Abstract
Innovations and discoveries in cancer therapeutics have improved survival rates in patients with various types of malignancies. At the same time, physicians are identifying an increased number of patients with treatment-related cardiotoxicity. It is imperative that physicians recognize early treatment-related adverse effects to determine the safest therapeutic options for patients with cancer. This manuscript evaluates the role of cardiovascular imaging and biomarkers in identifying cardiotoxicity trigged by various chemotherapeutic agents and summarizes expert consensus statements regarding cardiotoxicity monitoring.
Keywords: cardiotoxicity, anthracyclines, immune checkpoint inhibitors, echocardiogram, cardiac magnetic resonance, CMR, biomarkers
INTRODUCTION
New discoveries and innovation in cancer therapy have improved the survival of patients with many types of malignancies. However, these agents are not without the potential for adverse cardiovascular effects. For example, anthracyclines, anti-HER2 agents, and immune checkpoint inhibitors are effective for treating many malignancies but are also known to be cardiotoxic. It is essential that physicians be able to identify and monitor chemotherapy-related cardiotoxicity at the earliest onset to ensure patient safety and determine the safest therapeutic options. This manuscript discusses the role of various imaging techniques—including 2-dimensional transthoracic echocardiogram (2D TTE), 3-dimensional transthoracic echocardiogram (3D TTE), radionuclide angiography (MUGA), cardiac magnetic resonance (CMR), and cardiac biomarkers (eg, troponin, BNP)—to help diagnose and track therapy-related cardiotoxicity. We also highlight differences and similarities in expert consensus statements related to cardiotoxicity monitoring.
DEFINING CARDIOTOXICITY
In 2014, the American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) described cardiotoxicity, or cancer therapeutics-related cardiac dysfunction (CTRCD), as a ≥ 10% decrease from baseline left ventricular ejection fraction (LVEF) to a value < 53% as measured by 2D TTE.1 CTRCD can be further defined based on the presence of symptoms (hypoxia, shortness of breath, lower extremity edema) and reversibility; it is considered reversible if LVEF returns to within 5% of baseline.
It is important to note that cardiotoxicity from anthracyclines is potentially reversible if detected early and managed appropriately,2 whereas cardiotoxicity from trastuzumab is usually reversible only after cessation of chemotherapy.3 Resuming trastuzumab after the patient recovers LVEF is feasible because most patients do not demonstrate a subsequent reduction in LV systolic function when rechallenged while on heart failure medications.3
IMAGING MODALITIES
Echocardiography
I. Two-Dimensional Transthoracic Echocardiography
Its wide availability, portability, low cost, and lack of radiation exposure make 2D TTE a convenient method for monitoring the cardiotoxicity of chemotherapeutic agents and the ideal choice for imaging at baseline.4 Although there are multiple methods to estimate chamber sizes and LVEF, the ASE/EACVI recommend using the biplane method of disks (modified Simpson's rule).5 However, given the discrepancies between guidelines and consensus statements, we feel that a simplified recommendation of anthracycline- and trastuzumab-related cardiotoxicity monitoring is needed. Therefore, we suggest an easy algorithm to follow in asymptomatic patients (Figure 1).
Figure 1.
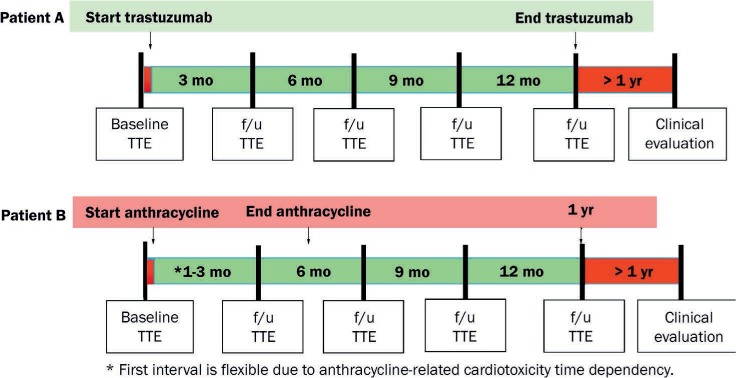
Proposed timing of cardiotoxicity monitoring with transthoracic echocardiogram for anthracyclines and anti-HER2 therapies in patients with breast cancer without heart failure symptoms. TTE: transthoracic echocardiogram
Chemotherapeutic agents rarely cause valvular disease, yet many of these patients undergo concomitant radiation therapy that may lead to valvular disease.6 Marantic endocarditis also may cause valvular disease in some patients, such as those with solid tumor malignancies.7 In addition to assessing valve structure and function, 2D TTE may also be used to evaluate the pericardium because anthracyclines, other chemotherapeutic agents, and immunotherapies have been associated with pericarditis and/or the development of pericardial effusions.8,9
II. Three-Dimensional Transthoracic Echocardiography
Three-dimensional TTE offers distinct advantages over 2D TTE, such as more accurate assessment of LV volumes.10 Also, 3D TTE is more accurate than other echocardiographic techniques at detecting LVEF < 50% (using cardiac magnetic resonance as the gold standard) in survivors of childhood cancer, presumably because 3D TTE measurements are not skewed by geometric assumptions of LV shape.11 Furthermore, LVEF measurements on 3D TTE demonstrate less variability at 1-year follow-up, and less inter- and intraobserver variability, compared to 2D TTE.12 This is essential because serial echocardiographic measurements of LVEF may determine if a chemotherapeutic agent can be resumed or discontinued during treatment. Although preferable to 2D TTE, the use of 3D TTE is somewhat restricted by limited availability, increased cost, and the need for an experienced operator to obtain high-quality images.
III. Speckle-Tracking Global Longitudinal Strain
Although great emphasis is placed on monitoring the ejection fraction (EF) of cancer patients on chemotherapeutic agents, a decrease in EF may not be evident until substantial myocardial injury has taken place (Figure 2). Conversely, measurements of strain and strain rate may allow for earlier and more sensitive detection of myocardial injury among patients receiving chemotherapeutic agents, particularly anthracyclines.13
Figure 2.
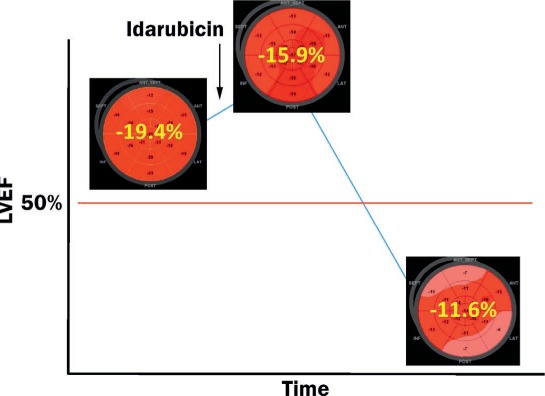
An example of subclinical cardiotoxicity related to anthracycline detected by global longitudinal strain before a decrease in left ventricular ejection fraction (LVEF).
Based on one major study, patients with < 8% reduction in global longitudinal strain (GLS) are not likely to have meaningful cardiotoxicity, whereas those with a GLS reduction > 15% during chemotherapy may have clinically significant cardiotoxicity.14 Also, a GLS less negative than −17.5% may identify patients at higher risk of developing cardiotoxicity related to anthracyclines. (See the American College of Cardiology website for more information on GLS).15–17
As useful as they are, strain measurements do have limitations, such as variability in measurements between different ages, genders, and imaging vendors.16,18
First-Pass or Radionuclide Multiple-Gated Acquisition Scan
First-pass or radionuclide multiple-gated acquisition (MUGA) scan was one of the initial tests used to identify anthracycline toxicity among cancer patients; in fact, some of the original guidelines for monitoring and potentially withholding anthracycline chemotherapy in the setting of reduced LVEF were based on this imaging modality.18 MUGA estimates of LVEF have been reported to be more accurate and reproducible than 2D TTE but not necessarily when compared to other imaging modalities.19 In a single-center study among 75 patients with different cancers, MUGA demonstrated a wide range in limits of agreement and less reproducibility, causing misclassification of LVEF in patients with cancer when compared to cardiac magnetic resonance (the gold standard for LVEF measurement).20 Additionally, in a study with 50 breast cancer patients receiving trastuzumab, 3D TTE was as accurate and reproducible as MUGA for serial monitoring of LVEF.21
Although MUGA is widely available, one disadvantage is patient exposure to a minimal amount of radiation.
Cardiac Magnetic Resonance
Cardiac magnetic resonance (CMR) is the gold standard for obtaining accurate and reproducible measurements of LV volume and EF and is superior to echocardiography in this regard.22,23
CMR does not rely on geometric assumptions regarding LV shape; this is particularly important in patients who have undergone chemotherapy/radiation therapy, which may cause remodeling.
Accurately estimating EF is particularly important when patients reach thresholds that may predicate the cessation of chemotherapy. In one study, CMR was superior to 2D and 3D TTE at identifying patients with an EF < 50%.24 Thus, CMR may be a robust alternative to echo when chemotherapy cessation is being considered (Figure 3). There is evidence of changes in myocardial T1 mapping in patients who have received anthracyclines in the past, as with childhood cancer survivors. These changes suggest fibrosis and seem to correlate well with impaired functional capacity by cardiopulmonary exercise testing.25 In another study, increased cumulative anthracycline dose was associated with decreased LV mass on CMR, and both were associated with an increased risk of adverse cardiovascular events.26
Figure 3.
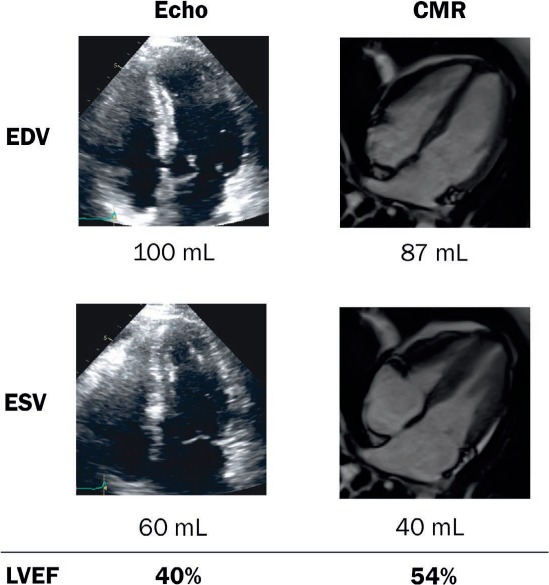
Illustrative case of a 37-year-old woman with lymphoma undergoing chemotherapy. She had a decrease in left ventricular (LV) systolic function by echocardiogram (echo) biplane LV ejection fraction (LVEF) quantification, which was verified by cardiac magnetic resonance imaging, and LVEF > 50%. Treatment with anthracycline was resumed without any consequences in LV systolic function. CMR: cardiac magnetic resonance; EDV: end diastolic volume; ESV: end systolic volume
Immune Checkpoint Inhibitors (ICIs) are increasingly being used as an immunotherapy treatment for cancer, but they are known to potentially cause myocarditis. CMR has been used to help in noninvasively diagnosing ICI-associated myocarditis.27
BIOMARKERS
Testing with cardiac biomarkers is a highly sensitive, widely available, and relatively cost-effective method of testing for myocardial injury among patients undergoing chemotherapy.28 The following describes the biomarkers used to detect cardiotoxicity.
Troponin I
Cardinale et al. studied 703 cancer patients with aggressive malignancies who underwent high-dose chemotherapy and found no significant reductions in EF and only a 1% rate of cardiac events in those with no troponin I elevation ≤ 1 month after chemotherapy. This suggests that a lack of troponin I elevation among patients undergoing chemotherapy has a high negative predictive value for cardiotoxicity. There was a higher rate of cardiac events among those with troponin I elevation soon after chemotherapy and an even higher rate of cardiac events among those with troponin I elevation both immediately and 1 month after chemotherapy.28
Cardiotoxicity after trastuzumab chemotherapy is often reversible. However, in another study by Cardinale, patients with troponin I elevations during chemotherapy were more likely to undergo trastuzumab-associated cardiotoxicity and less likely to achieve LVEF recovery.29 A more recent study showed that patients with a normal GLS and negative ultrasensitive troponin I measured at the end of anthracycline treatment are less likely to develop cardiotoxicity.30 Elevations in ultrasensitive troponin I may identify breast cancer patients who will develop cardiotoxicity and heart failure if treated serially with anthracycline and trastuzumab.31
Troponin T
Among patients receiving trastuzumab, troponin T elevation was not shown to be a predictor of cardiotoxicity.32 On the other hand, among those treated with ICIs, a troponin T level of ≥ 1.5 ng/mL was associated with a 4-fold increase in the likelihood of a major adverse cardiac event.27
High-Sensitivity Troponins
When compared with several biomarkers such as myeloperoxidase, PIGF, and GDF-15, elevations of high-sensitivity (hs) troponin I during treatment do not predict cardiotoxicity.33 In a study by Pourier et al. of childhood cancer survivors, hs troponin T was not a sensitive marker for late-onset cardiotoxicity.34 Of note, hs troponin T was elevated in 11% of cancer patients without a diagnosis of myocardial infarction who were receiving clinical care in our center.35 This could be a limiting factor in establishing a diagnosis of CTRCD. Overall, hs troponin assays have limited value for identifying cardiotoxicity risk.
Brain Natriuretic Peptide (BNP) and NT-pro BNP
The role of natriuretic peptides in predicting cardiotoxicity is less clear. One small study demonstrated a rise in BNP after chemotherapy in patients who eventually developed anthracycline cardiotoxicity compared to those who did not.36 However, other studies have shown no association between NT-pro BNP elevations and future systolic or diastolic dysfunction.37 Although BNP levels may reflect filling pressures, the volume status of a patient undergoing chemotherapy rapidly fluctuates (as a result of poor oral intake and/or vomiting), and this biomarker may not be as reliable.
Other Biomarkers
In addition to troponin, BNP, and NT-pro BNP, other biomarkers have been examined as potential predictors of cardiotoxicity. Galectin-3 is a biomarker associated with cardiac inflammation and fibrosis,38 whereas biomarker ST2 is elevated in cardiac remodeling.30 However, neither of these biomarkers have been shown to predict future cardiotoxicity among patients treated with cardiotoxic agents.30,39 Elevations in myeloperoxidase have been shown to predict cardiotoxicity.39
Limitations of Biomarkers
Different biomarker assays used at different hospitals may make it difficult to standardize cutoffs that are deemed predictive of cardiotoxicity and may warrant cessation of chemotherapy. Also, other confounding factors—such as infection, arrhythmias, hypertensive events, pulmonary embolism—could cause an elevation in troponin assays. Lack of validation from large prospective trials is one of the main limitations when considering biomarkers. Table 1 outlines the utility of various biomarkers in detecting cardiotoxicity.28,30,32–34,36,37,39
Table 1.
Summary of studies assessing the role of biomarkers for predicting cardiotoxicity.28,30,32–34,36,37,39
| SERUM BIOMARKER | AUTHORS | SAMPLE SIZE (N) | RESULT |
|---|---|---|---|
| hs troponin I | Putt et al.33 | 78 | Not predictive of cardiotoxicity |
| hs troponin T | Pourier et al.34 | 64 | Not predictive of late cardiotoxicity in survivors |
| Troponin I | Cardinale et al.28 | 703 | Predictive of adverse cardiac events |
| Troponin T | Fallah-Rad et al.32 | 42 | Not predictive of cardiotoxicity |
| BNP | Okumura et al.36 | 13 | May predict cardiotoxicity |
| NT-proBNP | Dodos et al.37 | 100 | Not predictive of cardiotoxicity |
| ST-2 | Sawaya et al.30 | 81 | Not predictive of cardiotoxicity |
| Galectin-3 | Ky et al.39 | 78 | Not predictive of cardiotoxicity |
| MPO | Ky et al.39 | 78 | May predict cardiotoxicity |
hs: high sensitivity; NT-proBNP: N-terminal pro b-type natriuretic peptide; MPO: myeloperoxidase
MONITORING DURING TREATMENT: EXPERT CONSENSUS
Early detection of cardiotoxicity is essential, not only because treatment may need to be held but also because the response to medical management for heart failure among those who have received anthracyclines is time sensitive.40 Table 2 summarizes the most prominent consensus statements for the detection and monitoring of cardiotoxicity.1,18,41,42
Table 2.
| POSITION PAPER/GUIDELINE | BASELINE TTE (YES/NO) | FREQUENCY OF MONITORING (AC) | FREQUENCY OF MONITORING (T) | FOLLOW-UP AFTER TREATMENT |
|---|---|---|---|---|
| MUGA18 (1987) | No, MUGA instead | At baseline, at the end of treatment, when cumulative dose reaches 250–500 mg/m2, and at 450 mg/m2 (and each subsequent dose) | N/A | N/A |
| ASE/EACVI1 (2014) | Yes | At baseline, at the end of treatment, when cumulative dose reaches 240 mg/m2, and each additional 50 mg/m2 dose thereafter | At baseline and every 3 months | 6 months after completion of chemotherapy |
| ESC41 (2016) | Yes | At baseline, at the end of treatment, when cumulative dose reaches 240 mg/m2 | At baseline and every 3 months | Long-term surveillance |
| ASCO42 (2017) | Yes | N/A | N/A | 6–12 months after completion |
TTE: transthoracic echocardiography; MUGA: multiple-gated acquisition scan; AC: anthracycline; T: trastuzumab; N/A: not available or not commented; ASE: American Society of Echocardiography; EACVI: European Association of Cardiovascular Imaging; ESC: European Society of Cardiology; ASCO: American Society of Clinical Oncology
In the 1970s, MUGA was the primary method of detecting cardiotoxicity among those receiving anthracyclines. In 1987, Schwartz et al. proposed the following MUGA-based guidelines18:
Perform baseline MUGA for LVEF calculation. Subsequent studies should be performed at least 3 weeks after completing 100 mg/m2 of doxorubicin and before the next dose.
- Patients with normal baseline LVEF (≥ 50%):
- Perform the second study after 250 to 300 mg/m2.
- Repeat study after 400 mg/m2 in patients with known heart disease, radiation exposure, abnormal electrocardiographic results, or cyclophosphamide therapy, or repeat after 450 mg/m2 in the absence of any of these risk factors.
- Perform sequential studies thereafter before each dose.
- Discontinue doxorubicin therapy once functional criteria for cardiotoxicity develops (ie, absolute decrease in LVEF ≥ 10% associated with a decline to ≤ 50%).
- Patients with abnormal baseline LVEF (< 50%):
- Doxorubicin therapy should not be initiated with baseline LVEF ≤ 30%.
- In patients with LVEF > 30% and < 50%, sequential studies should be obtained before each dose.
Discontinue doxorubicin with cardiotoxicity (absolute decrease in LVEF ≥ 10% and/or final LVEF ≤ 30%).
The ASE/EACVI Expert Consensus Statement provides recommendations for cardiotoxicity monitoring. Prior to anthracycline administration, a baseline measurement of LVEF and GLS via echocardiogram in addition to troponin is recommended. These measurements should be repeated immediately and 6 months after anthracycline therapy. However, if a dose exceeds 240 mg/m2 during treatment, then measurements at this point and before each additional 50 mg/m2 dose are recommended. During trastuzumab therapy, measurements of LVEF, GLS, and troponin are recommended at baseline and every 3 months.1
The European Society of Cardiology (ESC) position paper on cancer treatments and cardiovascular toxicity provides similar recommendations.41 Before anthracycline therapy, it also recommends assessing baseline cardiac function and considering an alternate chemotherapeutic agent if systolic dysfunction is noted. In addition, it recommends repeat assessment after anthracycline chemotherapy, or earlier assessment if a dose of 240 mg/m2 is exceeded during therapy. Measuring at least one cardiac biomarker, in addition to high-sensitivity troponin I, is recommended with each cycle of anthracycline chemotherapy. For trastuzumab, the ESC recommends cardiac monitoring every 3 months during chemotherapy and once after it is completed. It also recommends a repeat echocardiogram 3 weeks after an initial LVEF decrease in asymptomatic patients as well as measuring troponin with every cycle among patients with high baseline risk.41
The American Society of Clinical Oncology 2017 guidelines recommend performing a baseline TTE depending on the dose of anthracycline received, the number of cardiovascular risk factors, and whether or not the patient will receive sequential therapy (ie, lower-dose anthracycline followed by trastuzumab). They also recommend that a follow-up TTE be performed 6 to 12 months after completing chemotherapy in asymptomatic patients who are considered to have an increased cardiac risk.42
Despite the frequency of monitoring during therapy, large trials for both anthracyclines and trastuzumab demonstrate that a vast majority of cardiotoxicity cases occur within 1 year of chemotherapy cessation.2,43 Thus, monitoring up to 1 year after treatment may be reasonable.
CONCLUSION
Advancements in cancer therapies have led to an improved prognosis for many patients. However, the potential for therapy-related cardiotoxicity still exists, most notably from anthracyclines and anti-HER2 agents. Recently, ICIs have been associated with myocarditis.27 Table 3 summarizes the chemotherapeutic agents discussed in this manuscript, their associated cardiotoxicities, and possible treatments.44
Table 3.
Summary of cardiotoxicity from chemotherapeutic agents.
| CANCER THERAPY | TOXICITY | TREATMENT |
|---|---|---|
| Anthracyclines (doxorubicin, idarubicin) | Cardiomyopathy | Dexrazoxane, dose limitation, prolonged infusion, early initiation of guideline-directed medical therapy for heart failure |
| Anti-HER2 therapies (trastuzumab) | Cardiomyopathy | Guideline-directed medical therapy for heart failure, rechallenge (while on heart failure therapy) if ejection fraction recovers to > 50% |
| Immune checkpoint inhibitors | Myocarditis | High-dose intravenous corticosteroids, plasmapheresis, immunoglobulins, other immunomodulators |
Imaging modalities such as echocardiography and CMR may result in earlier detection of cardiotoxicity, allowing physicians to promptly address common manifestations such as a decrease in LVEF. Early detection is essential for minimizing the risks of cardiotoxicity, since early initiation of heart failure therapy (including beta blockers and angiotensin-converting enzyme inhibitors) has been shown to increase the likelihood of reversing anthracycline-induced cardiotoxicity.40 An integrated approach using both advanced cardiovascular imaging techniques and biomarkers may allow for the timely detection of cardiotoxicity.
KEY POINTS
Cardiovascular imaging plays an essential role in monitoring for cardiotoxicity subsequent to cardiotoxic chemotherapeutic administration.
Although serum biomarkers can aid in the diagnosis of cardiotoxicity, there is insufficient data to support their use as a sole diagnostic tool.
An integrated approach using both cardiovascular imaging and serum biomarkers may be the best method to monitor for cardiotoxicity.
Footnotes
Conflict of Interest Disclosure:
All authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Plana JC, Galderisi M, Barac A et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014 Oct;15(10):1063–93. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardinale D, Colombo A, Bacchiani G et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015 Jun 2;131(22):1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 3.Ewer MS, Vooletich MT, Durand JB et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005 Nov 1;23(31):7820–6. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 4.Eschenhagen T, Force T, Ewer MS et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011 Jan;13(1):1–10. doi: 10.1093/eurjhf/hfq213. [DOI] [PubMed] [Google Scholar]
- 5.Lang RM, Bierig M, Devereux RB et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. [DOI] [PubMed] [Google Scholar]
- 6.Hamza A, Tunick PA, Kronzon I. Echocardiographic manifestations of complications of radiation therapy. Echocardiography. 2009 Jul;26(6):724–8. doi: 10.1111/j.1540-8175.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 7.Edoute Y, Haim N, Rinkevich D, Brenner B, Reisner SA. Cardiac valvular vegetations in cancer patients: a prospective echocardiographic study of 200 patients. Am J Med. 1997 Mar;102(3):252–8. doi: 10.1016/S0002-9343(96)00457-3. [DOI] [PubMed] [Google Scholar]
- 8.Casey DJ, Kim AY, Olszewski AJ. Progressive pericardial effusion during chemotherapy for advanced Hodgkin lymphoma. Am J Hematol. 2012 May;87(5):521–4. doi: 10.1002/ajh.22239. [DOI] [PubMed] [Google Scholar]
- 9.Palaskas N, Morgan J, Daigle T et al. Targeted Cancer Therapies With Pericardial Effusions Requiring Pericardiocentesis Focusing on Immune Checkpoint Inhibitors. Am J Cardiol. 2019 Apr 15;123(8):1351–7. doi: 10.1016/j.amjcard.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Badano LP, Boccalini F, Muraru D et al. Current clinical applications of transthoracic three-dimensional echocardiography. J Cardiovasc Ultrasound. 2012 Mar;20(1):1–22. doi: 10.4250/jcu.2012.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong GT, Plana JC, Zhang N et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012 Aug 10;30(23):2876–84. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013 Jan 8;61(1):77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014 Jul 1;63(25 Pt A):2751–68. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 14.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013 May;26(5):493–8. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Ali MT, Yucel E, Bouras S et al. Myocardial Strain Is Associated with Adverse Clinical Cardiac Events in Patients Treated with Anthracyclines. J Am Soc Echocardiogr. 2016 Jun;29(6):522–527.e3. doi: 10.1016/j.echo.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Risum N, Ali S, Olsen NT et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J Am Soc Echocardiogr. 2012 Nov;25(11):1195–203. doi: 10.1016/j.echo.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 17.American College of Cardiology [Internet] Washington, DC: American College of Cardiology; 2018. Global Longitudinal Strain for LV Function; 2018 Aug 10 [cited 2019 Sep 11]. Available from: https://www.acc.org/latest-in-cardiology/ten-points-to-remember/2018/08/10/11/01/assessment-of-left-ventricular-global-longitudinal-strain. [Google Scholar]
- 18.Schwartz RG, McKenzie WB, Alexander J et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med. 1987 Jun;82(6):1109–18. doi: 10.1016/0002-9343(87)90212-9. [DOI] [PubMed] [Google Scholar]
- 19.Bellenger NG, Burgess MI, Ray SG et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000 Aug;21(16):1387–96. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Nijjar PS, Misialek JR et al. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: comparison with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017 Mar 24;19(1):34. doi: 10.1186/s12968-017-0348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker J, Bhullar N, Fallah-Rad N et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol. 2010 Jul 20;28(21):3429–36. doi: 10.1200/JCO.2009.26.7294. [DOI] [PubMed] [Google Scholar]
- 22.The clinical role of magnetic resonance in cardiovascular disease. Task Force of the European Society of Cardiology, in collaboration with the Association of European Paediatric Cardiologists. Eur Heart J. 1998 Jan;19(1):19–39. [PubMed] [Google Scholar]
- 23.Grothues F, Smith GC, Moon JC et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002 Jul 1;90(1):29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong AC, Gidding S, Gjesdal O, Wu C, Bluemke DA, Lima JA. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc Imaging. 2012 Aug;5(8):837–48. doi: 10.1016/j.jcmg.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tham EB, Haykowsky MJ, Chow K et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013 Jun 10;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilan TG, Coelho-Filho OR, Pena-Herrera D et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012 Dec 1;110(11):1679–86. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmood SS, Fradley MG, Cohen JV et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018 Apr 24;71(16):1755–64. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardinale D, Sandri MT, Colombo A et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004 Jun 8;109(22):2749–54. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 29.Cardinale D, Colombo A, Torrisi R et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010 Sep 1;28(25):3910–6. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 30.Sawaya H, Sebag IA, Plana JC et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012 Sep 1;5(5):596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ky B, Putt M, Sawaya H et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014 Mar 4;63(8):809–16. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallah-Rad N, Walker JR, Wassef A et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011 May 31;57(22):2263–70. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 33.Putt M, Hahn VS, Januzzi JL et al. Longitudinal Changes in Multiple Biomarkers Are Associated with Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. Clin Chem. 2015 Sep;61(9):1164–72. doi: 10.1373/clinchem.2015.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pourier MS, Kapusta L, van Gennip A et al. Values of high sensitive troponin T in long-term survivors of childhood cancer treated with anthracyclines. Clin Chim Acta. 2015 Feb 20;441:29–32. doi: 10.1016/j.cca.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Upadhyay A, Lopez-Mattei J, Kim P et al. Diagnostic performance of high sensitivity troponin in cancer patients. J Am Coll Cardiol. 2019 Mar;73(9 Suppl 1):209. [Google Scholar]
- 36.Okumura H, Iuchi K, Yoshida T et al. Brain natriuretic peptide is a predictor of anthracycline-induced cardiotoxicity. Acta Haematol. 2000;104(4):158–63. doi: 10.1159/000046508. [DOI] [PubMed] [Google Scholar]
- 37.Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol. 2008 May;97(5):318–26. doi: 10.1007/s00392-007-0633-6. [DOI] [PubMed] [Google Scholar]
- 38.Eliaz I. The Role of Galectin-3 as a Marker of Cancer and Inflammation in a Stage IV Ovarian Cancer Patient with Underlying Pro-Inflammatory Comorbidities. Case Rep Oncol. 2013 Jul 3;6(2):343–9. doi: 10.1159/000353574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ky B, Putt M, Sawaya H et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014 Mar 4;63(8):809–16. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardinale D, Colombo A, Lamantia G et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010 Jan 19;55(3):213–20. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 41.Zamorano JL, Lancellotti P, Rodriguez Muñoz D et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016 Sep 21;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211. ESC Scientific Document Group. [DOI] [PubMed] [Google Scholar]
- 42.Armenian SH, Lacchetti C, Barac A et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017 Mar 10;35(8):893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 43.Cameron D, Piccart-Gebhart MJ, Gelber RD et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017 Mar 25;389(10075):1195–1205. doi: 10.1016/S0140-6736(16)32616-2. Herceptin Adjuvant (HERA) Trial Study Team. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarifa A, Albittar A, Kim PY et al. Cardiac toxicities of anticancer treatments: chemotherapy, targeted therapy and immunotherapy. Curr Opin Cardiol. 2019 Jul;34(4):441–450. doi: 10.1097/HCO.0000000000000641. [DOI] [PubMed] [Google Scholar]


