Abstract
Thomson navel orange peel is a by‐product of citrus processing, which contains high levels of bioactive compounds advantageous to human health, nevertheless due to its high moisture content it is exceedingly perishable. Drying is among the most common preservation methods, which could prolong the plants shelf‐life via reducing their moisture value. Taking this into account, depending on their type and conditions, drying techniques could degrade plant heat‐sensitive metabolites and lead to quality decline. Therefore, the goal of this paper was to investigate the influence of seven drying methods named sun, shade, oven, vacuum oven, microwave, and freeze‐drying with different drying conditions on the physical properties, for example, bulk density and color (L*, a*, b*, ΔE, and browning index (BI)) and essential oil characteristics such as extraction yield, chemical composition, antioxidant (total phenolic content (TPC), DPPH, and FRAP essays), and antimicrobial (MIC and MBC) activities of Thomson peel and determine the superior drying procedure. Results showed that freeze‐dried sample had the highest retention of L* (48.54) and b* (49.00) values, lowest BI (216.11) as well as highest EO extraction yield (6.90%), TPC (60.10 GAE/100 g), FRAP (0.52% at 80 mg/ml), and lowest IC50 (5.00 mg/ml), MIC and MBC compared with other drying treatments. Therefore, it could be inferred that freeze‐drying is the most efficient drying approach in respect of preserving both physical and EO attributes of Thomson peel.
Keywords: antimicrobial, antioxidant, drying, essential oil, Thomson peel
In this research, chemical composition and biological activity of Thomson navel orange peel were analyzed under different drying conditions. Various drying methods increased the monoterpene hydrocarbons of essential oil. Freeze‐dried peels had the highest retention of L* and the lowest formation of brown pigments. Freeze‐dried peels is the best method to maintain antioxidant and antimicrobial activity.
![]()
1. INTRODUCTION
Orange, Citrus sinensis L. of the family Rutaceae, is one of the most abundant fruit crops in the world and is well‐received by consumers due to its attractive color, pleasant aroma, and flavor (Juhaimi, Matthäus, Özcan, & Ghafoor, 2016; Matthaus & Özcan, 2012; Smeriglio et al., 2019). Among the most popular orange varieties, Thomson navel (Citrus sinensis L. Osbeck) is the one that has good economic value and is widely cultivated in countries like Iran, China, India, USA, and Brazil (Faostat, 2018; de la Torre et al., 2019). On a worldwide scale, around 40% of the orange production is utilized in orange juice processing, which generates an enormous amount of wastes (mostly peels) on an average mass of 0.5 kg/kg of raw orange (Smeriglio et al., 2019). Therefore, orange peels as the primary waste have been either discarded which may cause environmental pollutions or used as molasses for animal feed (Gavahian, Chu, & Mousavi Khaneghah, 2019). However, in the outer layer of the orange peels known as flavedo, within a large number of very small glands, essential oils (EOs) are placed (de la Torre et al., 2019). Over the past few years, the biological activities (e.g., antioxidant, anti‐inflammatory, antiaging, antibacterial, antifungal, and anti‐aflatoxigenic activities) of orange peel EOs have been specified (Barreca et al., 2017; Celano et al., 2019; Hasija, Ibrahim, & Wadia, 2015; Kamal, Ashraf, Hussain, Shahzadi, & Chughtai, 2013) which are strongly related to various constituents of these volatile oils including hydrocarbons, alcohols, esters and aldehydes (Geraci, Di Stefano, Di Martino, Schillaci, & Schicchi, 2017). Therefore, the problem of wasted orange peels could be turned into an asset, if potentially marketable proceedings such as EO extraction occurs (Gavahian et al., 2019). Nevertheless, the most critical challenge in regard to orange peels is their high moisture content (75%–90%), which makes them highly perishable with very low storage life (de la Torre et al., 2019). Hence, in order to preserve them for future utilization, their water levels required to diminish. Drying is commonly used to preserve fresh plant materials, and it can be carried out at different temperatures and relative humidity conditions (Samadi, Larijani, Naghdi Badi, & Mehrafarin, 2018). The removal of moisture from plants, primarily retards many of the moisture‐mediated deteriorative reactions and prevents the growth and reproduction of microorganisms (Naidu et al., 2016). Likewise, it has been acknowledged that moisture reduction could cause a remarkable boost in extraction yield of plant's EOs (Franco‐Vega, Ramírez‐Corona, Palou, & López‐Malo, 2016). It is worth noting that dried orange peels could also be employed in food formulation applications such as dairy products, beverages, bakery products, and candy industries (Ghanem, Mihoubi, Kechaou, & Mihoubi, 2012). There are different drying processes such as natural sun drying (SD), shade drying (ShD), oven drying (OD), vacuum oven drying (VOD), microwave drying (MW), and freeze‐drying (FD) (Xing et al., 2017). Each has a different mechanism by transferring different energies at various speeds and times into the product, which would lead to multiple irreversible chemical and biological reactions accompanied by several structural, physical, and mechanical alterations (Xing et al., 2017). Therefore, it is necessary to evaluate the influence of different drying methods on Thomson peels before selecting a desirable method for commercial drying.
So far as we know, there are several studies on the chemical identification and antioxidant abilities of EOs retrieved from fresh Thomson peels (Kamal et al., 2013; Kirbaslar, Kirbaslar, Pozan, & Boz, 2009; Nekoei & Mohammadhosseini, 2014; Njoroge, Phi, & Sawamura, 2009). However, no information is available concerning the effect of various drying methods on the physical characteristics of dried Thomson peel and its EOs quality and quantity. Thus, this work was carried out to determine the possible effect of drying techniques on the physical aspects of dried matter, and consequently on the chemical profile, antioxidant and antibacterial activities of EOs extracted from this valuable by‐product.
2. MATERIALS AND METHODS
2.1. Materials
The study was carried out in October 2018 from one selected orchards of Amol, Northern Iran (36◦46'N and 52◦35'E, around 76 m above sea level), and fruits were harvested at commercially mature stage (about 7.5–8.0 months after flowering, fully colored) from approximately 12‐to 15‐year‐old trees on the third week of October. After collection, oranges were brought to a laboratory at Sari Agricultural Sciences and Natural Resources University, Mazandaran province and were rapidly processed on the same day. They were washed under running tap water and patted dry in an attentive manner. The flavedo of fruits was carefully removed with a manual peeler and cut into small pieces. With the intention of preserving their original freshness, collected samples were stored in a refrigerator at 4°C until used in the drying experiments. All chemicals and solvents applied in this research were purchased from Merck (Darmstadt, Germany).
2.2. Drying of fresh orange peels
Samples were randomly divided into ten batches each containing 50 g orange peels. One was used for fresh analysis, and the remaining parts were dried by using the following techniques: (a) shade‐dried samples were attained under natural air flow at room temperature (20°C ± 5°C) up to 60 hr; (b) for the purpose of obtaining sun‐dried samples, the plants were left under direct sun/day light at temperatures between 25 and 37°C for 36 hr; (c) conventional oven was applied in a laboratory oven (BM55E, Fan Azma Gostar Co.) at two temperatures of 45 and 60°C for 5 and 4 hr, respectively; (d) a vacuum oven (VS‐1202, Vision Scientific Co. Ltd.) was utilized for VOD at 45°C and 60°C up to 48 and 36 hr, respectively; (e) a microwave oven (MA3884VC, LG Electronics Co.) with a maximum power output of 900 W, which was equipped with a swivel tray plus digital setting for power and time, was applied for drying the samples. The samples were placed in a commercial microwave oven, and drying was done at two different microwave power levels of 360 and 600 W for 35 and 20 min, respectively; (f) the samples were dried by a freeze‐drier (Vaco 2 zirbus) at a temperature of −50°C and pressure of 0.125 mbar till 24 hr for the sake of obtaining freeze‐dried samples. Finally, samples were ground into a fine powder by a Bosch MKM6000 laboratory mill (Bosch Instruments) and passed through a laboratory screen mesh no.16 and stored in bags at −18°C until analyzed. The initial moisture content of fresh Thomson peels was 77.3 ± 0.9% (wet basis), and all samples were dried to reach a constant weight.
2.3. Physical properties of dried powders
2.3.1. Bulk density
Bulk density was determined by pouring 5 g of dried Thomson peel powder into an empty 25 ml glass cylinder, and then a gentle tapping of the cylinder was applied until a negligible difference in volume of samples was observed. Bulk density was determined by dividing the weight of sample to its volume and expressed as grams per milliliter (g/ml) (Razavi & Farahmandfar, 2008).
2.3.2. Color measurement
Color alterations in dried Thomson peel samples were analyzed by measuring the parameters of L* a* b* using the IMG‐Pardazesh Cam‐System colorimeter, which was calibrated by a standard calibration plate of a white surface provided by the manufacturer. L* indicates the darkness‐lightness, a* greenness‐redness, and b* blueness‐yellowness of samples. The total color variation index (ΔE) was determined by Equation (1), where the subscript “0” in equation refers to the color of fresh Thomson peel (Cserhalmi, Sass‐Kiss, Tóth‐Markus, & Lechner, 2006):
| (1) |
Variations in perceptible color, expressed as ΔE, can be categorized analytically as not notable (0–0.5), slightly notable (0.5–1.5), notable (1.5–3.0), readily visible (3.0–6.0), and great (6.0–12.0).
Furthermore, browning index (BI) was calculated using measured L*, a*, and b* values according to Equation (2) (Pathare, Opara, & Al‐Said, 2013):
| (2) |
Where
| (3) |
2.4. Essential oils analysis
2.4.1. Essential oil extraction
For the intention of extracting EOs via hydrodistillation process under optimal operating conditions, 50 g of Thomson peels was added to 150 ml distilled water in a 500‐ml glass boiling bottle. Next, the clevenger apparatus was placed in a balloon heater and the set was attached to a cold water flow with a view to ensure condensation of EOs. Extraction process was carried out up to 3 hr which after completion, two phases were observed, an organic yellowish phase (EO) with lower density than water and an aqueous phase (aromatic water). Eventually, the EOs were collected, dried under anhydrous sodium sulfate and stored in sealed vials in the dark at 4°C till analysis. Experiments were conducted three times for each treatment. The extraction yield of Thomson peel EOs was calculated according to Equation (4), and it was expressed as ml/g sample (Farahmandfar, Asnaashari, Pourshayegan, Maghsoudi, & Moniri, 2018):
| (4) |
2.4.2. GC‐MS volatile compounds determination
A gas chromatograph (Model 7890A), fitted with an HP‐5 capillary column (0.25 mm i.d. × 30 m length × 25 mm film thickness) coupled to a 5975C mass selective detector quadrupole (Agilent Technologies), was utilized for GC‐MS analysis. The conditions for GC‐MS analysis were in the following terms: helium as carrier gas with a flow rate of 1 ml/min, injection volume of 1 μl, injection temperature of 240°C, split ratio of 70:1, a temperature program, starting at 60°C, hold for 2 min, then increased at 5°C/min till reached 220°C, and ionization energy of 70 eV. The identification of EO constituents was performed by comparison of their kovats retention indices (RI) and retention times (RT) with National Institute of Standards and Technology (NIST 11.0) mass‐spectral libraries and previous literatures. The kovats retention indices were determined using the Dool (1963) equation (Equation 5), and a homologous series of n‐alkanes (C8–C18) injected under the chromatography conditions described above. The quantitative analysis of EO compounds, expressed in percentage, was carried out via the normalization method of the FID peak areas as indicated by Zhang, Chen, Wang, and Yao (2006).
| (5) |
where RI is retention index, x is the target compound, n 0 is n‐alkane directly eluting before x, n 1 is n‐alkane directly eluting after x and RT is the retention time.
2.4.3. Determination of total phenolic content
Total phenol content (TPC) was evaluated by using the method of Farahmandfar et al. (2018). Briefly, 15 μL of appropriately diluted sample was mixed with 75 μL of Folin‐Ciocalteu reagent and 1,185 μL of distilled water in a falcon tube, and after standing for 2–3 min at room temperature, 225 μL of sodium carbonate solution (20%) was added. After incubation of the mixture at room temperature for 20 min in the dark, the absorbance was then read at 750 nm using a spectrophotometer (T80+, PG Instruments Ltd.). Gallic acid was utilized for standard calibration, and the amount of total phenolic was displayed as milligrams of gallic acid equivalents (GAE)/ 100 g sample.
2.4.4. Determination of DPPH radical scavenging activity
This method is based on the measurement of the scavenging ability of antioxidants toward the stable radical DPPH (1,1‐diphenyl‐2‐picrylhydrazyl), and it was conducted according to Farahmandfar, Asnaashari, and Bakhshandeh (2019) with slight modification. Briefly, 2 ml of various dilutions of the test samples was mixed with 2 ml of a 100 μM methanolic DPPH solution. The solution kept for 20 min in the dark, and then its absorbance was measured at 517 nm using a spectrophotometer (T80+, PG Instruments Ltd.). Inhibition of free radical DPPH was calculated using following equation:
| (6) |
where A sample is the absorbance of the solution when the EOs have been added at different concentrations and A blank is the absorbance of the DPPH solution. Likewise, IC50 values of treatments which denote the required concentration of a sample to scavenge 50% of DPPH free radicals, were measured.
2.4.5. Determination of ferric reducing antioxidant potential
The Ferric reducing antioxidant potential (FRAP) of the EOs was measured according to the method of Maurya and Devasagayam (2010). Sample solution (2.5 ml) at different concentrations was combined with 2.5 ml of 200 mmol/L sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide. Then, the mixture was incubated at 50°C for 20 min. After that, 2.5 ml of 10% trichloroacetic acid (w/v) was added to the mixture and then was centrifuged at 3,000 rpm for 8 min (HERMEL Z 9 200A centrifuge). Five milliliters of the upper layer was mixed with 5 ml of deionized water and 1 ml of 0.1% ferric chloride. Finally, the absorbance values of the solutions were read spectrophotometrically at 700 nm. The solution with the higher absorbance value shows higher reducing potential.
2.4.6. Determination of minimum inhibitory concentration and minimum bactericide concentration
The antimicrobial activity of Thomson peel EOs was examined against two gram‐negative bacterial strains (Pseudomonas aeruginosa ATCC 9,027 and Escherichia coli ATCC 35,218) and two gram‐positive bacterial strains (Staphylococcus aureus ATCC 25,904 and Listeria monocytogenes ATCC 19,115). Bacterial strains were suspended in ringer solution until the turbidity equal to 0.5 McFarland (1.5 × 108 CFU/ml). The MIC value was determined by the protocols of National Committee for Clinical Laboratory Standards (NCCLS). The broth microdilution method was done using a sterilized 96‐well plate and triphenyl tetrazolium chloride indicator. Microplates containing serial dilutions of peel EOs (0.62, 1.25, 2.5, 5, 10, 20, 40, 80 mg/ml) were prepared, and each well was inoculated with bacterial strains to yield the appropriate density in 100 μl Mueller‐Hinton broth. Then, the 96‐well plate was incubated at 37°C for 24 hr. Minimum inhibitory concentration (MIC) was reported as the lowest concentration of EOs which bacterial growth did not occur and no visible changes being detected in the broth medium (Behbahani, Shahidi, Yazdi, Mortazavi, & Mohebbi, 2017). In relation to minimum bactericidal concentration (MBC) evaluation, all wells in which microbial growth was not observed (opacity‐free), were cultured on Mueller‐Hinton agar and incubated at 37°C for 24 hr. MBC was the lowest concentration in which an antimicrobial agent would kill a particular microorganism (Humeera et al., 2013).
2.5. Statistical analysis
Experiments were performed in triplicates, and the results are expressed as means ± standard deviation. Results were subjected to one‐way analysis of variance (ANOVA) using the SPSS version 24.0 software (SPSS Inc.). Statistical significance of differences between samples was accepted at p < .05 using the Duncan's multiple range test. In addition, a correlation analysis between total phenolic content and each of the antioxidant capacity assays was performed with the Pearson's test.
3. RESULTS AND DISCUSSION
3.1. Physical properties
3.1.1. Bulk density
Bulk density is an important indicator of transport cost and packaging considerations. A dry product with high bulk density can be stored in smaller containers than a similar product with lower density (Razavi & Farahmandfar, 2008). Bulk density is well correlated with particle size of dried powders, where smaller powder particles would reduce the porosity and enhance the coherence of dried sample which would lead to a denser product (Sogi, Garg, & Bawa, 2002). According to Table 1, the amount of bulk density in Thomson peel dried powders varied from 1.79 to 2.69 g/ml, highest belonged to MW 360 W and OD 45°C samples and the lowest to FD sample. A similar result in the study of Michalska, Wojdyło, Lech, Łysiak, and Figiel (2016) was observed where MW and OD samples had higher bulk density than other treatments. It was explained that in case of MW, high temperature would cause partial carbonizing of the product, which results in a higher particle/air ratio expressed as bulk density. As far as OD is concerned, application of hot air would cause a considerable shrinkage and collapse of the cell walls which would induce tensions in cellular structure, causing a decline in cell size, roundness, compactness and an increase in elongation (Karam, Petit, Zimmer, Djantou, & Scher, 2016). Though, there was no significant different between bulk densities of OD 45°C and VOD 45°C samples (p > .05), applying vacuum in higher drying temperature (60°C) created product with 5.5% lower bulk density than OD 60°C sample (Table 1). Vacuum would allow faster moisture transfer to the surrounding of material and would prevent structural collapse. This process, well‐known as the puffing phenomenon, engenders a porous texture, and thereby it could reduce the material's density (Chong, Figiel, Law, & Wojdyło, 2014). The content of bulk density (1.79 g/ml) in FD powder was the lowest observed among the samples (Table 1). FD would cause the ice in the fresh sample to sublime directly to vapor, which protect the primary structure and shape with minimal shrinkage. Generally, there is a negative correlation between drying temperature and the bulk density as the temperate raises bulk density would reduce (Karam et al., 2016). That was the case for MW as the power increased from 360 to 600 W, bulk density decreased from 2.69 to 2.53 g/ml (Table 1). Similar results were obtained by Horuz and Maskan (2015), which they clarified that, increment in microwave power generally reduces the bulk density of dried products through enhancement of puffing. However, that was not the case in OD and VOD treatments where increase in temperature (from 45 to 60°C) enhanced the bulk density up to 8.09% and 4.54%, respectively. This indicates that higher temperatures not always reduces the density of a material, in some cases depending on plant physiology it would lead to higher shrinkage and cell expansion and eventually a denser product (Argyropoulos, Heindl, & Müller, 2011).
Table 1.
Bulk density and color values of dried and fresh samples of Thomson peel
| Bulk density (g/ml) | Color measurement | |||||
|---|---|---|---|---|---|---|
| L | a | b | ΔE | BI | ||
| SD | 2.39 ± 0.03c | 41.24 ± 4.72cd | 8.86 ± 0.58b | 42.94 ± 2.91c | 23.46 ± 3.84bcd | 244.40 ± 21.95abc |
| ShD | 2.10 ± 0.08d | 37.84 ± 1.23cd | 7.09 ± 0.61c | 40.50 ± 1.37cd | 27.78 ± 2.63bc | 254.58 ± 10.62ab |
| OD 45 | 2.47 ± 0.05bc | 38.17 ± 1.24c | 5.60 ± 0.39e | 40.80 ± 0.84c | 28.16 ± 2.42b | 251.59 ± 8.49b |
| OD 60 | 2.67 ± 0.03a | 40.03 ± 1.63c | 8.61 ± 0.35b | 41.67 ± 1.05c | 25.06 ± 0.40c | 242.22 ± 9.26b |
| VOD 45 | 2.42 ± 0.03c | 30.36 ± 0.67e | 7.93 ± 0.80bc | 31.04 ± 0.88e | 38.41 ± 1.42a | 236.10 ± 5.55bc |
| VOD 60 | 2.53 ± 0.03b | 28.36 ± 0.96f | 8.13 ± 0.60bc | 30.28 ± 0.80e | 40.16 ± 1.38a | 260.79 ± 32.56abc |
| MW 360 | 2.69 ± 0.03a | 37.57 ± 1.77cd | 5.84 ± 0.61de | 39.71 ± 1.10cd | 29.05 ± 2.74b | 246.36 ± 11.98b |
| MW 600 | 2.53 ± 0.04b | 35.37 ± 1.49d | 7.03 ± 0.71cd | 38.91 ± 1.00d | 30.32 ± 1.40b | 271.14 ± 10.24a |
| FD | 1.79 ± 0.03e | 48.54 ± 0.69b | 1.79 ± 0.37f | 49.00 ± 1.10b | 22.04 ± 2.38d | 216.11 ± 16.64c |
| Fresh | – | 53.99 ± 0.91a | 20.95 ± 1.86a | 58.31 ± 1.10a | – | 270.34 ± 14.64ab |
All values are expressed as mean ± standard deviation. Means (n = 3) having different letters within the same column differ significantly at p < .05.
Abbreviations: –, not determined; BI, Browning index; SD, Sun drying; ShD, Shade drying; OD 45 and 60, Oven drying at 45°C and 60°C; VOD 45 and 60, Vacuum oven drying at 45°C and 60°C; MW 360 and 600, Microwave drying at 360 W and 600 W; FD, Freeze‐drying.
3.1.2. Color
Color is a critical quality index which influences consumer reception and the market value of the dried materials (M’hiri, Ghali, Nasr, & Boudhrioua, 2018). The color parameters (L*, a*, b*, ΔE and BI) and the appearance alterations of dried fruit peels are presented in Table 1 and Figure 1, respectively. The chromatic parameters L* (darkness/brightness), a* (greenness/redness), and b* (blueness/yellowness) values of fresh Thomson peel were 53.99, 20.95, and 58.31, respectively. All color values of all dried samples declined considerably in comparison with the fresh Thomson peel (Table 1). Among dried samples, VOD at 60°C was the darkest with lowest yellowness and highest ΔE value which is in agreement with results of Zhang, Liu, and Gao (2018), where least amount of L*, b*, and ΔE values were observed in VOD sample. This could be due to the low vacuum pressure of the operation, which caused longer drying times and as a result of exposing to relatively high temperature for a long period of time, higher color deterioration occurred in this method (Arslan, Özcan, & Mengeş, 2010). On the other hand, FD was the best option to preserve majority of color values with the highest lightness (L* = 48.54), yellowness (b* = 49.00) and lowest redness (a* = 1.79). This was in accordance with the findings of Zhang et al. (2018), where FD had the highest content of lightness and yellowness along with lowest redness in Angelica keiskei compared with other drying procedures. They acknowledged that FD is the most appropriate technique for retention of fruits and vegetables color quality due to its exceptionally low drying temperature which could preserve pigments responsible for the yellow color like carotenoids and flavonoids after thermal treatment (Ghanem, Mihoubi, Kechaou, & Mihoubi, 2012). ΔE is another color index, which shows the degree of overall color change in dried samples compared with the color of fresh Thomson peel. The last color parameter is BI which shows the purity of brown color and is reported as an important parameter in processes where enzymatic and nonenzymatic browning takes place (Saricoban & Yilmaz, 2010). Highest BI belonged to MW 600 W sample (271.14), which may be due to nonenzymatic Maillard browning, and formation of brown pigments at higher drying temperature of MW treatment (Bal, Kar, Satya, & Naik, 2011). FD sample indicated lowest BI value (216.11) which proved that it is the best drying treatment in terms of minimal color deterioration. It should be noted that BI content of fresh material was comparable with all dried powders (p > .05) with the exception of FD sample. Fresh Thomson peel contains high amount of moisture which due to its high availability it could promote enzymatic degradation and it might lead to enhance brown color in fresh material (Chua, Chong, Chua, & Figiel, 2019).
Figure 1.
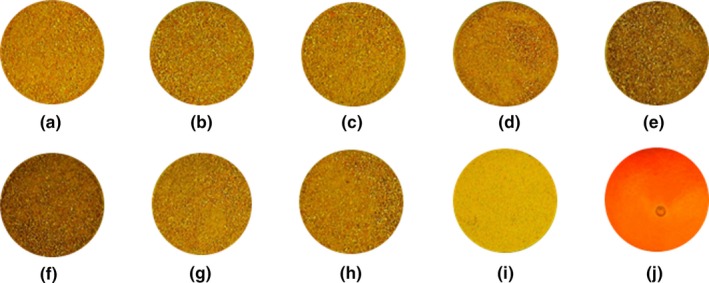
Appearance of Thomson peel samples: (a) sun drying; (b) shade drying; (c) oven drying 45°C; (d) oven drying 60°C; (e) vacuum oven drying 45°C; (f) vacuum oven drying 60°C; (g) microwave drying 360 W; (h) microwave drying 600 W; (i) freeze‐drying and (j) fresh
3.2. Essential oils characteristics
3.2.1. Essential oil yield
The results showed that various drying methods notably effects the EO content of Thomson peel (Figure 2) and depending on the type of drying method, duration, and temperature both increment and reduction in the quantities of EO yields were observed (Rahimmalek & Goli, 2013). The highest EO yield (6.90% v/w) obtained via FD whereas the lowest EO yield (1.20% v/w) was noticed in fresh sample. These results were in agreement with the study of Rahimmalek and Goli (2013) where FD sample of Thymus daenensis subsp. daenensis had the highest EO yield compared with other drying techniques, and it can be explained by the FD temperature which is the lowest among drying treatments. Thus, it could maintain more EOs in the dried sample and preserve aromatic compounds from diffusion into the atmosphere (Rahimmalek & Goli, 2013). As far as fresh sample is concerned, the lowest extraction yield could be due to its large particle size. All dried samples were ground and screened by a standard sieve; however, fresh sample merely got chopped to small pieces and due to its high moisture content grinding process was not possible as a sticky paste would be formed. Accordingly, it has been acknowledged in previous studies that there is a negative correlation between particle size and extraction efficiency where in larger particle size, solvent diffusion in solid material would decrease due to low surface to volume ratio and it would reduce EO extraction (Eikani, Golmohammad, & Rowshanzamir, 2007). Moreover, increasing the OD temperature (from 45 to 60°C) significantly decreased the EO content (Figure 2). This would prove that drying temperature increment in OD would cause severe loss in EOs extraction, and it could be due to multiple impediments that come with oven drying, including vast ventilation where a large volume of air flowing through samples over a long duration allows volatile compounds to easily evaporate, in addition to creating an environment for high oxidation to occur (Chua et al., 2019; Figiel, Szumny, Gutiérrez‐Ortíz, & Carbonell‐Barrachina, 2010). Similar results on the higher loss of volatiles were also observed for the OD samples of oregano (Figiel et al., 2010) and Dracocephalum kotschyi Boiss. (Samadi et al., 2018). It was also reported in previous studies that temperature enhancement for VOD and MW treatments would cause higher rates of reduction in the EO content of some plant species such as Dracocephalum kotschyi Boiss. (Samadi et al., 2018), peppermint leaves (Salarikia, Miraei Ashtiani, & Golzarian, 2017), and Mentha longifolia L. (Saeidi, Ghafari, & Rostami, 2016). However, in our study, increasing temperature in VOD and MW methods enhanced the EO content of dried samples. This proves that the effects of higher drying temperature could be variable in different plants, and it might be emanated from physiological differences in plant species, secretory tissue, their localization and chemical composition of EO (Rahimmalek & Goli, 2013; Salarikia et al., 2017).
Figure 2.
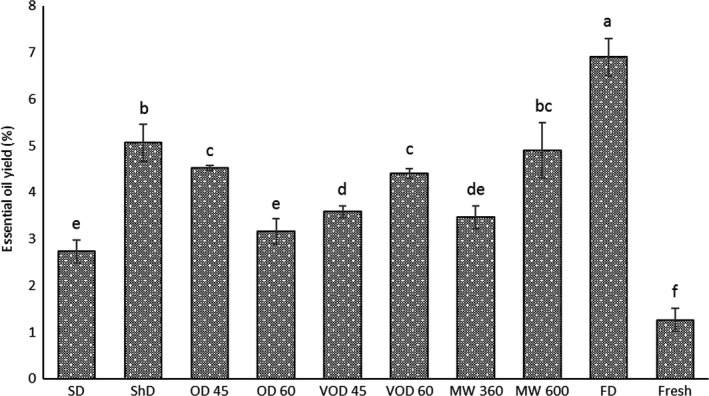
Effect of different drying methods on the essential oil yield of Thomson peels. The vertical bars represent standard deviation (±SD) of the means. SD, Sun drying; ShD, Shade drying; OD 45 and 60, Oven drying at 45°C and 60°C; VOD 45 and 60, Vacuum oven drying at 45°C and 60°C; MW 360 and 600, Microwave drying at 360 W and 600 W; FD, Freeze‐drying
3.2.2. GC‐MS compounds identification
In the current experiment, 39 components were identified in the EOs of dried and fresh Thomson peel samples representing 93.3%–100.2% of the total volatile oils (Table 2). The volatile compounds of essential oils could be categorized in the following main chemical groups: monoterpene hydrocarbons (83.55%–93.31%), oxygenated monoterpenes (5.70%–8.97%), and sesquiterpenes (0.63%–2.83%). In fresh Thomson peel, the major components of the EOs were limonene (71.54), β‐myrcene (7.20%), linalool (4.11%), α‐pinene (1.85%), sabinene (1.70%), and decanal (1.21%). As stated in previous reports, EO constituents of Thomson peel could be considerably influenced by intrinsic (genetics, subspecies, and plant age) or extrinsic (geographical origin, climate condition, and isolation methods) factors (Duman, Soltanbeigi, & Ozcan, 2016; Kirbaslar et al., 2009; Nekoei & Mohammadhosseini, 2014; Njoroge et al., 2009; Xiao, Ma, Niu, Chen, & Yu, 2016; Yang et al., 2017). By way of illustration, the quantity of major volatile compounds of Turkish Thomson peel EO in the study of Duman et al. (2016) were as follows: limonene (71.80%), β‐myrcene (4.55%), sabinene (1.39%), linalool (3.89%), and α‐pinene (1.17%), which were significantly different compared with present study.
Table 2.
Contents of volatile compounds in Thomson peel essential oils
| No. | Volatile compounds | RT (min) | RI | Pick area (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | ShD | OD 45 | OD 60 | VOD 45 | VOD 60 | MW 360 | MW 600 | FD | Fresh | ||||
| Monoterpenes | |||||||||||||
| 1 | α‐Pinene | 5.64 | – | 2.42b | 2.48b | 3.53a | 2.58b | 2.62b | 2.39b | 2.66b | 2.74b | 1.26d | 1.85c |
| 2 | β‐Phellandrene | 6.67 | 1,093 | 0.63cd | 0.88bc | 0.51d | 0.37e | 1.83a | 0.74c | 0.76c | 0.70c | 1.08b | 0.72c |
| 3 | Sabinene | 6.74 | 1,000 | 1.32b | 1.72ab | 1.20b | 1.03c | 1.30b | 1.38abc | 1.40ab | 1.79a | 0.80d | 1.70ab |
| 4 | β‐Myrcene | 7.23 | 1,025 | 8.69a | 7.33b | 6.62c | 8.07a | 5.54d | 6.78bc | 7.91ab | 8.19a | 4.91d | 7.20b |
| 5 | Limonene | 8.72 | 1,081 | 79.00bc | 79.58bc | 76.34d | 78.45c | 80.03b | 78.06c | 79.86bc | 79.42bc | 81.88a | 71.54e |
| 6 | β‐Ocimene | 9.14 | 1,200 | 0.16ab | 0.19ab | – | 0.21a | 0.16ab | 0.18a | 0.16ab | 0.18ab | 0.12b | 0.16ab |
| 7 | γ‐Terpinene | 9.35 | 1,091 | 0.31b | 0.18c | 0.32b | 0.47a | 0.29b | 0.25b | 0.17c | 0.12d | 0.15cd | 0.23bc |
| 8 | p‐Menthene | 10.05 | 855 | 0.18cd | 0.16d | 0.70a | 0.28b | 0.23bc | 0.21bc | 0.22bc | 0.17cd | 0.14d | 0.15cd |
| Total | 92.71b | 92.52b | 89.22c | 91.46abc | 92.00abc | 89.99bc | 93.14a | 93.31a | 89.62b | 83.55d | |||
| Oxygenated Monoterpenes | |||||||||||||
| 9 | 1‐Octanol | 9.58 | 1,031 | 0.07e | 0.09e | 0.20d | 0.23cd | 0.50b | 0.26c | 0.27c | 0.26c | 0.24cd | 0.92a |
| 10 | Linalool | 10.43 | 1,084 | 1.89d | 2.06d | 2.79b | 1.61e | 1.09g | 1.32f | 1.28f | 1.63e | 2.37c | 4.11a |
| 11 | Nonanal | 10.50 | 1,007 | 0.30a | 0.30a | – | 0.30a | 0.22b | 0.30a | 0.30a | 0.31a | 0.31a | – |
| 12 | trans‐Limonene oxide | 11.42 | 982 | – | 0.06b | 0.11a | – | – | – | – | – | – | – |
| 13 | β‐Terpineol | 11.61 | 1,045 | – | – | – | 0.10a | – | – | 0.09a | – | – | – |
| 14 | Citronellal | 11.84 | 1,042 | 0.11b | 0.16a | 0.20a | – | – | 0.11b | 0.07c | 0.12b | – | 0.07c |
| 15 | Isoneral | 12.15 | 1,058 | – | – | 0.05a | – | – | – | – | – | – | – |
| 16 | 1‐Nonanol | 12.37 | 1,100 | – | – | – | – | 0.07a | – | – | – | – | – |
| 17 | Terpinen‐4‐ol | 12.57 | 934 | 0.83b | 0.38d | 0.46c | 0.89a | 0.62b | 0.42cd | 0.38d | 0.24e | 0.27e | 0.38d |
| 18 | α‐Terpineol | 12.95 | 1,048 | 0.73b | 0.63c | 0.89a | 0.87a | 0.64c | 0.49d | 0.85a | 0.48d | 0.82a | 0.85a |
| 19 | Decanal | 13.36 | 1,053 | 1.01e | 1.12d | 1.48b | 1.23c | 1.21c | 1.49b | 1.21c | 1.22c | 1.80a | 1.21c |
| 20 | cis‐Carveol | 13.71 | 1,057 | 0.10b | 0.08b | 0.15a | – | 0.11ab | – | – | – | – | – |
| 21 | Nerol | 13.97 | 1,041 | – | – | 0.14b | 0.16b | 0.30a | 0.17b | 0.10c | 0.10c | – | 0.10c |
| 22 | Neral | 14.33 | 1,080 | 0.45b | 0.42bc | 0.63a | 0.28d | 0.12e | 0.30d | 0.37c | 0.42bc | 0.55a | 0.37c |
| 23 | Carvone | 14.42 | 1,025 | – | – | 0.06a | – | – | – | – | – | – | – |
| 24 | Geraniol | 14.69 | 1,036 | – | – | 0.06c | – | 0.27a | 0.12b | 0.07c | – | – | 0.07c |
| 25 | Geranial | 15.15 | 1,085 | 0.63c | 0.60c | 0.83a | 0.44e | 0.32f | 0.55cd | 0.53d | 0.59c | 0.73b | 0.53d |
| 26 | Phellandral | 15.23 | 1,008 | 0.11b | 0.10b | 0.15a | 0.08c | – | 0.08c | 0.10b | 0.09bc | – | 0.10b |
| 27 | Undecanal | 16.09 | 1,031 | – | – | 0.10a | – | 0.08ab | 0.11a | 0.06b | 0.06b | – | 0.06b |
| 28 | Dodecanal | 18.74 | 1554 | 0.13e | 0.16de | 0.28b | 0.21c | 0.26b | 0.35a | 0.20cd | 0.18c | 0.39a | 0.20c |
| Total | 6.36c | 6.16cd | 8.58a | 6.40c | 5.81cd | 6.07cd | 5.88cd | 5.70d | 7.48b | 8.97a | |||
| Sesquiterpenes | |||||||||||||
| 29 | α‐Copaene | 17.93 | 1,183 | 0.09d | 0.10d | 0.19b | 0.14c | 0.18b | 0.24a | 0.12cd | 0.11cd | 0.20b | 0.12cd |
| 30 | β‐Cubebene | 18.30 | 1588 | – | 0.07b | 0.15a | – | 0.08b | 0.15a | 0.01c | 0.02c | 0.17a | 0.01c |
| 31 | β‐Elemene | 18.35 | 1511 | – | 0.07b | 0.13a | 0.09b | 0.09b | 0.14a | – | – | – | – |
| 32 | β‐Caryophyllene | 19.06 | 1,257 | 0.10d | 0.12d | 0.20b | 0.16c | 0.16c | 0.23a | 0.10d | 0.11d | 0.24a | 0.10d |
| 33 | β‐Copaene | 19.30 | 1527 | 0.06e | 0.07e | 0.15b | 0.10d | 0.13c | 0.18a | 0.09de | 0.09d | 0.16ab | 0.09de |
| 34 | cis‐β‐Farnesene | 19.94 | 1549 | – | – | 0.12b | 0.11b | 0.10b | 0.16a | – | – | – | – |
| 35 | Germacrene | 20.59 | 1568 | 0.08e | 0.10de | 0.18b | 0.12d | 0.14c | 0.22a | 0.09e | 0.10de | 0.20ab | 0.09de |
| 36 | Valencene | 20.89 | 1549 | – | – | 0.40a | 0.25b | 0.26b | 0.36a | 0.14c | 0.17c | 0.36a | 0.14c |
| 37 | α‐Farnesene | 21.20 | 1542 | – | – | 0.12b | 0.11b | 0.10b | 0.16a | – | – | – | – |
| 38 | Δ‐Cadinene | 21.62 | 1505 | 0.17cd | 0.17cd | 0.29b | 0.26b | 0.27b | 0.43a | 0.18c | 0.15d | 0.38a | 0.18cd |
| 39 | β‐Sinensal | 28.87 | – | 0.13f | 0.21e | 0.34c | 0.30cd | 0.28d | 0.56b | 0.14f | 0.13f | 0.69a | 0.14f |
| Total | 0.63e | 0.91d | 2.27b | 1.64c | 1.79c | 2.83a | 0.87d | 0.88d | 2.40ab | 0.87d | |||
| Total | 99.70a | 99.59a | 100.07a | 99.50a | 99.60a | 98.89a | 99.89a | 99.89a | 99.50a | 93.39b | |||
Means (n = 3) having different letters within the same row differ significantly at p < .05. Major volatile components are shown in bold text.
Abbreviations: –, not detected; RT, Retention time; RI, Calculated retention index; SD, Sun drying; ShD, Shade drying; OD 45 and 60, Oven drying at 45°C and 60°C; VOD 45 and 60, Vacuum oven drying at 45°C and 60°C; MW 360 and 600, Microwave drying at 360 W and 600 W; FD, Freeze‐drying.
As shown in Table 2, different drying methods had notable effects on all major components identified in the EOs. Among the active ingredient groups, monoterpene hydrocarbons were the most important ones as they possessed the majority of main volatile components. Although the utmost content of monoterpene hydrocarbons was obtained by MW 600 W (93.31%), different components of this group were changed differently when using other drying methods (Table 2). The highest amount of limonene (81.88%), β‐myrcene (8.69%), and α‐pinene (3.53%) was noticed in FD, SD, and OD 45°C, respectively. In comparison with other drying treatments, VOD 45°C led to more contents of β‐phellandrene (1.83%). The highest amounts of β‐ocimene (0.21%) and γ‐terpinene (0.47%) was measured for OD 60°C, though the maximum amounts of p‐menthene (0.70%) and sabinene were obtained by OD 45°C and MW 600 W, respectively. Overall, the total amount of monoterpene hydrocarbons in dried samples (Table 2) were higher than fresh sample (p < .05). Primarily, monoterpene hydrocarbons are categorized as nonpolar compounds and it seems that these compounds have low affinity to the water fraction of fruit peels. Thereby, they would not be evaporated along with water during hydrodistillation process (Hazrati, Farnia, Habibzadeh, & Mollaei, 2018). On the other hand, drying processes can make a porous structure on plant materials and help availability of solutes, for example, essential oil, via increment of mass transfer coefficient during extraction, thus higher volatile compounds than fresh sample can be obtained (Feyzi, Eikani, Golmohammad, & Tafaghodinia, 2017). This was not in agreement with the results of prior work done by Samadi et al. (2018) and Pirbalouti, Mahdad, and Craker (2013) where dried samples of Dracocephalum kotschyi Boiss. and basil landrace, respectively, had lower amount of monoterpene hydrocarbons than fresh sample. The differences in the outcomes might be related to the type and origin of the plants as well as the conditions used for drying treatments (Shahhoseini, Estaji, Hosseini, Ghorbanpour, & Omidbaigi, 2013). Moreover, the changes in the oxygenated monoterpenes contents (specifically linalool and decanal as the major oxygenated monoterpenes) are displayed in Table 2. Although the highest content of linalool (4.11%) was obtained was observed in fresh sample, FD procedure showed higher content of decanal (1.80%) compared with other treatments. As indicated in Table 2, not merely drying processes triggered the elimination of some oxygenated monoterpenes and sesquiterpenes, likewise they caused the appearance of other volatile compounds which were absent in fresh peel EO. For instance, some components such as citronellal, nerol, geraniol, phellandral, undecanal, β‐cubebene, and valencene were present in fresh peels while they have been disappeared in several dried samples (Table 2), whereas nonanal, trans‐limonene oxide, β‐terpineol, isoneral, 1‐nonanol, cis‐carveol, carvone, β‐elemene, cis‐β‐farnesene, and α‐farnesene were absent in fresh fruit peels but they appeared in several dried samples. Similar findings were reported by researchers (Rahimmalek & Goli, 2013; Samadi et al., 2018), which they clarified that this phenomenon may be attributed to the formation or removal of volatile compounds by esterification, oxidation, glycoside hydrolysis, and other processes during drying. The total amount of sesquiterpenes was influenced by the drying treatments as well. VOD sample at 60°C led to more amount of sesquiterpenes than other drying procedures. As is shown in Table 2, the highest amounts of β‐sinensal (0.69%) and Δ‐cadinene (0.43%) (as the main sesquiterpenes of EO) were determined in samples dried by FD and VOD (60°C) methods, respectively. Overall, sesquiterpenes have higher molecular weight than monoterpenes, and thus, they are less volatile and hardly removed from the plant material; however, they are susceptible to oxidation reaction and exposing the samples for extended drying times would reduce the sesquiterpene compounds (Chua et al., 2019). That could explain the highest retention of these components by VOD 60°C where oxygen is minimized and in contrast the greatest loss of these compounds by SD which has the highest drying time in atmospheric condition. Similar results were observed in the study of Samadi et al. (2018) where sun drying had the highest sesquiterpenes loss, while VOD at 50°C treatment was among the best methods of preserving sesquiterpenes.
3.2.3. Total phenol content
TPC of Thomson peel EOs were shown in Figure 3. As demonstrated, the amount of TPC in SD, ShD, OD (45 and 60°C) MW (360 W), and VOD (45°C) samples were lower than their fresh counterpart. In case of SD and ShD, usually their lower drying temperature could be beneficial in preserving heat‐labile antioxidant compounds; however, unexpected precipitation and cloud condition could influence the drying rate and due to extended drying duration, increase in enzymatic degradation could occur (Chua et al., 2019). Similar reduction in TPC of SD and ShD samples was observed in ginger, tomato (Gümüşay, Borazan, Ercal, & Demirkol, 2015), Vitex negundo and Vitex trifolia (Chong & Lim, 2012). Concerning to OD samples the lower TPC could be the result of the high oxidation process which has taken place during the prolong exposure of samples to hot air (Chong & Lim, 2012). In the study of Yi and Wetzstein (2011), exposure to 70°C OD caused highest TPC loss in rosemary, motherwort and peppermint leaves. Furthermore, the TPC of other drying treatments were as following order: FD > VOD 60°C > MW 600W which the amounts of TPC in all of them were higher than fresh sample (p < .05). There are three explanations for this situation. First of all, highest TPC of FD sample is due to lower drying temperature of FD process where utmost retention of heat‐sensitive compounds is attainable (Chong & Lim, 2012). This was asserted in previous studies where FD samples showed highest TPC content in ginger (Gümüşay et al., 2015), Vitex negundo and Vitex trifolia (Chong & Lim, 2012). Secondly, when plants are under drying treatments, their tissues are more fragile and higher temperatures in MW or VOD would cause an easier breakdown of cell walls which would boost the extractability of antioxidants during extraction (Hossain, Barry‐Ryan, Martin‐Diana, & Brunton, 2010). On the other hand, total drying time would be reduced and samples would be less subjected to destructive effects of high drying temperatures (Chua et al., 2019). Thirdly, lower TPC in fresh sample could be due to its high moisture content, which promotes enzymatic reaction and that may result in the loss of antioxidant compounds (Chua et al., 2019; Hossain et al., 2010).
Figure 3.
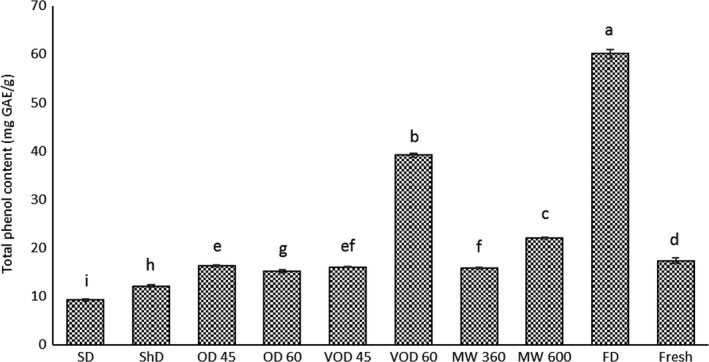
Effects of different drying methods on total phenol content of Thomson peel essential oils. The vertical bars represent standard deviation (±SD) of the means. SD, Sun drying; ShD, Shade drying; OD 45 and 60, Oven drying at 45°C and 60°C; VOD 45 and 60, Vacuum oven drying at 45°C and 60°C; MW 360 and 600, Microwave drying at 360 W and 600 W; FD, Freeze‐drying
3.2.4. Antioxidant activity
In this study, the antioxidant activity of Thomson peel EOs was investigated by two distinct methods: DPPH˙ scavenging test and ferric reducing/antioxidant power assay. In the DPPH test, the stable free radical with dark violet color interact with the phenolic compounds and immediately after receiving proton from them, it instantly loses its chromophore and becomes yellow (Farahmandfar et al., 2018; İnan, Özcan, & Aljuhaimi, 2018). Therefore, the discoloration degree would indicate the potentials of free radical scavenging of the analyzed compound (Sayyad & Farahmandfar, 2017). Likewise, reducing potential of antioxidants is an important indicator of their activity, and it could be measured through FRAP test which evaluates the reduction in ferric ion (Fe3+)–ligand complex to the intensely blue colored ferrous (Fe2+) complex by antioxidants in acidic media (Shahidi & Zhong, 2015). Antioxidant activities (DPPH and FRAP tests) of Thomson peel EOs at concentrations of 5000–80,000 ppm are given in Tables 3 and 4, respectively. In both experiments, with increase in concentrations of the EOs, the scavenging of free radicals and reducing power of these compounds increased, which is due to the increasing amount of phenolic compounds at higher concentrations of the EOs, similar to the results of previously published studies of researchers (Farahmandfar et al., 2018; Sayyad & Farahmandfar, 2017). As indicated in Table 3, different drying methods with varied conditions would significantly influence the DPPH scavenging activity of EOs. In DPPH test, the highest antioxidant activity (lowest IC50) was observed in FD sample, followed by VOD 60°C, MW 600W, fresh, VOD 45°C, OD 45°C, MW 360W, ShD, OD 60°C, and SD samples (Table 3). We found a quite strong negative correlation (Table 5) between total phenol content and IC50 concentration (R 2 = 0.704), which proved that higher phenolic compounds would enhance scavenging activity (reduce IC50) of Thomson peel EOs, as FD, VOD 60°C, and MW 600 W samples with highest TPCs showed highest antioxidant activities (lowest IC50) and SD sample with lowest TPC had lowest scavenging activity (highest IC50). Similar results were observed in the prior published work of An et al. (2016) on Chinese ginger where the DPPH scavenging ability had high correlation with TPC (R 2 = 0.866), with highest TPC and lowest IC50 belonged to FD sample.
Table 3.
DPPH radical scavenging activities of Thomson peel essential oils
| Treatments | Inhibition (%) | IC 50 (mg/ml) | ||||
|---|---|---|---|---|---|---|
| 5 mg/ml | 10 mg/ml | 20 mg/ml | 40 mg/ml | 80 mg/ml | ||
| SD | 30.30 ± 0.52i | 39.40 ± 0.52i | 50.00 ± 0.50i | 56.96 ± 0.25g | 59.06 ± 0.40i | 20.00a |
| ShD | 35.46 ± 0.59h | 48.43 ± 0.51e | 51.30 ± 0.36h | 67.90 ± 0.55c | 70.46 ± 0.45c | 15.04c |
| OD 45 | 40.66 ± 0.41e | 47.36 ± 0.40f | 58.00 ± 0.40e | 62.33 ± 0.35e | 68.36 ± 0.35e | 14.23e |
| OD 60 | 37.20 ± 0.25g | 41.20 ± 0.20h | 52.14 ± 0.22g | 54.53 ± 0.47h | 65.30 ± 0.36g | 16.07b |
| VOD 45 | 43.30 ± 0.36d | 50.51 ± 0.41d | 65.03 ± 0.04c | 70.10 ± 0.10b | 78.16 ± 0.20b | 7.99f |
| VOD 60 | 47.20 ± 1.02b | 56.30 ± 0.36a | 67.30 ± 0.26b | 69.53 ± 0.45b | 78.73 ± 0.37b | 7.24i |
| MW 360 | 39.30 ± 0.26f | 45.20 ± 0.45g | 56.36 ± 0.15f | 59.43 ± 0.73f | 67.60 ± 0.45f | 14.76d |
| MW 600 | 45.60 ± 0.40c | 54.36 ± 0.30b | 60.23 ± 1.07d | 63.56 ± 0.11d | 69.50 ± 0.55d | 7.50h |
| FD | 50.00 ± 1.20a | 56.19 ± 0.26a | 68.90 ± 1.41a | 74.20 ± 0.30a | 87.90 ± 0.36a | 5.00 j |
| Fresh | 42.73 ± 0.75d | 52.66 ± 0.87c | 58.10 ± 0.55e | 62.40 ± 0.52e | 64.26 ± 0.25h | 7.86g |
All values are expressed as mean ± standard deviation. Means (n = 3) having different letters within the same column differ significantly at p < .05.
Abbreviations: SD, Sun drying; ShD, Shade drying; OD 45 and 60, Oven drying at 45°C and 60°C; VOD 45 and 60, Vacuum oven drying at 45°C and 60°C; MW 360 and 600, Microwave drying at 360 W and 600 W; FD, Freeze‐drying.
Table 4.
Ferric reducing/antioxidant power of Thomson peel essential oil
| Treatments | Inhibition (%) | ||||
|---|---|---|---|---|---|
| 5 mg/ml | 10 mg/ml | 20 mg/ml | 40 mg/ml | 80 mg/ml | |
| SD | 0.03 ± 0.00gh | 0.05 ± 0.01f | 0.07 ± 0.01e | 0.15 ± 0.00g | 0.23 ± 0.00g |
| ShD | 0.01 ± 0.00h | 0.16 ± 0.00d | 0.21 ± 0.00c | 0.27 ± 0.00 f | 0.32 ± 0.01f |
| OD 45 | 0.09 ± 0.00f | 0.16 ± 0.02d | 0.21 ± 0.01c | 0.33 ± 0.01c | 0.38 ± 0.01d |
| OD 60 | 0.04 ± 0.01g | 0.08 ± 0.01e | 0.15 ± 0.01d | 0.29 ± 0.01ef | 0.35 ± 0.01e |
| VOD 45 | 0.12 ± 0.01e | 0.18 ± 0.01cd | 0.23 ± 0.02c | 0.3 ± 0.00de | 0.41 ± 0.02c |
| VOD 60 | 0.22 ± 0.01b | 0.26 ± 0.06b | 0.30 ± 0.01b | 0.36 ± 0.01b | 0.48 ± 0.03b |
| MW 360 | 0.03 ± 0.01gh | 0.16 ± 0.02d | 0.23 ± 0.01c | 0.31 ± 0.01cd | 0.36 ± 0.01de |
| MW 600 | 0.09 ± 0.00f | 0.16 ± 0.02d | 0.21 ± 0.01c | 0.33 ± 0.01c | 0.38 ± 0.01d |
| FD | 0.21 ± 0.01ab | 0.25 ± 0.02 b | 0.34 ± 0.08 ab | 0.37 ± 0.01 a | 0.52 ± 0.12 a |
| Fresh | 0.15 ± 0.01d | 0.20 ± 0.00c | 0.22 ± 0.01c | 0.31 ± 0.01cde | 0.42 ± 0.02c |
All values are expressed as mean ± standard deviation. Means (n = 3) having different letters within the same column differ significantly at p < .05.
Abbreviations: SD, Sun drying; ShD, Shade drying; OD 45 and 60, Oven drying at 45°C and 60°C; VOD 45 and 60, Vacuum oven drying at 45°C and 60°C; MW 360 and 600, Microwave drying at 360 W and 600 W; FD, Freeze‐drying.
Table 5.
Correlation analysis between the measured antioxidant parameters of the experiment
| Parameters | TPC | DPPH (IC50) | FRAP (80 mg/ml) |
|---|---|---|---|
| TPC | 1.000 | ||
| DPPH (IC50) | −0.704* | 1.000 | |
| FRAP (80 mg/ml) | 0.836** | −0.897** | 1.000 |
Abbreviations: TPC, total phenol content, DPPH, 2, 2‐diphenyl‐1‐picrylhydrazyl assay; FRAP, ferric reducing antioxidant power assay.
Indicate significant correlation at the 0.05 level.
Indicate significant correlation at the 0.01 level.
The results of FRAP test was quite similar to DPPH essay, since there was a relatively strong correlation between TPC and FRAP value (R 2 = 0.836), as well (Table 5). At the highest EO concentration (80,000 ppm) FD was the dominant drying technique in respect of reducing activity due to its higher phenolic content, on the other hand, SD sample with lowest TPC was merely the weakest treatment with regard to FRAP value. This was in accordance to the study of Gümüşay et al. (2015) on tomato and ginger, where a similar trend was observed, as FD and SD had the highest and lowest reducing capacity compared with other drying treatments. Effect of temperature raise in FRAP method was not as clear as DPPH test, since there was no significant difference between microwave treatments (360 and 600W) (p > .05); however, much like the DPPH assay, a slight increase and decrease in FRAP value of VOD and OD samples was observed respectively, as temperature raised from 45 to 60°C, which is undoubtedly related to their phenolic contents.
3.2.5. Antibacterial activity
The MICs and MBCs of Thomson peel EOs against gram‐negative and gram‐positive bacteria are reported in Table 6. As illustrated, different drying treatments showed various degrees of activity against the tested strains. All EOs were more effective against gram‐positive bacteria than gram‐negative bacteria in the present study. This was in agreement with previous studies of researchers (Burt, 2004; Geraci et al., 2017), and this can be attributed to diversities in their cell structure. The outer peptidoglycan layer in gram‐negative bacteria is an ineffective permeability barrier, since the prions present in these types of bacteria would limit the entry of solutes and make them less susceptible to antibacterial components (Singh, Negi, & Radha, 2013). According to Table 6, S. aureus and E. coli were the most sensitive tested strains and the variation in MIC and MBC values were more clarified in these bacteria than L. monocytogenes and P. aeruginosa. The best treatment in terms of antibacterial activities was FD followed by VOD at 60°C and MW at 600 W which the amount of MIC and MBC values were much lower than fresh sample, in contrast the weakest ones (highest MIC and MBC values) were SD and ShD (Table 6). It was reported in prior studies that antibacterial activity is well correlated with the amount of phenolic compounds within Eos (Singh et al., 2013), specifically by virtue of containing the most well‐known and characterized compounds of the citrus EOs such as limonene, α‐pinene, sabinene, β‐myrcene, and linalool which due to their synergistic effect, they can apply a strong and vast spectrum of antimicrobial activities. However, since these compounds are very hydrophobic and are difficult to disperse in water, high concentrations must be applied in order for them to be effective antimicrobial components (Calo, Crandall, O'Bryan, & Ricke, 2015). That is why, FD, VOD (60°C) and MW (600 W) samples with higher TPC (Figure 3) had higher antimicrobial activities and contrarily SD and ShD samples with lower TPC showed feeble antibacterial activities compared to other treatments.
Table 6.
Antimicrobial activity of the Thomson navel orange essential oils against selected bacterial strains
| Treatments | Test microorganisms | |||||||
|---|---|---|---|---|---|---|---|---|
| Gram‐negative | Gram‐positive | |||||||
| Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | Listeria monocytogenes | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| SD | 20 | 40 | 80< | 80< | 5 | 10 | 80< | 80< |
| ShD | 10 | 20 | 80< | 80< | 5 | 20 | 80< | 80< |
| OD 45 | 5 | 20 | 80 | 80< | 2.50 | 5 | 40 | 40 |
| OD 60 | 5 | 10 | 80< | 80< | 2.50 | 10 | 80< | 80< |
| VOD 45 | 10 | 20 | 80< | 80< | 2.50 | 5 | 40 | 80 |
| VOD 60 | 1.25 | 2.50 | 20 | 80 | 0.62 | 1.25 | 10 | 20 |
| MW 360 | 2.50 | 10 | 80< | 80< | 2.50 | 10 | 80 | 80 |
| MW 600 | 1.25 | 2.50 | 20 | 40 | 1.25 | 2.50 | 10 | 20 |
| FD | 1.25 | 1.25 | 10 | 20 | 0.31 | 0.62 | 5 | 10 |
| Fresh | 5 | 20 | 40 | 80< | 2.50 | 5 | 20 | 40 |
Abbreviations: FD, Freeze‐drying; MBC, minimum bactericidal concentration (μg/mL); MIC, minimum inhibitory concentration (μg/mL); MW 360 and 600, Microwave drying at 360 W and 600 W; OD 45 and 60, Oven drying at 45°C and 60°C; SD, Sun drying; ShD, Shade drying; VOD 45 and 60, Vacuum oven drying at 45°C and 60°C.
4. CONCLUSION
The current study is the first to report the effects of six drying methods with varied conditions on the physical and EO properties of Thomson peel. In summary, highest amount of color lightness and yellowness as well as lowest content of ΔE and BI was observed in FD sample. The EO extracted via FD sample had higher values of extraction yield, limonene (the major volatile compound of Thomson peel EO), TPC, DPPH scavenging, reducing power along with lowest MIC and MBC against four bacteria strains compared with other drying treatments of this study. Our findings could provide a valuable data base for developing a process for dehydration of Thomson peel, and FD could be the potential method for producing an excellent dry product with highest quality EOs.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ETHICAL APPROVAL
This study does not involve any human or animal testing.
ACKNOWLEDGMENTS
We are grateful to Sari Agricultural Sciences & Natural Resources University (SANRU) for support under Project No. 02‐1398‐06.
Farahmandfar R, Tirgarian B, Dehghan B, Nemati A. Changes in chemical composition and biological activity of essential oil from Thomson navel orange (Citrus sinensis L. Osbeck) peel under freezing, convective, vacuum, and microwave drying methods. Food Sci Nutr. 2020;8:124–138. 10.1002/fsn3.1279
REFERENCES
- An, K. , Zhao, D. , Wang, Z. , Wu, J. , Xu, Y. , & Xiao, G. (2016). Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chemistry, 197, 1292–1300. 10.1016/j.foodchem.2015.11.033 [DOI] [PubMed] [Google Scholar]
- Argyropoulos, D. , Heindl, A. , & Müller, J. (2011). Assessment of convection, hot‐air combined with microwave‐vacuum and freeze‐drying methods for mushrooms with regard to product quality. International Journal of Food Science & Technology, 46(2), 333–342. 10.1111/j.1365-2621.2010.02500.x [DOI] [Google Scholar]
- Arslan, D. , Özcan, M. M. , & Mengeş, H. O. (2010). Evaluation of drying methods with respect to drying parameters, some nutritional and colour characteristics of peppermint (Mentha x piperita L.). Energy Conversion and Management, 51(12), 2769–2775. 10.1016/j.enconman.2010.06.013 [DOI] [Google Scholar]
- Bal, L. M. , Kar, A. , Satya, S. , & Naik, S. N. (2011). Kinetics of colour change of bamboo shoot slices during microwave drying. International Journal of Food Science & Technology, 46(4), 827–833. 10.1111/j.1365-2621.2011.02553.x [DOI] [Google Scholar]
- Barreca, D. , Gattuso, G. , Bellocco, E. , Calderaro, A. , Trombetta, D. , Smeriglio, A. , … Nabavi, S. M. (2017). Flavanones: Citrus phytochemical with health‐promoting properties. BioFactors, 43(4), 495–506. 10.1002/biof.1363 [DOI] [PubMed] [Google Scholar]
- Behbahani, B. A. , Shahidi, F. , Yazdi, F. T. , Mortazavi, S. A. , & Mohebbi, M. (2017). Use of Plantago major seed mucilage as a novel edible coating incorporated with Anethum graveolens essential oil on shelf life extension of beef in refrigerated storage. International Journal of Biological Macromolecules, 94, 515–526. 10.1016/j.ijbiomac.2016.10.055 [DOI] [PubMed] [Google Scholar]
- Burt, S. (2004). Essential oils: Their antibacterial properties and potential applications in foods—a review. International Journal of Food Microbiology, 94(3), 223–253. 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- Calo, J. R. , Crandall, P. G. , O'Bryan, C. A. , & Ricke, S. C. (2015). Essential oils as antimicrobials in food systems–A review. Food Control, 54, 111–119. 10.1016/j.foodcont.2014.12.040 [DOI] [Google Scholar]
- Celano, R. , Campone, L. , Pagano, I. , Carabetta, S. , Di Sanzo, R. , Rastrelli, L. , … Russo, M. (2019). Characterisation of nutraceutical compounds from different parts of particular species of Citrus sinensis ‘Ovale Calabrese’by UHPLC‐UV‐ESI‐HRMS. Natural Product Research, 33(2), 244–251. [DOI] [PubMed] [Google Scholar]
- Chong, C. H. , Figiel, A. , Law, C. L. , & Wojdyło, A. (2014). Combined drying of apple cubes by using of heat pump, vacuum‐microwave, and intermittent techniques. Food and Bioprocess Technology, 7(4), 975–989. 10.1007/s11947-013-1123-7 [DOI] [Google Scholar]
- Chong, K. , & Lim, Y. (2012). Effects of drying on the antioxidant properties of herbal tea from selected Vitex species. Journal of Food Quality, 35(1), 51–59. 10.1111/j.1745-4557.2011.00422.x [DOI] [Google Scholar]
- Chua, L. Y. , Chong, C. H. , Chua, B. L. , & Figiel, A. (2019). Influence of drying methods on the antibacterial, antioxidant and essential oil volatile composition of herbs: A review. Food and Bioprocess Technology, 12(3), 450–476. 10.1007/s11947-018-2227-x [DOI] [Google Scholar]
- Cserhalmi, Z. , Sass‐Kiss, A. , Tóth‐Markus, M. , & Lechner, N. (2006). Study of pulsed electric field treated citrus juices. Innovative Food Science & Emerging Technologies, 7(1–2), 49–54. 10.1016/j.ifset.2005.07.001 [DOI] [Google Scholar]
- de la Torre, I. , Martin‐Dominguez, V. , Acedos, M. , Esteban, J. , Santos, V. , & Ladero, M. (2019). Utilisation/upgrading of orange peel waste from a biological biorefinery perspective. Applied Microbiology and Biotechnology, 1–17. [DOI] [PubMed] [Google Scholar]
- Duman, E. , Soltanbeigi, A. , & Ozcan, M. M. (2016). Chemical compositions of essential oil of some Citrus spp. (Sour, Lemon, Kumquat, Mandarin and Orange) peels. Journal of Medicinal & Spice Plants, 21(4), 153–159. [Google Scholar]
- Eikani, M. H. , Golmohammad, F. , & Rowshanzamir, S. (2007). Subcritical water extraction of essential oils from coriander seeds (Coriandrum sativum L.). Journal of Food Engineering, 80(2), 735–740. 10.1016/j.jfoodeng.2006.05.015 [DOI] [Google Scholar]
- Faostat, F. (2018). Statistical databases. Food and Agriculture Organization of the United Nations.
- Farahmandfar, R. , Asnaashari, M. , & Bakhshandeh, T. (2019). Influence of ultrasound‐assist and classical extractions on total phenolic, tannin, flavonoids, tocopherol and antioxidant characteristics of Teucrium polium aerial parts. Journal of Food Measurement and Characterization, 13(2), 1357–1363. 10.1007/s11694-019-00051-5 [DOI] [Google Scholar]
- Farahmandfar, R. , Asnaashari, M. , Pourshayegan, M. , Maghsoudi, S. , & Moniri, H. (2018). Evaluation of antioxidant properties of lemon verbena (Lippia citriodora) essential oil and its capacity in sunflower oil stabilization during storage time. Food Science & Nutrition, 6(4), 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyzi, E. , Eikani, M. H. , Golmohammad, F. , & Tafaghodinia, B. (2017). Extraction of essential oil from Bunium persicum (boiss.) by instant controlled pressure drop. Journal of Chromatography A, 1530, 59–67. 10.1016/j.chroma.2017.11.033 [DOI] [PubMed] [Google Scholar]
- Figiel, A. , Szumny, A. , Gutiérrez‐Ortíz, A. , & Carbonell‐Barrachina, Á. A. (2010). Composition of oregano essential oil (Origanum vulgare) as affected by drying method. Journal of Food Engineering, 98(2), 240–247. 10.1016/j.jfoodeng.2010.01.002 [DOI] [Google Scholar]
- Franco‐Vega, A. , Ramírez‐Corona, N. , Palou, E. , & López‐Malo, A. (2016). Estimation of mass transfer coefficients of the extraction process of essential oil from orange peel using microwave assisted extraction. Journal of Food Engineering, 170, 136–143. 10.1016/j.jfoodeng.2015.09.025 [DOI] [Google Scholar]
- Gavahian, M. , Chu, Y. H. , & Mousavi Khaneghah, A. (2019). Recent advances in orange oil extraction: An opportunity for the valorisation of orange peel waste a review. International Journal of Food Science & Technology, 54(4), 925–932. 10.1111/ijfs.13987 [DOI] [Google Scholar]
- Geraci, A. , Di Stefano, V. , Di Martino, E. , Schillaci, D. , & Schicchi, R. (2017). Essential oil components of orange peels and antimicrobial activity. Natural Product Research, 31(6), 653–659. 10.1080/14786419.2016.1219860 [DOI] [PubMed] [Google Scholar]
- Ghanem, N. , Mihoubi, D. , Kechaou, N. , & Mihoubi, N. B. (2012). Microwave dehydration of three citrus peel cultivars: Effect on water and oil retention capacities, color, shrinkage and total phenols content. Industrial Crops and Products, 40, 167–177. 10.1016/j.indcrop.2012.03.009 [DOI] [Google Scholar]
- Gümüşay, Ö. A. , Borazan, A. A. , Ercal, N. , & Demirkol, O. (2015). Drying effects on the antioxidant properties of tomatoes and ginger. Food Chemistry, 173, 156–162. 10.1016/j.foodchem.2014.09.162 [DOI] [PubMed] [Google Scholar]
- Hasija, S. , Ibrahim, G. , & Wadia, A. (2015). Antimicrobial activity of Citrus sinensis (Orange), Citrus limetta (Sweet lime) and Citrus limon (lemon) peel oil on selected food borne pathogens. International Journal of Life Science Research, 3, 35–39. [Google Scholar]
- Hazrati, S. , Farnia, P. , Habibzadeh, F. , & Mollaei, S. (2018). Effect of different drying techniques on qualitative and quantitative properties of Stachys Schtschegleevii essential oil. Journal of Food Processing and Preservation, 42(8), e13686. [Google Scholar]
- Hossain, M. , Barry‐Ryan, C. , Martin‐Diana, A. B. , & Brunton, N. (2010). Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chemistry, 123(1), 85–91. 10.1016/j.foodchem.2010.04.003 [DOI] [Google Scholar]
- Humeera, N. , Kamili, A. N. , Bandh, S. A. , Amin, S.‐U. , Lone, B. A. , & Gousia, N. (2013). Antimicrobial and antioxidant activities of alcoholic extracts of Rumex dentatus L. Microbial Pathogenesis, 57, 17–20. 10.1016/j.micpath.2013.02.001 [DOI] [PubMed] [Google Scholar]
- İnan, Ö. , Özcan, M. M. , & Aljuhaimi, F. (2018). Effect of location and Citrus species on total phenolic, antioxidant, and radical scavenging activities of some Citrus seed and oils. Journal of Food Processing and Preservation, 42(3), e13555. [Google Scholar]
- Juhaimi, F. A. , Matthäus, B. , Özcan, M. M. , & Ghafoor, K. (2016). The physico‐chemical properties of some citrus seeds and seed oils. Zeitschrift Für Naturforschung C, 71(3–4), 79–85. 10.1515/znc-2016-0004 [DOI] [PubMed] [Google Scholar]
- Kamal, G. M. , Ashraf, M. Y. , Hussain, A. I. , Shahzadi, A. , & Chughtai, M. I. (2013). Antioxidant potential of peel essential oils of three Pakistani citrus species: Citrus reticulata, Citrus sinensis and Citrus paradisii . Pakistan Journal of Botany, 45(4), 1449–1454. [Google Scholar]
- Karam, M. C. , Petit, J. , Zimmer, D. , Djantou, E. B. , & Scher, J. (2016). Effects of drying and grinding in production of fruit and vegetable powders: A review. Journal of Food Engineering, 188, 32–49. 10.1016/j.jfoodeng.2016.05.001 [DOI] [Google Scholar]
- Kirbaslar, F. G. , Kirbaslar, S. I. , Pozan, G. , & Boz, I. (2009). Volatile constituents of Turkish orange (Citrus sinensis (L.) Osbeck) peel oils. Journal of Essential Oil Bearing Plants, 12(5), 586–604. [Google Scholar]
- M’hiri, N. , Ghali, R. , Ben Nasr, I. , & Boudhrioua, N. (2018). Effect of different drying processes on functional properties of industrial lemon byproduct. Process Safety and Environmental Protection, 116, 450–460. 10.1016/j.psep.2018.03.004 [DOI] [Google Scholar]
- Matthaus, B. , & Özcan, M. (2012). Chemical evaluation of citrus seeds, an agro‐industrial waste, as a new potential source of vegetable oils. Grasas y Aceites, 63(3), 313–320. 10.3989/gya.118411 [DOI] [Google Scholar]
- Maurya, D. K. , & Devasagayam, T. P. A. (2010). Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food and Chemical Toxicology, 48(12), 3369–3373. 10.1016/j.fct.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Michalska, A. , Wojdyło, A. , Lech, K. , Łysiak, G. P. , & Figiel, A. (2016). Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chemistry, 207, 223–232. 10.1016/j.foodchem.2016.03.075 [DOI] [PubMed] [Google Scholar]
- Naidu, M. M. , Vedashree, M. , Satapathy, P. , Khanum, H. , Ramsamy, R. , & Hebbar, H. U. (2016). Effect of drying methods on the quality characteristics of dill (Anethum graveolens) greens. Food Chemistry, 192, 849–856. 10.1016/j.foodchem.2015.07.076 [DOI] [PubMed] [Google Scholar]
- Nekoei, M. , & Mohammadhosseini, M. (2014). Application of HS‐SPME, SDME and cold‐press coupled to GC/MS to analysis the essential oils of Citrus sinensis CV. Thomson Navel and QSRR study for prediction of retention indices by stepwise and genetic algorithm‐multiple linear regression approaches. Analytical Chemistry Letters, 4(2), 93–103. [Google Scholar]
- Njoroge, S. M. , Phi, N. T. L. , & Sawamura, M. (2009). Chemical composition of peel essential oils of sweet oranges (Citrus sinensis) from Uganda and Rwanda. Journal of Essential Oil Bearing Plants, 12(1), 26–33. [Google Scholar]
- Pathare, P. B. , Opara, U. L. , & Al‐Said, F.‐ A.‐J. (2013). Colour measurement and analysis in fresh and processed foods: A review. Food and Bioprocess Technology, 6(1), 36–60. 10.1007/s11947-012-0867-9 [DOI] [Google Scholar]
- Pirbalouti, A. G. , Mahdad, E. , & Craker, L. (2013). Effects of drying methods on qualitative and quantitative properties of essential oil of two basil landraces. Food Chemistry, 141(3), 2440–2449. 10.1016/j.foodchem.2013.05.098 [DOI] [PubMed] [Google Scholar]
- Rahimmalek, M. , & Goli, S. A. H. (2013). Evaluation of six drying treatments with respect to essential oil yield, composition and color characteristics of Thymys daenensis subsp. daenensis. Celak leaves. Industrial Crops and Products, 42, 613–619. 10.1016/j.indcrop.2012.06.012 [DOI] [Google Scholar]
- Razavi, S. M. A. , & Farahmandfar, R. (2008). Effect of hulling and milling on the physical properties of rice grains. International Agrophysics, 22(4), 353–359. [Google Scholar]
- Saeidi, K. , Ghafari, Z. , & Rostami, S. (2016). Effect of drying methods on essential oil content and composition of Mentha longifolia (L.). Hudson. Journal of Essential Oil Bearing Plants, 19(2), 391–396. [Google Scholar]
- Salarikia, A. , Miraei Ashtiani, S. H. , & Golzarian, M. R. (2017). Comparison of drying characteristics and quality of peppermint leaves using different drying methods. Journal of Food Processing and Preservation, 41(3), e12930 10.1111/jfpp.12930 [DOI] [Google Scholar]
- Samadi, L. , Larijani, K. , Naghdi Badi, H. , & Mehrafarin, A. (2018). Qualitative and quantitative variations of the essential oils of Dracocephalum kotschyi Boiss. as affected by different drying methods. Journal of Food Processing and Preservation, 42(11), e13816. [Google Scholar]
- Saricoban, C. , & Yilmaz, M. T. (2010). Modelling the effects of processing factors on the changes in colour parameters of cooked meatballs using response surface methodology. World Applied Sciences Journal, 9(1), 14–22. [Google Scholar]
- Sayyad, R. , & Farahmandfar, R. (2017). Influence of Teucrium polium L. essential oil on the oxidative stability of canola oil during storage. Journal of Food Science and Technology, 54(10), 3073–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahhoseini, R. , Estaji, A. , Hosseini, N. , Ghorbanpour, M. , & Omidbaigi, R. (2013). The effect of different drying methods on the content and chemical composition of essential oil of Lemon verbena (Lippia citriodora). Journal of Essential Oil Bearing Plants, 16(4), 474–481. [Google Scholar]
- Shahidi, F. , & Zhong, Y. (2015). Measurement of antioxidant activity. Journal of Functional Foods, 18, 757–781. 10.1016/j.jff.2015.01.047 [DOI] [Google Scholar]
- Singh, R. G. , Negi, P. S. , & Radha, C. (2013). Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. Journal of Functional Foods, 5(4), 1883–1891. 10.1016/j.jff.2013.09.009 [DOI] [Google Scholar]
- Smeriglio, A. , Cornara, L. , Denaro, M. , Barreca, D. , Burlando, B. , Xiao, J. , & Trombetta, D. (2019). Antioxidant and cytoprotective activities of an ancient Mediterranean citrus (Citrus lumia Risso) albedo extract: Microscopic observations and polyphenol characterization. Food Chemistry, 279, 347–355. 10.1016/j.foodchem.2018.11.138 [DOI] [PubMed] [Google Scholar]
- Sogi, D. , Garg, S. , & Bawa, A. (2002). Functional properties of seed meals and protein concentrates from tomato‐processing waste. Journal of Food Science, 67(8), 2997–3001. 10.1111/j.1365-2621.2002.tb08850.x [DOI] [Google Scholar]
- van Den Dool, H. , & Dec. Kratz, P. (1963). A generalization of the retention index system including linear temperature programmed gas‐liquid partition chromatography. Journal of Chromatography, 11, 463–471. 10.1016/S0021-9673(01)80947-X [DOI] [PubMed] [Google Scholar]
- Xiao, Z. , Ma, S. , Niu, Y. , Chen, F. , & Yu, D. (2016). Characterization of odour‐active compounds of sweet orange essential oils of different regions by gas chromatography‐mass spectrometry, gas chromatography‐olfactometry and their correlation with sensory attributes. Flavour and Fragrance Journal, 31(1), 41–50. 10.1002/ffj.3268 [DOI] [Google Scholar]
- Xing, Y. , Lei, H. , Wang, J. , Wang, Y. , Wang, J. , & Xu, H. (2017). Effects of different drying methods on the total phenolic, rosmarinic acid and essential oil of purple perilla leaves. Journal of Essential Oil Bearing Plants, 20(6), 1594–1606. 10.1080/0972060X.2017.1413957 [DOI] [Google Scholar]
- Yang, C. , Chen, H. , Chen, H. , Zhong, B. , Luo, X. , & Chun, J. (2017). Antioxidant and anticancer activities of essential oil from Gannan navel orange peel. Molecules, 22(8), 1391 10.3390/molecules22081391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, W. , & Wetzstein, H. Y. (2011). Effects of drying and extraction conditions on the biochemical activity of selected herbs. HortScience, 46(1), 70–73. 10.21273/HORTSCI.46.1.70 [DOI] [Google Scholar]
- Zhang, C. , Liu, D. , & Gao, H. (2018). Kinetics, physicochemical properties, and antioxidant activities of Angelica keiskei processed under four drying conditions. LWT, 98, 349–357. 10.1016/j.lwt.2018.08.054 [DOI] [Google Scholar]
- Zhang, H. , Chen, F. , Wang, X. , & Yao, H.‐Y. (2006). Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Research International, 39(8), 833–839. 10.1016/j.foodres.2006.03.007 [DOI] [Google Scholar]


