“If the thunder don't get you then the lightning will.”
“The Wheel” Grateful Dead
Ten years ago, few people would have imagined an entire issue of a journal devoted to the field of cardio-oncology or dreamt that there would be entire journals—such as Cardio-Oncology or JACC Cardio-Oncology—dedicated solely to this field. Cancer and cardiac disease race neck and neck as the leading causes of death, which makes the field of cardio-oncology almost an inevitable amalgam. While anthracyclines are the forerunners of this field, novel cancer therapies with potential for cardiotoxicity are being created on a weekly basis. Scientific vigilance and clinical experience have led to discoveries that many of these “targeted” therapies have off-target cardiac side effects. In fact, recent discoveries have been made regarding coexisting genetic and environmental risk factors for both cancer and cardiac disease. For example, age-related clonal hematopoiesis, a common condition associated with an increased risk of malignancy, has also been associated with an increased risk of cardiovascular disease.1
THERAPIES ASSOCIATED WITH CARDIOTOXICITY
As the field of cardio-oncology has progressed beyond just anthracyclines, the adverse cardiovascular effects of other cancer therapies, such as immune checkpoint inhibitors, have generated a great deal of interest. The meteoric rise and therapeutic benefit of immune checkpoint inhibitors and other immunotherapies has led to expanded indications for different malignancy types every year. While we are learning more about which types of immunotherapies work for which patients and malignancies, we are only beginning to understand their role in immune-related adverse cardiovascular events such as myocarditis and pericarditis. This is an area ripe for discovery, particularly related to pressing questions such as (1) what are the true risk factors and incidence of immune-related adverse cardiovascular events, (2) is there any specific biomarker or cardiac test that can predict the onset of myocarditis, (3) what is the ideal treatment, and (4) can survivors be re-challenged with immunotherapy if they recover from myocarditis? Ongoing multicenter studies will hopefully begin to answer these questions. One thing is certain, however: immunotherapy is here to stay, and indications for its use will continue to expand.
In addition to immunotherapies, the use of tyrosine kinase inhibitors and vascular endothelial growth factor inhibitors is also rapidly increasing. These drugs are critical for the treatment of various cancers, including renal, lung, and gastrointestinal cancers and certain hematological malignancies. As with anthracyclines and immunotherapies, these treatments are increasingly being associated with heart failure and other cardiovascular side effects, and efforts are ongoing to define the true incidence and associated risks.
GENETIC FACTORS IN CARDIOTOXICITY
The role of genetic risk factors in determining risk of cardiotoxicity is actively being investigated (Figure 1). Genetics have long been postulated to be one of the reasons why some patients develop cardiotoxicity while others with the same apparent risk factors do not. There are numerous retrospective studies suggesting that pharmacogenetic testing may be beneficial prior to starting anthracycline-based treatment, but there is not enough data to guide clinical decision making.2 In a recent study that sequenced DNA for genes that cause dilated cardiomyopathy, 12% of patients with anthracycline cardiotoxicity had mutations, particularly truncating variants in the titin (TTN) gene.3 In addition, the study developed a mouse model of TTN cardiomyopathy, wherein TTN-variant mice and wild-type mice were administered doxorubicin. TTN-variant mice without doxorubicin exposure and wild-type mice exposed to doxorubicin maintained normal cardiac function over time, whereas TTN-variant mice exposed to doxorubicin had cardiac dysfunction. This proof of concept suggests that patients may have predisposing genetic risks that are triggered by the “second hit” of chemotherapy. Ongoing studies, such as the Dilated Cardiomyopathy Discovery trial, are exploring this concept in a multicenter prospective study.
Figure 1.
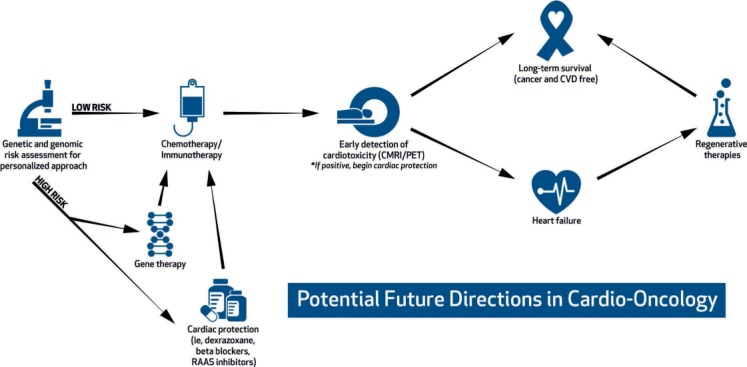
Potential future paradigm for cardiotoxicity risk assessment, prevention, and treatment. CMR: cardiac magnetic resonance imaging; PET: positron emission tomography; CVD: cardiovascular disease
BIOMARKERS AND IMAGING IN PREDICTING CARDIOTOXICITY
In addition to genetic variants, researchers are exploring genomic and proteomic biomarkers for disease. Pilot trials have shown that circulating microRNA biomarkers may be used to predict subsequent cardiotoxicity, and further studies in this area are ongoing.4 The use of transcriptome profiling also has been used to elucidate mechanisms of disease and potential therapeutic targets. For example, Gupta et al. used transcriptome from murine myocardium to show that the RNA-binding protein QKI plays a role in anthracycline-induced cardiomyopathy and may be a therapeutic target.5 Preliminary data also supports the use of human-induced pluripotent stem cell-derived cardiomyocytes in vivo to predict patient-specific risk of developing cardiotoxicity, showing potential for a tailored approach to treatment.6 Additionally, it has been shown that anthracycline-related cardiotoxicity is dependent on topoisomerase II beta in the heart, and perhaps levels of topoisomerase II beta gene expression will help predict who will get cardiotoxicity.7
The role of state-of-the-art imaging techniques such as cardiac magnetic resonance imaging (CMR) or positon emission tomography (PET) in detecting cardiotoxicity is just beginning to be elucidated. Pigs that received doxorubicin had an early increase in T2 mapping (in the absence of other abnormal CMR findings) that correlated with cardiomyocyte edema on autopsy. Stopping doxorubicin at this early stage reversed the cardiomyocyte edema, suggesting that CMR may be able to detect a reversible and early form of cardiotoxicity; however, human data is lacking regarding this novel early CMR marker.8 CMR is useful for patients with poor acoustic windows—including women with bilateral mastectomies and breast implants—as well as patients with limited windows or discrepant ejection fractions (EF) on echocardiogram. In addition to EF, volume measurements and aortic pulse wave velocity can be early markers of cardiotoxicity; in fact, a recent study showed that a drop in left ventricular mass can occur and is independently associated with cardiotoxicity.9,10 CMR strain is also under investigation to determine its role as a potential early marker. Finally, there is evidence that various PET imaging protocols may play a role in early detection of cardiotoxicity and in predicting who may be at risk.11,12
REGENERATIVE THERAPY
There is considerable interest in regenerative therapies for cardiomyopathy caused by anthracyclines, and animal models have shown some promise. For example, in a study of sheep with anthracycline cardiotoxicity, those receiving transendocardial injection of allogeneic mesenchymal stem cells (MSC) showed attenuation of fibrosis and ventricular remodeling compared to controls.13 The National Heart, Lung, and Blood Institute recently completed the SENECA trial, which evaluated the safety and feasibility of delivering allogeneic MSCs via direct cardiac injection in patients with anthracycline cardiotoxicity, and results are forthcoming.14
Education and training in cardio-oncology is also burgeoning. There are several scientific meetings wholly devoted to the field, and there is a growing presence of cardio-oncology topics at major national oncology and cardiology meetings. The number of fellowship programs dedicated to training the next generation of cardio-oncologists is also increasing. In summary, the field of cardio-oncology is experiencing tremendous recognition and growth as a scientific study. Hopefully, these efforts will translate into long-term cancer survivorship without cardiac compromise, so we can focus on the thunder without fearing the lightning.
Footnotes
Conflict of Interest Disclosure:
The author has completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Jaiswal S, Natarajan P, Silver AJ et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017 Jul 13;377(2):111–21. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aminkeng F, Ross CJ, Rassekh SR et al. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol. 2016 Sep;82(3):683–95. doi: 10.1111/bcp.13008. ; CPNDS Clinical Practice Recommendations Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Pavia P, Kim Y, Alejandra Restrepo-Cordoba M et al. Genetic Variants Associated with Cancer Therapy-Induced Cardiomyopathy. Circulation. 2019 Jul 2;140(1):21–41. doi: 10.1161/CIRCULATIONAHA.118.037934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todorova VK, Makhoul I, Wei J, Klimberg VS. Circulating miRNA Profiles of Doxorubicin-induced Cardiotoxicity in Breast Cancer Patients. Ann Clin Lab Sci. 2017 Mar;47(2):115–119. [PubMed] [Google Scholar]
- 5.Gupta SK, Garg A, Bar C et al. Quaking Inhibits Doxorubicin-Mediated Cardiotoxicity Through Regulation of Cardiac Circular RNA Expression. Circ Res. 2018 Jan 19;122(2):246–254. doi: 10.1161/CIRCRESAHA.117.311335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burridge PW, Li YF, Matsa E et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016 May;22(5):547–56. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh ETH, Chang HM. Oncocardiology—Past, Present, and Future. JAMA Cardiol. 2016 Dec 1;1(9):1066–72. doi: 10.1001/jamacardio.2016.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galan-Arriola C, Lobo M, Vilchez-Tschischke JP et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2019 Feb 26;73(7):779–791. doi: 10.1016/j.jacc.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Drafts BC, Twomley KM, D'Agostino R, Jr et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013 Aug;6(8):877–85. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan JH, Castellino SM, Melendez GC et al. Left ventricular mass change after anthracycline chemotherapy. Circ Heart Fail. 2018 Jul;11(7) doi: 10.1161/CIRCHEARTFAILURE.117.004560. e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarocchi M, Bauckneht M, Arboscello E et al. An increase in myocardial 18-fluorodeoxyglucose uptake is associated with left ventricular ejection fraction decline in Hodgkin lymphoma patients treated with anthracycline. J Transl Med. 2018 Oct 25;16(1):295. doi: 10.1186/s12967-018-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauckneht M, Ferrarazzo G, Fiz F et al. Doxorubicin Effect on Myocardial Metabolism as a Prerequisite for Subsequent Development of Cardiac Toxicity: A Translational 18F-FDG PET/CT Observation. J Nucl Med. 2017 Oct;58(10):1638–1645. doi: 10.2967/jnumed.117.191122. [DOI] [PubMed] [Google Scholar]
- 13.Psaltis PJ, Carbone A, Nelson AJ et al. Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. JACC Cardiovasc Interv. 2010 Sep;3(9):974–83. doi: 10.1016/j.jcin.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Bolli R, Hare JM, Henry TD et al. Rationale and Design of the SENECA (StEm cell iNjECtion in cAncer survivors) Trial. Am Heart J. 2018 Jul;201:54–62. doi: 10.1016/j.ahj.2018.02.009. Cardiovascular Cell Therapy Research Network (CCTRN) [DOI] [PMC free article] [PubMed] [Google Scholar]


