Abstract
Patient: Female, 68-year-old
Final Diagnosis: Well-differentiated papillary mesothelioma (WDPM)
Symptoms: abdominal discomfort • abdominal pain • bloating • chronic abdominal pain • diffuse abdominal pain • recurrent abdominal pain
Medication: —
Clinical Procedure: Elective abdominal surgery
Specialty: Oncology
Objective:
Rare co-existance of disease or pathology
Background:
Omental calcifications of the peritoneum are typically small and asymptomatic. However, larger psammomatous bodies that cause symptoms such as abdominal pain and bloating are often associated with tumors such as primary serous papillary carcinoma, mesothelioma, or metastatic ovarian cancer.
Case Report:
We describe omental calcifications in a 68-year-old woman who had been asymptomatic for the last 10 years. The case details the histomorphologic features and immunohistochemical signature of a 4.0×3.5×1.0 cm mass consisting of mature adipose tissue that was surgically removed together with an 8.5×6.5×1.8 cm irregular intra-abdominal/mesenteric mass composed of yellow-red fatty tissue. Microscopic sections contained fat with variable clustered classic/psammomatous calcifications, some with a thin epithelioid periphery, in association with a very focal and subtle papillary surface epithelial/mesothelial proliferation. Tumor cell invasion was not observed during examination. Immunohistochemical staining showed that mesothelial cells in the mass were strongly positive for calretinin and focally positive for EMA, CK903, and vimentin. Strong nuclear positivity for PAX8 was also reported. Additional stains were added in response to this pattern, showing strong positivity for CK8, moderate positivity for BAP1, focal positivity for ER, minimal positivity for CD56, and negativity for CK5/6 and D2-40. Three possible explanations are suggested for the phenomenon observed in the pathology slides: reactive mesothelial hyperplasia, well-differentiated papillary mesothelioma, or serous papillary carcinoma of the peritoneum.
Conclusions:
Findings suggest that these calcifications are a benign, reactive phenomenon, and that the abundance of psammoma bodies may be related to ongoing crops of papillary mesothelial hyperplasia or benign well-differentiated papillary mesothelioma.
MeSH Keywords: Calcification, Physiologic; Carcinoma, Papillary; Hyperplasia; Immunohistochemistry; Neoplasms, Mesothelial; Omentum
Background
Peritoneal and omental calcification, including those of the psammomatous variety, are associated with a wide range of both benign and malignant abdominal pathologies. On occasion, psammomatous calcifications are associated with tumors such as metastatic ovarian cancer and phenotypically identical primary peritoneal serous papillary carcinoma or mesothelioma. Calcification can occur in primary tumors in peritoneal or omental tissues, as well as metastases [1]. Conditions that systematically dysregulate calcium and mineral homeostasis, particularly hyperparathyroidism, can also cause development of calcified masses [2].
A deposition of a mineral substance consisting of calcium phosphate molecules located outside of normal bone tissue is known as “heterotopic” or “dystrophic” calcification. Small calcified masses in the abdomen are generally asymptomatic and detectable through radiological methods such as contrast-enhanced CT scans. However, to identify their source, biopsy and histological analysis may be necessary [2]. Larger masses that cause symptoms such as abdominal pain, discomfort, or bloating are more typically associated with malignant tumors than with benign processes [3].
We present a unique case of chronic omental calcifications that persisted in a patient for more than 10 years before she became symptomatic. The onset of symptoms typical of malignant abdominal lesions, and the presence of a large, calcification-containing mass in the abdominal wall, raised the possibility that a benign or low-grade neoplasm had transformed into an overtly malignant tumor. Immunohistochemical staining of calcified sections revealed an expression profile consistent with well-differentiated papillary mesothelioma (WDPM) or papillary serous carcinoma (PSC), requiring more thorough analysis.
Case Report
A 68-year-old woman presented with abdominal discomfort and recurrent diffuse abdominal pains associated with increasing omental calcifications. The patient had a 10-year history of omental calcifications, which had significantly increased in number and density in recent months. Her medical history included a hysterectomy due to a large, “grapefruit-like” mass (likely a leiomyoma), an appendectomy, and resection of a benign breast mass. The patient’s mother had undergone a radical thyroidectomy due to “excessive growth”, the precise nature of which was unknown to the patient. Additionally, the patient reported that her mother experienced episodic symptoms of diffuse abdominal pain and a feeling of fullness. This family history raised concerns about possible metastatic papillary thyroid carcinoma, which is known to be associated with psammomatous calcifications. The patient had no history of tobacco smoking, alcohol, or substance abuse.
An extensive workup was performed, including upper and lower gastrointestinal endoscopy to identify any obvious causes for her symptoms. A modification of the patient’s diet and bowel regime failed to alleviate the symptoms, and there were no identified aggravating or relieving factors for the pain. In January 2019, after a worsening of symptoms and progressively increasing calcifications captured on imaging, the patient underwent elective abdominal surgery. During the operation, severe adhesions were observed in the right upper quadrant, likely as a result of her previous surgical procedures. A 4.0×3.5×1.0 cm mass comprised of mature adipose tissue was discovered in the abdominal wall. The mass was focally bounded by a thin fibrous layer, compatible with an encapsulated lipoma.
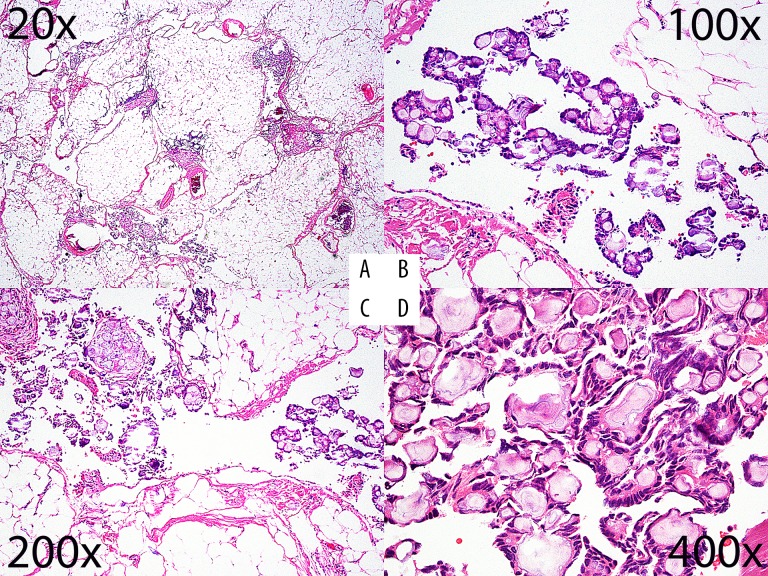
On further inspection, an 8.5×6.5×1.8 cm irregular intra-abdominal/mesenteric mass composed of yellow-red fatty tissue was discovered and excised. Microscopic sections contained fat with variable clustered classic/psammomatous calcifications. Some sections demonstrated a thin epithelioid periphery, in association with a very focal and subtle papillary surface epithelial/mesothelial proliferation (Figure 1).
Figure 1.
H&E photomicrographs: (A) 20×, (B) 100×, (C) 200×, and (D) 400× magnification. The lower powers highlight the dispersed nature of the papillary aggregates and the higher powers show their architectural detail.
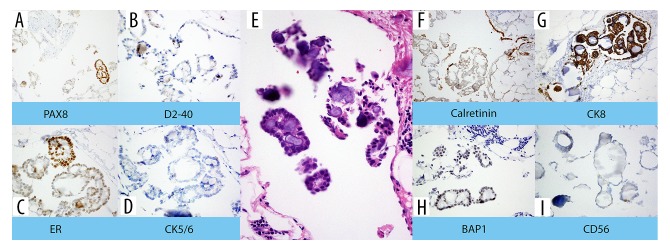
Immunohistochemical staining showed that mesothelial cells in the mass presented as strongly positive for calretinin (Figure 2) and were focally positive for epithelial membrane antigen (EMA), CK903 (high-molecular keratin), and vimentin. Mesothelial cells also demonstrated strong nuclear positivity for the paired box 8 protein (PAX8) (Figure 2). Additional stains added in response to this pattern showed strong positivity for CK8, moderate positivity for BAP1, focal positivity for ER, minimal positivity for CD56, and negativity for CK5/6 and D2-40 (Figure 2).
Figure 2.
H&E with immunohistochemical stains, all 200× magnification. Central H&E image (E) was originally 100×, digitally expanded to 1550×. This particular proliferation has a propensity for lineage ambiguity, being positive for some stains expected in mesothelial proliferations, such as calretinin (F) and CK8 (G), and negative for D2-40 (B), CK5/6 (D), and negative (as expected) for CD56 (I). It was also positive for ER (C) and PAX-8 (A), which are commonly expressed in gynecologic papillary serous carcinoma, and positive for BAP1 (H), which is more commonly expressed in papillary serous carcinomas rather than mesothelial proliferations.
The proliferative cells were strongly positive for some antibodies typically associated with mesothelial derivation but negative for others. Given this result and the fact that the cells were variably positive for antibodies conventionally associated with gynecologic malignancy, the case was seen by an expert consultant. The conferred differential diagnosis strongly favored papillary mesothelial hyperplasia/benign well-differentiated papillary mesothelioma, which is a benign, reactive phenomenon. The worrisome positive stains were noted to be positive at a low rate in most of this lesion. Despite the presence of calcifications lasting for at least 10 years, the new symptoms and laboratory findings raised the possibility of serous papillary carcinoma. Other diagnoses suggested by the immunohistochemistry and histological findings included reactive mesothelial hyperplasia (RMH) or WDPM. To help differentiate between these diagnoses, we conducted a literature search for WDPM case reports or case series. Table 1 is a summary of the observations from the literature search. Table 2 compares the immunohistochemical findings from this case versus WDPM, RMH, and PSC cases detailed in the literature.
Table 1.
Well-differentiated papillary mesothelioma literature search.
| First author | Date | Median age | M/F | No. patients | Tumor site |
|---|---|---|---|---|---|
| Our case | 2019 | 68 | 0/1 | 1 | Omentum |
| Kim [4] | 2019 | 64 | 5/7 | 12 | Peritoneum, omentum, pelvic |
| Sun [5] | 2018 | 42 | 58/17 | 75 | Peritoneum, omentum, mesentery, Douglas pouch, ligament of the uterus, serosal surfaces of the ovary, fallopian tube, uterus, stomach, intestines, pleura, testicular tunica vaginalis, and inguinal hernia sac |
| Bazine [6] | 2017 | 36 | 1/0 | 1 | Peritoneum |
| Nasit [7] | 2014 | 28 | 0/1 | 1 | Peritoneum, omentum, urinary bladder |
| Irwin [8] | 2014 | 68 | 0/1 | 1 | Peritoneum, omentum |
| Washimi [9] | 2013 | 58 | 0/1 | 1 | Peritoneum |
| Ribeiro [10] | 2013 | 50 | 0/2 | 2 | Peritoneum, pleura |
| Malpica [11] | 2012 | 47 | 0/26 | 26 | Peritoneum, omentum, pelvis, fallopian tube, mesentery, cul-de-sac, colon, ovary, uterine serosa |
| Nemoto [12] | 2012 | 73 | 0/1 | 1 | Peritoneum, omentum, stomach, colon |
| Hatano [13] | 2012 | 45 | 1/0 | 1 | Peritoneum, omentum, stomach, colon |
| Ikeda [14] | 2010 | 73 | 0/1 | 1 | Peritoneum, omentum |
| Martinez-Consuegra [15] | 2008 | 46 | 0/1 | 1 | Peritoneum |
| Hoekstra [16] | 2005 | 74 | 0/1 | 1 | Peritoneum |
| Haba [17] | 2003 | 44 | 0/1 | 1 | Peritoneum |
| Diaz [18] | 2002 | 41 | 0/1 | 1 | Peritoneum, pelvis |
| Hoekman [19] | 1996 | 36 | 0/3 | 3 | Peritoneum |
| Daya [20] | 1990 | 41 | 4/18 | 22 | Omentum, pelvis |
| Lovell [21] | 1990 | 11 | 0/1 | 1 | Peritoneum |
| First Author | Other Conditions | Tumor size | Immunostains | Calcification | Psammomatous Masses |
|---|---|---|---|---|---|
| Our Case | Past history includes benign breast mass, hysterectomy due to leiomyoma, appendectomy | 8.5×6.5×1.8 cm | +Calretinin, +PAX8, +EMA, +vimentin, −CK903 | Yes | Yes |
| Kim [4] | Cecal carcinoma, uterine leiomyoma, hepatocellular carcinoma, gastric GIST, uterine cervical carcinoma, duct carcinoma, gastric carcinoma, colorectal carcinoma | 0.1–3 cm | 100% +calretinin, 28.6% +PAX8, 100% focally +EMA, 91.7% +CK5/6 | NO | NO |
| Sun [5] | 52% of WDPM lesions found during surgery for uterine leiomyoma, ovarian cysts endometriosis of ovary, caesarean delivery, uterine cervix carcinoma, endometrial carcinoma, endometrioid adenocarcinoma, pelvic endometriosis, ovarian teratoma, granulosa cell tumor of ovary. 47% found during surgery for lesions such as gastrointestinal stromal tumor, inguinal hernia, scrotal nodule, lung cancer | 0.2–6 cm, <2 cm in 80% of the pure WDPM | 100% +calretinin, 94% +PAX8, 35% +EMA | NR | NR |
| Bazine [6] | NR | 5 mm | +Calretinin | NR | NR |
| Nasit [7] | NR | 0.8–7.8cm | +Calretinin | NO | NO |
| Irwin [8] | Gastric cancer | NR | +calretinin | NR | Occasional |
| Washimi [9] | Rectal carcinoid | 5 mm (2004), 2cm in 2011 | +Calretinin, +EMA | NR | NR |
| Ribeiro [10] | Pleural diabetes, corneal ulcers | NR | +Calretinin (in one case), +BAP-1 | NR | NR |
| Malpica [11] | Pancreatic carcinoma, gallbladder carcinoma, urachal carcinoma, inguinal hernia, paraovarian cyst, ovarian serous cystadenofibroma, ovarian Mullerian tumor, colonic carcinoma, leiomyoma | 0.1–2 cm | 100% +calretinin, 3.8% of cases only 42% of cases had data | 3.8% | |
| Nemoto [12] | NR | 5 cm | +Calretinin | NR | NR |
| Hatano [13] | Adenomatoid tumor | 2.4 cm | +Vimentin, +calretinin, −EMA | NR | NR |
| Ikeda [14] | NR | NR | +Calretinin | NR | NR |
| Martinez-Consuegra [15] | Cholecystitis | 5 mm to 1.0 cm | +Calretinin | NR | Some |
| Hoekstra [16] | Ovarian serous cystadenoma, hepatic lesions | <1 cm peritoneum | +Calretinin | Yes | NR, 22% in lit. search |
| Haba [17] | Adenomyosis, hypermenorrhea | 0.5–2.0 cm | NR | NR | NR |
| Diaz [18] | Ovarian cyst | 5.0 cm | +Calretinin | NR | NR |
| Hoekman [19] | Appendicitis, hepatic lesion | NR | 100% +Keratin, 100% +vimentin, 33%+ EMA, 66% −EMA | NR | NR |
| Daya [20] | Small tumor foci on the ovarian surfaces, in addition to other tumor nodules in abdomen, ovarian masses, ovarian endometrioid carcinoma, benign cystic teratoma | 0.5–2.0 cm | NR | 4.5% of cases | 22% of cases |
| Lovell [21] | NR | NR | NR | Yes | Yes |
NR – not reported; NO – not observed
Table 2.
Immunohistochemical comparison for this case versus WDPM, RMH, and PSC.
| Antibody | Our case | WDPM | RMH | PSC |
|---|---|---|---|---|
| Calretinin | Positive | 100% positive [4–16,18] | 96% positive [22] | 0–38% positive [23], usually negative [5] |
| PAX8 | Positive | 29 to 94% positive [4,5] | 55% positive [24] | Mostly positive [23] |
| EMA | Positive (focally) | 35% positive [5], positive [4,5,7,9,18,19], negative [13] | 0% positive, [22,25], 20% positive [26] | Usually positive [27] |
| Vimentin | Focally positive | 100% positive [13,19] | Positive [25] | Usually positive [28] |
| Cytokeratin | CK5/6: negative | CK5/6: 92–93% positive [4,5], positive [6 9–11,15,18], negative [7, 16] | CK5/6: positive [25] | CK5/6: 22–35% positive [23] |
| BAP1 | Variable but moderately positive | 100% positive [29] 100% positive* [30], positive [10] |
86% positive [31] | 100% positive [24], 99.7% positive [32] |
| ER | Focally positive | Usually negative [33], negative [7, 8] | NA | 60–93% positive [23] |
| D2-40 | Negative | Positive [4,5,9,13,14] | Positive [25] | Negative [34], 23.2% positive [35] |
| CD56 | Minimal positivity | NA | Negative [25], 100% negative [36] | 33% positive** [37] |
WDPM – well-differentiated papillary mesothelioma; RMH – reactive mesothelial hyperplasia; PSC – papillary serous carcinoma.
Pure WDPM;
serous borderline tumors.
The patient tolerated surgery well and recovered quickly, reporting complete relief of her symptoms at her 5-month follow-up appointment. She was worried about the inconclusive pathology; therefore, a referral was made for an expert opinion, which also favored benign pathology (WDPM).
Discussion
Peritoneal and omental calcifications are broadly grouped into metastatic or heterotopic/dystrophic categories. Metastatic calcification is caused by systemic mineral imbalances throughout the body, whereas dystrophic calcification can arise from dead or damaged tissues as a result of injury, surgery, aging, or inflammation. It can also be associated with diseases, including infectious pathogens or malignancies (paraneoplastic) [1,2]. In either case, calcium phosphate minerals are deposited in soft tissues of the body. Certain medical treatments, such as abdominal surgery, can also lead to the development of these calcified masses. Typically, small and asymptomatic calcifications are identified by CT scans of the region.
Large calcified masses that cause symptoms, such as abdominal discomfort, pain, bloating, appetite changes, or a feeling of fullness, are commonly indicative of a malignant etiology. Calcification of nodules within organs, rather than in the form of a thin lining of vessels or cavities, can also be a sign of malignancy [3]. The most common diseases associated with malignant cases are ovarian cancer and tumors of the mesothelial or sub-mesothelial layers of the peritoneum. These include primary papillary serous peritoneal carcinoma or malignant mesothelioma [38].
In women with normal-sized ovaries, carcinomatosis in the peritoneum may occur due to serous papillary carcinoma of the surface of the ovary [39], or primary serous papillary carcinoma of the peritoneum. In addition, when differentiating between types of fat-containing tumors within the abdomen, the presence of calcified soft tissue can be a diagnostic clue. Synchronous fatty and calcified tissue can occur in teratomas, and, in rare cases, as calcification of lipomas [40]. Calcification is also occasionally associated with WDPM, which is usually considered a tumor of no-to-low malignant potential. This differentiation is made more difficult by an identification that is often ancillary [38,41].
WDPM is rare and distinct from malignant mesothelioma based on clinicopathology [19,20,38,42]. WDPM most commonly occurs in women, spanning a wide age range, but usually occurs during the reproductive years. Primarily arising from the peritoneal surfaces of the abdomen and pelvis, WDPM can also occur in the pleura, pericardium, and tunica vaginalis [5,38,43]. Clinically, it is often discovered incidentally during pelvic examination or surgery. Imaging features of WDPM are not well documented; however, peritoneal thickening, multiple peritoneal nodules (occasionally calcified), omental infiltration, and ascites have all been reported [44,45]. In contrast to mesothelioma, WDPM is not commonly associated with asbestos exposure [5,38]. The well-formed papillary architecture of WDPM superficially spreads and is lined by single layers of bland, cuboidal, or flattened mesothelial cells with little to no nuclear atypia, usually without mitoses [5,38]. In some instances of WDPM, psammomatous bodies [8,11,15,16,20,21] and invasive foci [5,6] have been reported. In a recent study of 75 patients with WDPM [5], the affected areas/nodules were generally less than 2 cm in diameter but ranged from 6 mm to 6.0 cm in diameter. Lesions greater than 2 cm were commonly hybrid tumors composed of WDPM combined with an adenomatoid tumor or multicystic mesothelioma [5].
Where complete resection is possible, the prognosis is usually good. Typically, this includes an indolent post-surgical course and long/unaffected survival [38,44]. However, based on the potential for recurrence or the risk of misdiagnosis of an under-sampled malignant mesothelioma, follow-up imaging is strongly recommended. There are rare case reports of WDPM progressing to true malignant peritoneal mesothelioma, which highlights the value of genetic (e.g., molecular) analysis of neoplasms in general, and WDPM/mesothelial proliferations in particular [11,12,41,45].
We put forward three possible explanations for the phenomenon observed in the pathology slides: RMH showing peculiar psammomatous calcifications, multiple microscopic foci of benign WDPM, or the emergence of a low-grade serous papillary carcinoma of the peritoneum. Differentiating WDPM from other histologically similar disease processes in the peritoneum, such as serous papillary carcinoma and borderline RMH, can be difficult, but the identification can be aided by immunohistochemistry. In contrast to WDPM, RMH commonly has reactive alterations, inflammatory alterations, or both in adjacent serosa. It is also associated with a history of immune, cardiovascular, inflammatory, or toxic diseases [5]. Sun et al. [5] reported that desmin may be a useful marker to differentiate RMH from WDPM. Only 1 (2.6%) of their WDPM cases showed positive focal staining for desmin, which has been shown in different studies to be more commonly positive in RMH [5,26,46].
In our case, focal EMA suggested a diagnosis of WDPM over RMH, as it is present in a higher percentage of cases of WDPM [4,5,7,9,18] than RMH [5,22,25,26]. Additionally, according to a study by Hoekman et al., EMA was focally present in all 3 of the cases of WDPM examined [19]. In a 2017 study by Nautiyal et al. [22], EMA was negative in all 11 RMH cases tested. Positive PAX8 staining is also more likely in WDPM [5,24], but also occurs in many RMH cases, rendering it of little use as a diagnostic tool in this case. Likewise, the presence of vimentin [13,19,25] and BAP-1 [10,29–31] could indicate either diagnosis, whereas the lack of D2-40 or CK5/6 and the presence of ER are counter to the usual immunohistochemistry of both WDPM and RMH.
Because the positive staining for EMA [4,5,7,9,18,19,27], vimentin [13,19,28], and BAP-1 [10,24,29,30,32] observed in our case is indicative of both WDPM and serous papillary carcinoma (Table 2), it did not assist our differential diagnosis. PAX8 has also been shown to be less useful in differentiating WDPM from serous papillary carcinoma due to crossover of the immunophenotypic patterns of PAX8 [5]. However, the presence of calretinin, as in our case, is much more likely in WDPM [4–16,18] and occurs in a smaller percentage of cases of serous papillary carcinoma [5,23] (Table 2). Interestingly, the calcification in our case was positive for ER, which occurs commonly in serous papillary carcinoma but generally not in WDPM [23,33]. In addition, the lack of D2-40 or CK5/6 would indicate serous papillary carcinoma over WDPM [4,5,34,35,37]. The minimal positivity for CD56 in our case also points towards PSC [37] versus WDPM or RMH [36] (Table 2). This case highlights the importance of the described stain selections. If a more limited diagnostic immunostain panel had been chosen in the current case, especially considering the patient’s clinical course, the ER, PAX-8, and BAP1 positivity in particular may have led an investigator toward a diagnosis of a PSC, potentially significantly affecting clinical care.
A combination of baseline cytomorphologic, immunohistochemical and other special stains, along with a genetic analysis are generally required to sort through this differential, often requiring the services of an expert who sees a significant number of these cases in their primary or expert consultancy practices. Even then, the morphologic and immunohistochemical appearance may not allow for such distinctions. Ongoing observation of the patient, combined with a radiographic survey for potential primary tumors, may be required, while noting, as above, that malignant neoplasms of these types may arise primarily in the peritoneal lining.
In the present patient’s case, positive staining for PAX8, EMA, and vimentin showed characteristics of carcinomas of mesenchymal origin from thyroid, urinary, reproductive, or kidney organ systems. However, the immunohistochemistry (Table 2) and characteristics of the calcifications did not rule out the potential for other diagnoses. To reliably determine the possibility of a malignant lesion, it was necessary to examine the tissue for tumor cell invasion. When this was not observed during the examination, it supported the benign diagnosis. While performing this examination, it was imperative to note any rare occurrences of invasive-appearing benign tumors, particularly after surgery or following any other interventional procedures [23,47].
Conclusions
A large fatty and calcified mass was located and excised from the abdominal cavity of a post-menopausal woman with a family history of nondescript thyroid disease, multiple abdominal surgeries, and a personal 10-year history of chronic omental calcification. The mass was removed only after she developed symptoms of recurrent diffuse abdominal pain. The large size of the mass, along with the symptoms of abdominal discomfort, raised the concern that the lesion was a new malignancy rather than one associated with benign calcification. Although immunostaining of the calcified adipose tissue revealed an expression pattern akin to papillary carcinoma, it did not rule out the possibility of other diagnoses. We suggest that these calcifications are a benign, reactive phenomenon and that the abundance of psammoma bodies may be related to ongoing crops of papillary mesothelial hyperplasia and/or benign well-differentiated papillary mesothelioma.
Acknowledgments
We want to thank the dedicated staff at Jewish Hospital-Mercy Health. Special thanks to Dr. Anna Krapivina, who proof-read this manuscript and generously gave technical advice. Thanks also to Beth Wayne, Clinical Research Educator at the Jewish Hospital, for proofreading this manuscript and Lisa McCormick, Hospital Librarian, for help finding and retrieving literature.
Abbreviations
- RMH
reactive mesothelial hyperplasia;
- WDPM
well-differentiated papillary mesothelioma;
- CK903
high-molecular-weight keratin;
- EMA
epithelial membrane antigen;
- PAX8
paired box immunostain 8;
- CT
computed tomography;
- EMM
epithelioid malignant mesothelioma;
- PSC
papillary serous carcinoma,
- PMM
peritoneal malignant mesothelioma.
References:
- 1.Cheng JM, Tirumani SH, Kim KW, et al. Malignant abdominal rocks: Where do they come from? Cancer Imaging. 2013;13(4):527–39. doi: 10.1102/1470-7330.2013.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Yeh BM, Breiman RS, et al. Peritoneal calcification: Causes and distinguishing features on CT. Am J Roentgenol. 2004;182(2):441–45. doi: 10.2214/ajr.182.2.1820441. [DOI] [PubMed] [Google Scholar]
- 3.Wojcik G, Piskorz J, Bulikowski W. Massive peritoneal cavity calcification in the course of advanced ovarian cancer: A case report. Prz Menopauzalny. 2015;14(2):149–51. doi: 10.5114/pm.2015.52156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim M, Kim HS. Clinicopathological characteristics of well-differentiated papillary mesothelioma of the peritoneum: A single-institutional experience of 12 cases. In Vivo. 2019;33(2):633–42. doi: 10.21873/invivo.11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun M, Zhao L, Weng Lao I, et al. Well-differentiated papillary mesothelioma: A 17-year single institution experience with a series of 75 cases. Ann Diagn Pathol. 2019;38:43–50. doi: 10.1016/j.anndiagpath.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Bazine A, Fetohi M, Namad T, et al. A case of well-differentiated papillary mesothelioma of the male peritoneum: Successful treatment by systemic chemotherapy. Cureus. 2017;9(3):e1104. doi: 10.7759/cureus.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasit JG, Dhruva G. Well-differentiated papillary mesothelioma of the peritoneum: A diagnostic dilemma on fine-needle aspiration cytology. Am J Clin Pathol. 2014;142(2):233–42. doi: 10.1309/AJCPOTO9LBB4UKWC. [DOI] [PubMed] [Google Scholar]
- 8.Irwin GW, Ervine A, Kennedy JA. Well-differentiated papillary mesothelioma: Peritoneal implants are not always metastases in the presence of cancer. Scott Med J. 2014;59(1):e18–21. doi: 10.1177/0036933013519030. [DOI] [PubMed] [Google Scholar]
- 9.Washimi K, Yokose T, Amitani Y, et al. Well-differentiated papillary mesothelioma, possibly giving rise to diffuse malignant mesothelioma: A case report. Pathol Int. 2013;63(4):220–25. doi: 10.1111/pin.12053. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro C, Campelos S, Moura CS, et al. Well-differentiated papillary mesothelioma: Clustering in a Portuguese family with a germline BAP1 mutation. Ann Oncol. 2013;24(8):2147–50. doi: 10.1093/annonc/mdt135. [DOI] [PubMed] [Google Scholar]
- 11.Malpica A, Sant’Ambrogio S, Deavers MT, Silva EG. Well-differentiated papillary mesothelioma of the female peritoneum: A clinicopathologic study of 26 cases. Am J Surg Pathol. 2012;36(1):117–27. doi: 10.1097/PAS.0b013e3182354a79. [DOI] [PubMed] [Google Scholar]
- 12.Nemoto H, Tate G, Kishimoto K, et al. Heterozygous loss of NF2 is an early molecular alteration in well-differentiated papillary mesothelioma of the peritoneum. Cancer Genet. 2012;205(11):594–98. doi: 10.1016/j.cancergen.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Hatano Y, Hirose Y, Matsunaga K, et al. Combined adenomatoid tumor and well differentiated papillary mesothelioma of the omentum. Pathol Int. 2011;61(11):681–85. doi: 10.1111/j.1440-1827.2011.02720.x. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda K, Suzuki T, Tate G, Mitsuya T. Cytomorphologic features of well-differentiated papillary mesothelioma in peritoneal effusion: A case report. Diagn Cytopathol. 2008;36(7):512–15. doi: 10.1002/dc.20824. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Consuegra N, Munoz-Juarez M, Ortiz-Hidalgo C. Unusual multifocal glomeruloid pattern in a well-differentiated papillary mesothelioma of the peritoneum. Int J Surg Pathol. 2008;16(4):426–27. doi: 10.1177/1066896908318450. [DOI] [PubMed] [Google Scholar]
- 16.Hoekstra AV, Riben MW, Frumovitz M, et al. Well-differentiated papillary mesothelioma of the peritoneum: A pathological analysis and review of the literature. Gynecol Oncol. 2005;98(1):161–67. doi: 10.1016/j.ygyno.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Haba T, Wakasa K, Sasaki M. Well-differentiated papillary mesothelioma in the pelvic cavity. A case report. Acta Cytol. 2003;47(1):88–92. doi: 10.1159/000326481. [DOI] [PubMed] [Google Scholar]
- 18.Diaz LK, Okonkwo A, Solans EP, et al. Extensive myxoid change in well-differentiated papillary mesothelioma of the pelvic peritoneum. Ann Diagn Pathol. 2002;6(3):164–67. doi: 10.1053/adpa.2002.33902. [DOI] [PubMed] [Google Scholar]
- 19.Hoekman K, Tognon G, Risse EK, et al. Well-differentiated papillary mesothelioma of the peritoneum: A separate entity. Eur J Cancer. 1996;32A(2):255–58. doi: 10.1016/0959-8049(95)00574-9. [DOI] [PubMed] [Google Scholar]
- 20.Daya D, McCaughey WT. Well-differentiated papillary mesothelioma of the peritoneum. A clinicopathologic study of 22 cases. Cancer. 1990;65(2):292–96. doi: 10.1002/1097-0142(19900115)65:2<292::aid-cncr2820650218>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Lovell FA, Cranston PE. Well-differentiated papillary mesothelioma of the peritoneum. Am J Roentgenol. 1990;155(6):1245–46. doi: 10.2214/ajr.155.6.2122674. [DOI] [PubMed] [Google Scholar]
- 22.Nautiyal N, Bhardwaj A, Acharya S, et al. Diagnostic utility of epithelial membrane antigen (EMA) and calretinin (CAL) in effusion cytology. J Clin Diagn Res. 2017;11(5):EC36–39. doi: 10.7860/JCDR/2017/24339.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husain AN, Colby TV, Ordonez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2018;142(1):89–108. doi: 10.5858/arpa.2017-0124-RA. [DOI] [PubMed] [Google Scholar]
- 24.Chapel DB, Husain AN, Krausz T, McGregor SM. PAX8 expression in a subset of malignant peritoneal mesotheliomas and benign mesothelium has diagnostic implications in the differential diagnosis of ovarian serous carcinoma. Am J Surg Pathol. 2017;41(12):1675–82. doi: 10.1097/PAS.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 25.Terada T. Immunohistochemical profile of normal mesothelium and histiocytic/methothelial hyperplasia: A case report. Int J Clin Exp Pathol. 2011;4(6):631–36. [PMC free article] [PubMed] [Google Scholar]
- 26.Attanoos R, Griffin A, Gibbs A. The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium. A novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet- derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology. 2003;43(3):231–38. doi: 10.1046/j.1365-2559.2003.01686.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaspar HG, Crum CP. The utility of immunohistochemistry in the differential diagnosis of gynecologic disorders. Arch Pathol Lab Med. 2015;139(1):39–54. doi: 10.5858/arpa.2014-0057-RA. [DOI] [PubMed] [Google Scholar]
- 28.Desouki MM. Serous carcinoma. PathologyOutlines.com, Inc; 2019. [cited 2019 September 7]; Available from: http://www.pathologyoutlines.com/topic/uterusserous.html. [Google Scholar]
- 29.Stevers M, Rabban JT, Garg K, et al. Well-differentiated papillary mesothelioma of the peritoneum is genetically defined by mutually exclusive mutations in TRAF7 and CDC42. Mod Pathol. 2019;32(1):88–99. doi: 10.1038/s41379-018-0127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HE, Molina JR, Sukov WR, et al. BAP1 loss is unusual in well-differentiated papillary mesothelioma and may predict development of malignant mesothelioma. Hum Pathol. 2018;79:168–76. doi: 10.1016/j.humpath.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol. 2015;28(8):1043–57. doi: 10.1038/modpathol.2015.65. [DOI] [PubMed] [Google Scholar]
- 32.Andrici J, Jung J, Sheen A, et al. Loss of BAP1 expression is very rare in peritoneal and gynecologic serous adenocarcinomas and can be useful in the differential diagnosis with abdominal mesothelioma. Hum Pathol. 2016;51:9–15. doi: 10.1016/j.humpath.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Ordonez NG. Value of estrogen and progesterone receptor immunostaining in distinguishing between peritoneal mesotheliomas and serous carcinomas. Hum Pathol. 2005;36(11):1163–67. doi: 10.1016/j.humpath.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Hutton RL, Dalton SR. Primary peritoneal serous borderline tumors. Arch Pathol Lab Med. 2007;131(1):138–44. doi: 10.5858/2007-131-138-PPSBT. [DOI] [PubMed] [Google Scholar]
- 35.Nofech-Mozes S, Khalifa MA, Ismiil N, et al. Immunophenotyping of serous carcinoma of the female genital tract. Modern Pathol. 2008;21(9):1147–55. doi: 10.1038/modpathol.2008.108. [DOI] [PubMed] [Google Scholar]
- 36.Kentrou NA, Tsagarakis NJ, Tzanetou K, et al. An improved flow cytometric assay for detection and discrimination between malignant cells and atypical mesothelial cells, in serous cavity effusions. Cytometry B Clin Cytom. 2011;80(5):324–34. doi: 10.1002/cyto.b.20608. [DOI] [PubMed] [Google Scholar]
- 37.Bosmuller HC, Wagner P, Pham DL, et al. CD56 (neural cell adhesion molecule) expression in ovarian carcinomas: Association with high-grade and advanced stage but not with neuroendocrine differentiation. Int J Gynecol Cancer. 2017;27(2):239–45. doi: 10.1097/IGC.0000000000000888. [DOI] [PubMed] [Google Scholar]
- 38.Levy AD, Arnaiz J, Shaw JC, Sobin LH. From the archives of the AFIP: Primary peritoneal tumors: imaging features with pathologic correlation. Radiographics. 2008;28(2):583–607. doi: 10.1148/rg.282075175. quiz 621–22. [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Kim JK, Cho KS. CT features of serous surface papillary carcinoma of the ovary. Am J Roentgenol. 2004;183(6):1721–24. doi: 10.2214/ajr.183.6.01831721. [DOI] [PubMed] [Google Scholar]
- 40.Shin NY, Kim MJ, Chung JJ, et al. The differential imaging features of fat-containing tumors in the peritoneal cavity and retroperitoneum: The radiologic-pathologic correlation. Korean J Radiol. 2010;11(3):333–45. doi: 10.3348/kjr.2010.11.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costanzo L, Scarlata S, Perrone G, et al. Malignant transformation of well-differentiated papillary mesothelioma 13 years after the diagnosis: A case report. Clin Respir J. 2014;8(1):124–29. doi: 10.1111/crj.12057. [DOI] [PubMed] [Google Scholar]
- 42.Goepel JR. Benign papillary mesothelioma of peritoneum: A histological, histochemical and ultrastructural study of six cases. Histopathology. 1981;5(1):21–30. doi: 10.1111/j.1365-2559.1981.tb01763.x. [DOI] [PubMed] [Google Scholar]
- 43.Churg A, Cagle PT, Roggli VL. Tumors of the serosal membranes. Amer Registry of Pathology. 2006 [Google Scholar]
- 44.McLaughlin PD, Filippone A, Maher MM. Neoplastic diseases of the peritoneum and mesentery. Am J Roentgenol. 2013;200(5):W420–30. doi: 10.2214/AJR.12.8494. [DOI] [PubMed] [Google Scholar]
- 45.Park JY, Kim KW, Kwon HJ, et al. Peritoneal mesotheliomas: Clinicopathologic features, CT findings, and differential diagnosis. Am J Roentgenol. 2008;191(3):814–25. doi: 10.2214/AJR.07.3628. [DOI] [PubMed] [Google Scholar]
- 46.Kawai T, Tominaga S, Hiroi S, et al. Peritoneal malignant mesothelioma (PMM), and primary peritoneal serous carcinoma (PPSC) and reactive mesothelial hyperplasia (RMH) of the peritoneum. Immunohistochemical and fluorescence in situ hybridisation (FISH) analyses. J Clin Pathol. 2016;69(8):706–12. doi: 10.1136/jclinpath-2015-203211. [DOI] [PubMed] [Google Scholar]
- 47.Churg A, Galateau-Salle F. The separation of benign and malignant mesothelial proliferations. Arch Pathol Lab Med. 2012;136(10):1217–26. doi: 10.5858/arpa.2012-0112-RA. [DOI] [PubMed] [Google Scholar]