Abstract
Background
Cerebral ischemia-reperfusion injury is a pivotal cause of deaths due to cerebrovascular accident. Increased research efforts are needed to reveal the mechanism underlying its aggravation or alleviation. In this study, the effects of dexmedetomidine post-conditioning on the HMGB1/TLR4/NF-κB signaling pathway in cerebral ischemia-reperfusion rats was explored.
Material/Methods
Ninety rats were randomly divided into 5 groups – a sham group (Sham), a model group (I/R), a dexmedetomidine post-conditioning group (Dex), a recombinant high mobility group protein B1 group (rHMGB1), and a recombinant HMGB1+dexmedetomidine post-conditioning group (rHMGB1+Dex) – with 18 rats in each group. Longa grading, wet-dry weighing, TTC staining, HE staining, and immunohistochemical staining were used to assess brain damage. ELISA, RT-PCR, and Western blot analyses were performed to assess expression of IL-1β, TNF-α, IL-6, IL-8, HMGB1, TLR4, and NF-κB.
Results
Compared with the I/R group, the neurological function score, brain water content, infarction area, and the number of COX-2- and IBA-1-positive cells in the Dex group were significantly lower, accompanied by downregulated expression of the HMGB1/TLR4/NF-κB pathway, alleviated inflammation, and oxidative stress injury in brain tissue. These trends were mostly reversed in the rHMGB1 group and rHMGB1+Dex group, but not in the Dex group. Furthermore, when compared to the Dex group, there were significant increases of H2O2, MDA, NO, IL-1β, TNF-α, IL-6, IL-8, HMGB1, TLR4, and p-P65 in the rHMGB1 group and rHMGB1+Dex group, in which a significant decrease of T-AOC, SOD, and p-IκBα was also detected.
Conclusions
Dexmedetomidine post-conditioning can alleviate cerebral ischemia-reperfusion injury in rats by inhibiting the HMGB1/TLR4/NF-κB signaling pathway.
MeSH Keywords: Dexmedetomidine, HMGB1 Protein, NF-kappa B, Toll-Like Receptor 4
Background
Cerebrovascular accident is one of the most pernicious neurological diseases, accounting for about 5.5 million deaths worldwide annually [1]. Although breakthroughs have been made with respect to treatment methods in recent years, the prognosis of cerebrovascular accidents remains unsatisfactory, especially in low- and middle-income countries [2]. Among all types of cerebrovascular accidents, cerebral ischemic disease is an enduring topic in clinical medicine due to its high incidence, high mortality, and serious sequelae [3]. Apart from ischemia-hypoxia damage, ischemia-reperfusion (I/R) injury also plays a pivotal role in cerebral ischemic disease via Ca2+overload, free radical damage, and inflammatory damage [4].
HMGB1 is a highly conserved DNA-binding protein found in almost all mammals [5]. As a key member of the HMGB family, HMGB1 can achieve immunoregulation by binding with receptors on the surface of immune cells, resulting in a major effect on inflammatory factors expression [6–8]. During inflammatory response, HMGB1, as an endogenous ligand of Toll-like receptors 4 (TLR4), can activate it and then nuclear factor kappa B (NF-κB) [9]. Thereafter, a series of inflammatory factors, including IL-1β, TNF-α, IL-6, and IL-8, form a cascade of secretion, leading to excessive and injurious inflammation, which then causes tissue damage [6]. Studies have shown that the HMGB1/TLR4/NF-κB pathway is activated during I/R in various organs, including myocardium and kidney, which leads to the cascade of various inflammatory factors and is an important part of the mechanism of tissue injury [4,7,9,10].
Dexmedetomidine (Dex is a potent short-term tranquilizer that exerts its sedative, anti-anxiety, anti-sympathetic, and analgesic effects mainly by stimulating the α2 adrenergic receptor [11–13]. However, in recent years, a variety of studies have revealed that Dex also has neuroprotective effects, thus attracting the attention of medical researchers [11,12,14,15]. Antioxidant and anti-inflammatory effects were hypothesized to account for the neuroprotective function of Dex, which may be directly associated with microglia and the HMGB1 pathway [16]. Thus far, the protective effect of Dex on tissue injury has been reported in the fields of renal I/R injury, acute lung injury, myocardial I/R injury, and spinal cord injury, but there has been relatively little research on its role in brain I/R injury [7,9,10,15,17–20]. Furthermore, the few studies on Dex, cerebral I/R injury, and HMGB1 also basically concentrated on preconditioning, lacking insights into post-conditioning administration, and being limited by fewer experimental observation indicators and fewer experimental methods. Therefore, the present study innovatively combined the HMGB1/TLR4/NF-κB pathway in I/R injury with Dex post-conditioning and microglia, and extensively assessed the indicators T-AOC, p-P65, p-IκBα, H2O2, MDA, SOD, NO, IL-1β, TNF-α, IL-6, IL-8, HMGB1, and TLR4 to comprehensively and systematically elucidate the mechanism underlying the neuroprotective effects of Dex.
Material and Methods
Experimental animal
Ninety SPF male SD rats (8–10 weeks old, 250±30 g) were purchased from Jinan Pengyue Laboratory Animal Breeding Co. Rats were fed a standard diet and housed with standard conditions with temperature 23±2°C and average humidity 55±5%. All animals were given free access to food and water and had a 12-h light/dark cycle. All animal experiments followed the NIH guidelines (NIH Pub. No. 85-23, revised 1996) and were approved by the Animal Protection and Use Committee of Linzi District People’s Hospital and Yantaishan Hospital.
Model of cerebral ischemia-reperfusion (MCAO)
Cerebral ischemia was induced by middle cerebral artery occlusion (MCAO), and the mortality rate after MCAO was 20%. The rats were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (50 mg/kg), then a midline incision was made to expose the left common carotid artery in the neck region. A 3-0 monofilament nylon suture (4.0 cm in length; Ethicon, USA) was inserted into the external carotid artery lumen through a small nick to block the middle cerebral artery. Two hours after the induction of ischemia, the filament was slowly withdrawn and the animals were then returned to their cages for a period of 22 h of reperfusion. All the procedures were performed with the exception of filament insertion in sham-operated rats.
Experimental protocols
Ninety rats were randomly divided into 1 of the following 5 groups (n=18 each group): the Dex post-treatment group received dexmedetomidine hydrochloride injection (Jiangsu Hengrui Pharmaceutical Co., Lianyungang, Jiangsu) into the tail vein at 3 ug/kg immediately after embolization for 5 min and then received pump injection through the tail vein at 3 ug/kg/h [21] for 2 h [22–24], the Model group (I/R group) received tail vein injection of an equal amount of saline, the Sham group received tail vein injection of an equal amount of saline, the recombinant high mobility group protein B1 group (rHMGB1 group) received rHMGB1 (KeyGEN BioTECH Corp., Nanjing, Jiangsu) injection into the tail vein at 8 ug/kg, and the rHMGB1+Dex group received rHMGB1 injection into the tail vein at 8 ug/kg and then we repeated the Dex group treatment method 15 min later.
Neurologic assessment
Using the Longa scoring method, the symptoms of neurological deficit were recorded 24 h after awakening from anesthesia [25–28]. The scoring criteria were as follows: 0 points, no neurological injury symptoms; 1 point, the contralateral forelimb of the lesion could not be fully straightened when lifting the tail; 2 points, turning to the paralysed side when walking; 3 points, falling to the opposite side of the lesion when walking; and 4 points, unable to walk spontaneously and lose consciousness. Neurological symptoms were scored by single-blind method.
Brain water content
Six rats in each group were sacrificed after anesthetization, and their brain tissue was quickly removed in an ice bath. Water and blood stains on the brain surface were blotted by filter paper. The wet weight of brain tissue was accurately weighed by electronic analysis balance. Then, the dry weight was determined after the brains were dried in an oven for 24 h to a constant weight. The water content measurement was calculated according to Elliott’s formula. The water content of brain tissue was calculated as: (wet weight–dry weight)/wet weight×100%. Brain water content represented the degree of brain edema.
TTC staining
After anesthesia, rats were killed by dislocation and the brains were taken from the ice. Brain tissues of 6 rats in each group were placed in liquid nitrogen for follow-up experiments, with the remaining brain tissue being frozen for 30 min at −20°C. Coronal slices were quickly placed in 2% TTC solution, incubated at 37°C for 15 min, fixed in 4% paraformaldehyde for 24 h, and photographed. The volume of cerebral infarction was measured using Image J 1.43 software (National Institutes of Health, Bethesda, MD, USA).
HE staining
The hippocampus of rats immobilized in 4% paraformaldehyde solution was dehydrated and embedded in paraffin and then sliced at a thickness 5 microns. These sections were dewaxed with xylene and hydrated with ethanol, stained by hematoxylin (Solarbio, Beijing, China) for 5 min, and rinsed with tap water. They are further treated with ethanol hydrochloride for 30 s, immersed in tap water for 15 min, and placed in eosin (Solarbio, Beijing, China) for 2 min. After routine dehydration, transparentizing, and sealing, the morphology of pyramidal cells in the hippocampal CA1 region was finally observed under x400 optical microscope (Olympus BX51, Olympus Company, Japan).
Immunohistochemistry of COX-2- and IBA-1-positive cells in hippocampal CA1 region
After routine slicing of brain tissue, baked slices were dewaxed with xylene and then hydrated with gradient ethanol solution. After 20 min of inactivation with 3% H2O2 methanol solution, they were heated for 10 min in citrate buffer (pH 6.0) to repair the high-temperature antigen, and then sealed with 5% BSA for 20 min. Rabbit anti-rat COX-2 (1: 500, orb193247, Biorbyt, Cambridge, UK) and anti-rat IBA-1 (1: 200, orb336635, Biorbyt, Cambridge, UK) were dripped onto the slices before incubation at 4°C overnight. After rewarming, they were incubated with the goat anti-rabbit IgG (1: 1000, ABIN101988, antibodies-online, Germany) labeled with horseradish peroxidase and then colored by DAB, re-dyed, dehydrated, transparentized, and sealed. Aperio Imagescope 11.1 software and ×400 optical microscope (Olympus, Japan) were used to count the positive cells and the results showed the percentage of positive cells.
Concentration of biochemical indicators
The brain tissue of the 6 remaining rats were taken from each group, and parts of them were placed frozen at −80°C, and the other part were weighed to prepare 10% tissue homogenate. The content of hydrogen peroxide in brain homogenate was determined by xylenol orange colorimetry, T-AOC was determined by the ABTS method, SOD activity was assessed by xanthine oxidase method, MDA was assessed by thiobarbituric acid colorimetry, and NO was assessed by the nitrate reductase method.
ELISA
Determination of inflammatory factors in brain tissue was performed using ELISA. PBS was added to brain tissue, then fully blended to form a homogenate. The supernatant was obtained after being centrifuged for 15 min at 3500 rpm. The contents of IL-1β (orb79117, Biorbyt, Cambridge, UK), TNF-α (orb79138-480, Biorbyt, Cambridge, UK), IL-6 (orb79123, Biorbyt, Cambridge, UK), and IL-8 (orb312288, Biorbyt, Cambridge, UK) were detected by ELISA, with their data being read at 450 nm by use of a microplate reader (RT-6100, Lei Du).
RT-PCR
The expression of HMGB1, TLR4, and NF-κB p65 mRNA in brain tissue was evaluated by RT-PCR. The brain tissue was homogenized after grinding and centrifuged at 4°C (12 000 rpm, 15 min). Total RNA (OD260/OD280 between 1.8 and 2.0 indicates that RNA purity was qualified) was extracted using a TRIzol kit (Takara, Dalian, China) and transcribed into cDNA by use of a reverse transcription kit (Applied Biosystems, Waltham, MA, USA). RT-PCR was performed using Mastercycler® nexus X2 (Eppendorf, Hamburg, Germany). The data were processed by 2−ΔΔCt method, with β-actin mRNA as an internal parameter.
The sequences of primers (Shanghai Biotechnology Service Co.) were as follows:
HMGB1, forward: 5′-TATGGCAAAGGCTGACAAGG-3′,
reverse: 5′-TTTCTTCGCAACATCACCAA-3′;
TLR4, forward: 5′-CCAGAGCCGTTGGTGTATCT-3′,
reverse: 5′-CAGAGCATTGTCCTCCCACT-3′;
NF-κB p65, forward: 5′-GGCAGCACTCCTTATCAAC-3′,
reverse: 5′-GGTGTCGTCCCATCGTAG-3′;
β-actin, forward: 5′-CACCCGCGAGTACAACCTTC-3′,
reverse: 5′-CCCATACCCACCATCACACC-3′.
Western blot analysis
The detection of HMGB1/TLR4/NF-κB pathway-related proteins was performed by Western blot analysis. We used a BCA kit (Solarbio, Beijing, China) to evaluate the protein concentration in brain homogenate. The protein sample (40 μg) was mixed with 10% SDS gel buffer at a 1: 1 ratio and then heated at 95°C for 5 min to degrade the protein. The sample was then transferred to a PVDF membrane (Merck, Darmstadt, Germany) at 80 V voltage for 30 min and sealed for 1 h in TBST solution containing 5% skimmed milk powder at 4°C. Rabbit anti-rat HMGB1 (1: 500, orb178187, Biorbyt, Cambridge, UK), TLR4 (1: 500, orb11489, Biorbyt, Cambridge, UK), P65 (1: 500, orb229138, Biorbyt, Cambridge, UK), p-P65 (1: 500, orb304662, Biorbyt, Cambridge, UK), IκBα (1: 500, orb223182, Biorbyt, Cambridge, UK), p-IκBα (1: 500, orb223035, Biorbyt, Cambridge, UK), and β-actin (1: 2000, orb178392, Biorbyt, Cambridge, UK) were diluted with TBST solution containing 3% bovine serum protein. After incubation overnight at 4°C and rewarming, goat anti-rabbit IgG (1: 1000, ABIN101988, antibodies-online, Aachen, Germany) labeled with horseradish peroxidase was used for 1-h incubation. The protein was colored for 3–5 min with ECL luminescent substrates after washing, with its expression level being standardized by β-actin, and the gray-scale quantification was carried out using Image J (NIH) software.
Statistical analysis
SPSS 19.0 statistical analysis software was used for data processing, with results being presented as mean±standard deviation. Single-factor analysis of variance (ANOVA) was used for data analysis among multiple groups, which was followed by LSD test. P values of <0.05 were considered to indicate a statistically significant result.
Results
Dex reduces neurological score and alleviates encephaledema
As shown in Figure 1A, the neurological function score of the Sham group was 0. When compared with the I/R group, the neurological function score of the Dex group was significantly lower, while that of the rHMGB1 group was significantly higher (p<0.05). In comparison with the Dex group, the scores of the rHMGB1 group and rHMGB1+Dex group were significantly higher (p<0.05). When compared to the Sham group, the brain water contents of the other groups were significantly higher (Figure 1B) (p<0.05). In contrast to the I/R group, the brain water content of the Dex group decreased significantly, while that of the rHMGB1 group increased significantly (p<0.05). Compared with the Dex group, the contents of the rHMGB1 group and rHMGB1+Dex group were significantly higher (p<0.05). These results show the neuroprotective effect of Dex.
Figure 1.
Neurologic assessment and brain water content measurement. (A) Neurologic assessment; (B) Measurement of water content in brain tissue. Compared with Sham group, * P<0.05; compared with I/R group, # P<0.05; compared with Dex group, & P<0.05.
Dex reduces cerebral infarction area
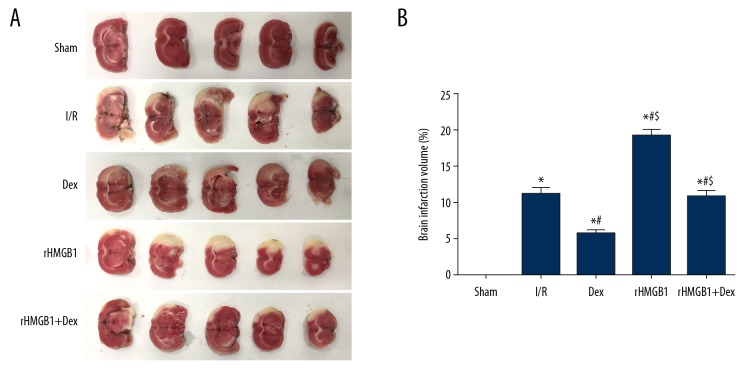
In this study, the infarcted area of brain tissue was stained white, with the non-infarcted area being red (Figure 2). Compared with the I/R group, the percentage of cerebral infarction volume in the Dex group was decreased significantly, while that in the rHMGB1 group increased significantly (p<0.05). It was also increased significantly (p<0.05) in the rHMGB1 group and rHMGB1+Dex group when compared to the Dex group, which confirmed the neuroprotective effects of Dex from the perspective of infarction protection.
Figure 2.
TTC staining of rat brain tissue. (A) TTC staining; (B) Percentage of cerebral infarction volume. Compared with Sham group, * P<0.05; compared with I/R group, # P<0.05; compared with Dex group, $ P<0.05.
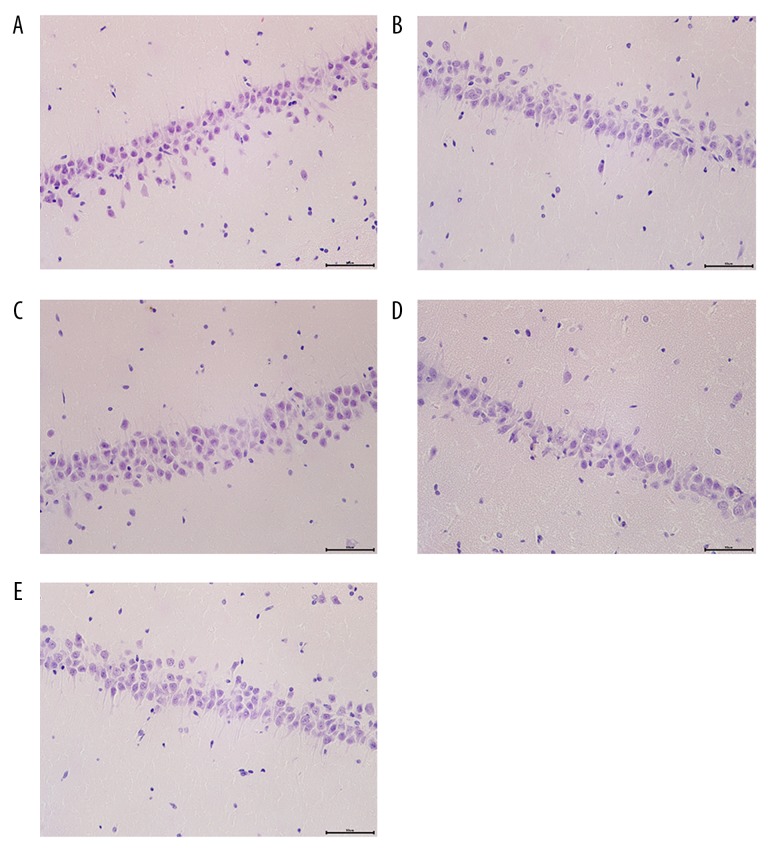
Dex alleviates the structural disorder of hippocampal cells
It was elucidated that the cells in hippocampal CA1 region of the Sham group were conical, uniform in size, neatly arranged, and had clear outlines (Figure 3). In the I/R group and rHMGB1+Dex group, cells were disorderly arranged, with unclear structure, shrinkage of cell, pyknosis of nuclei into triangles or polygons, disappearance of nucleoli, and strong eosinophilic cytoplasm. In the Dex group, the arrangement of hippocampal neurons was relatively neat but with mild swelling and degeneration. The damage of hippocampal neurons in the rHMGB1 group was more severe than that in the I/R group, further showing that Dex alleviated the nerve damage and protected the integrity of nerve function.
Figure 3.
HE staining (×400). (A) Sham group; (B) I/R group; (C) Dex group; (D) rHMGB1 group; (E) rHMGB1+Dex group.
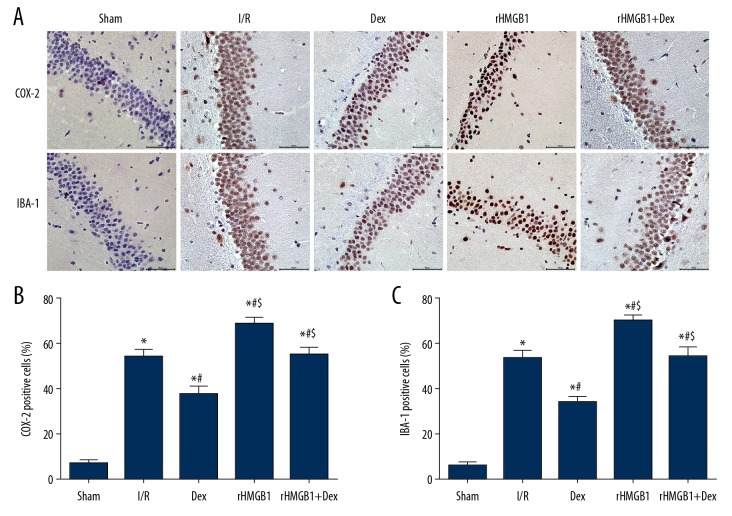
Dex reduces the number of microglia
In comparison with the Sham group, the number of COX-2- and IBA-1-positive cells in the hippocampal CA1 area in other groups increased significantly (Figure 4) (p<0.05). These cells were significantly decreased in the Dex group when compared with the I/R group (p<0.05), with the opposite trends presented in the rHMGB1 group and rHMGB1+Dex group when compared with the Dex group (p<0.05), which further revealed the potential involvement of microglia in the neuroprotective mechanism of Dex.
Figure 4.
Immunohistochemistry of COX-2 and IBA-1 positive cells in hippocampal CA1 region of rats (×400). (A) Immunohistochemical images; (B) Percentage of COX-2 positive cells (%); (C) Percentage of IBA-1 positive cells (%).Compared with Sham group, * P<0.05; compared with I/R group, # P<0.05; compared with Dex group, $ P<0.05.
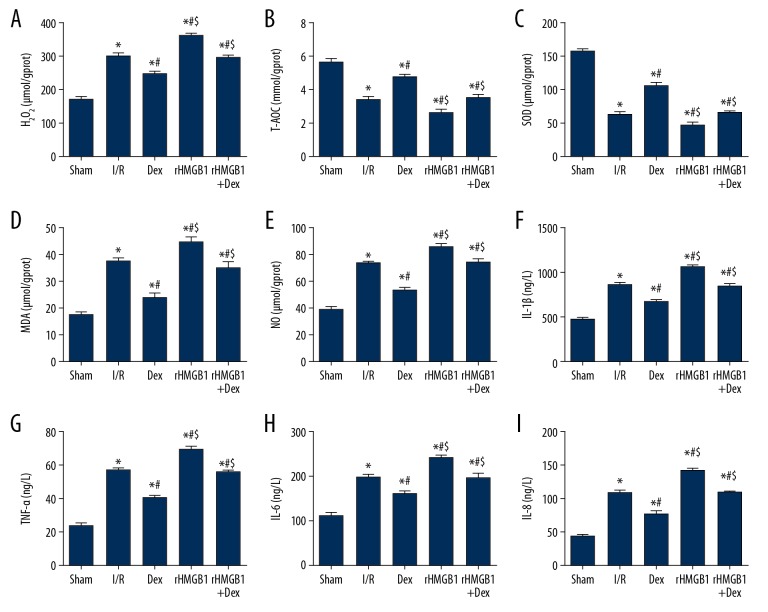
Dex inhibits inflammatory factor release and alleviates oxidative stress
When compared to the Sham group, the concentrations of H2O2, MDA, NO, IL-1β, TNF-α, IL-6, and IL-8 in brain homogenate of the other groups was significantly higher, while those of T-AOC and SOD were significantly lower (Figure 5) (p<0.05). Compared with the I/R group, the concentrations of H2O2, MDA, NO, IL-1β, TNF-α, IL-6, and IL-8 in the Dex group were significantly lower, while T-AOC and SOD were significantly higher (p<0.05). The concentrations of H2O2, MDA, NO, IL-1β, TNF-α, IL-6, and IL-8 in the rHMGB1 group were significantly higher, while T-AOC and SOD were significantly lower (p<0.05) when compared with the I/R group. Furthermore, the concentrations of H2O2, MDA, NO, IL-1β, TNF-α, IL-6, and IL-8 in the rHMGB1 group and rHMGB1+Dex group were significantly higher compared with the Dex group, while T-AOC and SOD were significantly lower (p<0.05), which suggested that Dex exerts its neuroprotective function through anti-inflammatory and anti-oxidative pathways.
Figure 5.
Determination of biochemical indicators and ELISA in brain homogenate. (A) H2O2; (B) T-AOC; (C) SOD; (D) MDA; (E) NO; (F) IL-1β; (G) TNF-α; (H) IL-6; (I) IL-8. Compared with Sham group, * P<0.05; compared with I/R group, # P<0.05; compared with Dex group, $ P<0.05.
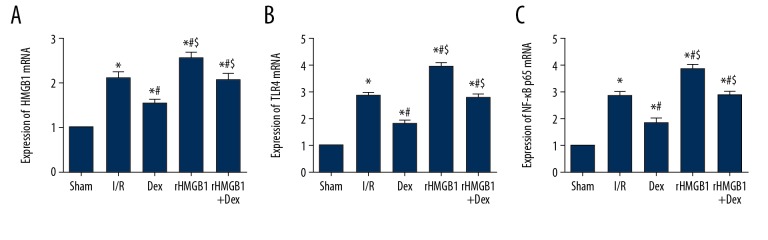
Dex decreases the concentrations of HMGB1, TLR4, and NF-κB p65 mRNA
In contrast to the expressions of HMGB1, TLR4 and NF-κB p65 in other groups were significantly higher than those in the Sham group (Figure 6) (p<0.05). There was a significant decrease (p<0.05) with respect to expressions of HMGB1, TLR4, and NF-κB p65 in the Dex group when compared with the I/R group, rHMGB1 group, and rHMGB1+Dex group. The concentrations were also higher in the rHMGB1 group compared with the I/R group, collectively indicating that the HMGB1 signal pathway is closely involved in the neuroprotective function of Dex.
Figure 6.
RT-PCR of HMGB1, TLR4 and NF-κB p65 mRNA in brain tissue. (A) HMGB1 mRNA; (B) TLR4 mRNA; (C) NF-κB p65 mRNA. Compared with Sham group, * P<0.05; compared with I/R group, # P<0.05; compared with Dex group, $ P<0.05.
Dex decreases the concentrations of proteins related to HMGB1/TLR4/NF-κB pathway
As shown in Figure 7, compared with the Sham group, the expression of HMGB1, TLR4, and p-P65 protein in the brain tissues of other groups increased significantly (p<0.05). We found a significant decrease (p<0.05) in the Dex group when compared with the I/R group, rHMGB1 group, and rHMGB1+Dex group. It was also higher in the rHMGB1 group compared with the I/R group. In terms of the expression of p-IκBα, a significant increase (p<0.05) was found in the Dex group when compared with the I/R group, rHMGB1 group, and rHMGB1+Dex group. There was also a significant decrease (p < 0.05) noted in the rHMGB1 group when compared with the I/R group, collectively confirming that Dex has a neuroprotective function in brain I/R injury by regulating the HMGB1/TLR4/NF-κB pathway.
Figure 7.
Western blot of HMGB1/TLR4/NF-κB pathway-related proteins in brain tissue. (A) Protein band image; (B) HMGB1; (C) TLR4; (D) p-P65/P65; (E) p-IκBα/IκBα. Compared with Sham group, * P<0.05; compared with I/R group, # P<0.05; compared with Dex group, $ P<0.05.
Discussion
The neuroprotective function of Dex is intriguing. In this study, the post-conditioning of Dex in cerebral I/R injury was explored, showing a strong relationship with the HMGB1/TLR4/NF-κB pathway.
It was confirmed that I/R can damage brain tissue, which further accounts for the increase of neurological score, deterioration of brain edema, enlargement of cerebral infarction area, and disorder of hippocampal cells. The above multiple signs of injury were significantly alleviated by Dex, which can be evidently antagonized by rHMGB1. Being an α2 adrenergic receptor agonist, the neuroprotective effect of Dex is relevant to the critical role of adrenaline in the blood–brain barrier (BBB) [14]. The regulation of related microcirculation is closely associated with the locus coeruleus. Studies have shown that electrical stimulation of the locus coeruleus can significantly increase the level of noradrenaline in the central nervous system, thus increasing BBB permeability [14]. Dex attenuates tissue damage by stabilizing the barrier between nerve tissues by alleviating encephaledema [5].
Injurious inflammation and oxidative damage were speculated to be prominent mechanisms in I/R injury, in which the dynamic changes of H2O2, MDA, NO, IL-1β, TNF-α, IL-6,and IL-8 provided us with notable insights into the neuroprotective effect of Dex [4,7,10,15,29–34]. In this study, the expressions of IL-1β, TNF-α, IL-6, and IL-8 were significantly suppressed in the Dex group when compared to the I/R group, indicating that Dex plays a clear anti-inflammatory role in amelioration of cerebral I/R injury.
Glial cells are also closely involved in cerebral I/R injury. Strong activation of microglia and astrocytes hinder nerve repair, while glial cells aggravate nerve injury by releasing inflammatory mediators [35–37]. The TLR4-microglia-NF-kB/IL-1β pathway forms positive feedback loop in inflammation [5]. In this study, considering that the hippocampal CA1 region is particularly sensitive to hypoxia and other injuries, immunohistochemical staining was applied to analyze the changes of microglia in the hippocampal CA1 region [11]. COX-2- and IBA-1-positive cells in the hippocampal CA1 region in the IR group were significantly more abundant than in the sham group, indicating that microglia are indeed involved in I/R injury. Furthermore, they were significantly decreased in the Dex group compared with the IR group, and this trend was eliminated in the rHMGB1 group, suggesting that Dex can achieve neuroprotective effects by inhibiting the over-activation of glial cells. Similar phenomena have also been reported in spinal cord injury [11].
Oxidative stress is also an important mechanism of I/R injury. Studies on oxidative stress in various organs suggested that it plays a critical role in this pathological process [11,38,39]. Cerebral ischemia can upregulate reactive oxygen species (ROS) and ROS generation can be promoted by the reperfusion procedure, causing double-damage by lipid peroxidation, oxidation of proteins, and DNA fragmentation [40]. The HMGB1/TLR4/NF-κB pathway is closely related to changes in SOD, NO, H2O2, and MDA [41–43]. MDA is an end-product of the unsaturated fatty acids metabolism, indicating the peroxidation status of cells, while SOD indicates peroxide scavenging capacity [18]. In the present study, when compared to sham group, the concentrations of MDA, NO, and H2O2 in the I/R group were significantly higher, while SOD and T-AOC were significantly lower. These trends were reversed in the Dex group. Thus, it was confirmed that oxidative stress occurs during cerebral I/R injury, and that post-conditioning with Dex inhibits this process, thus producing neuroprotective effects.
HMGB1 is closely related to immune cell secretion, cell injury, and cell death [37]. By binding to DNA to promote transcription, HMGB1 participates in DNA repair under physiological conditions [5]. In contrast, the concentration of HMGB1 is often excessively elevated in pathological conditions. The increase of HMGB1 activates the HMGB1/TLR4/NF-κB pathway, which results in secondary tissue damage, making HMGB1 and its downstream pathway key links in various pathological procedures [6,9,17,44]. Studies have shown that knockout of TLR4 can significantly downregulate downstream inflammatory pathways and notably reduce the secretion of inflammatory factors [45]. NF-κB belongs to the transcription factor protein family, which can affect secondary tissue damage by modulating the expression of inflammatory factors [43]. HMGB1 interacts with TLR4 to induce activation and relieve the inhibitory effect of I-κBα on NF-κB, subsequently upregulating the downstream products [6,19,42]. After activation of the HMGB1/TLR4/NF-κB pathway induced by I/R injury, IL-1β, TNF-α, IL-6, and IL-8 are secreted to mediate injurious inflammation. Similar phenomena have also been confirmed in lung, kidney, spinal cord, and myocardium [7,9,10,46–49]. In this study, the expression of HMGB1, TLR4, and NF-κB were significantly decreased in the Dex group, but were significantly increased in the rHMGB1 and rHMGB1+Dex groups, suggesting that this pathway indeed participates in cerebral I/R injury, and can be inhibited by the post-conditioning of Dex. I-κBα is a potent antagonistic protein of NF-κB and may provide us with critical insights into nerve injury in cerebral I/R [19]. In this study, the concentration of I-κBα decreased significantly in the IR group and increased with the administration of Dex, which was antagonized by rHMGB1, suggesting that I-κBα participated in the cerebral I/R injury and can be increased by Dex, which resulted in the inhibition of the downstream expression of the HMGB1/TLR4/NF-κB pathway. In conclusion, the present study suggests that Dex post-conditioning can alleviate cerebral I/R injury in rats by inhibiting HMGB1/TLR4/NF-κB pathway signaling.
This present study has some limitations. Initially, this study mainly focused on Dex post-conditioning and consequently drew a conclusion without comparison with Dex preconditioning. Although Dex post-conditioning is more feasible in clinical application, related studies showed that Dex preconditioning is superior to post-conditioning in anti-apoptosis and anti-oxidative effects, making the comparative experiment with preconditioning of Dex warranted [11]. Secondly, this study treated a rat model with a single dose of Dex, lacking the comparison of neuroprotective effects between different doses. The dose-dependent effects of Dex can be focused on in follow-up studies. Thirdly, PI3K/Akt/mTOR is also an pivotal downstream pathway in I/R injury modulated by HMBG1 and can be further inhibited by Dex [17,20]. Its role remains to be explored in our subsequent research to illuminate the holistic mechanism underlying the neuroprotective effects of Dex. Finally, BDNF is one of the protective factors for the repair of cerebral I/R injury [50]. Dex can achieve neuroprotective function by increasing the concentration of BDNF, suggesting that BDNF can be used as a complementary indicator in follow-up studies to further elucidate the mechanism involved [11].
Conclusions
Dexmedetomidine post-conditioning can alleviate cerebral ischemia-reperfusion injury in rats by inhibiting HMGB1/TLR4/NF-κB pathway signaling.
Abbreviations
- RT-PCR
reverse transcription-polymerase chain reaction
- TTC
2,3,5-triphenyltetrazolium chloride
Footnotes
Source of support: Departmental sources
References
- 1.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6 Suppl):S85–90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Azarpazhooh MR, Mandzia JL, Thrift AG, et al. Age, sex, and setting in the etiology of stroke study (ASSESS): Study design and protocol. J Neurol Sci. 2019;399:209–13. doi: 10.1016/j.jns.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Engelhard K. Anaesthetic techniques to prevent perioperative stroke. Curr Opin Anaesthesiol. 2013;26(3):368–74. doi: 10.1097/ACO.0b013e3283608239. [DOI] [PubMed] [Google Scholar]
- 4.El-Sahar AE, Safar MM, Zaki HF, et al. Neuroprotective effects of pioglitazone against transient cerebral ischemic reperfusion injury in diabetic rats: Modulation of antioxidant, anti-inflammatory, and anti-apoptotic biomarkers. Pharmacol Rep. 2015;67(5):901–6. doi: 10.1016/j.pharep.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Zhang S, Fan X, et al. Dexmedetomidine preconditioning ameliorates inflammation and blood-spinal cord barrier damage after spinal cord ischemia-reperfusion injury by down-regulation high mobility group box 1-toll-like receptor 4-nuclear factor kappaB signaling pathway. Spine (Phila Pa 1976) 2019;44(2):E74–81. doi: 10.1097/BRS.0000000000002772. [DOI] [PubMed] [Google Scholar]
- 6.Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G, Song H, Qiu L, et al. Dexmedetomidine preconditioning inhibits the long term inflammation induced by renal ischemia/reperfusion injury in rats. Acta Cir Bras. 2016;31(1):8–14. doi: 10.1590/S0102-865020160010000002. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93(6):865–73. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rong H, Zhao Z, Feng J, et al. The effects of dexmedetomidine pretreatment on the pro- and anti-inflammation systems after spinal cord injury in rats. Brain Behav Immun. 2017;64:195–207. doi: 10.1016/j.bbi.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Yang YF, Peng K, Liu H, et al. Dexmedetomidine preconditioning for myocardial protection in ischaemia-reperfusion injury in rats by downregulation of the high mobility group box 1-toll-like receptor 4-nuclear factor kappaB signalling pathway. Clin Exp Pharmacol Physiol. 2017;44(3):353–61. doi: 10.1111/1440-1681.12711. [DOI] [PubMed] [Google Scholar]
- 11.Kjell J, Olson L. Rat models of spinal cord injury: From pathology to potential therapies. Dis Model Mech. 2016;9(10):1125–37. doi: 10.1242/dmm.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benggon M, Chen H, Applegate R, et al. Effect of dexmedetomidine on brain edema and neurological outcomes in surgical brain injury in rats. Anesth Analg. 2012;115(1):154–59. doi: 10.1213/ANE.0b013e31824e2b86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weerink MAS, Struys M, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarmento A, Borges N, Lima D. Influence of electrical stimulation of locus coeruleus on the rat blood-brain barrier permeability to sodium fluorescein. Acta Neurochir (Wien) 1994;127(3–4):215–19. doi: 10.1007/BF01808769. [DOI] [PubMed] [Google Scholar]
- 15.Fang B, Li XQ, Bi B, et al. Dexmedetomidine attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia-reperfusion injury in rats. Cell Physiol Biochem. 2015;36(1):373–83. doi: 10.1159/000430107. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Huang X, Liu Z, et al. Dexmedetomidine inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated macrophages in vitro. J Surg Res. 2013;181(2):308–14. doi: 10.1016/j.jss.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Meng L, Li L, Lu S, et al. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-kappaB and PI3K/Akt/mTOR pathways. Mol Immunol. 2018;94:7–17. doi: 10.1016/j.molimm.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W, Luo F, Lu Q, et al. The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state. Chem Biol Interact. 2016;243:127–34. doi: 10.1016/j.cbi.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Q, Yi M, Guo Q, et al. Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-kappaB pathway. Int Immunopharmacol. 2015;29(2):370–76. doi: 10.1016/j.intimp.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Hu X, Wang J, et al. Short-term hesperidin pretreatment attenuates rat myocardial ischemia/reperfusion injury by inhibiting high mobility group box 1 protein expression via the PI3K/Akt pathway. Cell Physiol Biochem. 2016;39(5):1850–62. doi: 10.1159/000447884. [DOI] [PubMed] [Google Scholar]
- 21.Liang S, Wang Y, Liu Y. Dexmedetomidine alleviates lung ischemia-reperfusion injury in rats by activating PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 2019;23(1):370–77. doi: 10.26355/eurrev_201901_16785. [DOI] [PubMed] [Google Scholar]
- 22.McCarter KD, Li C, Jiang Z, et al. Effect of low-dose alcohol consumption on inflammation following transient focal cerebral ischemia in rats. Sci Rep. 2017;7(1):12547. doi: 10.1038/s41598-017-12720-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin XL, Li PF, Zhang CB, et al. Electroacupuncture alleviates cerebral ischemia and reperfusion injury via modulation of the ERK1/2 signaling pathway. Neural Regen Res. 2016;11(7):1090–98. doi: 10.4103/1673-5374.187041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Mayhan WG, Arrick DM, et al. Dose-related influence of chronic alcohol consumption on cerebral ischemia/reperfusion injury. Alcohol Clin Exp Res. 2011;35(7):1265–69. doi: 10.1111/j.1530-0277.2011.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 26.McCarter KD, Li C, Jiang Z, et al. Effect of low-dose alcohol consumption on inflammation following transient focal cerebral ischemia in rats. Sci Rep. 2017;7(1):12547. doi: 10.1038/s41598-017-12720-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin XL, Li PF, Zhang CB, et al. Electroacupuncture alleviates cerebral ischemia and reperfusion injury via modulation of the ERK1/2 signaling pathway. Neural Regen Res. 2016;11(7):1090–98. doi: 10.4103/1673-5374.187041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao H, Mayhan WG, Arrick DM, et al. Dose-related influence of chronic alcohol consumption on cerebral ischemia/reperfusion injury. Alcohol Clin Exp Res. 2011;35(7):1265–69. doi: 10.1111/j.1530-0277.2011.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li XQ, Chen FS, Tan WF, et al. Elevated microRNA-129-5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. J Neuroinflammation. 2017;14(1):205. doi: 10.1186/s12974-017-0977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Can M, Gul S, Bektas S, et al. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesthesiol Scand. 2009;53(8):1068–72. doi: 10.1111/j.1399-6576.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Wang C, Zhang D, et al. Dexmedetomidine protects against traumatic brain injury-induced acute lung injury in mice. Med Sci Monit. 2018;24:4961–67. doi: 10.12659/MSM.908133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Yang B, Hu Y, et al. Wogonin prevents TLR4-NF-kappaB-medicated neuro-inflammation and improves retinal ganglion cells survival in retina after optic nerve crush. Oncotarget. 2016;7(45):72503–17. doi: 10.18632/oncotarget.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell MT, Puskas F, Bennett DT, et al. Dexmedetomidine, an alpha-2a adrenergic agonist, promotes ischemic tolerance in a murine model of spinal cord ischemia-reperfusion. J Thorac Cardiovasc Surg. 2014;147(1):500–6. doi: 10.1016/j.jtcvs.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 34.Bekker A, Haile M, Kline R, et al. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol. 2013;25(1):16–24. doi: 10.1097/ANA.0b013e31826318af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang SL, Duan L, Xia B, et al. Dexmedetomidine preconditioning plays a neuroprotective role and suppresses TLR4/NF-kappaB pathways model of cerebral ischemia-reperfusion. Biomed Pharmacother. 2017;93:1337–42. doi: 10.1016/j.biopha.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 36.Zhu YJ, Peng K, Meng XW, Ji FH. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res. 2016;1644:1–8. doi: 10.1016/j.brainres.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 37.Smith PD, Puskas F, Meng X, et al. The evolution of chemokine release supports a bimodal mechanism of spinal cord ischemia and reperfusion injury. Circulation. 2012;126(11 Suppl 1):S110–17. doi: 10.1161/CIRCULATIONAHA.111.080275. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigo R, Fernandez-Gajardo R, Gutierrez R, et al. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013;12(5):698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- 39.Cekic B, Besir A, Yulug E, et al. Protective effects of dexmedetomidine in pneumoperitoneum-related ischaemia-reperfusion injury in rat ovarian tissue. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):343–46. doi: 10.1016/j.ejogrb.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Zeng X, Wang H, Xing X, et al. Dexmedetomidine protects against transient global cerebral ischemia/reperfusion induced oxidative stress and inflammation in diabetic rats. PLoS One. 2016;11(3):e0151620. doi: 10.1371/journal.pone.0151620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y, Tang D, Kang R. Oxidative stress-mediated HMGB1 biology. Front Physiol. 2015;6:93. doi: 10.3389/fphys.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun N, Wang H, Wang L. Protective effects of ghrelin against oxidative stress, inducible nitric oxide synthase and inflammation in a mouse model of myocardial ischemia/reperfusion injury via the HMGB1 and TLR4/NF-kappaB pathway. Mol Med Rep. 2016;14(3):2764–70. doi: 10.3892/mmr.2016.5535. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Su B, Zhu H, et al. Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-kappaB and p38 MAPK. Exp Ther Med. 2016;12(6):3607–13. doi: 10.3892/etm.2016.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papatheodorou A, Stein A, Bank M, et al. High-mobility group box 1 (HMGB1) is elevated systemically in persons with acute or chronic traumatic spinal cord injury. J Neurotrauma. 2017;34(3):746–54. doi: 10.1089/neu.2016.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao H, Chen Z, Xie LJ, Liu GF. Suppression of TLR4/NF-kappaB signaling pathway improves cerebral ischemia-reperfusion injury in rats. Mol Neurobiol. 2018;55(5):4311–19. doi: 10.1007/s12035-017-0552-0. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Zhang X, Liu L, et al. Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-kappaB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res. 2010;1321:143–51. doi: 10.1016/j.brainres.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 47.Zhang JJ, Peng K, Zhang J, et al. Dexmedetomidine preconditioning may attenuate myocardial ischemia/reperfusion injury by down-regulating the HMGB1-TLR4-MyD88-NF-small ka, CyrillicB signaling pathway. PLoS One. 2017;12(2):e0172006. doi: 10.1371/journal.pone.0172006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu XL, Chen X, Wang W, et al. Electroacupuncture pretreatment attenuates spinal cord ischemia-reperfusion injury via inhibition of high-mobility group box 1 production in a LXA4 receptor-dependent manner. Brain Res. 2017;1659:113–20. doi: 10.1016/j.brainres.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15(3):R153. doi: 10.1186/cc10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Kanegan MJ, He DN, Dunn DE, et al. BDNF mediates neuroprotection against oxygen-glucose deprivation by the cardiac glycoside oleandrin. J Neurosci. 2014;34(3):963–68. doi: 10.1523/JNEUROSCI.2700-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]