Abstract
Background
The aim of this study was to investigate the clinical significance of NLRP3 and HMGB1 in patients with active ulcerative colitis.
Material/Methods
This was a prospective observational study which included a total of 62 cases with active ulcerative colitis during January 2017 to December 2018. The patients were divided into a mild/moderate group or a severe group according to Sutherland Disease Activity Index (DAI) score. Clinical activity index and endoscopic index were used to determine the severity of UC. Serum levels of NLRP3, HMGB1, endothelin-1, IL-1β, and TNF-α were determined by enzyme-linked immunosorbent assay (ELISA).
Results
Sutherland DAI score, clinical activity index, and endoscopic index were all significantly higher in severe patients than in the mild/moderate group. NLRP3, HMGB1, endothelin-1, IL-1β, and TNF-α were significantly higher in severe UC patients. NLRP3 level was positively correlated with HMGB1, ET-1, IL-1β, and TNF-α levels. Both NLRP3 and HMGB1 were positively correlated with Sutherland DAI score, clinical activity index, and endoscopic index.
Conclusions
Both serum NLRP3 and HMGB1 were elevated in UC patients, and the serum levels of NLRP3 were positively correlated with serum levels of HMGB1, ET-1, IL-1β, and TNF-α, as well as severity of UC patients.
MeSH Keywords: Colitis, Ulcerative; Hemostasis, Endoscopic; HMGB1 Protein
Background
Ulcerative colitis (UC) a chronic intestinal tract inflammatory disorder that is a type of inflammatory bowel disease (IBD) [1,2]. UC usually causes bloody diarrhea, abdominal pain, and extraintestinal manifestations [3,4]. According to data of the Fifth International Meeting on inflammatory bowel diseases, about 0.4% of people in the United States and 0.5% of people in Canada have IBD [5]. Moreover, the incidence of UC has increased during the past 20 years [6]. However, despite numerous studies on UC, the underlying mechanisms are still unclear.
Nod-like receptor protein 3 (NLRP3) is a newly-found inflammation-related factor, which is involved in inflammatory response of many diseases [7]. It was reported that NLRP3 promoted angiogenesis in early stages of wound healing [8]. A recent study also showed the inhibition of NLRP3 could protect against peritonitis [9]. Studies showed high-mobility group box 1 protein (HMGB1) could promote the activation of severe NLRP3 bioprocesses [10]. Several studies also found NLRP3 and HMGB1 were abnormally expressed in IBD, and it was found that fecal HMGB1 was elevated in patients with UC and Crohn’s disease [11]. NLRP3 was also found to be increased in a mouse UC model [12].
However, despite these studies, clinical evidence for NLRP3 and HMGB1 is still inadequate and few studies have reported on the relationship between NLRP3 and HMGB1 in UC patients. In the present study, we demonstrated that both serum NLRP3 and HMGB1 were elevated in UC patients. The serum levels of NLRP3 were positively correlated with serum levels of HMGB1, ET-1, IL-1β, and TNF-α, as well as severity of UC patients. This research might give more clinical evidence for NLRP3 and HMGB1 in UC and might provide potential new biomarkers for UC diagnosis.
Material and Methods
Patients
This prospective observational study included a total of 62 cases with active ulcerative colitis who came to our hospital during January 2017 to December 2018. All patients who met the inclusion criteria were consecutively enrolled into the research. The diagnosis of ulcerative colitis was confirmed by colonoscopy according to the standards of the Digestive Society of the Chinese Medical Association and British Society of Gastroenterology [13,14]. None of the patients had received any related treatment before the study. The patients were divided into a mild/moderate group or a severe group according to Sutherland Disease Activity Index (DAI) score, in which 3~5 represents mild, 6~10 represents moderate, and 11~12 represents severe UC DAI [15]. The following patients were excluded: patients <18 years or >70 years; patients with other severe intestinal disease such as Crohn’s disease, local stenosis, intestinal obstruction, intestinal perforation, rectal polyps, toxic colonic dilatation, and colorectal cancer; patients with other severe liver, renal, cardiovascular, or inflammatory diseases; and patient who were pregnant. All patients received standard treatment according to the Chinese Medical Association after diagnosis. Written informed consent was obtained from all patients. The present study was approved by HwaMei Hospital, University of the Chinese Academy of Sciences.
Clinical activity index and endoscopic index
The clinical activity index and endoscopic index were measured to determine the severity of patients [16]. The clinical activity index was recorded for the first week after admission, and the endoscopic index was recorded at colonoscopy.
Measurement of serum inflammatory factors
Serum levels of inflammatory factors NLRP3 (kit: NLRP3 ELISA kit, LifeSpan Biosciences, LS-F31954), HMGB1 (kit: HMGB1 ELISA kit, LifeSpan Biosciences, LS-F26519), endothelin-1 (ET-1, kit: ET-1 ELISA kit, R&D Systems, QET00B), IL-1β (kit: IL-1β ELISA kit, Abcam, ab46052), and TNF-α (kit: IL-1β ELISA kit, Abcam, ab181421) were determined by enzyme-linked immunosorbent assay (ELISA) using commercial kits according to the manufacturer’s instructions.
Statistical analysis
Measurement data are expressed as mean±SD. Comparison between 2 groups was performed using the t test. Correlations were analyzed using Pearson’s analysis. P<0.05 was considered as statistically significant. All calculations were made using SPSS 20.0.
Results
Basic characteristics for mild/moderate and severe UC patients
In all patients, 41 cases were diagnosed as mild/moderate UC and 21 cases were diagnosed as severe UC. The mean age of all patients was 40.68±13.72, with a male: female sex ratio of 37: 25. The Sutherland DAI score, clinical activity index, and endoscopic index were all significantly higher in severe patients than in the mild/moderate group (Table 1, P<0.05). No significant difference was found in age of sex.
Table 1.
Basic clinical information for all patients.
| Variables | Mild/moderate, n=41 | Severe, n=21 | P |
|---|---|---|---|
| Age, year | 39.07±14.11 | 43.81±12.66 | 0.201 |
| Gender, Female (%) | 24: 17 | 13: 8 | 0.623 |
| Sutherland DAI score | 7.05±2.12 | 11.48±0.51 | 0.000 |
| Clinical activity index | 8.24±1.79 | 13.57±3.61 | 0.000 |
| Endoscopic index | 6.41±1.77 | 9.38±1.91 | 0.000 |
Relationship between serum NLRP3, HMGB1, and other inflammatory factors
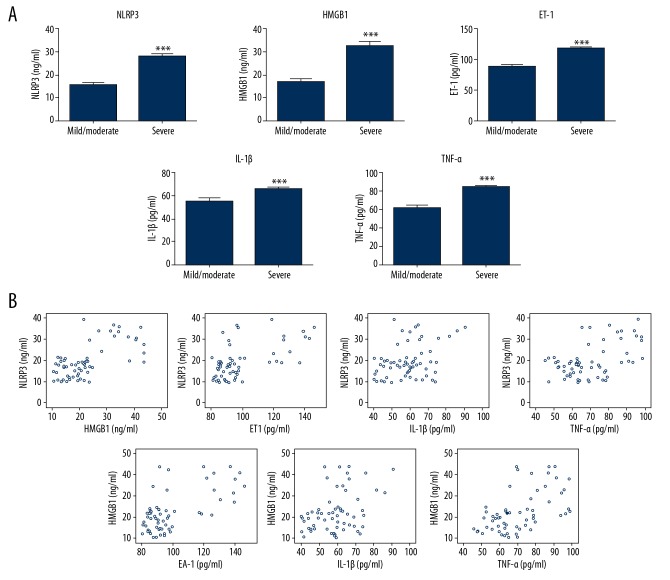
Serum levels of NLRP3, HMGB1, endothelin-1, IL-1β, and TNF-α were determined by ELISA. Results showed all factors were significantly higher in severe UC patients (P<0.05, Figure 1). Pearson’s analysis was used to determine the correlation among factors. It was found NLRP3 level was positively correlated with HMGB1, ET-1, IL-1β, and TNF-α levels (all P<0.05). Similar results were also found for HMGB1.
Figure 1.
(A) Serum levels of NLRP3, HMGB1, endothelin-1, IL-1β, and TNF-α in different groups of patients. (B) Correlation analysis among different factors was conducted by Pearson’s analysis.
Relationship between serum NLRP3, HMGB1, and clinical outcomes
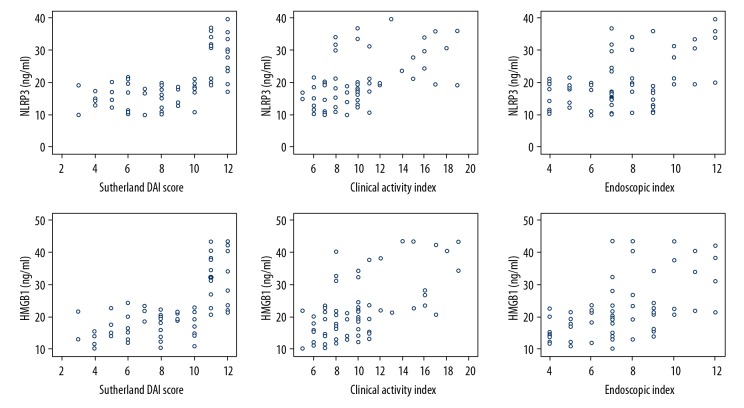
We used Pearson’s analysis to assess whether serum levels of NLRP3 and HMGB1 were correlated with Sutherland DAI score, clinical activity index, and endoscopic index. As shown in Figure 2, both NLRP3 and HMGB1 were positively correlated with Sutherland DAI score, clinical activity index, and endoscopic index, indicating both factors were positively correlated with UC severity.
Figure 2.
Correlation analysis among NLRP3, HMGB1, Sutherland DAI score, clinical activity index, and endoscopic index was conducted by Pearson’s analysis.
Discussion
Although there have been numerous studies on ulcerative colitis, the diagnosis of UC still needs more effective biomarkers, and the molecular mechanisms of UC remain unclear. In recent years, the NLRP3/HMGB1 axis was shown to be involved in inflammatory response in many diseases. Some studies also found NLRP3 and HMGB1 might be associated with UC [11,12]. However, the relationship between NLRP3 and HMGB1 in UC patients has seldom been reported, and the clinical significance of NLRP3 and HMGB1 is unclear. In the present study, we further confirmed that serum levels of BLRP3 and HMGB1 were upregulated in UC patients. We found a positive correlation between NLRP3 and HMGB1, as well as between NLRP3/HMGB1 and other inflammatory factors of ET-1, IL-1β, and TNF-α. We also found NLRP3 and HMGB1 were associated with severity of UC.
NLRP3 was reported to be associated with inflammatory response in many studies. Coll et al. found a kind of NLRP3 inhibitor, MCC950, and demonstrated that inhibition of NLRP3 by MCC950 could significantly improve autoinflammatory and autoimmune diseases and reduced IL-1β level [17]. Wu et al. demonstrated that NLRP3 was elevated in a lung inflammation model and that activation of NLRP3 contributed to lung inflammation-induced injury [18]. A relationship has also been reported between NLRP3 and ulcerative colitis. Itani et al. demonstrated that NLRP3 level in colon tissues of IBD patients was correlated with histological scores [12]. It was also found the NLRP3 inflammasome was activated in UC patients with long-standing disease [19]. However, the clinical evidence for NLRP3 in UC is still inadequate. In the present research, we demonstrated that the elevated NLRP3 level was positively correlated with levels of HMGB1, ET-1, IL-1β, and TNF-α, as well as UC severity.
The role of HMGB1 and its relationship with NLRP3 in inflammation have also been demonstrated. Ana et al. showed that HMGB1-NLRP3 signaling was activated in Aβ-mediated microglial inflammation [20]. It was also found that HMGB1 can activate the NLRP3 inflammasome and further increased IL-1β production in vascular smooth muscle cells [21]. It was also found that HMGB1 was upregulated in stools of UC patients and could be considered as a novel biomarker for intestinal inflammation [11]. In a recent study, Palone confirmed that fecal HMGB1 was remarkably increased in pediatric and adult patients with Crohn’s disease and UC [22]. However, up to now, no study has focused on the relationship between NLRP3 and HMGB1 in UC patients. In this research, we showed that HMGB1 level was positively correlated with NLRP3, as well as ET-1, IL-1β, and TNF-α, and could be used as a biomarker for UC severity.
The present study has some limitations. First, the study sample size is small and from a single center. Secondly, molecular mechanisms for NLRP3 and HMGB1 in UC development are still unclear. All these topics need further research.
Conclusions
The present prospective observational study demonstrated that NLRP3 and HMGB1 were elevated in serum of UC patients. The serum levels of NLRP3 were positively correlated with serum levels of HMGB1, ET-1, IL-1β, and TNF-α, and both NLRP3 and HMGB1 were associated with UC severity. This provides clinical evidence for the role of NLRP3 and HMGB1 in UC and might provide potential new biomarkers for UC diagnosis.
Abbreviations
- DAI
Disease Activity Index
- ELISA
enzyme-linked immunosorbent assay
- UC
ulcerative colitis
- IBD
inflammatory bowel disease
- NLRP3
nod-like receptor protein 3
- HMGB1
high-mobility group box 1 protein
Footnotes
Source of support: Departmental sources
References
- 1.Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380(9853):1606–19. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756–70. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: A genetic association study. Lancet. 2016;387(10014):156–67. doi: 10.1016/S0140-6736(15)00465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papamichael K, Lin S, Moore M, et al. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis. 2019;10:2040622319838443. doi: 10.1177/2040622319838443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latella G, Caprili R. News from the “5th international meeting on inflammatory bowel diseases” CAPRI 2010. J Crohns Colitis. 2010;4(6):690–702. doi: 10.1016/j.crohns.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Nie JY. Beverage consumption and risk of ulcerative colitis. Medicine. 2017;96(49):e9070. doi: 10.1097/MD.0000000000009070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265(1):35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinheimer-Haus EM, Mirza RE, Koh TJ. Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing. PLoS One. 2015;10(3):e0119106. doi: 10.1371/journal.pone.0119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Shi Q, Dou S, et al. Negative regulation of Nod-like receptor protein 3 inflammasome activation by T cell Ig mucin-3 protects against peritonitis. Immunology. 2018;153(1):71–83. doi: 10.1111/imm.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi W, Chen H, Li F, et al. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma. J Neuroinflammation. 2015;12:137. doi: 10.1186/s12974-015-0360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitali R, Stronati L, Negroni A, et al. Fecal hmgb1 is a novel marker of intestinal mucosal inflammation in pediatric inflammatory bowel disease. Am J Gastroenterol. 2011;106(11):2029–40. doi: 10.1038/ajg.2011.231. [DOI] [PubMed] [Google Scholar]
- 12.Itani S, Watanabe T, Nadatani Y, et al. Nlrp3 inflammasome has a protective effect against oxazolone-induced colitis: A possible role in ulcerative colitis. Sci Rep. 2016;6(1):39075. doi: 10.1038/srep39075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branch of Gastrointestinal Diseases, China Association of Chinese Medicine. [Consensus on Chinese medical diagnosis and treatment of ulcerative colitis (2009)]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30(5):527–32. [in Chinese] [PubMed] [Google Scholar]
- 14.Carter MJ, Travis SP IBD Section, British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai YC, Zheng L, Zhang YL, et al. Effects of Jianpi Qingchang decoction on the quality of life of patients with ulcerative colitis A randomized controlled trial. Medicine. 2017;96(16):e6651. doi: 10.1097/MD.0000000000006651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoepfer AM, Straumann A, Trummler M, et al. Ulcerative colitis: Correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15(12):1851–58. doi: 10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- 17.Coll RC, Robertson AAB, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–55. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Yan Z, Schwartz DE, et al. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol. 2013;190(7):3590–99. doi: 10.4049/jimmunol.1200860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazaridis LD, Pistiki A, Giamarellos-Bourboulis EJ, et al. Activation of NLRP3 inflammasome in inflammatory bowel disease: Differences between Crohn’s disease and ulcerative colitis. Dig Dis Sci. 2017;62(9):2348–56. doi: 10.1007/s10620-017-4609-8. [DOI] [PubMed] [Google Scholar]
- 20.Falcão AS, Carvalho LA, Lidónio G, et al. Dipeptidyl vinyl sulfone as a novel chemical tool to inhibit hmgb1/nlrp3-inflammasome and inflamma-mirs in Aβ-mediated microglial inflammation. Acs Chem Neurosci. 2016;8(1):89–99. doi: 10.1021/acschemneuro.6b00250. [DOI] [PubMed] [Google Scholar]
- 21.Kim EJ, Park SY, Baek SE, et al. HMGB1 Increases IL-1β production in vascular smooth muscle cells via NLRP3 inflammasome. Front Physiol. 2018;9:313. doi: 10.3389/fphys.2018.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palone F, Vitali R, Mennini M, et al. Fecal hmgb1 reveals microscopic inflammation in adult and pediatric patients with inflammatory bowel disease who are in clinical and endoscopic remission. Inflamm Bowel Dis. 2016;22(12):2886–93. doi: 10.1097/MIB.0000000000000938. [DOI] [PubMed] [Google Scholar]