Abstract
Anthracyclines are the cornerstone of therapy for a wide range of solid and hematologic malignancies; however, their use is limited by the risk of chemotherapy-induced cardiotoxicity leading to cardiomyopathy and heart failure. The incidence of cardiotoxicity in the literature depends on the definition being used, anthracycline dose, duration of follow-up, and surveillance methods used to identify cardiac injury. The reported risk of clinical heart failure has been around 2% to 4% with low-dose anthracycline regimens, whereas the incidence of cardiac injury defined by an abnormal increase in cardiac biomarkers has been reported as high as 35%.
Multiple mechanisms have been proposed for anthracycline cardiotoxicity, including the deleterious effects of oxidative stress and reactive oxygen species and the inhibition of topoisomerase II beta, which leads to cardiomyocyte death. In addition, genetic susceptibility is an emerging field that is currently generating active research. The risk factors associated with anthracycline cardiotoxicity include lifetime cumulative dose, age, prior cardiac dysfunction, and the presence of cardiovascular risk factors, in particular hypertension. In this review, we summarize the incidence, mechanisms, and risk factors for anthracycline-mediated left ventricular dysfunction and discuss the role of risk stratification and early detection in patient management.
Keywords: cardiotoxicity, anthracyclines, cardiomyopathy, heart failure, topoisomerase-II beta
INTRODUCTION
Anthracyclines have successfully been used for several decades to treat a wide range of malignancies and currently play a key role in the treatment of both hematologic and solid tumor cancers. The discovery of the anthracycline family of drugs dates back to the 1950s with the identification of daunorubicin from the soil bacterium Streptomyces peucetius.1 Since then, anthracyclines have been used in combination with other chemotherapeutics and targeted therapies to treat malignancies including lymphoma, sarcoma, breast cancer, and pediatric leukemia.2 The first reports of anthracycline-related left ventricular (LV) dysfunction and heart failure (HF) marked a series of changes in oncology and cardio-oncology practices, including the limitation of dose, assessment of cardiac function before anthracycline administration, and investigations of preventive strategies to reduce cardiotoxicity.3 In this review, we summarize the incidence, mechanisms, and risk factors of anthracycline-related cardiomyopathy and discuss the importance of risk stratification and early detection in patient management.
INCIDENCE
Chemotherapy-induced cardiomyopathy is a disease spectrum ranging from cardiac injury—detected by biomarkers and asymptomatic decline in systolic function as measured by left ventricular ejection fraction (LVEF)—to the development of overt symptoms and clinical signs of HF.4 The incidence of cardiomyopathy and HF in those treated with anthracyclines is variable. Von Hoff et al. were the first to note a continuum of increasing risk as the cumulative amount of drug increased—with an HF incidence of 3% at 400 mg/m2, 7% at 550 mg/m2, and 18% at doses of 700 mg/m2.5 A retrospective analysis by Swain et al.3 that included 630 patients treated with doxorubicin for breast cancer and small cell lung cancer reported that 26% of patients experienced anthracycline-related HF at a cumulative dose of 550 mg/m2. Since the 1990s, the doses of anthracycline used in the treatment of many cancers have continued to decline, and in some malignancies, such as subgroups of breast cancer, anthracyclines are being replaced as first-line therapy.6
In a Medicare study of 43,338 patients with breast cancer treated with either anthracyclines or another chemotherapy and followed over a median of 53 months, anthracycline-based chemotherapy was associated with an adjusted HR of 1.26 (CI 1.12–1.42) for development of congestive HF in women aged 66 to 70.7 In the pediatric and young adult population, survivors of childhood cancer who were treated with anthracyclines and/or mediastinal radiotherapy were reported to have a 15-fold increased lifetime risk of HF compared with matched controls.8
Among patients older than 65 years who were treated with commonly used anthracycline doses, the rate of anthracycline-associated HF is reported to be as high as 10%.9 The short-term risk for developing HF is also increased in older patients with pre-existing cardiovascular risk, with one study reporting a 17% incidence of clinical HF at 5 years among survivors of aggressive non-Hodgkin's lymphoma.9
The incidence of cardiotoxicity also varies according to how it is defined. Cardiotoxicity spans the continuum of severity, ranging from asymptomatic detectable structural changes on cardiac imaging, or the detection of measurable biomarkers before structural or electrical change is evident, to overt clinical symptoms requiring urgent hospitalization, cardiogenic shock, and/or refractory HF treated with advanced HF therapies.
The incidence of different subtypes of cardiotoxicity (mostly from low-dose anthracyclines) has been reported in the range of 2% to 4% for clinical HF decompensation, 9% to 11% for subclinical change identified on cardiac imaging, and 30% to 35% for cardiac injury defined as biomarker increase.10 With novel generations of more sensitive biomarkers, the threshold for detecting cardiac injury has been lowered, although the relevance of these findings in clinical practice remains to be elucidated.
A distinction has also been made between the timing of cardiotoxicity, with early toxicity occurring within the first year of treatment and late cardiotoxicity defined as toxicity that manifests after several years (median of 7 years post-treatment).5,11 This classification was based on retrospective studies in which the decline in LV function was determined either after HF developed or on random evaluations in pediatric cancer patients. A recent study by Cardinale et al. that involved 2,625 patients demonstrated an overall incidence of cardiotoxicity of 9% after anthracycline treatment. In this study, 98% of the cases occurred within the first year and were asymptomatic.12,13
MECHANISMS
Despite our ever-increasing understanding of the molecular basis of anthracycline-induced cardiotoxicity, the exact mechanism of action remains unknown. Several theories have been proposed over the years, and our current knowledge suggests that the deleterious effects of anthracyclines affect cardiac myocytes at various cellular levels.14 The following pathways have been implicated in anthracycline cardiotoxicity (Figure 1).
Figure 1.
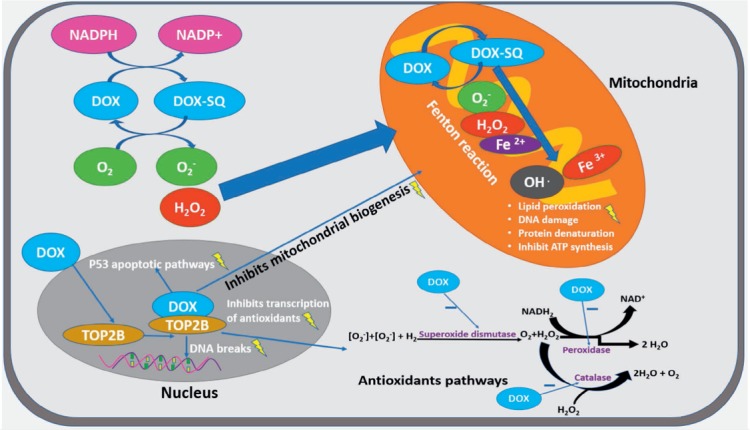
Cellular pathways involved in anthracycline-induced cardiotoxicity using doxorubicin as an example. NADPH: nicotinamide adenine dinucleotide phosphate; DOX: doxorubicin; TOP2B: topoisomerase II beta isoform
Oxidative Stress and Reactive Oxygen Species
The most-widely accepted hypothesis for the mechanism of anthracycline-induced cardiomyopathy is the generation of excess reactive oxygen species (ROS), which occurs by electron exchange between oxygen molecules and the anthracycline quinone moiety and other cellular electron donors.15 An imbalance of increased downstream oxidative stress products and reduced upstream antioxidants is thought to result in lipid peroxidation, cellular membrane disruption, and DNA/RNA damage leading to cellular apoptosis and fibrous tissue formation.14,16 In cardiomyocytes, the quinone moiety of anthracyclines is subject to univalent reduction to a semiquinone radical, which auto-oxidizes in the presence of molecular oxygen to re-form the quinone of the parent anthracycline and the free radical superoxide anion. This auto-oxidation process forms a repeating cycle, resulting in continued production and accumulation of superoxide anions.17 In turn, the paucity of free radical scavenging enzymes in cardiomyocytes leads to oxidative stress that may result in cardiomyocyte injury and/or cell death.15,18 The generated ROS can also interact with mitochondrial DNA, lipids, and proteins, disrupting the normal mitochondrial function and reducing cellular energy reserves.19 The high concentration of mitochondria in cardiac myocytes and the ability to upregulate their production in response to hypertrophy may predispose the heart to anthracycline toxicity more than other body tissues.20,21 The free radical hypothesis has led investigators to study the role of antioxidants in preventing or reversing anthracycline cardiomyopathy, but data on clinical or subclinical benefits remain elusive.22–24 Reactive oxygen species may also be generated by the formation of anthracycline-iron complexes that undergo redox cycling.25 A novel study, however, showed that the accumulation of mitochondrial rather than cytosolic/extracellular iron potentiates the cardiotoxicity.26 Transgenic mice with upregulated mitochondrial iron exporters were found in one study to be protected from anthracycline cardiotoxicity, and these findings have supported the mechanism of action of dexrazoxane, a cardioprotective iron chelator that reduces mitochondrial iron levels.27
Topoisomerase II Beta
Recent years have yielded a better understanding of the role of topoisomerase II beta (TOP2B) inhibition, making it one of the most conceivable mechanisms of anthracycline cardiomyopathy. DNA topoisomerases, collectively, are classes of enzymes that prevent the supercoiling of DNA during replication, transcription, and recombination.28 In cancer cells, anthracycline binds with TOP2B and DNA to form a complex that inhibits cellular replication and triggers cell death.29 Topoisomerase II exists in two isoenzymes in humans, topoisomerase II alpha (TOP2A) and TOP2B. TOP2A is largely expressed in replicating cells, whereas TOP2B exists in quiescent cells such as cardiomyocytes. It was proposed that the interaction between anthracycline and TOP2B in cardiac myocytes is responsible for anthracycline cardiotoxicity by causing DNA breaks and inhibiting mitochondrial biogenesis.2 Evidence supporting this theory comes from animal studies in which mice embryonic fibroblasts with TOP2B deletion were resistant to doxorubicin-induced DNA damage.30 Mice with cardiomyocyte-specific TOP2B gene deletion have also demonstrated protection from DNA strand breaks, defective mitochondrial biogenesis, and ROS formation when exposed to anthracycline, preventing the development of HF.31 Cardiomyocyte exposure to anthracyclines and TOP2B inhibition also results in impaired mitochondrial biogenesis and activation of p53, all of which lead to cardiomyocyte injury and death.31
RISK FACTORS
Cardiac complications of anthracyclines were initially reported a few years after the introduction of daunorubicin.2 As mentioned earlier, Von Hof et al.5 was the first to identify total cumulative dose as a major risk factor for doxorubicin-related HF.3 Through retrospective analysis, they generated a dose toxicity curve by plotting the incidence of HF (defined clinically) against the total anthracycline dose used across many studies and reported that there was a continuum of increasing risk with increasing total anthracycline dose.3 A subsequent study by Swain et al. found a higher HF incidence than reported by the Von Hoff group, with 5%, 16%, 26%, and 48% of patients experiencing doxorubicin-related congestive HF at cumulative doses of 400 mg/m2, 500 mg/m2, 550 mg/m2, and 700 mg/m2, respectively.3 With improved understanding of HF pathophysiology, the term “congestive heart failure” is now rarely used in recent literature; however, it still persists in oncology reports and in general indicates symptomatic HF with volume overload. The Swain group performed a retrospective analysis that included data from three prospective studies involving 630 patients that were randomized to doxorubicin plus placebo, with 32 of the patients developing HF. Age was found to be an important risk factor for anthracycline toxicity, as was cumulative doxorubicin dose above 400 mg/m2, with the greatest toxicity being seen among those older than 65 years of age. It was also noted that doxorubicin-related HF may be schedule dependent, with a lower incidence seen in those treated once per week compared to patients treated once every 3 weeks.3,5,32 The most important take-home message of these trials is that HF could occur at relatively low doses, such as ≤ 300 mg/m2, a notion that led to changes in oncology practice and reduction in anthracycline doses across many cancer treatment regimens.
Other risk factors in addition to cumulative anthracycline dose include female gender, African-American race, extremes of age (> 65 years or < 18 years), and concomitant cardiac exposure to radiation therapy, all of which have been shown to increase the risk of cardiotoxicity5,6,33–35 and have been incorporated into risk scores developed for survivors of childhood cancer. Childhood risk scores can be used to predict the risk of developing heart failure, ischemic heart disease, and stroke by age 50 among survivors of childhood cancer, particularly those within 5 years of their cancer diagnosis.36,37 More recent studies also reported associations between cardiovascular risk factors such as arterial hypertension, hyperlipidemia, and existing myocardial disease with anthracycline cardiomyopathy, leading to interest in cardiovascular risk factor treatment before, during, and after anthracycline therapy.12,38
GENETIC SUSCEPTIBILITY: AN EMERGING CONCEPT
The first genome-wide association study performed in patients with anthracycline toxicity supported the role of the anthracyclines–TOP2B interaction.39 This study identified a highly significant polymorphism in retinoic acid receptor-gamma (RARG), a protein known to play a role in the transcriptional regulation of TOP2B. In vitro studies showed that RARG represses the expression of TOP2B, and rat cardiomyoblasts carrying this polymorphism expressed higher basal levels of TOP2B, which may explain the increased risk of anthracycline toxicity in carriers of that allele.40 Variants in genes encoding for membrane transporters that regulate anthracycline influx (SLC28A3) and efflux (ABCC1, ABCC2, ABCC5) have also been found to associate with anthracycline toxicity in several studies.39–43 The most extensive evidence exists for one of the polymorphisms in the SLC28A3 gene, which showed a protective effect in three pediatric cohorts; the data suggest that a lower influx of anthracyclines into cardiomyocytes results in less cardiotoxicity.40,42,44 Another gene, UGT1A6, is associated with the drug detoxification glucuronidation pathway.45 It has been proposed that reduced UGT1A6-mediated glucuronidation of anthracycline metabolites may lead to the accumulation of toxic metabolites in variant gene carriers, which can then result in the observed anthracycline toxicity risk.46 There is still much to be learned about the role of genetic susceptibility in anthracycline toxicity, and investigations are underway.
A recent study by Labeit et al. suggested that rare variants in genes known to be associated with cardiomyopathies, particularly titin, can increase the risk of anthracycline cardiotoxicity.47 In another recent study, rare titin-truncating variants were found to be more prevalent in adult and childhood cancer patients who experienced anthracycline cardiotoxicity than in the general population.48 This study also found that anthracyclines were associated with protracted LV dysfunction in mice with a titin truncation compared to the wild-type mice. As more information becomes available, the clinical implications/applications of these finding will need to be determined.
CLINICAL PRACTICE
Risk stratification and early detection of anthracycline cardiotoxicity are two important aspects of cardio-oncology care because they provide an opportunity to initiate cardioprotective strategies and ultimately mitigate the risk of myocardial injury and HF.49 Current clinical practice is focused on identifying those at increased risk of anthracycline toxicity, implementing strategies to minimize the risk, treating those with clinical or subclinical cardiotoxicity, and developing appropriate surveillance strategies for long-term monitoring.
The clinical practice guideline that was developed by the American Society of Clinical Oncology provides a roadmap for practitioners in this area by guiding them through five clinical questions geared toward identifying risk, preventive strategies, monitoring, and treatment of cardiac dysfunction.50 The guideline provides an excellent summary of reported evidence and identifies three broad categories of risk:
Treatment that includes high doses of anthracyclines (eg, doxorubicin ≥ 250 mg/m2, epirubicin ≥ 600 mg/m2), high doses of radiation therapy (≥ 30 Gy) involving the heart, or lower doses of anthracyclines combined with lower doses of radiation therapy;
Treatment with lower doses of anthracyclines (eg, doxorubicin < 250 mg/m2, epirubicin < 600 mg/m2) or trastuzumab alone with risk factors that include older age, abnormal baseline LV function, or the presence of multiple other known cardiovascular risk factors such as hypertension and diabetes; and
Sequential therapy with lower doses of anthracyclines followed by trastuzumab.
Risk stratification is important because it determines the approach to prevention, management, and surveillance.50 The recent guidelines from the American College of Cardiology/American Heart Association define patients with cardiotoxicity as having stage A disease, which places them in a category where they are “at risk” for developing HF.51 Treatment recommendations for this stage include aggressive management of cardiovascular risk factors such as hypertension, hyperlipidemia, and diabetes.51 Managing risk factors is particularly important for oncologic patients during the pretreatment and treatment assessment given the associations between cardiovascular risk factors and the risk of cardiac toxicity. As these areas of clinical need continue to grow, ongoing research and improved understanding of risk and best strategies to minimize toxicity will be needed.
CONCLUSION
Our understanding of anthracycline-related cardiotoxicity has grown significantly in conjunction with improved cancer survival. Ongoing research to improve risk stratification will need to happen concurrently with the development of new therapeutic options, mechanistic studies to understand the association with other therapies, and clinical approaches to hasten diagnoses, treat cardiotoxicity, and improve outcomes.
KEY POINTS
Anthracyclines have a proven beneficial role in the treatment of multiple malignancies but can have cardiotoxic effects that result in left ventricular dysfunction and heart failure.
The proposed mechanisms of anthracycline cardiotoxicity, although not fully elucidated, include oxidative stress signaling and reactive oxygen species as well as the contribution of topoisomerase II-beta.
Risk factors for anthracycline cardiotoxicity include the lifetime cumulative dose, age (very young and old), female sex, cardiovascular risk factors, prior cardiac dysfunction, and concomitant treatment with trastuzumab. New data indicate an association between rare genetic variants in cardiomyopathy-associated genes and anthracycline cardiotoxicity.
Current clinical practice includes assessment of cardiac function before administration and with high doses of anthracyclines.
Footnotes
Conflict of Interest Disclosure:
Dr. Barac is a consultant for Bristol-Myers Squibb.
REFERENCES
- 1.Di Marco A, Cassinelli G, Arcamone F. The discovery of daunorubicin. Cancer Treat Rep. 1981;65(Suppl 4):3–8. [PubMed] [Google Scholar]
- 2.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014 Sep 2;64(9):938–45. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 3.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003 Jun 1;97(11):2869–79. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 4.Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018 Jun;104(12):971–7. doi: 10.1136/heartjnl-2017-312103. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Layard MW, Basa P et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979 Nov;91(5):710–7. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 6.Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012 Jun 20;30(18):2232–9. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007 Sep 1;25(25):3808–15. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 8.Oeffinger KC, Mertens AC, Sklar CA et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006 Oct 12;355(15):1572–82. doi: 10.1056/NEJMsa060185. Childhood Cancer Survivor Study. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong GT, Oeffinger KC, Chen Y et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013 Oct 10;31(29):3673–80. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther. 2017 Feb;31(1):63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991 Sep 25;266(12):1672–7. [PubMed] [Google Scholar]
- 12.Zamorano JL, Lancellotti P, Rodriguez Muñoz D et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016 Sep 21;37(36):2768–801. doi: 10.1093/eurheartj/ehw211. ESC Scientific Document Group. [DOI] [PubMed] [Google Scholar]
- 13.Cardinale D, Colombo A, Bacchiani G et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015 Jun 2;131(22):1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 14.Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019 Jun 1;307:41–8. doi: 10.1016/j.toxlet.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Doroshow JH. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 1983 Feb;43(2):460–72. [PubMed] [Google Scholar]
- 16.Singal PK, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J. 1997 Oct;11(12):931–6. doi: 10.1096/fasebj.11.12.9337145. [DOI] [PubMed] [Google Scholar]
- 17.Menna P, Salvatorelli E. Primary Prevention Strategies for Anthracycline Cardiotoxicity: A Brief Overview. Chemotherapy. 2017;62(3):159–68. doi: 10.1159/000455823. [DOI] [PubMed] [Google Scholar]
- 18.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999 Apr 1;57(7):727–41. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 19.Eder AR, Arriaga EA. Capillary electrophoresis monitors enhancement in subcellular reactive oxygen species production upon treatment with doxorubicin. Chem Res Toxicol. 2006 Sep;19(9):1151–9. doi: 10.1021/tx060083i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goffart S, von Kleist-Retzow JC, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: power-plant failure contributes to cardiac failure in hypertrophy. Cardiovasc Res. 2004 Nov 1;64(2):198–207. doi: 10.1016/j.cardiores.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Goormaghtigh E, Huart P, Praet M, Brasseur R, Ruysschaert JM. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys Chem. 1990 Apr;35(2–3):247–57. doi: 10.1016/0301-4622(90)80012-v. [DOI] [PubMed] [Google Scholar]
- 22.Dresdale AR, Barr LH, Bonow RO et al. Prospective randomized study of the role of N-acetyl cysteine in reversing doxorubicin-induced cardiomyopathy. Am J Clin Oncol. 1982 Dec;5(6):657–63. doi: 10.1097/00000421-198212000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Myers C, Bonow R, Palmeri S et al. A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N-acetylcysteine. Semin Oncol. 1983 Mar;10(1 Suppl 1):53–5. [PubMed] [Google Scholar]
- 24.Iarussi D, Auricchio U, Agretto A et al. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med. 1994;15(Suppl):s207–12. doi: 10.1016/0098-2997(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 25.Minotti G, Salvatorelli E, Menna P. Pharmacological foundations of cardio-oncology. J Pharmacol Exp Ther. 2010 Jul;334(1):2–8. doi: 10.1124/jpet.110.165860. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa Y, Ghanefar M, Bayeva M et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014 Feb;124(2):617–30. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swain SM, Vici P. The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review. J Cancer Res Clin Oncol. 2004 Jan;130(1):1–7. doi: 10.1007/s00432-003-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardaway BW. Adriamycin-associated cardiomyopathy: where are we now? updates in pathophysiology, dose recommendations, prognosis, and outcomes. Curr Opin Cardiol. 2019 May;34(3):289–95. doi: 10.1097/HCO.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 29.Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–8. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- 30.Lyu YL, Kerrigan JE, Lin CP et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007 Sep 15;67(18):8839–46. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Liu X, Bawa-Khalfe T et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012 Nov;18(11):1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 32.Torti FM, Bristow MR, Howes AE et al. Reduced cardiotoxicity of doxorubicin delivered on a weekly schedule. Assessment by endomyocardial biopsy. Ann Intern Med. 1983 Dec;99(6):745–9. doi: 10.7326/0003-4819-99-6-745. [DOI] [PubMed] [Google Scholar]
- 33.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991 Mar 21;324(12):808–15. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 34.Billingham ME, Bristow MR, Glatstein E, Mason JW, Masek MA, Daniels JR. Adriamycin cardiotoxicity: endomyocardial biopsy evidence of enhancement by irradiation. Am J Surg Pathol. 1977 Mar;1(1):17–23. [PubMed] [Google Scholar]
- 35.Hasan S, Dinh K, Lombardo F, Kark J. Doxorubicin cardiotoxicity in African Americans. J Natl Med Assoc. 2004 Feb;96(2):196–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Chow EJ, Chen Y, Kremer LC et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015 Feb 10;33(5):394–402. doi: 10.1200/JCO.2014.56.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chow EJ, Chen Y, Hudson MM et al. Prediction of Ischemic Heart Disease and Stroke in Survivors of Childhood Cancer. J Clin Oncol. 2018 Jan 1;36(1):44–52. doi: 10.1200/JCO.2017.74.8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szmit S, Jurczak W, Zaucha JM et al. Pre-existing arterial hypertension as a risk factor for early left ventricular systolic dysfunction following (R)-CHOP chemotherapy in patients with lymphoma. J Am Soc Hypertens. 2014 Nov;8(11):791–9. doi: 10.1016/j.jash.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Aminkeng F, Bhavsar AP, Visscher H et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet. 2015 Sep;47(9):1079–84. doi: 10.1038/ng.3374. Canadian Pharmacogenomics Network for Drug Safety Consortium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linschoten M, Teske AJ, Cramer MJ, van der Wall E, Asselbergs FW. Chemotherapy-Related Cardiac Dysfunction: A Systematic Review of Genetic Variants Modulating Individual Risk. Circ Genom Precis Med. 2018 Jan;11(1) doi: 10.1161/CIRCGEN.117.001753. e001753. [DOI] [PubMed] [Google Scholar]
- 41.Semsei AF, Erdelyi DJ, Ungvari I et al. ABCC1 polymorphisms in anthracycline-induced cardiotoxicity in childhood acute lymphoblastic leukaemia. Cell Biol Int. 2012 Jan;36(1):79–86. doi: 10.1042/CBI20110264. [DOI] [PubMed] [Google Scholar]
- 42.Visscher H, Ross CJ, Rassekh SR et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012 May 1;30(13):1422–8. doi: 10.1200/JCO.2010.34.3467. Canadian Pharmacogenomics Network for Drug Safety Consortium. [DOI] [PubMed] [Google Scholar]
- 43.Vulsteke C, Pfeil AM, Maggen C et al. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Res Treat. 2015 Jul;152(1):67–76. doi: 10.1007/s10549-015-3437-9. [DOI] [PubMed] [Google Scholar]
- 44.Visscher H, Ross CJ, Rassekh SR et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer. 2013 Aug;60(8):1375–81. doi: 10.1002/pbc.24505. CPNDS Consortium. [DOI] [PubMed] [Google Scholar]
- 45.Bock KW, Köhle C. UDP-glucuronosyltransferase 1A6: structural, functional, and regulatory aspects. Methods Enzymol. 2005;400:57–75. doi: 10.1016/S0076-6879(05)00004-2. [DOI] [PubMed] [Google Scholar]
- 46.Aminkeng F, Ross CJ, Rassekh SR et al. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol. 2016 Sep;82(3):683–95. doi: 10.1111/bcp.13008. CPNDS Clinical Practice Recommendations Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995 Oct 13;270(5234):293–6. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA et al. Genetic Variants Associated With Cancer Therapy-Induced Cardiomyopathy. Circulation. 2019 Jul 2;140(1):31–41. doi: 10.1161/CIRCULATIONAHA.118.037934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu AF, Chan AT, Steingart RM. Cardiac Magnetic Resonance and Cardio-Oncology: Does T2 Signal the End of Anthracycline Cardiotoxicity? J Am Coll Cardiol. 2019 Feb 26;73(7):792–4. doi: 10.1016/j.jacc.2018.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armenian SH, Lacchetti C, Barac A et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017 Mar 10;35(8):893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 51.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Oct 15;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. [DOI] [PubMed] [Google Scholar]


