Abstract
Background
The treatment of elderly patients with severe burns is difficult and the mortality rate is high. The aim of this study was to investigate the epidemiological features of elderly patients with severe burns.
Material/Methods
Data from 109 elderly patients with severe burns between January 2009 and December 2018 were retrospectively analyzed. Demographic data, clinical characteristics, treatments, and outcomes were statistically analyzed.
Results
Among the 109 elderly patients with severe burns, the male-to-female ratio was 1.73: 1.0. The median age of the elderly patients was 67 years, and the median total body surface area (TBSA) burned was 42%. Notably, 67.9% of burns occurred at home and most frequently occurred in summer (38.5%) and winter (28.4%); flame and flash burns predominated (83.4%). The incidence of inhalation injury was 35.8%, and pre-existing comorbidities were observed in approximately 51.4% of the patients. The median length of stay in the hospital per TBSA burned was 0.4 days. The mortality rate in the elderly patients was 24.8%, and the mortality rates in the ≥70% TBSA group, inhalation injury group, and patients with 3 or more pre-existing comorbidities were significantly higher than in the other groups. The risk of death increased with an increase in the number of pre-existing comorbidities (odds ratio: 2.222; 95% confidence interval: 1.174–4.205).
Conclusions
At a major burn center in Southwest China, the incidence and mortality of elderly patients with severe burns displayed no downward trend. There are etiological characteristics of these age groups that should be considered for prevention. Meanwhile, multidisciplinary treatment in a hospital and an increase in the social support for the elderly population might improve outcomes.
MeSH Keywords: Accident Prevention, Burns, Epidemiology, Frail Elderly
Background
Burns are a common form of trauma in production and in life, with high morbidity and mortality rates. According to the Global Burden of Diseases Study (GBD), the number of burn injuries worldwide was as high as 360 000 in 2017 [1]. As the population ages, the elderly population is growing rapidly. At the same time, the elderly often display characteristics such as slowness of movement, loss of vision and sensation, and thin skin, and are more vulnerable to burns [2,3]. In the past few decades, significant progress has been made in reducing the incidence and mortality of burns in children and adults, with the exception of the elderly population [3,4]. Some scholars have predicted that in the near future, the treatment model for burns will also change as the number of elderly patients with burns increases [3].
Although “the burns in the elderly population are more aggressive” is not a new claim, the discussion is still relatively limited. As an accidental injury, burns are preventable. Once we understand the cause and eliminate the hidden dangers, the sources of burns will be reduced or avoided. An investigation of burn epidemics can reflect the basic situation of burn prevention and management in different periods and provide a theoretical basis for prevention and research. Some countries and regions have made substantial progress in reducing the incidence and mortality of burns by implementing measures related to prevention and treatment [5–7]. Therefore, an analysis of the epidemiological characteristics of elderly patients with burns, particularly elderly patients with severe burns, is of great significance for prevention and treatment.
As the largest burn center in Southwest China, more than 1500 hospitalized patients with burns are treated at the Institute of Burns Research of Southwest Hospital annually, providing us the unique opportunity to investigate the epidemiological characteristics and trends of elderly patients with severe burns. Thus, the present study retrospectively analyzed the etiology, clinical characteristics and therapeutic efficiency in elderly patients with severe burns who were admitted to and treated at the burn center from January 2009 to December 2018, providing experience to improve preventative measures and treatment for elderly patients with severe burns.
Material and Methods
Patients
Data were collected from 109 elderly patients with severe burns who were admitted to the Institute of Burn Research, Southwest Hospital, the Third Military Medical University between January 2009 and December 2018. A severe burn is defined as a total burn area of no less than 30% of the total body surface area (TBSA), and elderly patients are defined as aged greater than or equal to 60 years. The study protocol was approved by the Ethics Committee of Southwest Hospital, Third Military Medical University.
Data collection
The following factors were considered in the statistical analysis: 1) demographic data, including sex, age at burn, and residence; 2) general information about the burn, including the location, etiology, and time of burn; 3) clinical characteristics, including the total burn area, third degree burn area, severity score for the burn, inhalation injury, time of admission after the burn and complications before the burn; and 4) treatment methods and prognosis, mainly including operations, hospitalization days (length of stay, LOS), main causes of death and outcomes of disease. The abbreviated burn severity index (ABSI) score [8], the modified Baux score [9] and the burn index (BI) score [10] were calculated for each patient as follows: ABSI=sex (female=1 and male=0)+age (0–20 years=1, 21–40 years=2, 41–60 years=3, 61–80 years=4, and 80–100 years=5)+inhalation injury (yes=1 and no=0)+full-thickness burns (yes=1 and no=0)+TBSA (1–10%=1, 11–20%=2, 21–30%=3, 31–40%=4, 41–50%=5, 51–60%=6, 61–70%=7, 71–80%=8, 81–90%=9, and 91–100=10). The modified Baux score=age+%TBSA+17×(inhalation injury, 1=yes and 0=no). BI=%TBSA of the full-thickness burn+1/2×%TBSA of the deep partial thickness burn.
Statistical analyses
All data are presented as medians and interquartile ranges (IQR) or proportions, as appropriate. All statistical analyses were performed using SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). Categorical variables were compared using the chi-square test or Fisher’s exact test, and continuous non-parametric variables were compared using the Mann-Whitney U-test or the Kruskal-Wallis test. The associations between mortality and demographic and clinical characteristics variables were assessed using a univariate analysis (Table 1). After univariate analysis, variables with P<0.05 (including inhalation injury, pre-existing comorbidities, TBSA, and full-thickness burns) were included in a multivariate logistic regression model (forward: LR method, entry: P=0.05; removal: P=0.10) to screen the independent risk factors for mortality. P<0.05 was considered statistically significant.
Table 1.
Comparison of characteristics between survivors and non-survivors among elderly patients with severe burns.
| Parameter | Survivors (n=82) | Non-survivors (n=27) | P-value | |
|---|---|---|---|---|
| Male: Female | n: n | 53: 29 | 16: 11 | 0.615 |
| Age | Years | 67.0 (62.0–71.0) | 71.0 (64.0–76.0) | 0.110 |
| Rural residence | n (%) | 69 (84.1) | 21 (77.8) | 0.643 |
| Admission within 6 hours after injury | n (%) | 33 (40.2) | 9 (33.3) | 0.522 |
| With pre-existing comorbidities (no ≥3) | n (%) | 6 (7.3) | 9 (33.3) | 0.002* |
| Inhalation injury | n (%) | 24 (29.3) | 15 (55.6) | 0.013* |
| With full-thickness burns | n (%) | 57 (69.5) | 26 (96.3) | 0.005* |
| Full-thickness burns | %TBSA | 25.0 (8.5–36.0) | 24.5 (15.8–44.3) | 0.261 |
| TBSA | % | 40.0 (34.8–47.3) | 45.0 (40.0–68.0) | 0.040* |
| ABSI score | /18 | 10.0 (9.0–11.0) | 11.0 (10.0–12.0) | 0.006* |
| Baux score | 115.0 (102.8–127.5) | 129.0 (115.0–143.0) | 0.001* | |
| Burn index | 23.3 (9.9–37.5) | 31.0 (27.5–55.5) | 0.004* | |
| Patients treated surgically | n (%) | 43 (52.4) | 14 (51.9) | 0.958 |
All values are presented as medians with inter-quartile ranges (IQR), or numbers and percentages in parentheses. TBSA – total body surface area; ABSI – abbreviated burn severity index.
P<0.05.
Results
Demographic characteristics
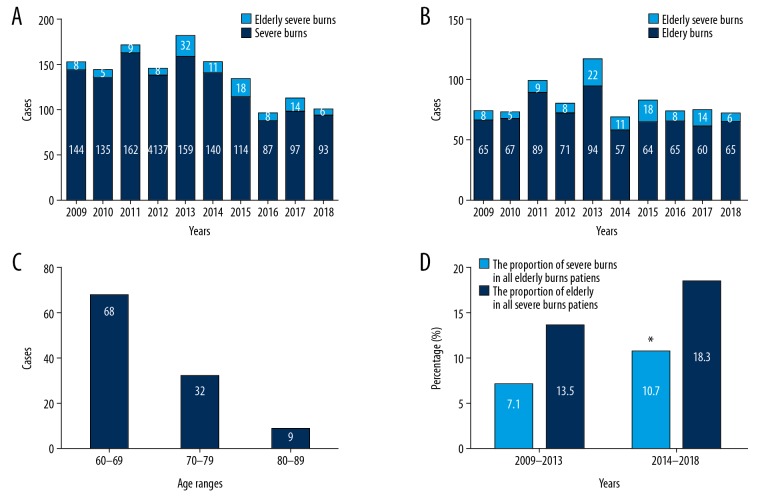
The 109 elderly patients with severe burns included 69 males (63.3%) and 40 females (36.7%); the male-to-female ratio was approximately 1.73: 1.0. In the same period, 1268 patients with severe burns and 697 elderly patients with burns were admitted to and treated in the same department. The proportions of elderly patients with severe burns among the total patients with severe burns and elderly patients with any burn showed large variations in different years (Figure 1A, 1B). All elderly patients were aged from 60 to 89 years, with a median age of 67.0 years (range, 62.5 to 72.5 years); the median ages of both males and females were 67 years. The age range with the highest burn incidence was 60–69 years (n=68, 62.4%), followed by 70–79 years (n=32, 29.4%), and 80–89 years had the lowest incidence (n=9, 8.2%). No elderly patients with severe burns were aged greater than 90 years (Figure 1C).
Figure 1.
The proportions of elderly patients with severe burns among patients with severe burns (A) and elderly patients with burns (B) in different years. Distribution of elderly patients with severe burns in different age groups (C). The proportion of elderly patients with severe burns among all patients with severe burns and all elderly patients with burns in the 2009–2013 and 2014–2018 cohorts (D). * P<0.05 compared with the 2009–2013 cohort.
In addition, the 109 patients were divided into 2 periods (2009–2013 and 2014–2018) according to the time of admission. In the 2014–2018 cohort, the elderly patients accounted for 10.7% of all patients with severe burns in the same period, and the proportion was significantly higher than the value of 7.1% observed in the previous 2009–2013 cohort (P<0.05). The incidence of severe burns in all elderly patients with burns who were admitted to our burn center also increased (18.3% versus 13.5%), although the difference was not significant (Figure 1D). The clinical characteristics, treatments, and outcomes of elderly patients with severe burns were not significantly different between the 2 periods, except for a higher proportion of full-thickness burns in the 2014–2018 cohort (P<0.05) (Table 2).
Table 2.
A Comparison of the characteristics of elderly patients with severe burns between the 2009–2013 and 2014–2018 cohorts.
| Parameter | 2009–2013 (n=52) | 2014–2018 (n=57) | P-value | |
|---|---|---|---|---|
| Male | n (%) | 30 (57.7) | 39 (68.4) | 0.246 |
| Age | Years | 69.0 (63.0–72.8) | 67.0 (62.0–73.5) | 0.310 |
| Rural residence | n (%) | 45 (86.5) | 45 (78.9) | 0.297 |
| Admission within 6 h after injury | n (%) | 20 (38.5) | 22 (38.6) | 0.988 |
| With a pre-existing comorbidity | n (%) | 26 (50.0) | 32 (56.1) | 0.521 |
| With full-thickness burns | n (%) | 35 (67.3) | 48 (84.2) | 0.039* |
| Inhalation injury | n (%) | 19 (36.5) | 20 (35.1) | 0.875 |
| TBSA | % | 41.5 (35.0–55.0) | 42.0 (34.0–51.5) | 0.600 |
| ABSI score | /18 | 10.0 (9.0–11.8) | 10.0 (9.0–12.0) | 0.664 |
| Baux score | 121.0 (105.3–131.0) | 117.0 (102.5–129.0) | 0.397 | |
| Burn index | 29.3 (9.0–41.4) | 28.0 (15.0–37.3) | 0.860 | |
| Patients treated surgically | n (%) | 24 (46.2) | 33 (57.9) | 0.220 |
| LOS survivors | Days | 16.5 (7.0–55.0) | 31.5 (7.5–55.8) | 0.577 |
| LOS survivors/TBSA | Days | 0.4 (0.1–1.4) | 0.8 (0.1–1.6) | 0.561 |
| Cure rate | n (%) | 17 (32.7) | 24 (42.1) | 0.311 |
| Mortality rate | n (%) | 12 (23.1) | 15 (26.3) | 0.696 |
All values are presented as medians with inter-quartile ranges (IQR), or numbers and percentages in parentheses. h – hours; TBSA – total body surface area; ABSI – abbreviated burn severity index; LOS – length of stay.
P<0.05.
Location, etiology, and time of admission of burns
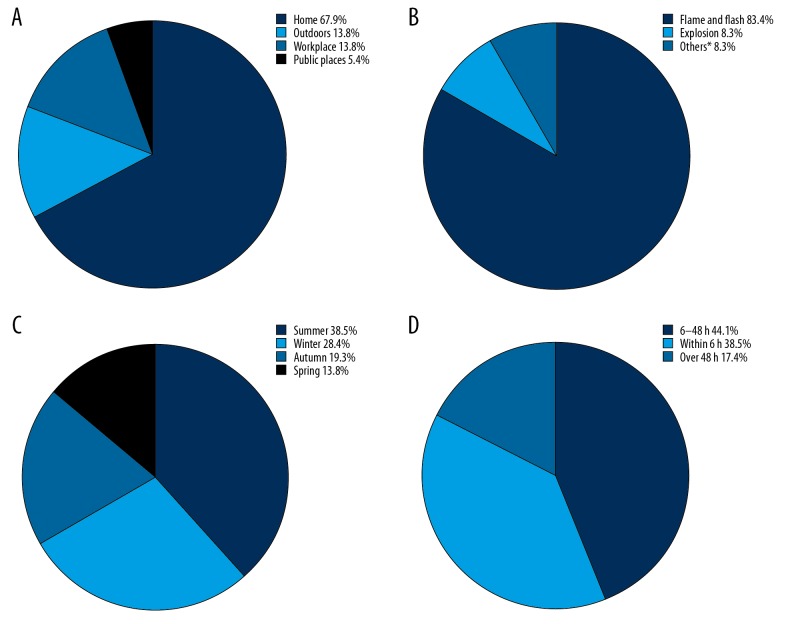
In our study, 74 burns (67.9%) occurred at home, and 35 burns (32.1%) occurred outside the home, for a 2.1: 1 ratio (Figure 2A). Flame and flash burns predominated (83.4%), followed by explosion burns (Figure 2B). Severe burns could occur in elderly individuals at any time of the year. Nevertheless, the incidence in summer (38.5%) and winter (28.4%) increased compared to the incidence in autumn (19.3%) or spring (13.8%) (Figure 2C). The time of admission to the hospital ranged from 2 hours to 30 days after injury. Only 42 patients (38.5%) were transported to our burn center for treatment within 6 hours after injury (Figure 2D). In addition, in a more detailed investigation of the month of injury, the 3 months in which burns frequently occurred were July (17.4%), August (11.9%), and December (10.1%) (Figure 3).
Figure 2.
Distribution of the location (A), etiology (B), seasons (C), and time to hospitalization (D) in elderly patients with severe burns from 2009 to 2018. * Scald, electricity, and contact burns.
Figure 3.
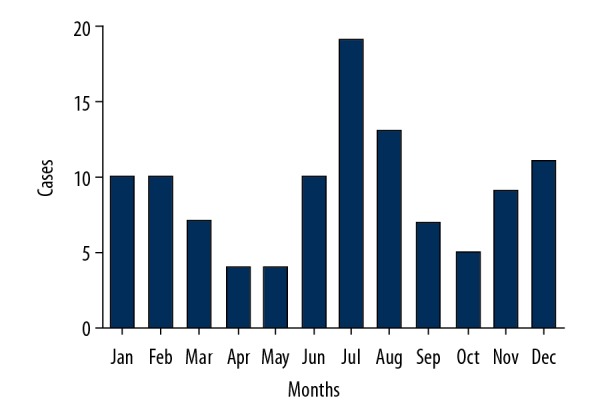
Monthly distribution of elderly patients with severe burns.
In an analysis stratified according to geographic characteristics, differences in the burn parameters mentioned above were not observed between the rural and urban areas (Table 3).
Table 3.
Comparison of burn parameters between rural and urban areas.
| Parameter | Rural areas (n=90) | Urban areas (n=19) | P-value | |
|---|---|---|---|---|
| Location of burns | ||||
| Home | n (%) | 62 (68.9) | 12 (63.2) | 0.627 |
| Outdoors | n (%) | 14 (15.6) | 1 (5.2) | |
| Workplace | n (%) | 12 (13.3) | 3 (15.8) | |
| Public places | n (%) | 2 (2.2) | 3 (15.8) | |
| Etiology of burns | ||||
| Flame and flash | n (%) | 73 (81.1) | 18 (94.7) | 0.236 |
| Explosion | n (%) | 9 (10.0) | 0 | |
| Others* | n (%) | 8 (8.9) | 1 (5.3) | |
| Seasonal distribution of burns | ||||
| Spring | n (%) | 11 (12.2) | 4 (21.1) | |
| Summer | n (%) | 34 (37.8) | 8 (42.1) | 0.725 |
| Autumn | n (%) | 19 (21.1) | 2 (10.5) | |
| Winter | n (%) | 26 (28.9) | 5 (26.3) | |
| Time to hospitalization | ||||
| within 6 hours | n (%) | 33 (36.7) | 9 (47.4) | 0.384 |
| 6–48 hours | n (%) | 41 (45.5) | 7 (36.8) | |
| Over 48 hours | n (%) | 16 (17.8) | 3 (15.8) | |
All values are presented as numbers and percentages in parentheses.
Scald, electricity, and contact burns.
Severity of burns and pre-existing comorbidities
The median burn area was 42% (35–52%) TBSA (range: 30–92% TBSA). Patients with TBSAs of 30–49%, 50–69%, and ≥70% accounted for 72.5%, 19.3%, and 8.3% of all cases, respectively. The majority of these patients had full-thickness burns (76.1%). The associations between the sex, age, and burn severity are presented in Table 4. The ABSI scores for females were significantly higher than those of males (P<0.01). Female also had higher TBSAs, Baux scores and BIs, but the differences were not significant. The Baux score was significantly increased with age in the age groups of 60–69 years, 70–79 years, and 80–89 years (P<0.01). No significant differences in the TBSAs, ABSIs, and BIs were detected in the 3 age groups.
Table 4.
Burn severity analysis.
| Parameter | TBSA median (IQR) | ABSI median (IQR) | Baux score median (IQR) | Burn Index Median (IQR) |
|---|---|---|---|---|
| Sex | ||||
| Male | 40.0 (35.0–52.0) | 10.0 (9.0–11.0) | 103.0 (97.0–117.0) | 27.5 (12.8–37.5) |
| Female | 45.0 (32.3–54.3) | 11.0 (10.0–12.0) | 106.3 (98.1–118.5) | 29.5 (10.4–44.4) |
| P-value | 0.940 | 0.002* | 0.945 | 0.676 |
| Age (years) | ||||
| 60–69 | 45.0 (35.0–54.8) | 10.0 (9.0–11.8) | 114.0 (101.3–127.0) | 27.3 (11.5–39.6) |
| 70–79 | 40.0 (34.0–55.0) | 10.0 (9.0–11.8) | 126.5 (107.0–138.5) | 28.8 (16.3–42.9) |
| 80–89 | 40.0 (37.5–44.0) | 11.0 (9.5–12.0) | 127.0 (123.0–142.5) | 32.5 (20.0–37.3) |
| P-value | 0.708 | 0.500 | 0.002* | 0.792 |
All values are presented as medians and inter-quartile ranges (IQR) in parentheses.
P<0.05.
TBSA – total body surface area; ABSI – abbreviated burn severity index.
In our study, 39 patients (35.8%) were complicated with inhalation injuries. Fifty-six patients (51.4%) who were elderly patients with severe burn had at least 1 of 24 pre-existing comorbidities, and the most common pre-existing comorbidities were hypertension (25.7%), diabetes mellitus (11.1%), ischemic heart disease (8.3%), and chronic obstructive pulmonary disease (8.3%) (Figure 4).
Figure 4.
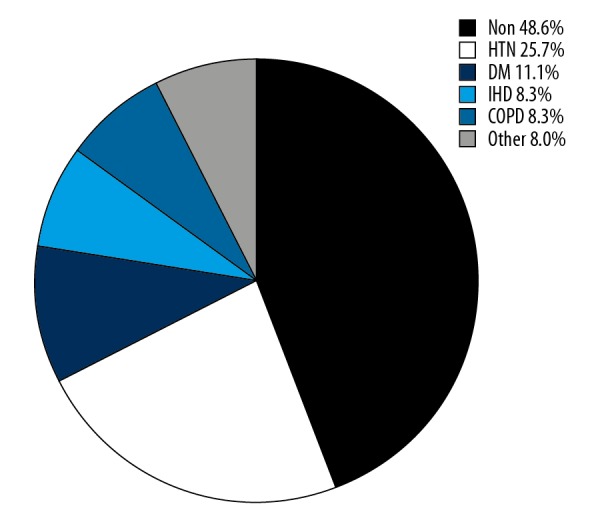
The most common pre-existing comorbidities. HTN – hypertension; DM – diabetes mellitus; IHD – ischemic heart disease; COPD – chronic obstructive pulmonary disease.
Treatment in the hospital
The median LOS and LOS/TBSA of the 109 patients were 19.0 days (range 5.5–49.5 days) and 0.4 days (range 0.1–1.2), respectively. Table 5 illustrates the distribution of the LOS, LOS/TBSA, and outcomes of disease in groups stratified by sex, age, and TBSA. Males had higher LOS, LOS/TBSA, and cure rate, as well as a lower mortality rate, but the differences were not significant. The enthusiasm for treatment reflected in the LOS, LOS/TBSA, and cure rate among the age groups of 60–69 years, 70–79 years, and 80–89 years inversely correlated with age (P<0.05 or P<0.01). When patients were stratified into burn size groups (30–49% TBSA, 50–69% TBSA, and ≥70% TBSA), marked reductions in LOS, LOS/TBSA, and the cure rate were observed in the 30–49% TBSA and ≥70% TBSA groups (P<0.05 or P<0.01).
Table 5.
LOS and outcome distributions.
| LOS (days) median (IQR) | LOS/TBSA (days) median (IQR) | Cured n (%) | Died n (%) | |
|---|---|---|---|---|
| Sex | ||||
| Male | 24.0 (7.5–51.0) | 0.6 (0.1–1.2) | 29 (42.0) | 16 (23.2) |
| Female | 12.0 (2.3–40.8) | 0.3 (0.1–1.4) | 12 (30.0) | 11 (27.5) |
| P-value | 0.174 | 0.265 | 0.211 | 0.615 |
| Age (years) | ||||
| 60–69 | 34.0 (8.5–58.8) | 0.7 (0.2–1.4) | 32 (47.1) | 13 (19.1) |
| 70–79 | 11.5 (4.0–30.8) | 0.2 (0.1–0.8) | 9 (28.1) | 11 (34.4) |
| 80–89 | 3.0 (1.0–12.5) | 0.1 (0–0.4) | 0 | 3 (33.3) |
| P-value | 0.001* | 0.004* | 0.010* | 0.212 |
| TBSA (%) | ||||
| 30–49 | 28.0 (8.0–53.0) | 0.7 (0.2–1.4) | 37 (46.8) | 17 (21.5) |
| 50–70 | 8.0 (3.5–20.5) | 0.1 (0.1–0.4) | 3 (14.3) | 4 (19.0) |
| ≥70 | 12.0 (1.0–47.0) | 0.1 (0–0.7) | 1 (11.1) | 6 (66.7) |
| P-value | 0.047* | 0.002* | 0.005* | 0.010* |
All values are presented as medians with inter-quartile ranges (IQR), or numbers and percentage in parentheses. TBSA – total body surface area; LOS – length of stay.
P<0.05.
Approximately equal numbers of patients underwent surgical treatment (n=57) with excision and skin grafting compared to non-surgical management (n=52), as summarized in Table 6. Patients who did not undergo surgery were significantly older (P<0.01), had a higher proportion of pre-existing comorbidities (P<0.05) and a lower proportion of full-thickness burns (P<0.01), and had significantly lower LOS, LOS/TBSA, and cure rates (P<0.01).
Table 6.
Comparison of elderly patients with severe burns treated with and without surgical management.
| Parameter | Surgical management (n=57) | Non-surgical management (n=52) | P-value | |
|---|---|---|---|---|
| Male: Female | n: n | 41: 16 | 28: 24 | 0.050 |
| Age | Years | 66.0 (62.0–70.0) | 69.0 (65.3–77.8) | 0.005* |
| Rural residence | n (%) | 46 (80.7) | 44 (84.6) | 0.591 |
| With a pre-existing comorbidity | n (%) | 23 (40.4) | 33 (63.5) | 0.016* |
| With full-thickness burns | n (%) | 51 (89.5) | 32 (61.5) | 0.001* |
| Inhalation injury | n (%) | 22 (38.6) | 17 (32.7) | 0.521 |
| TBSA | % | 40 (35–53) | 43 (35–52) | 0.708 |
| Full-thickness burns | %TBSA | 18.0 (6.0–32.5) | 6.0 (0–34.5) | 0.103 |
| ABSI score | /18 | 10.0 (9.0–11.0) | 10.5 (9.0–12.0) | 0.696 |
| Baux score | 115.0 (103.0–129.0) | 122.0 (105.3–133.3) | 0.250 | |
| Burn index | 29.5 (18.0–38.0) | 25.3 (8.6–40.8) | 0.113 | |
| LOS | Days | 37.0 (13.0–63.5) | 7.0 (1.0–22.3) | <0.01* |
| LOS/TBSA | Days | 0.9 (0.3–1.8) | 0.15 (0–0.58) | <0.01* |
| Cured | n (%) | 29 (50.9) | 12 (23.1) | 0.003* |
| Died | n (%) | 14 (24.6) | 13 (25.0) | 0.958 |
All values are presented as medians and inter-quartile ranges (IQR), or numbers and percentages in parentheses. TBSA – total body surface area; ABSI – abbreviated burn severity index; LOS – length of stay.
P<0.05.
The outcomes, mortality rates and clinical characteristics of death
Twenty-seven deaths were recorded among the 109 patients, including 16 males and 11 females. The overall mortality rate was 24.8%. Non-survivors had a significantly larger overall TBSA, and a greater proportion presented with 3 or more pre-existing comorbidities, sustained full-thickness burns and inhalation injury compared to the survivors (P<0.05 or P<0.01) (Table 1).
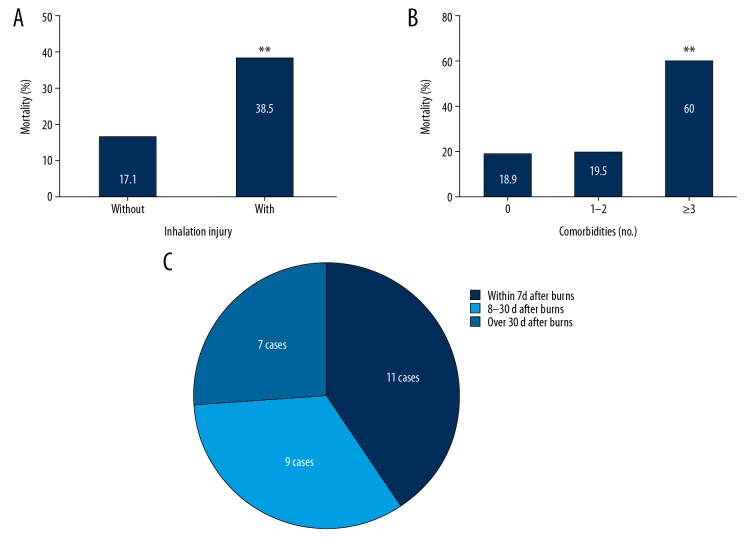
When stratified into burn size groups, marked increases in mortality were observed in the ≥70% TBSA group (P<0.05). However, no differences were observed in mortality in groups stratified by sex and age (Table 5). A higher mortality rate was observed in the inhalation injury group (38.5%) than in the non-inhalation injury group (17.1%, P<0.05) (Figure 5A). Furthermore, the mortality rate of the patients with 3 or more pre-existing comorbidities was as high as 60%, compared with a mortality rate of 18.9% in patients without pre-existing comorbidities (P<0.05) (Figure 5B). An association between a greater number of pre-existing comorbidities and the mortality rate was observed with the multivariate model, with an odds ratio (OR) of 2.222 (95% confidence interval [CI]: 1.174–4.205, P<0.05) (Table 7).
Figure 5.
Mortality rates of elderly patients with severe burns presenting with and without inhalation injury (A). Mortality rates of elderly patients with severe burns stratified according to the number of pre-existing comorbidities (B). Distribution of the time of death after burns in elderly patients with severe burns (C). * P<0.05 compared with patients without inhalation injury and ** P<0.05 compared patients with 0 and 1–2 comorbidities.
Table 7.
Independent risk factors for mortality of elderly patients with severe burns.
| Factors | B | OR | 95% CI | P-value |
|---|---|---|---|---|
| Pre-existing comorbidities (no.)# | 0.799 | 2.222 | 1.174–4.205 | 0.014* |
| Full-thickness burns (%TBSA) | 0.034 | 1.035 | 1.010–1.060 | 0.006* |
According to the number of pre-existing comorbidities, it is divided into 0, 1–2 and ≥3, respectively.
P<0.05.
OR – odds ratio; CI – confidence interval; TBSA – total body surface area.
After investigating the medical notes regarding the cause of death in more detail, sepsis, major burns and acute renal failure were the most common causes of death (Table 8). The median time of death was 14 days (range 3–35 days) after injury, and the most common time of death was within 7 days after injury (40.7%) (Figure 5C).
Table 8.
The reported causes of death in elderly severe burns patients with severe burns from 2009 to 2018.
| Primary cause of death | Number |
|---|---|
| Sepsis | 7 |
| Major burns | 5 |
| Acute renal failure | 3 |
| Pneumonia | 3 |
| Multiple organ failure | 2 |
| Unknown | 2 |
| Respiratory failure | 1 |
| Stroke | 1 |
| Cardiac failure | 1 |
| Myocardial infarction | 1 |
| Upper alimentary tract hemorrhaging | 1 |
Discussion
Although the overall incidence of burns is decreasing [11], some studies have reported increasing trends of burn admissions or burn incidence rates in the elderly population [12–15]. The results of our study also revealed a similar increasing trend. The proportion of elderly patients with severe burns among all patients with severe burns (10.7% versus 7.1%) was higher in the second 5-year cohort (2014–2018) than in the previous 5-year cohort (2009–2013). As a result, the rapid growth of the elderly population and the vulnerability of this population to injury led to an increase in the number of elderly patients with severe burns [12,16].
Most of the elderly patients with severe burns in this study were aged 60 to 69 years old, which was similar to other reports [2]. Some studies reported a higher risk of burns in older women [12,14,17,18]. However, most of the patients included in this study were male. This discrepancy may be related to the differences in sex distribution among different countries and regions and the different divisions of labor between males and females within the family [2,12]. In the present study, the majority of burns occurred at home, were relatively common in summer and winter, and flame and flash burns were the major risk factors for elderly patients. These findings are consistent with previous reports [2,12,14,18–20]. Therefore, strengthening the investigation of burn hazards in the homes of elderly patients with burns and increasing preventive measures, such as installing smoke alarms and improving wire quality, are important measures to prevent burns in the elderly population [21]. In addition, differences in the occurrence and prognosis of burns have been reported in elderly patients residing in rural and urban households [22,23]. However, no significant differences were observed between rural and urban households in the present study. Thus, with the increase in rural urbanization construction and the development of the social economy in China, burn-related lifestyle factors were relatively similar between rural and urban residents.
Elderly people often live alone, and flame burns are common; thin skin, a limited ability to escape harm and other factors easily lead to deeper burns [24]. Cheng et al. [14] observed that compared with children and middle-aged and young patients, the highest incidence of full-thickness burns was observed in elderly patients. In the present study, the proportion of elderly patients presenting with full-thickness burns was 76.1%. In addition, females appeared to have more severe burns and higher mortality rates than males, although the differences were not significant. Chang et al. [25] reported that although a lower burn severity was observed in women than in men, the number of deaths was the same, indicating that women had worse outcomes after burns. However, we did not observe any difference in the severity of burns among different age ranges. This finding differs from the results of some studies [26–28] and might be related to the small sample size and the insufficient numbers of patients in each age range in the present study.
The prevalence of pre-existing comorbidities is a unique feature of the elderly population and increases the vulnerability and mortality of burns in the elderly population [29–31]. In the present study, approximately half of the patients had at least one pre-existing comorbidity, which is less than the reported 69–82.4% rate in the literature [18,32,33]. The major pre-existing comorbidities were hypertension, diabetes mellitus, ischemic heart disease, and chronic obstructive pulmonary disease, similar to some previous findings [2,34,35]. Currently, researchers generally believe that burns further exacerbate the pre-existing comorbidities, and the underlying diseases existing in the elderly before injury might in turn affect the recovery from burns. The interaction between the 2 would prolong the hospital stay, consume more medical resources and result in a poor prognosis [36,37]. For instance, Lara et al. [30] observed that in elderly patients with the same severity of burns, patients with dementia were hospitalized for twice as long as patients without dementia, the 30-day mortality rate was 3 times higher than patients without dementia, and more medical resources were consumed.
Since Janzekovic [38] first proposed the concept of early excision and skin grafting in 1970, this technique has been shown to reduce the hospitalization time and mortality of children and adults [39]. Half of the patients in the present study underwent surgical treatment, and older patients with pre-existing comorbidities were more likely to choose conservative treatment. However, surgical treatment did not improve the number of hospitalization days and mortality rates in elderly patients, which may be related to more complications after surgery [40]. Elderly patients with burns are often characterized by a pre-existing comorbidity, critical conditions, numerous complications and a weak wound healing ability [3]. Therefore, the hospitalization time should exceed the overall hospitalization time of patients with burns. However, the median hospitalization period for the patients included in this study was only 0.4 days/% TBSA, which was lower than the usual hospitalization period (1 day/%) for the total burn population [41], and much lower than the rate of 2.4 days/% TBSA for elderly patients with burns in some countries, such as the UK [32]. In the present study, the treatment received by elderly patients with severe burns was obviously insufficient, and with aging and an increase in the burn area, the enthusiasm for treatment was lower. Similarly, studies by Cheng et al. [14] and Tang et al. [42] also reported this phenomenon. Tang et al. [42] reported a proportion of elderly patients with severe burns who refused treatment and were discharged as high as 57.3%, whereas the proportion of young and middle-aged patients was only 7.8%; the reason why the elderly patients refused treatment was mainly insufficient funds.
The mortality rate is the focus of epidemiological investigations. Even with the same degree of burns, the prognosis of the elderly is often worse [3]. The literature published over the last decade reports a mortality rate of burns in the elderly ranging from 8% to 67%, and the average is approximately 15–20% in most reports [14,18–20,32,34,35]. In the present study, the mortality rate of the elderly patients with severe burns was 24.8%. However, when comparing the data from the same institute from 1999 to 2006, the mortality rate of the elderly patients in both the 30–50% TBSA group and the >50% group increased [22]. Meanwhile, the mortality rate of the last 5-year cohort (2014–2018) was also higher than the previous 5-year cohort (2009–2013) (26.3% versus 23.1%) in this study, although this increase had no statistically significant. Undoubtedly, the treatment of burns is improving. Why is the mortality rate of the elderly patients with severe burns not declining? This topic deserves additional consideration and further research. In addition, regarding the mortality rate of elderly patients with burns, the actual number of deaths caused by burns might be higher than reported in the literature because most studies only analyzed the mortality rate during hospitalization, and a certain proportion of the injured patients died at the scene of the burn or outside the hospital after completing treatment [43]. Duke et al. [37] also observed an increase in the long-term mortality of elderly patients associated with burns, and the true mortality rate associated with burns would be underestimated if the rate only relies on hospital death data.
The mortality rate of patients with burns is mainly affected by age, the burn area and inhalation injury [2,12,19,22,39,44]. In the present study, the mortality rate did not increase with age and burn area, which might be related to the insufficient sample size. However, we observed a significantly higher mortality rate in patients with inhalation injury (38.5% versus 17.1%), consistent with previous reports [19,20]. Meanwhile, significant difference in the mortality rates was not observed between patients without a pre-existing comorbidity and patients with 1 or 2 pre-existing comorbidities (18.9% versus 19.5%); however, when older patients had 3 or more pre-existing comorbidities, the mortality rate was significantly higher, up to 60%. The multivariate regression analysis also showed that the number of pre-existing comorbidities was an independent risk factor for death, similar to results reported by Stylianou et al. [45]. In addition, the causes of death in elderly patients with severe burns were sepsis, major burns, acute renal failure and pneumonia in previous studies, and the median time of death was 14 days after injury [12,32,46]. We obtained similar results in the present study.
Conclusions
In this study, the incidence and mortality of severe burns in the elderly in our department have no sign of declining. Men aged 60–69 years are the key populations to target preventative measures for burns in the elderly. Most elderly patients with severe burns experienced flame and flash burns, and burns frequently occurred in summer and winter. Meanwhile, multidisciplinary treatment in a hospital and an increase in the social support for the elderly population may improve outcomes.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the National Natural Science Foundation of China (grant no. 81571898) and the Military Medical Innovation Improvement Program of Southwest Hospital (grant no. SWH2018QNLC-13)
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;10159:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayuo J, Botchway AE. Burns among older persons: A narrative review. Burns Open. 2017 S246891221730010X. [Google Scholar]
- 3.Jeschke MG, Peck MD. Burn care of the elderly. J Burn Care Res. 2017;38(3):e625–28. doi: 10.1097/BCR.0000000000000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraft R, Herndon DN, Al-Mousawi AM, et al. Burn size and survival probability in paediatric patients in modern burn care: A prospective observational cohort study. Lancet. 2012;379(9820):1013–21. doi: 10.1016/S0140-6736(11)61345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mistry RM, Pasisi L, Chong S, et al. Socioeconomic deprivation and burns. Burns. 2010;36(3):403–8. doi: 10.1016/j.burns.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Sarma BP. Prevention of burns: 13 years’ experience in Northeastern India. Burns. 2011;37(2):265–72. doi: 10.1016/j.burns.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Lee KC, Joory K, Moiemen NS. History of burns: The past, present and the future. Burns Trauma. 2014;2(4):169–80. doi: 10.4103/2321-3868.143620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forster NA, Zingg M, Haile SR, et al. 30 years later – does the ABSI need revision? Burns. 2011;37(6):958–63. doi: 10.1016/j.burns.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: Extending and updating the Baux Score. J Trauma. 2010;68(3):690–97. doi: 10.1097/TA.0b013e3181c453b3. [DOI] [PubMed] [Google Scholar]
- 10.Nakae H, Wada H. Characteristics of burn patients transported by ambulance to treatment facilities in Akita Prefecture, Japan. Burns. 2002;28(1):73–79. doi: 10.1016/s0305-4179(01)00063-8. [DOI] [PubMed] [Google Scholar]
- 11.Smolle C, Cambiasodaniel J, Forbes AA, et al. Recent trends in burn epidemiology worldwide: A systematic review. Burns. 2017;43(2):249–57. doi: 10.1016/j.burns.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brusselaers N, Monstrey S, Vogelaers D, et al. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit Care. 2010;14(5):R188. doi: 10.1186/cc9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen CI, Chiou MJ, Kuo CF, et al. Determination of risk factors for burn mortality based on a regional population study in Taiwan. Burns. 2018;44(6):1591–601. doi: 10.1016/j.burns.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Cheng W, Shen C, Zhao D, et al. The epidemiology and prognosis of patients with massive burns: A multicenter study of 2483 cases. Burns. 2019;45(3):705–16. doi: 10.1016/j.burns.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Duan WQ, Xu XW, Cen Y, et al. Epidemiologic investigation of burn patients in Sichuan Province, China. Med Sci Monit. 2019;25:872–79. doi: 10.12659/MSM.912821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamidi M, Zeeshan M, Leon-Risemberg V, et al. Frailty as a prognostic factor for the critically ill older adult trauma patients. Am J Surg. 2019;218(3):484–89. doi: 10.1016/j.amjsurg.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Rybarczyk MM, Schafer JM, Elm CM, et al. A systematic review of burn injuries in low- and middle-income countries: Epidemiology in the WHO-defined African Region. Afr J Emerg Med. 2017;7(1):30–37. doi: 10.1016/j.afjem.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caetano P, Brand OC, Pascoal A, et al. Aging and burn: A five-year retrospective study in a major burn centre in Portugal. Annals of Physical and Rehabilitation Medicine. 2018;61:e64. [PMC free article] [PubMed] [Google Scholar]
- 19.Hao T, Liangxi W, Weiguo X, et al. Epidemiologic and clinical characteristics of severe burn patients: Results of a retrospective multicenter study in China, 2011–2015. Burns Trauma. 2018;6(1):14. doi: 10.1186/s41038-018-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toppi J, Cleland H, Gabbe B. Severe burns in Australian and New Zealand adults: Epidemiology and burn centre care. Burns. 2019;45(6):1456–61. doi: 10.1016/j.burns.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Gielen AC, McDonald EM, Shields W. Unintentional home injuries across the life span: problems and solutions. Annu Rev Public Health. 2015;36:231–53. doi: 10.1146/annurev-publhealth-031914-122722. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Peng Y, Shang X, et al. Epidemiologic investigation of geriatric burns in Southwest China. Burns. 2009;35(5):714–18. doi: 10.1016/j.burns.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Hendrix L, Charles A, Buchholz V, et al. Influence of race and neighborhood on the risk for and outcomes of burns in the elderly in North Carolina. Burns. 2011;37(5):762–69. doi: 10.1016/j.burns.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keck M, Lumenta DB, Andel H, et al. Burn treatment in the elderly. Burns. 2009;35(8):1071–79. doi: 10.1016/j.burns.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Chang EJ, Edelman LS, Morris SE, et al. Gender influences on burn outcomes in the elderly. Burns. 2005;31(1):31–35. doi: 10.1016/j.burns.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Scheven D, Barker P, Govindasamy J. Burns in rural Kwa-Zulu Natal: Epidemiology and the need for community health education. Burns. 2012;38(8):1224–30. doi: 10.1016/j.burns.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Otteni CR, Saruni SI, Duron VP. Baseline assessment of inpatient burn care at Tenwek Hospital, Bomet, Kenya. World Journal Surg. 2013;37(7):1530–35. doi: 10.1007/s00268-013-2045-2. [DOI] [PubMed] [Google Scholar]
- 28.Gevaart-Durkin A, Swart D, Chowdhury Z. A study of energy-related injuries from hospital admissions among children and adults in South Africa. Burns. 2014;40(6):1209–18. doi: 10.1016/j.burns.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Lundgren RS, Kramer CB, Rivara FP, et al. Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res. 2009;30(2):307–14. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey L, Mitchell R, Brodaty H, et al. Dementia: A risk factor for burns in the elderly. Burns. 2016;42(2):282–90. doi: 10.1016/j.burns.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Zavlin D, Chegireddy V, Boukovalas S, et al. Multi-institutional analysis of independent predictors for burn mortality in the United States. Burns Trauma. 2018;6(1):24. doi: 10.1186/s41038-018-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wearn C, Hardwicke J, Kitsios A, et al. Outcomes of burns in the elderly: Revised estimates from the Birmingham Burn Centre. Burns. 2015;41(6):1161–68. doi: 10.1016/j.burns.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Rehou S, Shahrokhi S, Thai J, et al. Acute phase response in critically ill elderly burn patients. Crit Care Med. 2019;47(2):201–9. doi: 10.1097/CCM.0000000000003516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin Z, Qin Z, Xin W, et al. The characteristics of elderly burns in Shanghai. Burns. 2010;36(3):430–35. doi: 10.1016/j.burns.2009.06.204. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Chen JJ, Crook N, et al. Epidemiologic investigation of burns in the elderly in Sichuan Province. Burns. 2013;39(3):389–94. doi: 10.1016/j.burns.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Campbell J, Degolia P, Fallon WF, Rader EL. In harm’s way: Moving the older trauma patient toward a better outcome. Geriatrics. 2009;64(1):8–13. [PubMed] [Google Scholar]
- 37.Duke JM, Boyd JH, Rea S, et al. Long-term mortality among older adults with burn injury: A population-based study in Australia. Bull World Health Organ. 2015;93(6):400–6. doi: 10.2471/BLT.14.149146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janžekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10(12):1103–8. [PubMed] [Google Scholar]
- 39.Orgill, Dennis P. Excision and skin grafting of thermal burns. N Engl J Med. 2009;360(9):893–901. doi: 10.1056/NEJMct0804451. [DOI] [PubMed] [Google Scholar]
- 40.Khadim MF, Rashid A, Fogarty B, et al. Mortality estimates in the elderly burn patients: the Northern Ireland experience. Burns. 2009;35(1):107–13. doi: 10.1016/j.burns.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Peck MD. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns. 2011;37(7):1087–100. doi: 10.1016/j.burns.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Tang Y, Wang LX, Xie WG, et al. [Multicenter epidemiological investigation of hospitalized elderly, young and middle-aged patients with severe burn]. Zhonghua Shao Shang Za Zhi. 2017;33(9):537–44. doi: 10.3760/cma.j.issn.1009-2587.2017.09.003. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 43.Navarrete N, Rodriguez N. Epidemiologic characteristics of death by burn injury from 2000 to 2009 in Colombia, South America: A population-based study. Burns Trauma. 2016;4:8. doi: 10.1186/s41038-016-0033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeschke MG, Pinto R, Costford SR, et al. Threshold age and burn size associated with poor outcomes in the elderly after burn injury. Burns. 2016;42(2):276–81. doi: 10.1016/j.burns.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stylianou N, Buchan I, Dunn KW. A model of British in-hospital mortality among burns patients. Burns. 2014;40(7):1316–21. doi: 10.1016/j.burns.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Albornoz CR, Villegas J, Sylvester M, et al. Burns are more aggressive in the elderly: Proportion of deep burn area/total burn area might have a role in mortality. Burns. 2011;37(6):1058–61. doi: 10.1016/j.burns.2011.03.006. [DOI] [PubMed] [Google Scholar]