diuretics |
|||
| • ACE inhibitors/angiotensin receptor blocker |
|||
| • Narcotics |
|||
| • Neuroleptics |
|||
| • Sedatives |
Treatment
Prevention.
Please refer to Prevention on page 136.
Recommendations
Optimize glucose control as early as possible to prevent or delay the development of cardiovascular autonomic neuropathy in people with type 1 diabetes. A
Consider a multifactorial approach targeting glycemia among other risk factors to prevent cardiovascular autonomic neuropathy in people with type 2 diabetes. C
Consider lifestyle modifications to improve cardiovascular autonomic neuropathy in patients with prediabetes. C
As with DSPN, multiple other therapies targeting various pathogenetic mechanisms have failed to reverse established CAN. CAN treatment is generally focused on alleviating symptoms and should be targeted to the specific clinical manifestation.
Symptomatic Treatment of Orthostatic Hypotension.
Treatment for orthostatic hypotension is challenging and usually involves both pharmacological and nonpharmacological interventions. Physical activity and exercise should be encouraged to avoid deconditioning, which is known to exacerbate orthostatic intolerance. Volume repletion with fluids and salt is central to the management of orthostatic hypotension. Low-dose fludrocortisone may be beneficial in supplementing volume repletion in some patients, although there are growing concerns on risk of supine hypertension.
As neurogenic orthostatic hypotension is in large part a consequence of the failure of norepinephrine release from sympathetic neurons, the administration of sympathomimetic medications is central to the care of patients whose symptoms are not controlled with other measures (173). Midodrine, a peripheral, selective, direct α1-adrenoreceptor agonist, is an FDA-approved drug for the treatment of orthostatic hypotension (175). Midodrine should be titrated gradually to efficacy. It should be used only when patients intend to be upright or seated to minimize supine hypertension (173). Recently, droxidopa was approved by the FDA for the treatment of neurogenic orthostatic hypotension but not specifically for patients with orthostatic hypotension due to diabetes (176).
Gastrointestinal Neuropathies
Gastrointestinal neuropathies may involve any portion of the gastrointestinal tract with manifestations including esophageal dysmotility, gastroparesis (delayed gastric emptying), constipation, diarrhea, and fecal incontinence. The prevalence data on gastroparesis are limited, as most reports were from selected case series rather than larger populations, and there was inconsistency in the outcome measures used (177). In the only community-based study, the cumulative incidence of gastroparesis over 10 years was higher in type 1 diabetes (5%) than in type 2 diabetes (1%) and in control subjects (1%) (178).
Gastroparesis may directly affect glycemic management (e.g., insulin dose or other antidiabetes agents) and may be a cause of glucose variability and unexplained hypoglycemia due to the dissociation between food absorption and the pharmacokinetic profiles of insulin and other agents (12,179–182). Gastroparesis is mainly found in patients with long-standing diabetes (183).
Screening and Diagnosis
Recommendations
Evaluate for gastroparesis in people with diabetic neuropathy, retinopathy, and/or nephropathy by assessing for symptoms of unexpected glycemic variability, early satiety, bloating, nausea, and vomiting. C
Exclusion of other causes documented to alter gastric emptying, such as use of opioids or glucagon-like peptide 1 receptor agonists and organic gastric outlet obstruction, is needed before performing specialized testing for gastroparesis. C
To test for gastroparesis, either measure gastric emptying with scintigraphy of digestible solids at 15-min intervals for 4 h after food intake or use a 13C-octanoic acid breath test. B
Gastroparesis may manifest with a broad spectrum of symptoms and signs (12,177,179,181). As part of a medical history, providers are encouraged to document symptoms of gastroparesis, such as early satiety, fullness, bloating, nausea, vomiting, dyspepsia, and abdominal pain. However, gastroparesis may be clinically silent in the majority of cases, and symptoms do not necessarily correspond with severity of gastroparesis and are poorly associated with abnormal gastric emptying (184,185). Symptoms such as anorexia, nausea, vomiting, and dyspepsia are nonspecific and resemble many other conditions (186) and may just be associated with the presence of diabetes (181). Importantly, hyperglycemia, hypoglycemia, and acute changes in blood glucose are well documented to alter gastric emptying (182,187,188), as are some medications, especially opioids, other pain management agents, and glucagon-like peptide 1 receptor agonists (189,190). Therefore, all these factors known to affect gastric emptying should always be considered before a firm diagnosis is established.
Exclusion of organic causes of gastric outlet obstruction or peptic ulcer disease (with esophagogastroduodenoscopy or a barium study of the stomach) is needed before considering specialized testing for gastroparesis. The diagnostic gold standard is the measurement of gastric emptying with scintigraphy of digestible solids at 15-min intervals for 4 h after food intake; the use of 13C-octanoic acid breath test is emerging as a viable alternative (12,179). Optimization of glucose levels prior to scanning is needed (182,186–188) to avoid false-positive results.
Treatment
Recommendation
Consider short-term metoclopramide in the treatment of diabetic gastroparesis. E
Treatment for diabetic gastroparesis may be very challenging. Dietary changes may be useful, such as eating multiple small meals and decreasing dietary fat and fiber intake. Withdrawing drugs with effects on gastrointestinal motility, such as opioids, anticholinergics, tricyclic antidepressants, glucagon-like peptide 1 receptor agonists, pramlintide, and possibly dipeptidyl peptidase 4 inhibitors, may also improve intestinal motility (180,191). In cases of severe gastroparesis, pharmacological interventions are needed. Only metoclopramide, a prokinetic agent, is approved by the FDA for the treatment of gastroparesis. However, the level of evidence regarding the benefits of metoclopramide for the management of gastroparesis is weak, and given the risk for serious adverse effects (extrapyramidal symptoms, such as acute dystonic reactions; drug-induced parkinsonism; akathisia; and tardive dyskinesia), its use in the treatment of gastroparesis beyond 5 days is no longer recommended by the FDA and the European Medicines Agency. It should be reserved for severe cases that are unresponsive to other therapies (191).
Urogenital Neuropathies
Diabetic autonomic neuropathy may also cause genitourinary disturbances, including sexual dysfunction and bladder dysfunction. In men, diabetic autonomic neuropathy may cause erectile dysfunction (ED) and/or retrograde ejaculation. ED is three times more common in men with diabetes than those without the disease (192–194). Sexual dysfunction is also more common in women with diabetes (195–199).
Recommendations
Consider screening men with other forms of diabetic neuropathy annually for erectile dysfunction with simple questions about a patient’s libido and ability to reach and maintain an erection. C
Consider screening patients with other forms of diabetic neuropathy for lower urinary tract symptoms and female sexual dysfunction in the presence of recurrent urinary tract infections using targeted questioning regarding symptoms, such as nocturia, pain during intercourse, and others. E
ED
ED may be a consequence of autonomic neuropathy, as autonomic neurotransmission controls the cavernosal and detrusor smooth muscle tone and function (200). The etiology, however, is multifactorial, and clinicians should also evaluate other vascular risk factors such as hypertension, hyperlipidemia, obesity, endothelial dysfunction, smoking, CVD, concomitant medication, and psychogenic factors (12). There is evidence of associations between ED and other diabetes complications, including CAN (201–203).
A diagnosis should be made after establishing the signs and symptoms of ED and after excluding alternate causes. Clinicians should consider performing hormonal evaluation (luteinizing hormone, testosterone, free testosterone, prolactin) to rule out hypogonadism. In addition, a variety of medications and organic causes should be excluded (12).
Glucose control was associated with a lower incidence of erectile dysfunction in men with type 1 diabetes (204,205). Evidence is less strong for type 2 diabetes. Control of other risk factors such as hypertension and hyperlipidemia may also improve the condition (12). Pharmacological treatment includes phosphodiesterase type 5 inhibitors as first-line therapy and transurethral prostaglandins, intracavernosal injections, vacuum devices, and penile prosthesis in more advanced cases.
Lower Urinary Tract Symptoms and Female Sexual Dysfunction
Lower urinary tract symptoms manifest as urinary incontinence and bladder dysfunction (nocturia, frequent urination, urination urgency, weak urinary stream) and is linked to the presence of diabetic neuropathy in both men and women (12,206). Female sexual dysfunction occurs more frequently in women with diabetes than in those without diabetes (196,207) and presents as decreased sexual desire, increased pain during intercourse, decreased sexual arousal, and inadequate lubrication.
Evaluation of bladder function should be performed for individuals with diabetes who have recurrent urinary tract infections, pyelonephritis, incontinence, or a palpable bladder. The medical history should include simple questions to unveil symptoms of lower urinary tract symptoms and female sexual dysfunction (196,207,208).
Sudomotor Dysfunction
Sudomotor dysfunction may manifest as dry skin, anhidrosis, or heat intolerance (209,210). A rare form of sudomotor dysfunction is gustatory sweating that comprises excessive sweating limited exclusively to the head and neck region triggered by food consumption or, in some cases, the smell of food. Originally described as being solely due to autonomic neuropathy, gustatory sweating is also described in patients with diabetic nephropathy on dialysis (211).
On the basis of the available evidence, the routine screening for sudomotor dysfunction in clinical practice is not recommended at this time. The efficacy of the topical antimuscarinic agent glycopyrrolate in the treatment of gustatory sweating was confirmed in a randomized controlled trial, and daily application attenuates this complication in most patients for at least 24 h (212).
ATYPICAL NEUROPATHIES
Mononeuropathies
Mononeuropathies occur more commonly in patients with diabetes than in those without diabetes (1) and can occur as a result of involvement of the median, ulnar, radial, and common peroneal nerves (213). Cranial neuropathies present acutely and are rare; primarily involve cranial nerves III, IV, VI, and VII; and usually resolve spontaneously over several months (213). Electrophysiological studies are most helpful in identifying nerve conduction slowing or conduction block at the site of nerve entrapment. Nerve entrapments may require surgical decompression. The improvement in symptom severity and functional status score is no different between patients with and without diabetes (213).
Diabetic Radiculoplexus Neuropathy
Diabetic radiculoplexus neuropathy, a.k.a. diabetic amyotrophy or diabetic polyradiculoneuropathy, typically involves the lumbosacral plexus (214–216). The complication occurs mostly in men with type 2 diabetes. People with the condition routinely present with extreme unilateral thigh pain and weight loss, followed by motor weakness. Electrophysiological assessment is required to document the extent of disease and alternative etiologies, including degenerative disc disease or neoplastic, infectious, and inflammatory spinal disease (215,216). The disorder is usually self-limiting, and patients improve over time with medical management and physical therapy (214,215). There is presently no evidence from randomized trials to support any recommendation on the use of any immunotherapy treatment in this condition (217).
Treatment-Induced Neuropathy
Treatment-induced neuropathy in diabetes (also referred to as insulin neuritis) is considered a rare iatrogenic small-fiber neuropathy caused by an abrupt improvement in glycemic control in the setting of chronic hyperglycemia, especially in patients with very poor glucose control (218). The prevalence and risk factors of this disorder are not known but are currently under study.
Neuropathy End Points for Research and Clinical Trials
There are currently no approved disease-modifying therapies for DSPN, CAN, or other forms of diabetic neuropathy, and multiple clinical trials for these conditions have failed. Important contributing factors include a lack of agreement and uniformity in the use of the most sensitive DSPN measures that capture the natural history of the disease and detect repair in the specific nerve fiber populations, as well as the inclusion of appropriate patient populations. Thus, a valid and careful diagnosis for DSPN in clinical research is critical for correctly identifying the appropriate patient population targeted for either a specific intervention or for prognostic implications.
The use of validated clinical instruments such as the Michigan Neuropathy Screening Instrument (MNSI) (most widely used in large cohorts of patients with type 1 and type 2 diabetes) (21,26,27,46,74,75), the modified Toronto Clinical Neuropathy Scale (mTCNS) (73), the Utah Early Neuropathy Scale (UENS) (77), or the Neuropathy Disability Score (NDS) (44) are recommended. These may be combined with electrophysiology; measures of small-fiber damage and repair, such as intraepidermal nerve fiber density (219–221) or corneal confocal microscopy (222); and objective measures of patient function in the design of DSPN trials.
The recommended CAN measures for clinical trials targeting either a specific intervention or for prognostic implications include 1) standardized cardiovascular autonomic reflex tests that are simple, sensitive, specific, reproducible, and assess the changes in the R-R interval on electrocardiogram recordings in response to simple clinical maneuvers (deep breathing, Valsalva, and standing) (13,81,191,223); 2) indices of HRV (see above) (11,151,169); and 3) resting heart rate and QTc (154,156,157). Other methods such as baroreflex sensitivity, cardiac sympathetic imaging, and microneurography require sophisticated infrastructure and highly trained personnel and are quite expensive and time-consuming (11,13,224).
Article Information
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
This position statement was reviewed and approved by the American Diabetes Association Professional Practice Committee in September 2016 and ratified by the American Diabetes Association Board of Directors in October 2016.
References
- 1.Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep 2014;14:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaghan BC, Kerber KA, Lisabeth LL, et al. Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol 2014;71:1143–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyck PJ, Albers JW, Andersen H, et al.; Toronto Expert Panel on Diabetic Neuropathy . Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 2011;27:620–628 [DOI] [PubMed] [Google Scholar]
- 4.Malik RA, Veves A, Tesfaye S, et al.; Toronto Consensus Panel on Diabetic Neuropathy . Small fibre neuropathy: role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab Res Rev 2011;27:678–684 [DOI] [PubMed] [Google Scholar]
- 5.Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn) 2012;18:60–84 [DOI] [PubMed] [Google Scholar]
- 6.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001;24:1448–1453 [DOI] [PubMed] [Google Scholar]
- 7.Asghar O, Petropoulos IN, Alam U, et al. Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care 2014;37:2643–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bongaerts BW, Rathmann W, Heier M, et al. Older subjects with diabetes and prediabetes are frequently unaware of having distal sensorimotor polyneuropathy: the KORA F4 study. Diabetes Care 2013;36:1141–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Im S, Kim SR, Park JH, Kim YS, Park GY. Assessment of the medial dorsal cutaneous, dorsal sural, and medial plantar nerves in impaired glucose tolerance and diabetic patients with normal sural and superficial peroneal nerve responses. Diabetes Care 2012;35:834–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group . Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 11.Bernardi L, Spallone V, Stevens M, et al.; Toronto Consensus Panel on Diabetic Neuropathy. . Investigation methods for cardiac autonomic function in human research studies. Diabetes Metab Res Rev 2011;27:639–65321695768 [Google Scholar]
- 12.Kempler P, Amarenco G, Freeman R, et al.; Toronto Consensus Panel on Diabetic Neuropathy. . Gastrointestinal autonomic neuropathy, erectile-, bladder- and sudomotor dysfunction in patients with diabetes mellitus: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011;27:665–677 [DOI] [PubMed] [Google Scholar]
- 13.Spallone V, Ziegler D, Freeman R, et al.; Toronto Consensus Panel on Diabetic Neuropathy . Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011;27:639–653 [DOI] [PubMed] [Google Scholar]
- 14.Tesfaye S, Boulton AJ, Dyck PJ, et al.; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bril V, England JD, Franklin GM, et al.; American Academy of Neurology; American Asociation of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation . Evidence-based guideline: treatment of painful diabetic neuropathy--report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation. Muscle Nerve 2011;43:910–917 [DOI] [PubMed] [Google Scholar]
- 17.Diabetes Control and Complications Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 18.Diabetes Control and Complications Research Group Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol 1995;38:869–880 [DOI] [PubMed] [Google Scholar]
- 19.Linn T, Ortac K, Laube H, Federlin K. Intensive therapy in adult insulin-dependent diabetes mellitus is associated with improved insulin sensitivity and reserve: a randomized, controlled, prospective study over 5 years in newly diagnosed patients. Metabolism 1996;45:1508–1513 [DOI] [PubMed] [Google Scholar]
- 20.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012;11:521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismail-Beigi F, Craven T, Banerji MA, et al.; ACCORD trial group . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117 [DOI] [PubMed] [Google Scholar]
- 23.Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care 2011;34:2244–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charles M, Fleischer J, Witte DR, et al. Impact of early detection and treatment of diabetes on the 6-year prevalence of cardiac autonomic neuropathy in people with screen-detected diabetes: ADDITION-Denmark, a cluster-randomised study. Diabetologia 2013;56:101–108 [DOI] [PubMed] [Google Scholar]
- 25.Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep 2014;14:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pop-Busui R, Lu J, Brooks MM, et al.; BARI 2D Study Group . Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care 2013;36:3208–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group . Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 29.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications 2006;20:216–223 [DOI] [PubMed] [Google Scholar]
- 31.Singleton JR, Marcus RL, Jackson JE, K Lessard M, Graham TE, Smith AG. Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann Clin Transl Neurol 2014;1:844–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME; Diabetes Prevention Program Research Group . The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care 2006;29:914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 2006;29:1294–1299 [DOI] [PubMed] [Google Scholar]
- 34.Biessels GJ, Bril V, Calcutt NA, et al. Phenotyping animal models of diabetic neuropathy: a consensus statement of the Diabetic Neuropathy Study Group of the EASD (Neurodiab). J Peripher Nerv Syst 2014;19:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien PD, Hinder LM, Sakowski SA, Feldman EL. ER stress in diabetic peripheral neuropathy: a new therapeutic target. Antioxid Redox Signal 2014;21:621–633 [DOI] [PubMed] [Google Scholar]
- 36.Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011;7:573–583 [DOI] [PubMed] [Google Scholar]
- 37.Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci 2013;36:439–449 [DOI] [PubMed] [Google Scholar]
- 38.Boulton A, Malik R. Diabetes mellitus: neuropathy. In Endocrinology: Adult and Pediatric. Jameson JL, De Groot LJ, Eds. Philadelphia, Saunders Elsevier, 2010, p. 984–998 [Google Scholar]
- 39.Boulton AJ, Valensi P, Tesfaye S.International Neuropathy Workshop of 2009: introduction to the final reports. Diabetes Metab Res Rev 2011;27:617–619 [DOI] [PubMed] [Google Scholar]
- 40.Boulton AJ, Vinik AI, Arezzo JC, et al.; American Diabetes Association . Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962 [DOI] [PubMed] [Google Scholar]
- 41.Maser RE, Steenkiste AR, Dorman JS, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1989;38:1456–1461 [DOI] [PubMed] [Google Scholar]
- 42.Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 1996;39:1377–1384 [DOI] [PubMed] [Google Scholar]
- 43.Albers JW, Herman WH, Pop-Busui R, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 2010;33:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–154 [DOI] [PubMed] [Google Scholar]
- 45.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 46.Jaiswal M, Lauer A, Martin CL, et al.; SEARCH for Diabetes in Youth Study Group . Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care 2013;36:3903–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sosenko JM, Gadia MT, Fournier AM, O’Connell MT, Aguiar MC, Skyler JS. Body stature as a risk factor for diabetic sensory neuropathy. Am J Med 1986;80:1031–1034 [DOI] [PubMed] [Google Scholar]
- 48.Clair C, Cohen MJ, Eichler F, Selby KJ, Rigotti NA. The effect of cigarette smoking on diabetic peripheral neuropathy: a systematic review and meta-analysis. J Gen Intern Med 2015;30:1193–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tesfaye S, Chaturvedi N, Eaton SE, et al.; EURODIAB Prospective Complications Study Group . Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 50.Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009;35:206–213 [DOI] [PubMed] [Google Scholar]
- 51.Callaghan BC, Xia R, Banerjee M, et al.; Health ABC Study . Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care 2016;39:801–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boulton AJ. The pathway to foot ulceration in diabetes. Med Clin North Am 2013;97:775–790 [DOI] [PubMed] [Google Scholar]
- 53.Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM; American Diabetes Association . Preventive foot care in people with diabetes. Diabetes Care 2003;26(Suppl. 1):S78–S79 [DOI] [PubMed] [Google Scholar]
- 54.Boulton AJ. Diabetic neuropathy and foot complications. Handb Clin Neurol 2014;126:97–107 [DOI] [PubMed] [Google Scholar]
- 55.Morrison S, Colberg SR, Parson HK, Vinik AI. Relation between risk of falling and postural sway complexity in diabetes. Gait Posture 2012;35:662–668 [DOI] [PubMed] [Google Scholar]
- 56.Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002;25:1749–1754 [DOI] [PubMed] [Google Scholar]
- 57.Wallace C, Reiber GE, LeMaster J, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care 2002;25:1983–1986 [DOI] [PubMed] [Google Scholar]
- 58.Brown SJ, Handsaker JC, Bowling FL, Boulton AJ, Reeves ND. Diabetic peripheral neuropathy compromises balance during daily activities. Diabetes Care 2015;38:1116–1122 [DOI] [PubMed] [Google Scholar]
- 59.Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: differences in demographic data and sensory symptoms. Pain 2009;146:34–40 [DOI] [PubMed] [Google Scholar]
- 60.Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain 2014;155:367–376 [DOI] [PubMed] [Google Scholar]
- 61.Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med 2004;21:976–982 [DOI] [PubMed] [Google Scholar]
- 62.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–1522 [DOI] [PubMed] [Google Scholar]
- 63.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group . Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10:393–400 [DOI] [PubMed] [Google Scholar]
- 64.Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care 2005;28:2378–2383 [DOI] [PubMed] [Google Scholar]
- 65.Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes Metab Res Rev 2004;20(Suppl. 1):S13–S18 [DOI] [PubMed] [Google Scholar]
- 66.Vinik E, Silva MP, Vinik AI. Measuring the relationship of quality of life and health status, including tumor burden, symptoms, and biochemical measures in patients with neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:97–109, viii [DOI] [PubMed] [Google Scholar]
- 67.Hogan P, Dall T, Nikolov P; American Diabetes Association . Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917–932 [DOI] [PubMed] [Google Scholar]
- 68.O’Brien JA, Patrick AR, Caro J. Estimates of direct medical costs for microvascular and macrovascular complications resulting from type 2 diabetes mellitus in the United States in 2000. Clin Ther 2003;25:1017–1038 [DOI] [PubMed] [Google Scholar]
- 69.O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 2009;27:95–112 [DOI] [PubMed] [Google Scholar]
- 70.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care 2004;27:1458–1486 [DOI] [PubMed] [Google Scholar]
- 71.Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001;24:250–256 [DOI] [PubMed] [Google Scholar]
- 72.Tan LS. The clinical use of the 10g monofilament and its limitations: a review. Diabetes Res Clin Pract 2010;90:1–7 [DOI] [PubMed] [Google Scholar]
- 73.Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 2002;25:2048–2052 [DOI] [PubMed] [Google Scholar]
- 74.Herman WH, Pop-Busui R, Braffett BH, et al.; DCCT/EDIC Research Group . Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin CL, Albers J, Herman WH, et al.; DCCT/EDIC Research Group . Neuropathy among the Diabetes Control and Complications Trial cohort 8 years after trial completion. Diabetes Care 2006;29:340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pop-Busui R, Lu J, Lopes N, Jones TL; BARI 2D Investigators . Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst 2009;14:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singleton JR, Bixby B, Russell JW, et al. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst 2008;13:218–227 [DOI] [PubMed] [Google Scholar]
- 78.Zilliox LA, Ruby SK, Singh S, Zhan M, Russell JW. Clinical neuropathy scales in neuropathy associated with impaired glucose tolerance. J Diabetes Complications 2015;29:372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herder C, Bongaerts BW, Rathmann W, et al. Association of subclinical inflammation with polyneuropathy in the older population: KORA F4 study. Diabetes Care 2013;36:3663–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boulton AJ, Armstrong DG, Albert SF, et al.; American Diabetes Association; American Association of Clinical Endocrinologists . Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31:1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ziegler D, Keller J, Maier C, Pannek J; German Diabetes Association . Diabetic neuropathy. Exp Clin Endocrinol Diabetes 2014;122:406–415 [DOI] [PubMed] [Google Scholar]
- 82.Miller JD, Carter E, Shih J, et al. How to do a 3-minute diabetic foot exam. J Fam Pract 2014;63:646–656 [PubMed] [Google Scholar]
- 83.Boulton AJ, Kempler P, Ametov A, Ziegler D. Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev 2013;29:327–333 [DOI] [PubMed] [Google Scholar]
- 84.Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care 2011;34:2054–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oyibo SO, Prasad YD, Jackson NJ, Jude EB, Boulton AJ. The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: a pilot study. Diabet Med 2002;19:870–873 [DOI] [PubMed] [Google Scholar]
- 86.Griebeler ML, Morey-Vargas OL, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med 2014;161:639–649 [DOI] [PubMed] [Google Scholar]
- 87.Moulin D, Boulanger A, Clark AJ, et al.; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag 2014;19:328–335 [DOI] [PMC free article] [PubMed]
- 88.Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care 2008;31:1448–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005;115:254–263 [DOI] [PubMed] [Google Scholar]
- 90.Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev 2009;3):CD007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 2004;110:628–638 [DOI] [PubMed] [Google Scholar]
- 92.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 2005;6:253–260 [DOI] [PubMed]
- 93.Raskin P, Huffman C, Toth C, et al. Pregabalin in patients with inadequately treated painful diabetic peripheral neuropathy: a randomized withdrawal trial. Clin J Pain 2014;30:379–390 [DOI] [PubMed] [Google Scholar]
- 94.Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”--a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain 2013;154:2616–2625 [DOI] [PubMed] [Google Scholar]
- 95.Ziegler D, Duan WR, An G, Thomas JW, Nothaft W. A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain. Pain 2015;156:2013–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quilici S, Chancellor J, Löthgren M, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol 2009;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dworkin RH, Jensen MP, Gammaitoni AR, Olaleye DO, Galer BS. Symptom profiles differ in patients with neuropathic versus non-neuropathic pain. J Pain 2007;8:118–126 [DOI] [PubMed]
- 98.Goldstein DJ, Lu Y, Detke MJ, Hudson J, Iyengar S, Demitrack MA. Effects of duloxetine on painful physical symptoms associated with depression. Psychosomatics 2004;45:17–28 [DOI] [PubMed] [Google Scholar]
- 99.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005;116:109–118 [DOI] [PubMed] [Google Scholar]
- 100.Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006;67:1411–1420 [DOI] [PubMed] [Google Scholar]
- 101.Raskin J, Wang F, Pritchett YL, Goldstein DJ. Duloxetine for patients with diabetic peripheral neuropathic pain: a 6-month open-label safety study. Pain Med 2006;7:373–385 [DOI] [PubMed] [Google Scholar]
- 102.Hardy T, Sachson R, Shen S, Armbruster M, Boulton AJ. Does treatment with duloxetine for neuropathic pain impact glycemic control? Diabetes Care 2007;30:21–26 [DOI] [PubMed] [Google Scholar]
- 103.Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin 2011;27:151–162 [DOI] [PubMed] [Google Scholar]
- 104.Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care 2014;37:2302–2309 [DOI] [PubMed] [Google Scholar]
- 105.Adriaensen H, Plaghki L, Mathieu C, Joffroy A, Vissers K. Critical review of oral drug treatments for diabetic neuropathic pain-clinical outcomes based on efficacy and safety data from placebo-controlled and direct comparative studies. Diabetes Metab Res Rev 2005;21:231–240 [DOI] [PubMed] [Google Scholar]
- 106.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 1998;280:1831–1836 [DOI] [PubMed] [Google Scholar]
- 107.Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther 2003;25:81–104 [DOI] [PubMed] [Google Scholar]
- 108.Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs. amitriptyline in painful diabetic neuropathy: an open-label pilot study. J Pain Symptom Manage 2000;20:280–285 [DOI] [PubMed] [Google Scholar]
- 109.Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007;132:237–251 [DOI] [PubMed] [Google Scholar]
- 110.Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med 1999;159:1931–1937 [DOI] [PubMed] [Google Scholar]
- 111.Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. J Clin Neuromuscul Dis 2001;3:53–62 [DOI] [PubMed] [Google Scholar]
- 112.Max MB, Culnane M, Schafer SC, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987;37:589–596 [DOI] [PubMed] [Google Scholar]
- 113.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992;326:1250–1256 [DOI] [PubMed] [Google Scholar]
- 114.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev 2007;4:CD005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Biesbroeck R, Bril V, Hollander P, et al. A double-blind comparison of topical capsaicin and oral amitriptyline in painful diabetic neuropathy. Adv Ther 1995;12:111–120 [PubMed] [Google Scholar]
- 116.Boyle J, Eriksson ME, Gribble L, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care 2012;35:2451–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015;7:CD008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain 1991;45:3–9; discussion 1–2 [DOI] [PubMed] [Google Scholar]
- 119.Sindrup SH, Ejlertsen B, Frøland A, Sindrup EH, Brøsen K, Gram LF. Imipramine treatment in diabetic neuropathy: relief of subjective symptoms without changes in peripheral and autonomic nerve function. Eur J Clin Pharmacol 1989;37:151–153 [DOI] [PubMed] [Google Scholar]
- 120.Sindrup SH, Jensen TS. Pharmacologic treatment of pain in polyneuropathy. Neurology 2000;55:915–920 [DOI] [PubMed] [Google Scholar]
- 121.Joss JD. Tricyclic antidepressant use in diabetic neuropathy. Ann Pharmacother 1999;33:996–1000 [DOI] [PubMed] [Google Scholar]
- 122.Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014;9:CD011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Derry S, Wiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015;1:CD011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Glassman AH, Roose SP, Bigger JT Jr. The safety of tricyclic antidepressants in cardiac patients. Risk-benefit reconsidered. JAMA 1993;269:2673–2675 [PubMed] [Google Scholar]
- 125.Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther 2004;75:234–241 [DOI] [PubMed] [Google Scholar]
- 126.Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain 2004;110:697–706 [DOI] [PubMed] [Google Scholar]
- 127.Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology 2003;60:1284–1289 [DOI] [PubMed] [Google Scholar]
- 128.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 1992;260:275–285 [PubMed] [Google Scholar]
- 129.Freeman R, Raskin P, Hewitt DJ, et al.; CAPSS-237 Study Group . Randomized study of tramadol/acetaminophen versus placebo in painful diabetic peripheral neuropathy. Curr Med Res Opin 2007;23:147–161 [DOI] [PubMed] [Google Scholar]
- 130.Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998;50:1842–1846 [DOI] [PubMed] [Google Scholar]
- 131.Harati Y, Gooch C, Swenson M, et al. Maintenance of the long-term effectiveness of tramadol in treatment of the pain of diabetic neuropathy. J Diabetes Complications 2000;14:65–70 [DOI] [PubMed] [Google Scholar]
- 132.Gimbel JS, Richards P, Portenoy RK. Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology 2003;60:927–934 [DOI] [PubMed] [Google Scholar]
- 133.Watson CP, Moulin D, Watt-Watson J, Gordon A, Eisenhoffer J. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain 2003;105:71–78 [DOI] [PubMed] [Google Scholar]
- 134.Hanna M, O’Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur J Pain 2008;12:804–813 [DOI] [PubMed] [Google Scholar]
- 135.Sommer C, Welsch P, Klose P, Schaefert R, Petzke F, Häuser W. Opioids in chronic neuropathic pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration [published correction appears in Schmerz 2015;29:308]. Schmerz 2015;29:35–46 [in German] [DOI] [PubMed] [Google Scholar]
- 136.Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain 2007;11:490–518 [DOI] [PubMed] [Google Scholar]
- 137.Katz NP, Adams EH, Benneyan JC, et al. Foundations of opioid risk management. Clin J Pain 2007;23:103–118 [DOI] [PubMed] [Google Scholar]
- 138.Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med 2007;146:116–127 [DOI] [PubMed] [Google Scholar]
- 139.Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev 2012;7:CD008943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vileikyte L, Gonzalez JS. Recognition and management of psychosocial issues in diabetic neuropathy. Handb Clin Neurol 2014;126:195–209 [DOI] [PubMed] [Google Scholar]
- 141.Vinik A, Emir B, Cheung R, Whalen E. Relationship between pain relief and improvements in patient function/quality of life in patients with painful diabetic peripheral neuropathy or postherpetic neuralgia treated with pregabalin. Clin Ther 2013;35:612–623 [DOI] [PubMed] [Google Scholar]
- 142.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med 2005;352:1324–1334 [DOI] [PubMed] [Google Scholar]
- 143.Vileikyte L, Peyrot M, Gonzalez JS, et al. Predictors of depressive symptoms in persons with diabetic peripheral neuropathy: a longitudinal study. Diabetologia 2009;52:1265–1273 [DOI] [PubMed] [Google Scholar]
- 144.Vileikyte L, Peyrot M, Bundy C, et al. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care 2003;26:2549–2555 [DOI] [PubMed] [Google Scholar]
- 145.Vinik EJ, Hayes RP, Oglesby A, et al. The development and validation of the Norfolk QOL-DN, a new measure of patients’ perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther 2005;7:497–508 [DOI] [PubMed] [Google Scholar]
- 146.DCCT The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998;41:416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pop-Busui R, Low PA, Waberski BH, et al.; DCCT/EDIC Research Group . Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009;119:2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Low PA. Diabetic autonomic neuropathy. Semin Neurol 1996;16:143–151 [DOI] [PubMed] [Google Scholar]
- 149.Low PA, Benrud-Larson LM, Sletten DM, et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 2004;27:2942–2947 [DOI] [PubMed] [Google Scholar]
- 150.Jaiswal M, Divers J, Isom S, et al. Prevalence and correlates of cardiovascular autonomic neuropathy in youth with type 1 diabetes: SEARCH for Diabetes in Youth Study [Abstract]. Diabetes 2014;63 (Suppl. 1):A145 [Google Scholar]
- 151.Ziegler D, Voss A, Rathmann W, et al.; KORA Study Group . Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: the KORA S4 survey. Diabetologia 2015;58:1118–1128 [DOI] [PubMed] [Google Scholar]
- 152.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003;26:1895–1901 [DOI] [PubMed] [Google Scholar]
- 153.Pop-Busui R, Cleary PA, Braffett BH, et al.; DCCT/EDIC Research Group . Association between cardiovascular autonomic neuropathy and left ventricular dysfunction: DCCT/EDIC study (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications). J Am Coll Cardiol 2013;61:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pop-Busui R, Evans GW, Gerstein HC, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:1578–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Young LH, Wackers FJ, Chyun DA, et al.; DIAD Investigators . Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009;301:1547–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ziegler D, Zentai CP, Perz S, et al.; KORA Study Group . Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care 2008;31:556–561 [DOI] [PubMed] [Google Scholar]
- 157.Lykke JA, Tarnow L, Parving HH, Hilsted J. A combined abnormality in heart rate variation and QT corrected interval is a strong predictor of cardiovascular death in type 1 diabetes. Scand J Clin Lab Invest 2008;68:654–659 [DOI] [PubMed] [Google Scholar]
- 158.Lonn EM, Rambihar S, Gao P, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol 2014;103:149–159 [DOI] [PubMed] [Google Scholar]
- 159.Kadoi Y. Perioperative considerations in diabetic patients. Curr Diabetes Rev 2010;6:236–246 [DOI] [PubMed] [Google Scholar]
- 160.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH; EURODIAB Prospective Complications Study Group . Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008;31:1360–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marques JL, George E, Peacey SR, et al. Altered ventricular repolarization during hypoglycaemia in patients with diabetes. Diabet Med 1997;14:648–654 [DOI] [PubMed] [Google Scholar]
- 162.Robinson RT, Harris ND, Ireland RH, Macdonald IA, Heller SR. Changes in cardiac repolarization during clinical episodes of nocturnal hypoglycaemia in adults with type 1 diabetes. Diabetologia 2004;47:312–315 [DOI] [PubMed] [Google Scholar]
- 163.Koivikko ML, Salmela PI, Airaksinen KE, et al. Effects of sustained insulin-induced hypoglycemia on cardiovascular autonomic regulation in type 1 diabetes. Diabetes 2005;54:744–750 [DOI] [PubMed] [Google Scholar]
- 164.Koivikko ML, Tulppo MP, Kiviniemi AM, et al. Autonomic cardiac regulation during spontaneous nocturnal hypoglycemia in patients with type 1 diabetes. Diabetes Care 2012;35:1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jaiswal M, McKeon K, Comment N, et al. Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care 2014;37:2616–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 2006;29:334–339 [DOI] [PubMed] [Google Scholar]
- 167.Orlov S, Cherney DZ, Pop-Busui R, et al. Cardiac autonomic neuropathy and early progressive renal decline in patients with nonmacroalbuminuric type 1 diabetes. Clin J Am Soc Nephrol 2015;10:1136–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wheelock KM, Jaiswal M, Martin CL, et al. Cardiovascular autonomic neuropathy associates with nephropathy lesions in American Indians with type 2 diabetes. J Diabetes Complications 2016;30:873–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–1065 [PubMed] [Google Scholar]
- 170.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 2010;33:434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O’Brien PC, Low PA. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology 1999;52:523–528 [DOI] [PubMed] [Google Scholar]
- 172.The Consensus Committee of the American Autonomic Society and the American Academy of Neurology Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996;46:1470. [DOI] [PubMed] [Google Scholar]
- 173.Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med 2008;358:615–624 [DOI] [PubMed] [Google Scholar]
- 174.Spallone V, Bellavere F, Scionti L, et al.; Diabetic Neuropathy Study Group of the Italian Society of Diabetology . Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis 2011;21:69–78 [DOI] [PubMed] [Google Scholar]
- 175.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA; Midodrine Study Group . Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. JAMA 1997;277:1046–1051 [PubMed] [Google Scholar]
- 176.Low PA, Tomalia VA. Orthostatic hypotension: mechanisms, causes, management. J Clin Neurol 2015;11:220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am 2015;44:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Melton LJ 3rd, Talley NJ. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol 2012;107:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med 2007;356:820–829 [DOI] [PubMed] [Google Scholar]
- 180.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology . Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013;108:18–37; quiz 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med 2001;161:1989–1996 [DOI] [PubMed] [Google Scholar]
- 182.Schvarcz E, Palmér M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology 1997;113:60–66 [DOI] [PubMed] [Google Scholar]
- 183.American Diabetes Association. Standards of medical care in diabetes-2015: summary of revisions. In Standards of Medical Care in Diabetes—2015 Diabetes Care 2015;38(Suppl. 1):S4. [DOI] [PubMed] [Google Scholar]
- 184.Hasler WL, Parkman HP, Wilson LA, et al.; NIDDK Gastroparesis Clinical Research Consortium . Psychological dysfunction is associated with symptom severity but not disease etiology or degree of gastric retention in patients with gastroparesis. Am J Gastroenterol 2010;105:2357–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Samsom M, Vermeijden JR, Smout AJ, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care 2003;26:3116–3122 [DOI] [PubMed] [Google Scholar]
- 186.Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil 2010;22:113–133 [DOI] [PMC free article] [PubMed]
- 187.Fraser R, Horowitz M, Dent J. Hyperglycaemia stimulates pyloric motility in normal subjects. Gut 1991;32:475–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schvarcz E, Palmér M, Aman J, Lindkvist B, Beckman KW. Hypoglycaemia increases the gastric emptying rate in patients with type 1 diabetes mellitus. Diabet Med 1993;10:660–663 [DOI] [PubMed] [Google Scholar]
- 189.Schirra J, Nicolaus M, Roggel R, et al. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 2006;55:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Murphy DB, Sutton JA, Prescott LF, Murphy MB. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology 1997;87:765–770 [DOI] [PubMed] [Google Scholar]
- 191.Pop-Busui R, Stevens M. Autonomic neuropathy in diabetes. In Therapy for Diabetes Mellitus and Related Disorders. 6th ed. Umpierrez GE, Ed. Alexandria, VA, American Diabetes Association, 2014, p. 834–863 [Google Scholar]
- 192.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54–61 [DOI] [PubMed] [Google Scholar]
- 193.Giugliano F, Maiorino M, Bellastella G, Gicchino M, Giugliano D, Esposito K. Determinants of erectile dysfunction in type 2 diabetes. Int J Impot Res 2010;22:204–209 [DOI] [PubMed] [Google Scholar]
- 194.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ; Urologic Diseases in America Project . Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med 2006;166:207–212 [DOI] [PubMed] [Google Scholar]
- 195.Enzlin P, Mathieu C, Van den Bruel A, Bosteels J, Vanderschueren D, Demyttenaere K. Sexual dysfunction in women with type 1 diabetes: a controlled study. Diabetes Care 2002;25:672–677 [DOI] [PubMed] [Google Scholar]
- 196.Enzlin P, Rosen R, Wiegel M, et al.; DCCT/EDIC Research Group . Sexual dysfunction in women with type 1 diabetes: long-term findings from the DCCT/EDIC study cohort. Diabetes Care 2009;32:780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Esposito K, Maiorino MI, Bellastella G, Giugliano F, Romano M, Giugliano D. Determinants of female sexual dysfunction in type 2 diabetes. Int J Impot Res 2010;22:179–184 [DOI] [PubMed] [Google Scholar]
- 198.Mazzilli R, Imbrogno N, Elia J, et al. Sexual dysfunction in diabetic women: prevalence and differences in type 1 and type 2 diabetes mellitus. Diabetes Metab Syndr Obes 2015;8:97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rutte A, van Splunter MM, van der Heijden AA, et al. Prevalence and correlates of sexual dysfunction in men and women with type 2 diabetes. J Sex Marital Ther 2015;41:680–690 [DOI] [PubMed] [Google Scholar]
- 200.Pop-Busui R, Hotaling J, Braffett BH, et al.; DCCT/EDIC Research Group . Cardiovascular autonomic neuropathy, erectile dysfunction and lower urinary tract symptoms in men with type 1 diabetes: findings from the DCCT/EDIC. J Urol 2015;193:2045–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fedele D, Coscelli C, Santeusanio F, et al. Erectile dysfunction in diabetic subjects in Italy. Gruppo Italiano Studio Deficit Erettile nei Diabetici. Diabetes Care 1998;21:1973–1977 [DOI] [PubMed] [Google Scholar]
- 202.Pop-Busui R, Braffett BH, Hotaling J, et al. Diabetic neuropathy and urologic complications in men with type 1 diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Study (DCCT/EDIC) [Abstract]. Diabetes 2014;63 (Suppl. 1):A149–A580 [Google Scholar]
- 203.Gazzaruso C, Giordanetti S, De Amici E, et al. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. Circulation 2004;110:22–26 [DOI] [PubMed] [Google Scholar]
- 204.Van Den Eeden SK, Sarma AV, Rutledge BN, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Research Group . Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 2009;32:664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wessells H, Penson DF, Cleary P, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Effect of intensive glycemic therapy on erectile function in men with type 1 diabetes. J Urol 2011;185:1828–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: relationship to autonomic neuropathy detected by sympathetic skin response. J Urol 1997;157:580–584 [DOI] [PubMed] [Google Scholar]
- 207.Pontiroli AE, Cortelazzi D, Morabito A. Female sexual dysfunction and diabetes: a systematic review and meta-analysis. J Sex Med 2013;10:1044–1051 [DOI] [PubMed] [Google Scholar]
- 208.Barry MJ, Fowler FJ Jr, O’Leary MP, et al.; The Measurement Committee of the American Urological Association . The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 1992;148:1549–1557; discussion 1564 [DOI] [PubMed] [Google Scholar]
- 209.Smith AG, Lessard M, Reyna S, Doudova M, Singleton JR. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications 2014;28:511–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther 2013;15:948–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shaw JE, Parker R, Hollis S, Gokal R, Boulton AJ. Gustatory sweating in diabetes mellitus. Diabet Med 1996;13:1033–1037 [DOI] [PubMed] [Google Scholar]
- 212.Shaw JE, Abbott CA, Tindle K, Hollis S, Boulton AJ. A randomised controlled trial of topical glycopyrrolate, the first specific treatment for diabetic gustatory sweating. Diabetologia 1997;40:299–301 [DOI] [PubMed] [Google Scholar]
- 213.Smith BE. Focal and entrapment neuropathies. Handb Clin Neurol 2014;126:31–43 [DOI] [PubMed] [Google Scholar]
- 214.Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve 2002;25:477–491 [DOI] [PubMed] [Google Scholar]
- 215.Massie R, Mauermann ML, Staff NP, et al. Diabetic cervical radiculoplexus neuropathy: a distinct syndrome expanding the spectrum of diabetic radiculoplexus neuropathies. Brain 2012;135:3074–3088 [DOI] [PubMed] [Google Scholar]
- 216.Laughlin RS, Dyck PJ. Electrodiagnostic testing in lumbosacral plexopathies. Phys Med Rehabil Clin N Am 2013;24:93–105 [DOI] [PubMed] [Google Scholar]
- 217.Chan YC, Lo YL, Chan ES. Immunotherapy for diabetic amyotrophy. Cochrane Database Syst Rev 2012;6:CD006521. [DOI] [PubMed] [Google Scholar]
- 218.Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015;138:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010;15:202–207 [DOI] [PubMed] [Google Scholar]
- 220.Lauria G, Devigili G. Skin biopsy as a diagnostic tool in peripheral neuropathy. Nat Clin Pract Neurol 2007;3:546–557 [DOI] [PubMed] [Google Scholar]
- 221.Lauria G, Hsieh ST, Johansson O, et al.; European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:903–912,e944–e909
- 222.Chen X, Graham J, Dabbah MA, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 2015;38:1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 1997;20:1561–1568 [DOI] [PubMed] [Google Scholar]
- 224.Pop-Busui R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. J Cardiovasc Transl Res 2012;5:463–478 [DOI] [PMC free article] [PubMed] [Google Scholar]