Abstract
Context
The time-to-glucose-peak following the oral glucose tolerance test (OGTT) is a highly reproducible marker for diabetes risk. In obese youths, we lack evidence for the mechanisms underlying the effects of the TCF7L2 rs7903146 variant on glucose peak.
Methods
We analyzed the metabolic phenotype and the genotype for the TCF7L2 rs7903146 in 630 obese youths with normal (NGT) and impaired (IGT) glucose tolerance. Participants underwent a 3-hour, 9-point OGTT to estimate, using the oral minimal model, the disposition index (DI), the static (φstatic) and dynamic (φdynamic) components β-cell responsiveness and insulin sensitivity (SI). In a subgroup (n = 241) longitudinally followed for 2 years, we estimated the effect of time-to-glucose-peak on glucose tolerance change.
Results
Participants were grouped into early (<30 minutes) and late (≥30 minutes) glucose peakers. A delayed glucose peak was featured by a decline in φstatic (P < .001) in the absence of a difference in φdynamic. The prevalence of T-risk allele for TCF7L2 rs7903146 variant significantly increased in the late peak group. A lower DI was correlated with higher glucose concentration at 1 and 2 hours, whereas SI was inversely associated with 1-hour glucose. Glucose peak <30 minutes was protective toward worsening of glucose tolerance overtime (odds ratio 0.35 [0.15–0.82]; P = .015), with no subjects progressing to NGT or persisting IGT, in contrast to the 40% of progressor in those with late glucose peak.
Conclusion
The prevalence of T-risk allele for the TCF7L2 rs7903146 prevailed in the late time-to-glucose peak group, which in turn is associated with impaired β-cell responsiveness to glucose (φ), thereby predisposing to prediabetes and diabetes in obese youths.
Keywords: time to glucose peak, pediatric obesity, prediabetes, TCF7L2
The 2-point oral glucose tolerance test (OGTT), with fasting and 2-hour blood sampling, remains the diagnostic gold standard for impaired glucose regulation in both youth and adults (1). However, its poor reproducibility over time (2) and the absence of a reliable estimate of β-cell function hinder its use as an outpatient test to individualize therapeutic options in youth with prediabetes and diabetes (3).
Although the use of a 3-hour, 9-point OGTT, with the simultaneous measure of insulin and C-peptide concentrations, is considered a robust test to estimate β-cell function by using the Oral Minimal Model (4–7), this approach is limited to research because of its cost and the relative invasive nature given the need for an IV line and of multiple sampling times. The 9-point OGTT, by using the Oral Minimal Model, describes the components of insulin secretion as static or dynamic, with the latter estimating the promptly released insulin and the former being the responsive to each glucose level reached during the oral load (8,9). Our group demonstrated that the static component, but not the dynamic one, of insulin secretion, is reduced in the presence of the risk genotype for the transcription factor 7 like 2 (TCF7L2) rs790315 in obese youths (10). TCF7L2 is 1 of the strongest genetic determinants of type 2 diabetes in both pediatric and adult longitudinal cohorts (11–13), and has been described to affect the incretin response in obese adults (14).
Herein we combined the model-based OGTT-derived estimate of insulin secretion and the genotyping for TCF7L2 to identify their effect on surrogate markers of glucose tolerance as time to glucose peak and glucose concentrations throughout the test. Therefore, we conducted a longitudinal study in a subgroup of patients to identify OGTT and TCF7L2 effect on glucose tolerance over time.
Research Design and Methods
We conducted a cross-sectional study in 630 youths from the Yale Pathophysiology of Youth Onset prediabetes/type 2 diabetes (T2D) study (NCT01967849), a large multiethnic pediatric cohort followed at the Yale Pediatric Obesity Clinic (15). The standard of care of the Yale Pathophysiology of Youth Onset prediabetes/T2D program includes a clinical and dietary evaluation every 6 months, with behavioral interventions, such as discontinuing sugar-laden drinks and advice aimed to decrease screen time and promote physical activity. Children are not started on a diet because the program is based on lifestyle changes that have been proven to be effective in this age group. (16)
The inclusion criteria for the study were body mass index (BMI) > 85th percentile for age and sex, and age between 8 and 21 years at the screening visit. Subjects using medications affecting glucose metabolism, diagnosed with syndromic obesity, or participating in other clinical trials were excluded from the study. Participants who tested positive for at least 1 of the autoantibodies associated with type 1 diabetes (anti-islet cell anti-insulin, antiglutamic acid decarboxylase, protein tyrosine phosphatase, and anti-Zinc transporter 2 in case of negativity of the previous ones) (17) were excluded.
Participants underwent a physical evaluation and an OGTT at baseline. The physical evaluation included weight and height measurements, Tanner stage evaluation of pubertal development, and a clinical and dietary encounter.
A longitudinal study was conducted on a subgroup of participants who returned after 2 ± 0.3 years for a physical and anthropometric examination and a second OGTT.
The longitudinal cohort presented in this study is part of a parent cohort of 307 youths previously described by Cropano et al (10). Herein we included subjects with 2-year OGTT to minimize the effect of different follow-up duration on the observed outcome (change in glucose tolerance). The study protocol was approved by the Human Investigations Committee of the Yale School of Medicine.
OGTT
Before the OGTT, all the subjects followed a weight-maintenance diet consisting of at least 250 g of carbohydrates per day for 7 days before the study and were instructed to avoid strenuous physical activity. Participants were studied in the Yale Center for Clinical Investigation at 8 am after a 12-hour overnight fast. After the local application of a topical anesthetic cream containing Emla (Astra Zeneca, Wilmington, DE), 1 antecubital IV catheter was inserted for blood sampling; its patency was maintained by slow infusion of normal saline. Each subject then rested, watching a videotape throughout the test. Two baseline samples were then obtained for measurements of plasma glucose, insulin, and c-peptide. Thereafter, flavored dextrose in a dose of 1.75 g/kg of body weight (up to a maximum of 75 g) was given orally, and blood samples were obtained at 10, 20, and 30 minutes, and then every 30 minutes for 180 minutes (for a total of 9 time points) for the measurement of plasma glucose, insulin, and c-peptide. Glucose was measured at the bedside using the YSI2700-STAT-Analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured by radioimmunoassay (Linco, St. Charles, MO) that has <1% cross-reactivity with c-peptide and proinsulin. Plasma c-peptide levels were determined by ELISA using ALPCO-Immunoassays (Salem, NH) with a 3.87% intra-assay variability.
In accordance with the American Diabetes Association (18), impaired glucose tolerance (IGT) was defined as a 2-hour plasma glucose level between 140 and 199 mg/dL; impaired fasting glucose (IFG) as glucose values of 100 to 125 mg/dL, T2D as a fasting glucose level of 126 mg/dL or higher or a 2-hour plasma glucose level >200 mg/dL confirmed in 2 OGTTs or an hemoglobin A1c ≥ 6.5% (48 mmol/mol). The diagnosis of T2D was confirmed by a second OGTT when based on the 2-hour glucose level (18,19).
OGTT-derived measures of insulin secretion and responsiveness
Time to glucose peak was defined as the time of the highest glucose value over a 3-hour OGTT out of the 9 time points sampled. Insulin sensitivity (SI) was estimated from plasma glucose and insulin concentrations measured during the 3-hour OGTT using the oral glucose minimal model (4,5,9). β-Cell secretion was estimated from the c-peptide measured during the 3-hour OGTT using the oral c-peptide minimal model (9,20). The model assumes that glucose-stimulated insulin secretion is made up of 2 components: 1) a dynamic component, representing secretion of promptly releasable insulin and proportional to the rate of glucose increase through the dynamic responsivity index (φd, 10–9); and 2) a static component, deriving from provision of new insulin to the releasable pool and characterized by a static responsivity index, φs (10–9 min-1), and by a delay time constant, T (min). From model parameters, the total responsivity index (φtotal) can be derived (8) and the disposition indices (DI = φtotal × SI, DIstatic = φstatic × SI, DIdynamic = φdynamic × SI), measuring the β-cell function, calculated as previously described (9).
Genotyping. Genomic DNA was extracted from peripheral blood leukocytes. Genotyping was performed with the use of a matrix-assisted–based laser desorption ionization time-of-flight mass spectrometry on the MassARRAY platform (Sequenom) through the Yale Center for Genome Analysis (13). Genotype for the single nucleotide polymorphism rs7903146 at TCFL2 was available for the entire cohort.
Statistical analysis
The cohort was grouped into early (<30 min) and late (≥3 min) glucose peak group based on the time for highest glucose concentration during the baseline OGTT out of the 9-point samples. A preliminary sensitivity analysis was conducted to define the cut-point for early/late time to peak among those who had a time to glucose peak <30 min, =30 min, =1 hour, >1 hour. The groups with a time to glucose peak ≥30 minutes exhibited a lower DIstatic than those with glucose peak <30 min in the absence of a difference of DIdynamic. Therefore, we adopted <30 minutes to group our cohort into early and late peakers.
To investigate the effect of β-cell function (DI) and SI on the glucose concentration at fasting, 1-hour, and 2-hour time point, we conducted 3 multivariate regression models, assuming age, BMI, ethnicity (non-Hispanic white, non-Hispanic black, Hispanic), and TCF7L2 genotype (presence or absence of the T risk allele) as covariates, with glucose concentration at each specified time point as continuous outcome measures. The choice of the timepoints at fasting, 1 hour, and 2 hours, was based on the available evidence for the role of fasting (21), 1-hour glucose (22–24), and 2-hour glucose (25) as predictors of changes in glucose tolerance over time (23,24,26). The relationship between β-cell function (DI) and glucose concentration at each time point was described by the slope of each regression curve; the 2-hour glucose concentration curve was adopted as comparison term. Not-normally distributed variables were naturally log-transformed before the analysis.
Kruskal-Wallis test was used to compare continuous variables, and categorical variables were compared using the chi-square test. Data were summarized using median (25th percentile, 75th percentile) for continuous variables and count (%) for categorical variables.
To assess the longitudinal effect of baseline clinical, metabolic, and genetic determinants on the binary outcome “glucose tolerance change,” we conducted a multivariate logistic regression analysis on the longitudinal cohort (n = 241). Glucose tolerance change was defined as progression from normal glucose tolerance (NGT) to IGT or persistence of IGT or progression from IGT to T2D (27). The following adjustment variables were selected (28): age, family history of diabetes in first- or second-degree relatives, BMI, ethnicity (non-Hispanic white, non-Hispanic black, Hispanic), time to peak less than 30 minutes, TCF7L2 genotype (CC, CT, or TT) (28–30). Before including the covariates into the 2 models, we examined them for multicollinearity and excluded variables with a variance inflation factor >5.
To exploit the differential effect of the same tested variables on the 2 outcomes (change to IGT or to T2D), we ran a confirmatory polytomous regression analysis for the 2 outcomes, adopting NGT as comparison term.
Statistical significance for the effect of each variable was established with α = 0.05. Results were expressed as odds ratio for the prespecified binary outcome with respect to the control group defined per each variable. Results were summarized as mean ± standard deviation or median [25th, 75th percentile] as appropriate. Analyses were performed using STATA.13 software (StataCorp 2013; Stata Statistical Software: Release 13;. College Station, TX).
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
Results
Baseline characteristics
We studied 630 participants (467 NGT and 163 IGT) who underwent a baseline OGTT and were genotyped for the TCF7L2 rs7903146 single nucleotide polymorphism. Participants were grouped by time to glucose peak into early (<30 minute) and late (≥30 minute) peakers. Baseline characteristics are described in Table 1. The 2 groups did not differ with respect to anthropometric characteristics and ethnic background; ~30% of the late peakers had IGT vs 5% in the early-peak group (P < .001), with a similar prevalence of IFG (P = .189). The percentage of homozygous carriers of the T risk allele for TCF7L2 was 5 times higher in the late peak group (P = .042) (Table 1).
Table 1.
Baseline Characteristics (n = 630)
| Early Peak (< 30 min) N = 83 | Late Peak (≥30 min) N = 547 | P | |
|---|---|---|---|
| Sex (female) | 53 (64) | 321 (59) | 0.403 |
| Family history T2D | 19 (23) | 160 (29) | 0.296 |
| Tanner stage | |||
| II-III | 37 (44) | 240 (44) | 0.906 |
| IV-V | 46 (56) | 307 (56) | |
| Ethnicity (NHW/NHB/H) | 34/28/21 (41/34/25) | 246/137/164 (45/25/30) | 0.258 |
| Age (y) | 13.5 (10.7, 16) | 13.7 (11.5, 15.7) | 0.535 |
| BMI (kg/m2) | 30.9 (27.8, 38.4) | 32.6 (28.1, 38) | 0.245 |
| HbA1c % [mmol/mol] | 5.4 (5.3, 5.7) [36 (34, 39)] | 5.5 (5.3, 5.7) [37 (34, 39)] | 0.585 |
| Fasting glucose (mg/dL) | 91 (86, 96) | 92 (87, 98) | 0.274 |
| 2h-glucose (mg/dL) | 102 (91, 116) | 124 (108, 142) | <0.001 |
| IGT, n (%) | 4 (5) | 160 (29) | <0.001 |
| IFG, n (%) | 12 (14) | 144 (26) | 0.189 |
| TCF7L2 rs7903146 genotype CC/CT/TT, n (%) | 48/33/2 (58/40/2) | 279/216/52 (51/39/10) | 0.042 |
NHW, non-Hispanic White; NHB, non-Hispanic Black; H, Hispanic; BMI, body mass index; IFG, impaired fasting glucose; IGT, impaired glucose tolerance. p<0.05 in bold
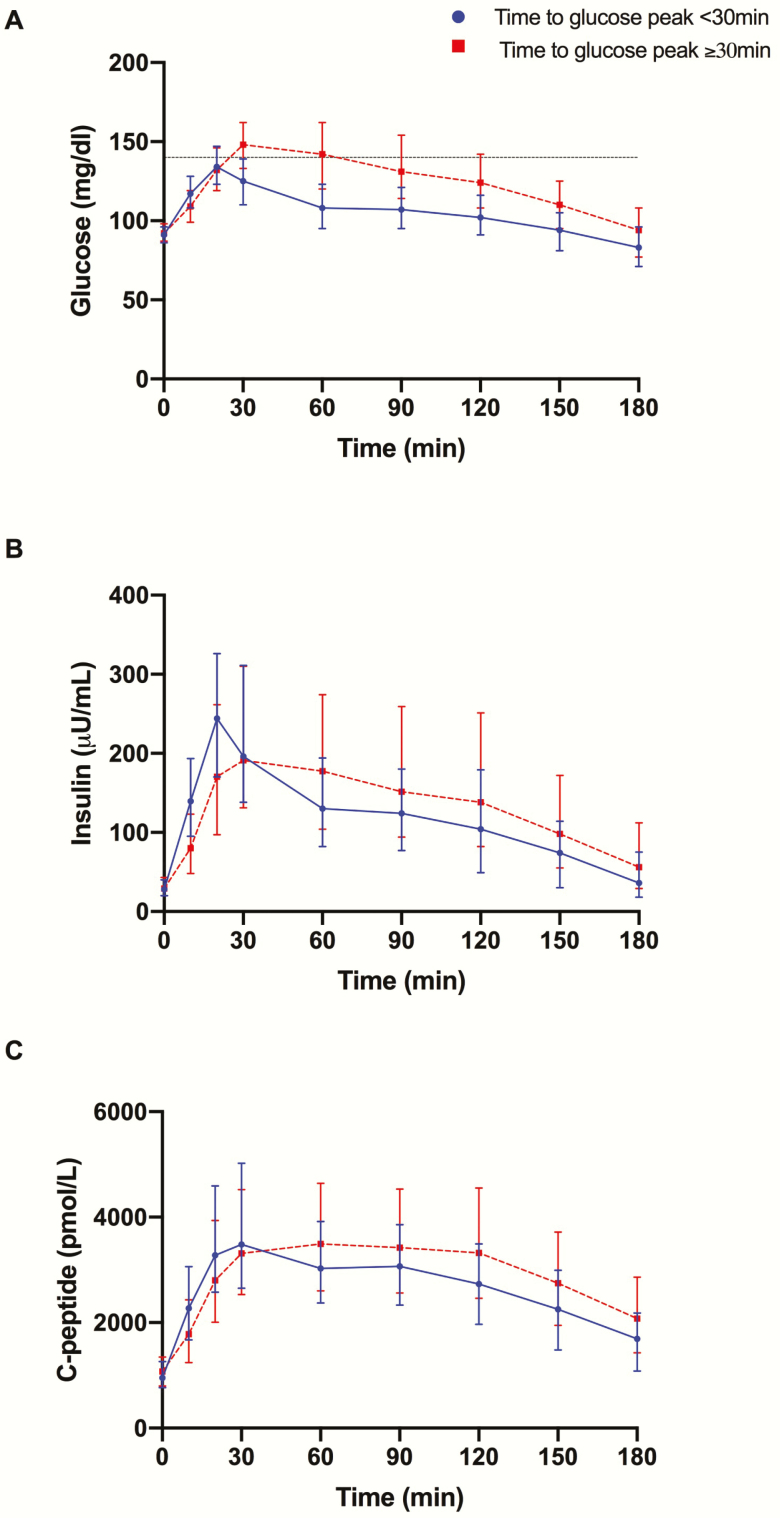
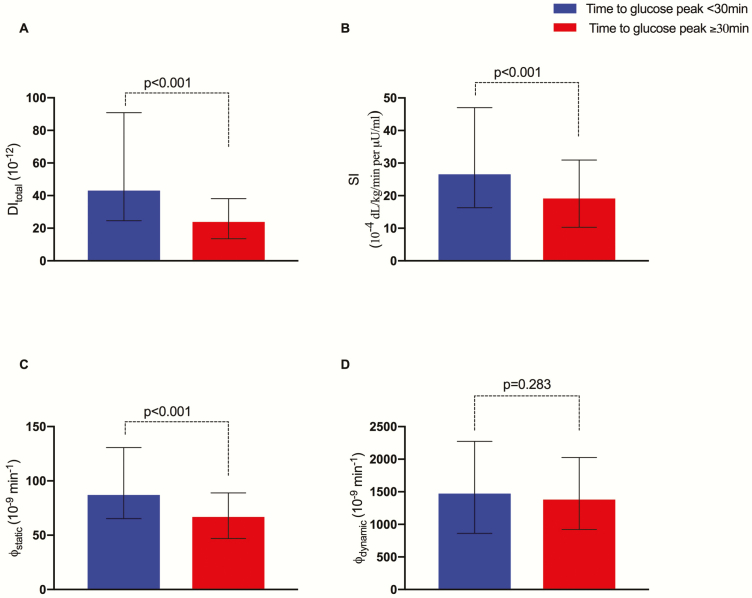
Insulin secretion and insulin sensitivity by time to glucose peak. Baseline glucose, insulin, and c-peptide concentrations did not differ between the 2 groups (P = .231, P = .781, and P = .554) (Fig. 1A–1C). Both DI and SI were significantly reduced in the late peak group (P < .001 for both) (Fig. 2A and 2B). By the use of the oral minimal model, we dissected the β-cell responsivity (φtotal) into its static (φstatic) and dynamic (φdynamic) components that mirror the 2 phases of insulin secretion. A blunted static insulin secretion (φstatic) featured the late peak participants (P < .001), in the absence of a difference in the φdynamic (P = .395) (Fig. 2C and 2D).
Figure 1.
(A) Glucose, (B) insulin, and (C) c-peptide profiles for the 4 groups based on time to glucose peak.
Figure 2.
Median and interquartile range for (A) DItotal, (B) SI, (C) φstatic, (D) φdynamic. The group with time to peak < 30 minutes has been adopted as comparison term. **P < .001; *P < .05. Abbreviations: ns, nonsignificant. DI, disposition index; SI, insulin sensitivity.
Glucose concentrations and β-cell function
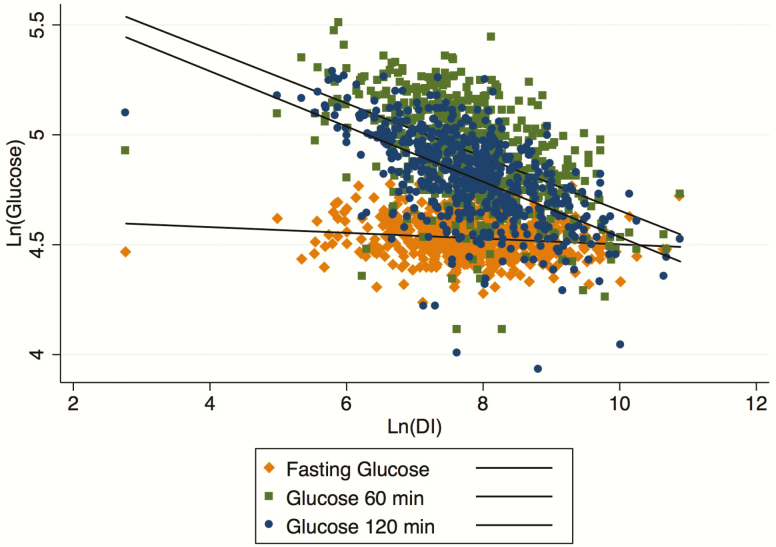
Therefore, we explored the determinants of glucose concentrations at fasting, 1 hour, and 2 hours. Naturally log-transformed DI resulted in having an inverse linear correlation with both 1- and 2-hour glucose concentration (r = -0.51 and -0.58, respectively, with P < .001), but not with fasting glucose (r = -0.13, P = NS). As described in Fig. 3, the slopes progressively decreased from the 2-hour glucose (upper line) to fasting glucose (bottom line). Although 2- and 1-hour glucose slopes did not differ from each other (P = .651), the slopes of the regression lines of DI and fasting glucose significantly diverged from that of 2-hour glucose (P < .001 for both), supporting the reduced variability of glucose concentrations at fasting upfront and a reduction of DI (Fig. 3).
Figure 3.
Relationship between Ln(DI) and Ln(Glucose) at 120 minutes (filled circle), 60 minutes (filled square), and fasting (filled diamond). Abbreviations: DI, disposition index.
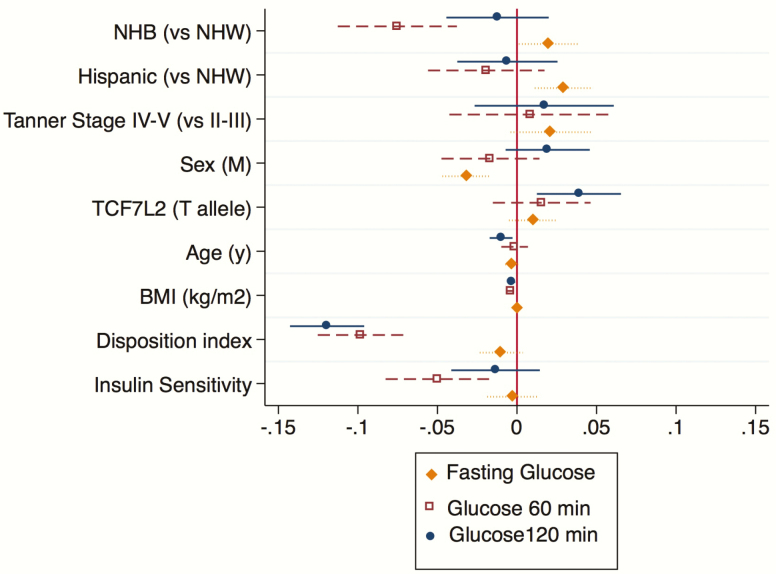
When adjusted for anthropometric and the presence of the risk allele T for TCF7L2 genotype, the decrease in the DI was still associated with an increase in 1- and 2-hour glucose (P < .001 for both, Fig. 4), whereas SI was inversely associated with 1-hour glucose (P = .023) in the absence of an effect on the 2-hour concentration (P = .342) (Fig. 4).
Figure 4.
β coefficient for multivariate regression analysis for the continuous outcomes glucose 120 minutes (filled circle), glucose 60 minutes (filled square), and fasting glucose (filled diamond). Data are expressed as median and 95% confidence interval. Abbreviations: BMI, body mass index; NHB, non-Hispanic black; NHW, non-Hispanic white.
Longitudinal predictors of glucose tolerance change
Thereafter, we analyzed the 241 participants who returned after a 2-year follow-up for a second OGTT. Of 156 participants with baseline NGT, 12% (n = 19) progressed to IGT; 38% of subjects with baseline IGT (27/85) persisted with IGT or progressed to T2D (5/85). The longitudinal cohort did not differ from the original cohort with respect to baseline metabolic and genetic characteristics, including the percentage of early/late time-to-glucose peakers (P = .378).
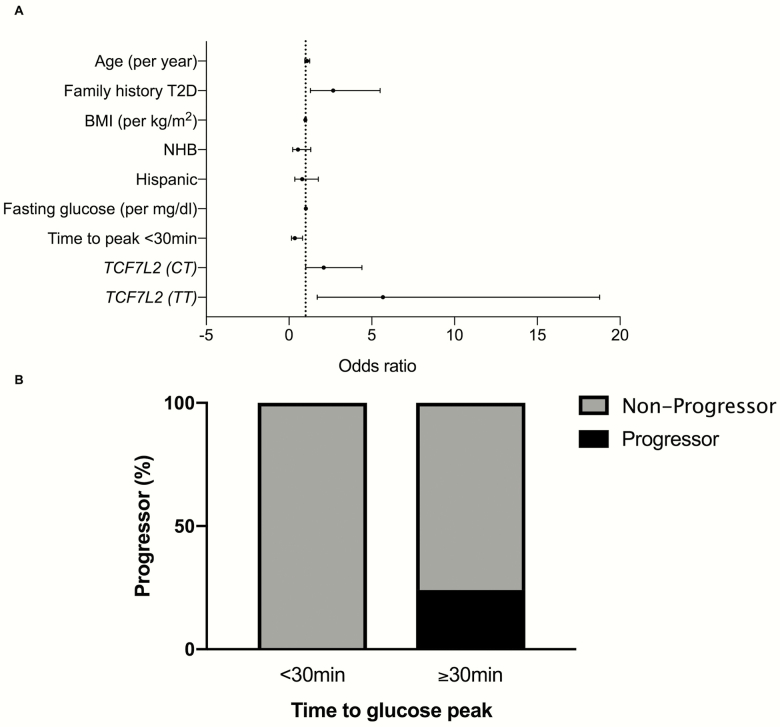
A time to glucose peak <30 minutes reduced the odds for progression from NGT to IGT or persistence in the IGT phenotype/progression to T2D (odds ratio, 0.35 [0.15–0.82]; P = .015) compared with the group with a time to peak ≥30 minutes; consistent with previous findings in the cross-sectional study, the T allele for TCF7L2 increased the risk for progression respect to the wild-type genotype (CC) with an odds of 5.67 ([1.7, 18.8]; P = .005) for the homozygous and 2.10 [1.01, 4.41]; P = .049) for the heterozygous carriers; a positive family history for T2D increased the odds for progression as well (2.67 [1.29, 5.51]; P = .008) (Fig. 5A) in the absence of an effect of the other clinical and metabolic variables (age, BMI, ethnicity, fasting glucose). These findings were confirmed by the absence of “progressors” among those with early peak (<30 minutes) (Fig. 5B), with similar percentage of progressors in those with time to glucose peak at 30 minutes and 1-hour (22% and 29% of progressor, respectively) and 68% progressor in those with time to glucose peak >1 hour.
Figure 5.
(A) Odds ratio for change in glucose tolerance, defined as change from NGT to IGT, or IGT > IGT or IGT > T2D. Data are expressed as median odds and 95% confidence intervals of the estimates. (B) Percentage of “progressor” from the longitudinal cohort (n = 241). Progressor are defined as NGT > IGT or IGT > IGT or IGT > T2D. Abbreviations: BMI, body mass index; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; NHB, non-Hispanic black; T2D, type 2 diabetes.
The polytomous regression analysis confirmed the reduction of the risk for IGT persistence or progression to IGT in those with glucose peak earlier than 30 minutes (relative risk reduction, 0.46 ± 0.17; P = .034) as compared to the late peak group, whereas and the homozygous risk genotype for TCF7L2 was confirmed to increase the relative risk (relative risk reduction, 5.31 ± 3.14; P = .005) for the IGT outcome. Thereby, the polytomous regression analysis did not confirm the significance of any of the tested variables as determinants of progression toward T2D, likely because of the limited number of participants (n = 5) who were diagnosed with T2D at the follow-up OGTT.
Discussion
By using the oral minimal model for insulin secretion, we described the metabolic and genetic determinants of several OGTT-derived measures associated with the risk of diabetes, the time to glucose peak, and the glucose concentrations at fasting, 1 hour, and 2 hours after the oral load. Therefore, we tested the longitudinal effect of the time to glucose peak, as well as of its metabolic and genetic determinants, on change in glucose tolerance over time. Thus, by this approach, the oral minimal model was adopted to validate time to glucose peak and glucose concentrations as measures of β-cell function.
First, a time to glucose peak ≥30 minutes was associated with a blunted static, but not dynamic, β-cell responsivity to glucose, as described by the oral minimal model (Fig. 2). The static component represents the delayed insulin release proportional to the difference between the actual glucose and the baseline (9,20). It has been referred to as β-cell “glucose responsiveness” and is influenced by the time of gastric emptying and the incretin response (31). In contrast, dynamic insulin secretion describes the early release of insulin, which did not change between early and late peakers (9). Consistent with the role of the incretin response in shaping the glucose profile via an effect on the static insulin release (32), we observed a higher prevalence of the T-risk allele of TCF7L2 rs7903146 in the late-peak group. The latter has been associated with impaired incretin response in adults (14,33,34) by influencing β-cell responsiveness to GLP-1 secretion (14).
Second, we demonstrated that glucose concentrations at both 1 and 2 hours, but not at fasting, act as a proxy of insulin secretion in the contest of individual insulin sensitivity, as described by the oral disposition index (Fig. 3). However, 2-hour glucose has been adopted as a glucose tolerance measure in the absence of a validation as surrogate for insulin secretion. We described that DI, and SI by itself, can differentially effect the glucose concentrations during each time point of the OGTT, with DI being the sole determinant of 2-hour glucose concentrations as described in Fig. 3, and SI contributing to the 1-hour glucose concentration but not to the 2-hour glucose level. Although DI and SI are intimately correlated, the absence of a direct effect of SI on 2-hour glucose level prompts the conclusion that glucose concentration at 2 hours is a result of β-cell failure and may occur later than the initial lowering of SI, as described previously (35). This observation is paradigm shifting for pediatric studies because it would contemplate the use of the 2-hour glucose level as surrogate measure for evaluating therapeutic interventions targeting insulin secretion, whereas earlier glucose concentrations could be retained a proxy of insulin sensitivity.
Third, both time to glucose peak and TCF7L2 genotype were significant determinants for progression from NGT to IGT or persistence in the IGT phenotype/progression to T2D. Although the role of TCF7L2 as a risk gene for diabetes progression is well known in both adult and pediatric cohorts (11,13,36), its effect on time to glucose peak and their interplay in determining diabetes progression has never been exploited. We can hypothesize that the time to glucose peak is the harbinger of an impaired β-cell function sustained, among others, from a TCF7L2-related mechanisms. In patients with T2D, the risk genotype of TCF7L2 rs7903146 variant has been associated with reduced insulin sensitivity (37), whereas in youth with prediabetes, we observed an additional effect on insulin secretion that could in turn be responsible for the higher percentage of progressor observed in the risk-genotype group.
Additionally, we provided a mechanism-based framework to define a threshold for delayed time to glucose peak in youth. Studies conducted in adults with normal or impaired glucose tolerance have, in turn, adopted 30 (38,39) or 60 minutes (40) as the cutoff for glucose peak to identify subjects at risk for prediabetes or diabetes (38,39). Such cut-points were lacking evidence in the pediatric population before this current study. Therefore, the 30-minute cut-point could be considered a functional threshold to define early and late time to peak. Besides its physiological implications, this observation supports the collection of at least 1 blood sample between the 0- and 30-minute time points of the OGTT, which is seldom performed for diagnostic purpose, to allow detection of an early glucose peak and an increased risk for dysglycemia.
A major limit of this study is the reduced number of progressors toward T2D (n = 5) because we could not exclude that the risk determinants driving the progression from NGT to IGT or the persistence of IGT could differ from those involved in overt diabetes.
The use of the oral minimal model and the availability of TCF7L2 genotype for the entire cohort are 2 points of strength of this study, along with the presence of a longitudinal cohort. A major limitation is the lack of incretin measures in the tested groups, which could have supported the mechanism by which TCF7L2 might affect the static insulin secretion. The longitudinal analysis was not conducted on the entire cohort, but on a subgroup that was similar to the original one with respect to clinical and metabolic phenotypes.
This study suggests that the use of an additional sample before 30 minutes during the OGTT to estimate the time to glucose peak may stratify obese youths by individuating specific impairment of β-cell function (φ), that could in turn be targeted by pathogenesis-based treatments.
Acknowledgments
The authors thank all volunteers for their participation in the study and Rachel Goldberg, Cindy Guandalini, and Mary Savoye for their help in the Yale Pediatric Clinic.
Financial Support: This study was supported by the National Institutes of Health, National Institute of Child Health and Human Development (grants R01-HD-40787, R01DK111038, R01-HD-28016, and K24-HD-01464 to S.C.), the National Center for Research Resources (Clinical and Translational Science Award [grant UL1-RR-0249139] to S.C.), the American Diabetes Association (Distinguished Clinical Scientist Award to S.C.), the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01-DK-111038 to S.C.; grant R01-DK-114504-01A1), the Robert Leet Patterson and Clara Guthrie Patterson Trust Mentored Research Award, and the European Medical Information Framework (EMIF 115372 to A.G.). This article’s contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Author Contributions: A.G. and S.C. designed the study; A.G. and D.T. analyzed the data and drafted the manuscript; C.C. and C.D.M. analyzed the data on insulin secretion using the oral minimal model; B.P. contributed to the data collection; L.G, B.G., and N.S. genotyped the TCF7L2 SNP; and L.G., C.C., and S.C. critically revised the manuscript. A.G. and S.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
Abbreviations
- BMI
body mass index
- DI
disposition index
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- SI
insulin sensitivity
- T2D
type 2 diabetes
Additional Information
Disclosure Summary: The authors have no conflict of interests with regard to the content of this study.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–s28. [DOI] [PubMed] [Google Scholar]
- 2. Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93(11):4231–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287(4):E637–E643. [DOI] [PubMed] [Google Scholar]
- 5. Dalla Man C, Yarasheski KE, Caumo A, et al. Insulin sensitivity by oral glucose minimal models: validation against clamp. Am J Physiol Endocrinol Metab. 2005;289(6):E954–E959. [DOI] [PubMed] [Google Scholar]
- 6. Dalla Man C, Campioni M, Polonsky KS, et al. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 2005;54(11):3265–3273. [DOI] [PubMed] [Google Scholar]
- 7. Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293(1):E1–E15. [DOI] [PubMed] [Google Scholar]
- 8. Bock G, Dalla Man C, Campioni M, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55(12):3536–3549. [DOI] [PubMed] [Google Scholar]
- 9. Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014;63(4):1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cropano C, Santoro N, Groop L, et al. The rs7903146 variant in the TCF7L2 gene increases the risk of prediabetes/type 2 diabetes in obese adolescents by impairing β-cell function and hepatic insulin sensitivity. Diabetes Care. 2017;40(8):1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Florez JC, Jablonski KA, Bayley N, et al. ; Diabetes Prevention Program Research Group TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355(3):241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cauchi S, El Achhab Y, Choquet H, et al. TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: a global meta-analysis. J Mol Med (Berl). 2007;85(7):777–782. [DOI] [PubMed] [Google Scholar]
- 13. Giannini C, Dalla Man C, Groop L, et al. Co-occurrence of risk alleles in or near genes modulating insulin secretion predisposes obese youth to prediabetes. Diabetes Care. 2014;37(2):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villareal DT, Robertson H, Bell GI, et al. TCF7L2 variant rs7903146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes. 2010;59(2):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346(11):802–810. [DOI] [PubMed] [Google Scholar]
- 16. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. Jama. 2007;297(24):2697–2704. [DOI] [PubMed] [Google Scholar]
- 17. Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95(7):3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Association AD. 15. Diabetes advocacy: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S152–S153. [DOI] [PubMed] [Google Scholar]
- 19. Zeitler P, Arslanian S, Fu J, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19 Suppl 27:28–46. [DOI] [PubMed] [Google Scholar]
- 20. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50(1):150–158. [DOI] [PubMed] [Google Scholar]
- 21. Tirosh A, Shai I, Tekes-Manova D, et al. ; Israeli Diabetes Research Group Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353(14):1454–1462. [DOI] [PubMed] [Google Scholar]
- 22. Alyass A, Almgren P, Akerlund M, et al. Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: results from two prospective cohorts. Diabetologia. 2015;58(1):87–97. [DOI] [PubMed] [Google Scholar]
- 23. Tfayli H, Lee SJ, Bacha F, Arslanian S. One-hour plasma glucose concentration during the OGTT: what does it tell about β-cell function relative to insulin sensitivity in overweight/obese children? Pediatr Diabetes. 2011;12(6):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tricò D, Galderisi A, Mari A, Santoro N, Caprio S. One-hour post-load plasma glucose predicts progression to prediabetes in a multi-ethnic cohort of obese youths. Diabetes Obes Metab. 2019;21(5):1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giannini C, Weiss R, Cali A, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes. 2012;61(3):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JY, Goran MI, Toledo-Corral CM, Weigensberg MJ, Choi M, Shaibi GQ. One-hour glucose during an oral glucose challenge prospectively predicts β-cell deterioration and prediabetes in obese Hispanic youth. Diabetes Care. 2013;36(6):1681–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galderisi A, Giannini C, Weiss R, et al. Trajectories of changes in glucose tolerance in a multiethnic cohort of obese youths: an observational prospective analysis. Lancet Child Adolesc Health. 2018;2(10):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyssenko V, Almgren P, Anevski D, et al. ; Botnia study group Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes. 2005;54(1):166–174. [DOI] [PubMed] [Google Scholar]
- 29. Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care. 2005;28(4):902–909. [DOI] [PubMed] [Google Scholar]
- 30. Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF; Diabetes Prevention Program Research Group Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care. 2009;32(9):1583–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. [DOI] [PubMed] [Google Scholar]
- 32. Dalla Man C, Micheletto F, Sathananthan A, Rizza RA, Vella A, Cobelli C. A model of GLP-1 action on insulin secretion in nondiabetic subjects. Am J Physiol Endocrinol Metab. 2010;298(6):E1115–E1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lyssenko V, Lupi R, Marchetti P, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest. 2007;117(8):2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Srinivasan S, Kaur V, Chamarthi B, et al. TCF7L2 genetic variation augments incretin resistance and influences response to a sulfonylurea and metformin: the study to understand the genetics of the acute response to metformin and glipizide in humans (SUGAR-MGH). Diabetes Care. 2018;41(3):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–S220. [DOI] [PubMed] [Google Scholar]
- 36. Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38(3):320–323. [DOI] [PubMed] [Google Scholar]
- 37. Ferreira MC, da Silva MER, Fukui RT, Arruda-Marques MDC, Dos Santos RF. TCF7L2 correlation in both insulin secretion and postprandial insulin sensitivity. Diabetol Metab Syndr. 2018;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hulman A, Witte DR, Vistisen D, et al. Pathophysiological characteristics underlying different glucose response curves: a latent class trajectory analysis from the prospective EGIR-RISC Study. Diabetes Care. 2018;41(8):1740–1748. [DOI] [PubMed] [Google Scholar]
- 39. Chung ST, Ha J, Onuzuruike AU, et al. Time to glucose peak during an oral glucose tolerance test identifies prediabetes risk. Clin Endocrinol. 2017;87(5):484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kramer CK, Ye C, Hanley AJ, et al. Delayed timing of post-challenge peak blood glucose predicts declining beta cell function and worsening glucose tolerance over time: insight from the first year postpartum. Diabetologia. 2015;58(6):1354–1362. [DOI] [PubMed] [Google Scholar]
- 41. Cree-Green M, Xie D, Rahat H, et al. Oral glucose tolerance test glucose peak time is most predictive of prediabetes and hepatic steatosis in obese girls. J Endocr Soc. 2018;2(6):547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim JY, Michaliszyn SF, Nasr A, et al. The shape of the glucose response curve during an oral glucose tolerance test Heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care. 2016;39(8):1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining β-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care. 2011;34(9):2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]