Abstract
Tumor-targeted therapies such as trastuzumab have led to significant improvements in survival of human epidermal growth factor receptor 2 (HER2)-positive breast cancer. However, these therapies have also been associated with significant left ventricular dysfunction. The incidence of trastuzumab-induced heart failure has decreased significantly since the initial reports, in large part due to improved screening, closer monitoring for early changes in left ventricular function, and a significant decrease in the concurrent administration of anthracyclines. The mechanism of trastuzumab cardiotoxicity is still not well understood, but current knowledge suggests that ErbB2 inhibition in cardiac myocytes plays a key role. In addition to trastuzumab and other HER2-targeted agents, vascular endothelial growth factor inhibitors, proteasome inhibitors, and immune checkpoint inhibitors are all additional classes of drugs used with great success in the treatment of solid tumors and hematologic malignancies. Yet these, too, have been associated with cardiac toxicity that ranges from a mild asymptomatic decrease in ejection fraction to fulminant myocarditis. In this review, we summarize the cardiotoxic effects of tumor-targeted and immunotherapies with a focus on HER2 antagonists.
Keywords: cardiotoxicity, immunotherapy, immune checkpoint inhibitors, heart failure, human epidermal growth factor receptor 2 antagonists
INTRODUCTION
Tumor-targeted cancer therapies act by disrupting pathways that regulate tumor growth and metastasis. These therapies can be broadly categorized based on the drug type (eg, monoclonal antibody, small molecule inhibitors) or the targeted pathway mechanism (eg, vascular endothelial growth factor, proteasome inhibitor). Early molecular therapies typically targeted a single molecule, such as imatinib targeting ABL kinase for the treatment of chronic myelogenous leukemia, or trastuzumab for the treatment of human epidermal growth factor receptor 2 (HER2)-positive cancers; however, the vast majority of new-generation kinase inhibitors act on many kinases, making the classifications increasingly challenging. This is relevant for anticipating and treating side effects, particularly those affecting the cardiovascular system, that may be recognized at different stages of drug development.1 In oncology, the term “immunotherapy” has most recently been reserved for strategies that target the immune system, typically by activating any of its components, thus triggering an immune response that acts against the tumor. Here, we highlight examples of tumor-targeted and immunotherapies with known cardiovascular effects.
HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2 RECEPTOR ANTAGONISTS
In women, breast cancer continues to be the most commonly diagnosed cancer and the second-leading cause of cancer death.2 Approximately 15% to 20% of patients with early breast cancers exhibit overexpression and/or amplification of the HER2 receptor or oncogene; consequently, adjuvant trastuzumab is now the standard of care for these patients.3 Whereas HER2-positive breast cancer was previously associated with high relapse and mortality rates, the development of trastuzumab, the first clinically used HER2-targeted monoclonal antibody, has revolutionized treatment for these patients. A number of trials have demonstrated the effectiveness of trastuzumab in improving survival of patients with HER2-positive breast cancer in both the metastatic and adjuvant setting.4,5 In addition to trastuzumab, HER2-targeted agents include lapatinib (small molecule kinase inhibitor against HER2), pertuzumab (monoclonal antibody directed against a different HER2 domain), and ado-trastuzumab emtansine (an antibody conjugate), all of which have been approved by the US Food and Drug Administration (FDA) for the treatment of breast cancer.
Despite its efficacy, trastuzumab is associated with cardiotoxicity, most often manifested by an asymptomatic decrease in left ventricular ejection fraction (LVEF) and less often by clinical heart failure.6–8 Recognition of trastuzumab-related cardiomyopathy is now an integral part of the oncology practice and is briefly summarized below.
TRASTUZUMAB-RELATED CARDIOTOXICITY
Incidence
Trastuzumab was initially thought to be noncardiotoxic based on early small trials; however, post-hoc analysis of the first large phase III clinical trial in patients with metastatic HER2-positive breast cancer demonstrated significant risk of heart failure (HF).9 In a retrospective analysis, cardiac dysfunction was identified in up to 27% of patients who received anthracycline, cyclophosphamide, and trastuzumab, with an incidence of New York Heart Association Class (NYHA) III or IV HF of up to 19%.10 At the same time, trastuzumab showed impressive promise by prolonging progression-free and overall survival, which led to its clinical approval for treatment of patients with metastatic HER2-positive breast cancer.9 To reduce the risk of cardiotoxicity, the design of ensuing clinical trials of patients with early HER2-positive breast cancer incorporated critical changes, including (1) separation of anthracyclines from trastuzumab treatment (only sequential and no concomitant treatment was allowed), (2) strict cardiac safety parameters at enrollment, and (3) cardiac monitoring during trastuzumab use with prespecified parameters for holding and stopping therapy based on HF symptoms or changes in LVEF.11 With these parameters, the incidence of severe HF (NYHA class III or IV) was dramatically reduced to between 0% and 3.9% in the trastuzumab arms versus 0% to 1.3% in the control arms in the five major randomized adjuvant trials.5,9,12 In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial, 5-year cumulative data showed a 3.9% incidence of NYHA class III or IV HF in the group who received doxorubicin and cyclophosphamide (AC) followed by paclitaxel and trastuzumab compared with 0.8% in the control group (AC followed by paclitaxel alone).5 Although symptomatic HF was reduced in this report, the burden of cardiotoxicity was not trivial; 31% of patients in the trastuzumab arm had the drug temporarily or permanently withheld for cardiac reasons, and 34% had an LVEF decrease of > 10% compared with 17% in the control arm.12 The HERceptin Adjuvant (HERA) trial reported even lower rates of cardiotoxicity, with severe HF occurring in 0.6% of patients treated with trastuzumab versus 0% in the observation arm, and a decrease in LVEF of > 10% was seen in 7.0% of patients in the trastuzumab arm versus 2.1% in the observation arm.4 The lower rate of cardiotoxicity was partially attributable to the higher LVEF cut-off used (only patients with LVEF > 55% were enrolled) and longer time from receipt of anthracycline therapy to initiation of trastuzumab.13
Mechanism
The pathophysiological mechanisms that mediate trastuzumab-induced cardiotoxicity are currently not well understood. Trastuzumab is a recombinant humanized monoclonal antibody directed against an extracellular region of HER2 or ErbB2, a tyrosine kinase receptor from the superfamily of epidermal growth factor (EGF) receptors.7 ErbB2 is a ligand-less receptor that heterodimerizes with other members of the receptor family that are able to bind the EGF or neuregulin (NRG).14
Binding of NRG to the ErbB receptors induces heterodimer formation and autophosphorylation, leading to activation of G proteins and stimulation of mitogen-activated protein kinase. Among other functions, this pathway mediates the hypertrophic response of myocytes to different stimuli and controls sarcomeric organization.15,16 Binding of trastuzumab to HER2 eventually inhibits downstream-associated signaling cascades of this receptor.17 The NRG–ErbB axis was found to be a critical component of the heart's stress response; in fact, cardiomyopathy developed by 8 to 12 weeks of life in cardiac myocyte-specific ErbB2- and ErbB4-conditional knockout mice.18–20 These mice adapted poorly to stresses, including anthracyclines and pressure overload. In addition, heterozygous Nrg1-knockout mice (in which NRG1 expression was low) have been shown to have increased sensitivity to anthracyclines.21
Overall, existing data suggests that ErbB2 plays an integral role in maintaining normal cardiac function and repair and in myocyte response to stress. A recent report by Mohan et al. suggests that the trastuzumab effects on cardiomyocytes may be mediated by the downstream activation of an autophagy-inhibitory signaling cascade that suppresses autophagy and triggers accumulation of toxic reactive oxygen species.22 It has also been suggested that trastuzumab, either alone or in combination with doxorubicin, strongly downregulates topoisomerase 2B (TOP2B), a major intracellular target in doxorubicin-induced cardiotoxicity.23 Furthermore, treatment of cardiomyocytes with a combination of trastuzumab and doxorubicin (either sequentially or concurrently) significantly enhances downregulation of TOP2B protein levels, increases apoptosis and cell growth inhibition, and promotes production of reactive oxidative and nitrative species in human cardiomyocytes compared with either trastuzumab or doxorubicin treatment.23 Figure 1 illustrates the signaling pathways for HER2 in both cancer cells and cardiac myocytes.
Figure 1.
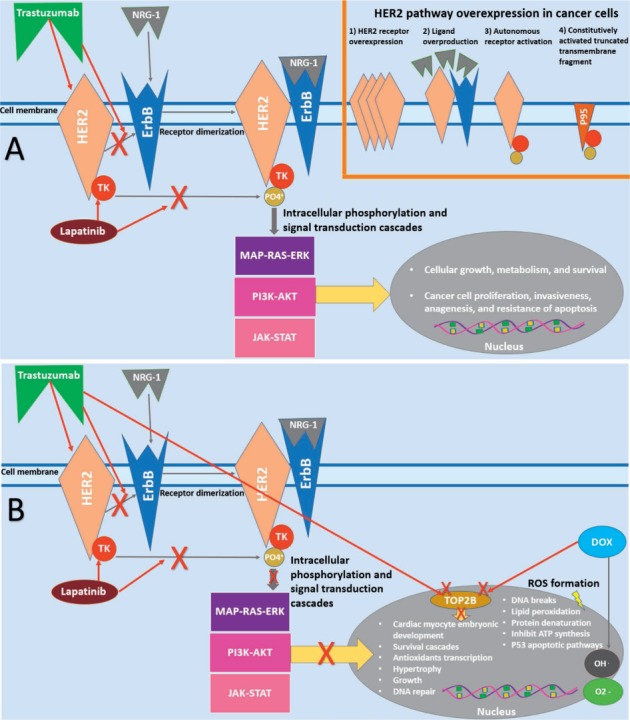
HER2 signaling pathways in cancer cell and cardiac myocyte. (A) In cancer cells, the HER2 signaling pathway is activated via different mechanisms. Trastuzumab and lapatinib block HER2 signaling at different levels. (B) In cardiac myocytes, HER2 signaling is involved in pro-survival pathways, which can be blocked by HER2-targeted therapies. HER2: human epidermal receptor 2; ErbB4: erythroblastic leukemia viral oncogene B receptors; NRG-1: neuregulin-1 ligand; TK: tyrosine kinase; MAP-RAS-ERK: mitogen-activated protein kinase-rate sarcoma virus oncogene-extracellular signal-regulated kinase; PI3K-AKT: phosphatidylinositol 3-kinase-protein kinase B; JAK-STAT: Janus kinase-signal transducer and activator of transcription protein; Top-2B: topoisomerase-2 beta isoform; DOX: doxorubicin; ROS: reactive oxygen species
Risk Factors
The risk of trastuzumab-related cardiac dysfunction is found to be highest among patients receiving concurrent anthracycline therapy, with the risk being greater with higher cumulative doses of anthracyclines (> 300 mg/m2).16,24 Modifiable risk factors such as smoking, hypertension, diabetes, and dyslipidemia are also found to be significantly associated with an increased risk of cardiac dysfunction in cancer patients treated with anthracyclines and/or trastuzumab, with the most consistent association seen with hypertension.24
In clinical practice, patients receiving trastuzumab or other HER2-targeted therapy commonly undergo routine assessment of LV systolic function at baseline and during treatment, largely following the design of breast cancer adjuvant clinical trials.
As a result, clinical cardiotoxicity in the form of symptomatic HF during treatment is rather rare. However, it has raised concerns about the consequences of holding or discontinuing HER2 therapy, particularly in the setting of asymptomatic LV dysfunction.25,26 Several primary prevention trials have tried to address this concern by using a neurohormonal antagonist when initiating and during HER2 treatment, and results have been promising; however, a very small effect size and use of multiple agents has limited their incorporation into clinical practice.27 The SAFE-HEART (Cardiac safety of HER2-targeted therapy in patients with reduced left ventricular function) trial was the first to prospectively test whether HER2-targeted therapies can be safely administered without interruptions in patients with reduced LVEF, and it met the primary outcome of successful completion of oncological therapy with concomitant HF therapy and cardiac monitoring.28 The SCHOLAR (Safety of continuing chemotherapy in overt left ventricular dysfunction using antibodies to human epidermal growth factor receptor-2) trial had a similar design and confirmed that continuation of trastuzumab may be feasible in the setting of mild cardiotoxicity, with an accepted risk of approximately 10% of patients developing symptomatic HF.29
OTHER ANTI-HER2 THERAPIES
Three additional HER2-targeted therapies have been approved for use in patients with HER2-positive breast cancer: lapatinib, pertuzumab, and ado-trastuzumab emtansine. The FDA currently recommends assessment of cardiac function in all patients prior to receiving HER2-targeted therapy, with periodic checks throughout treatment. Under these conditions, the combination of trastuzumab and pertuzumab, which at present is approved for both metastatic and adjuvant breast cancer treatment, does not appear to increase the risk of cardiotoxicity beyond the risk that is expected with trastuzumab treatment alone.30 Lapatinib is an orally active tyrosine kinase inhibitor that affects both human epidermal growth HER2 and the epidermal growth factor receptor ErbB1, and it may have a more favorable cardiac safety profile than trastuzumab, even among patients who have previously received anthracyclines, taxanes, and trastuzumab.31,32 The last available anti-HER2 agent, adotrastuzumab emtansine, is approved for use in metastatic HER2-positive disease and has been reported to have a favorable safety profile when given to patients with normal LV systolic function.33
TYROSINE KINASE INHIBITORS
Kinases are endogenous enzymes that regulate molecular signaling by phosphorylation, or the transfer of phosphate from ATP to proteins. Kinase inhibitors (KIs) and their subgroup, tyrosine kinase inhibitors (TKIs), are small molecules that exert their antitumor activity by binding to and inhibiting kinases. The success of imatinib, the first approved TKI for treatment of chronic myelogenous leukemia, has sparked widespread development of small molecules targeting different kinases. Importantly, contemporary KIs/TKIs are multitargeted, affecting multiple kinases and pathways to overcome tumor resistance and potentially interfering with physiologic processes and cellular homeostasis.34,35 This makes the classification of these cancer therapeutics and their adverse effects increasingly challenging. The TKIs most associated with HF are the ones targeting angiogenesis (described below). The adverse effects of other TKIs are diverse, often vascular and metabolic, and are described in the recent American Heart Association summary statement.36
VASCULAR ENDOTHELIAL GROWTH FACTOR AND ANGIOGENESIS INHIBITORS
Angiogenesis inhibitors targeting the vascular endothelial growth factor (VEGF) signaling pathway (VSP) have been important additions in the therapy of various cancers, especially renal cell carcinoma and colorectal cancer.37 This broad group of therapeutics—which includes monoclonal antibodies (eg, anti-VEGF antibody, bevacizumab) and TKIs (oral agents such as sunitinib, sorafenib)—has the predominant effect of VSP inhibition. Their most common side effect is hypertension, which is considered an on-target effect, although heart failure, arterial thromboembolism, and coronary/cerebrovascular ischemia have also been reported.38,39
Sunitinib, one of the first VSP TKIs used to treat metastatic renal cell cancer, has been associated with heart failure and decreases in LVEF in 15% to 28% of patients.40 A study by Di Lorenzo et al. identified coronary artery disease and hypertension as the most important predictors for the development of HF with sunitinib.41
Hypertension occurs in up to 70% of patients treated with angiogenesis inhibitors, and treatment is recommended.42 However, there has been no routine screening or monitoring of LV dysfunction in clinical oncology practice.11 This lack of prospective data on baseline and on-treatment cardiac function limits our understanding of true incidence and prognosis of VSP inhibitor-induced cardiotoxicity. In general, cardiac dysfunction is considered to be reversible with early recognition and cessation of VSP inhibitor therapy.43
PROTEASOME INHIBITORS
Proteasome inhibitors (PI) bortezomib and carfilzomib are cornerstone therapies for multiple myeloma.44 Bortezomib is a first-line PI approved by the FDA for the treatment of multiple myeloma and mantle cell lymphoma. It specifically targets the 26S proteasome and forms stable, reversible interactions with the chymotrypsin-like site of the complex, which leads to apoptosis of the affected cells.45 Malignant plasma cells have very high rates of proteasome activity and are therefore targeted for destruction by PIs. Cardiac myocytes are also known to have high rates of proteasome activity and protein turnover.46 Bortezomib and carfilzomib (a second-generation PI) have been proposed to cause cardiotoxicity through apoptosis, specifically through caspase-3/7 signaling, which is how they exert their oncologic effects.46 Large clinical trials involving bortezomib have not demonstrated significant cardiotoxicity,45,47 although smaller studies and case reports seem to suggest otherwise.44 Carfilzomib irreversibly inhibits the chymotryptic site of the proteasome and is currently used with increasing frequency for patients with relapsed and/or refractory multiple myeloma. Although carfilzomib has substantially increased survival in patients with relapsed or refractory multiple myeloma, there is increasing evidence that it may be associated with significant cardiac toxicity.44 A pooled analysis of carfilzomib safety data in 526 clinical trial patients reported that HF occurred in 7%, and there were 8 cardiac-related deaths ruled to be possibly related to the treatment.48 Another phase II trial of 266 patients treated with carfilzomib monotherapy for relapsed myeloma showed a lower rate of HF, only 3.8%.49 The risk factors for PI-associated cardiotoxicity remain unclear, with some authors reporting an additive effect when PIs are combined with anthracyclines and some also reporting a dose effect.45 Close clinical monitoring is recommended when using PIs, particularly carfilzomib given the increasing reports of cardiotoxicity.
IMMUNE CHECKPOINT INHIBITORS
The development of immunotherapies over the past decade has had a significant impact on the management of an increasing number of advanced-stage malignancies previously endowed with dismal prognoses.50 Monoclonal antibodies that target immune checkpoint molecules such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and its ligand (PD L-1) have shown unprecedented success in a broad spectrum of solid hematological tumors.50 Due to the pivotal role played by immune checkpoints in the maintenance of self-tolerance, their therapeutic blockade can alter immunological tolerance and give rise to autoimmune or inflammatory side effects, termed “immune-related adverse events.”50,51 Myocarditis has emerged as an uncommon but potentially life-threatening adverse effect of immune checkpoint inhibitors, but the identification and characterization of this toxicity has proven challenging due to its low incidence and varied presentations. Immune-mediated myocarditis has been reported in < 1% of patients receiving immune checkpoint inhibitors in clinical trials, but published cases are often characterized by fulminant progression, and combination therapy leads to increased risk of toxicity.52,53
The risk of cardiotoxicity from individual immune checkpoint inhibitors (ICIs) is unknown, and most of the data comes from case reports, pharmacovigilance studies, and small registries. The potential risk for fatal myocarditis, pericarditis, fatal arrhythmias, and cardiovascular death has been reported for ipilimumab (a CTLA-4 inhibitor), nivolumab and pembrolizumab (PD-1 inhibitors), and PD L-1 inhibitors including atezolizumab, avelumab, and durvalumab.54 The risk appears to be higher when ICIs are combined or used with other cardiotoxic chemotherapies, such as VEGF inhibitors.54 In clinical trials of ICIs, cardiotoxicity was reported to be higher (0.27%) in patients receiving a combination of nivolumab and ipilimumab compared to those receiving nivolumab alone (0.06%).52 Toxicity typically occurs early during therapy and, in cases of myocarditis, may progress rapidly with arrhythmias and hemodynamic compromise.55 Mahmood et al. found that the median time to onset of myocarditis was 34 days, with 81% presenting within 3 months of starting therapy.56 Steroids and immunosuppressive agents are a mainstay of therapy, with hemodynamic support and critical care involvement in cases of cardiogenic shock and advanced HF.
CONCLUSIONS: NEW PARADIGMS IN DIAGNOSIS, PROGNOSIS, AND MANAGEMENT OF CARDIOTOXICITY-RELATED HEART FAILURE
The surge in cancer therapies that target molecular pathways relevant to cardiovascular and cardiomyocyte homeostasis has led to a better understanding of their impact on cardiovascular health. The traditional definition of treatment-related cardiotoxicity focuses on a decline in LVEF and/or HF symptoms and is mostly based on cardiac dysfunction from anthracyclines and trastuzumab. Routine LVEF monitoring during treatment with these agents has significantly improved overall safety, and recent primary and secondary prevention trials promise to enhance our ability to avoid disruption and continue HER2-targeted therapy even in the setting of mild cardiac dysfunction.27,28
Nevertheless, there has been a widening gap in our understanding of the incidence and mechanisms of cardiotoxicity related to other targeted agents and immune therapies. Increased awareness and collaboration with oncologists are needed to identify risk predictors and diagnostic markers for HF associated with angiogenesis and proteasome inhibitors. Similarly, the development of multidisciplinary clinical pathways will be critical for early recognition and treatment of ICI-associated myocarditis.
KEY POINTS
HER2-targeted therapies (eg, trastuzumab) play a key role in the treatment of HER2-positive breast cancer but can also cause left ventricular dysfunction. Risk factors for trastuzumab-related cardiac dysfunction include concurrent exposure to anthracyclines, hypertension, smoking, diabetes, and dyslipidemia.
Routine monitoring of left ventricular function has reduced the frequency of symptomatic heart failure seen with trastuzumab, and clinical focus is increasingly shifting to primary and secondary cardiac prevention to avoid interruption and discontinuation of HER2 therapy.
Vascular endothelial growth factor and angiogenesis inhibitors are associated with varying degrees of hypertension and, less frequently, heart failure. The mechanism and incidence is less understood because routine cardiac function monitoring is not used with this class of agents. Monitoring and treatment of hypertension is recommended.
Immune checkpoint inhibitors (ICIs) activate the immune system by inhibiting the tumor-mediated downregulation of T-cells. Their main adverse effects are immune-related and typically present as myocarditis when affecting the heart. Although ICI-related myocarditis is rare, early diagnosis and treatment are important because of high mortality rates. The mainstay of treatment is high-dose steroids and hemodynamic support.
Footnotes
Conflict of Interest Disclosure:
Dr. Barac serves on the data and safety monitoring board for CTI BioPharma Corp and has received honoraria from Bristol-Myers Squibb.
REFERENCES
- 1.Seltzer JH, Gintant G, Amiri-Kordestani L et al. Assessing cardiac safety in oncology drug development. Am Heart J. 2019 Aug;214:125–33. doi: 10.1016/j.ahj.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society [Internet] Atlanta, GA: American Cancer Society; c2019. Cancer Facts and Figures 2019; 2019 [cited 2019 Aug 15]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html. [Google Scholar]
- 3.Cameron D, Piccart-Gebhart MJ, Gelber RD et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017 Mar 25;389(10075):1195–205. doi: 10.1016/S0140-6736(16)32616-2. Herceptin Adjuvant (HERA) Trial Study Team. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005 Oct 20;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J et al. Trastuzumab plus adjuvant chemo-therapy for operable HER2-positive breast cancer. N Engl J Med. 2005 Oct 20;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Perez EA, Rodeheffer R. Clinical cardiac tolerability of trastuzumab. J Clin Oncol. 2004 Jan 15;22(2):322–9. doi: 10.1200/JCO.2004.01.120. [DOI] [PubMed] [Google Scholar]
- 7.Kenigsberg B, Jain V, Barac A. Cardio-oncology Related to Heart Failure: Epidermal Growth Factor Receptor Target-Based Therapy. Heart Fail Clin. 2017 Apr;13(2):297–309. doi: 10.1016/j.hfc.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Barish R, Gates E, Barac A. Trastuzumab-Induced Cardiomyopathy. Cardiol Clin. 2019 Nov;37(4):407–418. doi: 10.1016/j.ccl.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001 Mar 15;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Seidman A, Hudis C, Pierri MK et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002 Mar 1;20(5):1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 11.Kenigsberg B, Wellstein A, Barac A. Left Ventricular Dysfunction in Cancer Treatment: Is it Relevant? JACC Heart Fail. 2018 Feb;6(2):87–95. doi: 10.1016/j.jchf.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf. 2008;31(6):459–67. doi: 10.2165/00002018-200831060-00002. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth BT, Varga ZV, Wu WJ, Pacher P. Trastuzumab cardiotoxicity: from clinical trials to experimental studies. Br J Pharmacol. 2017 Nov;174(21):3727–48. doi: 10.1111/bph.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemmens K, Segers VF, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006 Jul 14;281(28):19469–77. doi: 10.1074/jbc.M600399200. [DOI] [PubMed] [Google Scholar]
- 15.Clerk A, Michael A, Sugden PH. Stimulation of the p38 mitogen-activated protein kinase pathway in neonatal rat ventricular myocytes by the G protein-coupled receptor agonists, endothelin-1 and phenylephrine: a role in cardiac myocyte hypertrophy? J Cell Biol. 1998 Jul 27;142(2):523–35. doi: 10.1083/jcb.142.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suter TM, Procter M, van Veldhuisen DJ et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007 Sep 1;25(25):3859–65. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 17.Linschoten M, Teske AJ, Cramer MJ, van der Wall E, Asselbergs FW. Chemotherapy-Related Cardiac Dysfunction: A Systematic Review of Genetic Variants Modulating Individual Risk. Circ Genom Precis Med. 2018 Jan;11(1) doi: 10.1161/CIRCGEN.117.001753. e001753. [DOI] [PubMed] [Google Scholar]
- 18.García-Rivello H, Taranda J, Said M et al. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005 Sep;289(3):H1153–60. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 19.Ozcelik C, Erdmann B, Pilz B et al. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002 Jun 25;99(13):8880–5. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crone SA, Zhao YY, Fan L et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002 May;8(5):459–65. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 21.Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med. 2012 Nov 29;367(22):2150–3. doi: 10.1056/NEJMcibr1203156. [DOI] [PubMed] [Google Scholar]
- 22.Mohan N, Shen Y, Endo Y, ElZarrad MK, Wu WJ. Trastuzumab, but Not Pertuzumab, Dysregulates HER2 Signaling to Mediate Inhibition of Autophagy and Increase in Reactive Oxygen Species Production in Human Cardiomyocytes. Mol Cancer Ther. 2016 Jun;15(6):1321–31. doi: 10.1158/1535-7163.MCT-15-0741. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Mohan N, Endo Y, Shen Y, Wu WJ. Type IIB DNA topoisomerase is downregulated by trastuzumab and doxorubicin to synergize cardiotoxicity. Oncotarget. 2017 Dec 21;9(5):6095–108. doi: 10.18632/oncotarget.23543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armenian SH, Lacchetti C, Barac A. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017 Mar 10;35(8):893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 25.Barish R, Lynce F, Unger K, Barac A. Management of Cardiovascular Disease in Women With Breast Cancer. Circulation. 2019 Feb 19;139(8):1110–1120. doi: 10.1161/CIRCULATIONAHA.118.039371. [DOI] [PubMed] [Google Scholar]
- 26.Barac A. Quo Vadis Trastuzumab?: Navigating Cardiac Safety Risk Estimates With Complex Cancer Treatments. JACC Heart Fail. 2019 Mar;7(3):225–227. doi: 10.1016/j.jchf.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Barac A, Blaes A, Lynce F. Lessons From Primary Cardiac Prevention Trials During Trastuzumab Therapy: End of One Size Fits All. J Am Coll Cardiol. 2019 Jun 11;73(22):2869–2871. doi: 10.1016/j.jacc.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Lynce F, Barac A, Geng X et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019 Jun;175(3):595–603. doi: 10.1007/s10549-019-05191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leong DP, Cosman T, Alhussein MM et al. Safety of Continuing Trastuzumab Despite Mild Cardiotoxicity. A Phase I Trial. 2019;1(1):1–10. doi: 10.1016/j.jaccao.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baselga J, Cortés J, Kim SB et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012 Jan 12;366(2):109–19. doi: 10.1056/NEJMoa1113216. CLEOPATRA Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geyer CE, Forster J, Lindquist D et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006 Dec 28;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 32.Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008 Jun;83(6):679–86. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Miles D, Gianni L et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012 Nov 8;367(19):1783–91. doi: 10.1056/NEJMoa1209124. EMILIA Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen MH, Kerkela R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008 Jul 1;118(1):84–95. doi: 10.1161/CIRCULATIONAHA.108.776831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barac A. Yet another player in the cardio-oncology conundrum?: deciphering the role of FLT3. J Am Coll Cardiol. 2014 Mar 18;63(10):1020–1. doi: 10.1016/j.jacc.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 36.Campia U, Moslehi JJ, Amiri-Kordestani L et al. Cardio-Oncology: Vascular and Metabolic Perspectives: A Scientific Statement From the American Heart Association. Circulation. 2019 Mar 26;139(13):e579–e602. doi: 10.1161/CIR.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. 2018 May 8;2:13. doi: 10.1038/s41698-018-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang S, Zheng C, Tsai HT et al. Cardiovascular toxicity after antiangiogenic therapy in persons older than 65 years with advanced renal cell carcinoma. Cancer. 2016 Jan 1;122(1):124–30. doi: 10.1002/cncr.29728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdel-Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat Rev. 2017 Feb;53:120–127. doi: 10.1016/j.ctrv.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Chu TF, Rupnick MA, Kerkela R et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007 Dec 15;370(9604):2011–9. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Lorenzo G, Autorino R, Bruni G et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol. 2009 Sep;20(9):1535–42. doi: 10.1093/annonc/mdp025. [DOI] [PubMed] [Google Scholar]
- 42.Maitland ML, Bakris GL, Black HR et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010 May 5;102(9):596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steingart RM, Bakris GL, Chen HX et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Am Heart J. 2012 Feb;163(2):156–63. doi: 10.1016/j.ahj.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Grandin EW, Ky B, Cornell RF, Carver J, Lenihan DJ. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail. 2015 Feb;21(2):138–44. doi: 10.1016/j.cardfail.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Cole DC, Frishman WH. Cardiovascular Complications of Proteasome Inhibitors Used in Multiple Myeloma. Cardiol Rev. 2018 May-Jun;26(3):122–9. doi: 10.1097/CRD.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 46.Hasinoff BB, Patel D, Wu X. Molecular Mechanisms of the Cardiotoxicity of the Proteasomal-Targeted Drugs Bortezomib and Carfilzomib. Cardiovasc Toxicol. 2017 Jul;17(3):237–50. doi: 10.1007/s12012-016-9378-7. [DOI] [PubMed] [Google Scholar]
- 47.Richardson PG, Sonneveld P, Schuster MW et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005 Jun 16;352(24):2487–98. doi: 10.1056/NEJMoa043445. Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators. [DOI] [PubMed] [Google Scholar]
- 48.Siegel D, Martin T, Nooka A et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013 Nov;98(11):1753–61. doi: 10.3324/haematol.2013.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegel DS, Martin T, Wang M et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012 Oct 4;120(14):2817–25. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varricchi G, Galdiero MR, Marone G et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017 Oct 26;2(4) doi: 10.1136/esmoopen-2017-000247. e000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boutros C, Tarhini A, Routier E et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016 Aug;13(8):473–86. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 52.Johnson DB, Balko JM, Compton ML et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016 Nov 3;375(18):1749–55. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain V, Mohebtash M, Rodrigo ME, Ruiz G, Atkins MB, Barac A. Autoimmune Myocarditis Caused by Immune Checkpoint Inhibitors Treated With Antithymocyte Globulin. J Immunother. 2018 Sep;41(7):332–5. doi: 10.1097/CJI.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 54.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018 Sep;19(9):e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 55.Jain V, Bahia J, Mohebtash M, Barac A. Cardiovascular Complications Associated With Novel Cancer Immunotherapies. Curr Treat Options Cardiovasc Med. 2017 May;19(5):36. doi: 10.1007/s11936-017-0532-8. [DOI] [PubMed] [Google Scholar]
- 56.Mahmood SS, Fradley MG, Cohen JV et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018 Apr 24;71(16):1755–64. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


