Abstract
Background
S100 calcium-binding protein A16 (S100A16) is closely related to the onset and progression of tumors.
Material/Methods
In the research, the mainly purpose was to investigate the effect of S100A16 on the proliferation ability, invasion, and angiogenesis of HeLa cells. An adenoviral vector overexpressing S100A16 (Ad-S100A16) was constructed and transfected into HeLa cells, forming a stable cells line of overexpression. The effect of S100A16 on the proliferative capacity of HeLa cells was evaluated by a Cell Counting Kit-8 (CCK-8) assay. Cell migration capacity was determined by a Transwell migration assay. Changes in matrix metalloproteinase-2 (MMP-2), MMP-9, E-cadherin, and vimentin expression were evaluated by a cell-based immunofluorescence assay. The effect of S100A16 on angiogenesis was verified by knockout experiment.
Results
Overexpression of S100A16 significantly enhanced the proliferative and migratory capacities of HeLa cells (P<0.05), upregulated expression of matrix MMP-2, MMP-9, vimentin, phosphatidylinositol 3 kinase, and phosphorylated protein kinase B, and downregulated expression of E-cadherin. Vascular endothelial growth factor expression increased, phosphatase and tensin homolog expression decreased, and angiogenesis was positively correlated with S100A16 expression. These effects were largely mediated by the activation of the phosphatidylinositol 3 kinase/protein kinase B pathways.
Conclusions
S100A16 could promote the proliferation, migration, and tumor angiogenesis of HeLa cells by regulating the phosphatidylinositol 3 kinase/protein kinase B signaling pathways.
MeSH Keywords: Cell Migration Assays, Cell Proliferation, Phosphatidylinositol 3-Kinases, Uterine Cervical Neoplasms
Background
Cervical cancer, one of the most frequent malignant tumors, poses a serious threat to women’s health worldwide [1]. It is currently believed that persistent high-risk human papillomavirus (HPV) infection is the underlying cause of precancerous cervical lesions and cervical cancer. HPV infection is a prerequisite for the onset of cervical cancer, but not all women with HPV infection develop cervical cancer, indicating that other factors might contribute to the development of cervical cancer [2,3]. The picture is further complicated by a dramatic gap in the therapeutic approaches, such as surgery and chemotherapy, often accompanied by side effects and complications. Thus, the identification of specific diagnostic and prognostic markers, and the search of new therapeutic targets for cervical cancer, both of which are of paramount importance.
S100 calcium-binding protein A16 (S100A16) is a member of the S100 calcium-binding protein family, which is prone to chromosomal rearrangements and instability, leading to malignant transformation of cells [4,5]. The increased expression of S100A16 protein in various tumor cells reflects its close association with the onset and progression of tumors [6–10]. S100A16 was participated the adjusting of various signaling pathways, like extracellular signal regulated kinase, Notch, and nuclear factor kappa B pathways. The study by Zhu et al. [11] showed that the overexpression of S100A16 promotes cancer cell proliferation and invasion by Akt and extracellular signal regulated kinase signaling pathways. Enhanced S100A16 expression has also been associated with the expression of Notch1 in MCF-7 breast cancer cells, thereby promoting the onset of epithelial-mesenchymal transition [12,13]. Epithelial-mesenchymal transition is associated with the onset of tumors and may contribute to the transformation of primary tumors into metastatic tumors via various steps, such as invasion, migration, extravasation, and colonization [14]. The downregulation of E-cadherin and the upregulation of vimentin enable the tumor cells to invade the basement membrane, thus leading to metastasis [15,16].
The phosphatidylinositol 3 kinase/protein kinase B (PI3K/Akt) signaling pathway controls various cellular events, such as cell apoptosis, cell cycle progression [17]. This pathway has a relationship on the progression of tumor cells and plays a vital role in malignant proliferation, invasion and chemotherapy resistance. Therefore, the PI3K/Akt signaling pathway is expected to become a value target for tumor treatment [18]. However, the relationship between S100A16 and PI3K/Akt signaling pathway in these cells have not yet been studied. For these reasons, we explored the overexpression, silencing of S100A16 and the mechanism on HeLa cell proliferation, invasion, and angiogenesis.
Material and Methods
Cell culture and adenovirus infection
HeLa cells came from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s Modified Eagle Medium, high glucose (Gibco) containing 10% fetal bovine serum (FBS; Gibco) at 37°C in a 5% CO2 incubator. Adherent cells were passaged after being grown to 80% to 90% confluence and routinely harvested for storage.
Ad-S100A16, harboring the S100A16 gene, and Ad-GFP, harboring the green fluorescent protein (GFP) gene, were constructed by Sangon Biotech Co., Ltd. (Shanghai). Adherent HeLa cells were passaged and transfected with Ad-GFP or Ad-S100 A16 after being grown to 50% to 60% confluence. GFP expression in each group was observed and recorded after 24 hours of transfection.
Real time-polymerase chain reaction
RNA of HeLa cells was extracted using RNAiso Plus (Takara). A reverse transcription-polymerase chain reaction kit (Takara) and SYBR Premix Ex Taq II kit (TaKaRa) were used to detect the expression of S100A16. The relative expression level of each gene was calculated using the formula: F=2−ΔΔCt. Primer sequences as follows:
S100A16: forward: 5′-AGCAGGGAGATGTCAGACTGCTACACGGA-3′;
reverse 5′-AGGTGTGGCCAAAGGGGTCTAGCTG-3′;
GAPDH: forward: 5′-AAGGCTGTGGGCAAGG-3′;
reverse: 5′-TGGA GGAGTG GGT GTCG-3′.
Cell Counting Kit-8 (CCK-8) assay
Hela cells grown to logarithmic (log) phase were collected and suspended in serum-free medium. The cell suspension was added to 96-well plates and transfected with Ad-GFP and Ad-S100A16, respectively. At 72 hours post-transfection, CCK-8 solution (Beyotime) (10 μL/well) was added to plates and incubated in the dark at incubator (37°C) for 2 hours prior to measuring the absorbance at 490 nm using a microplate reader. The optical density value represented the level of cell proliferation.
Transwell migration assay
The cells were inoculated at 1.5×105/well and transfected with Ad-S100A16 for 72 hours, after which, cell suspensions were prepared (1.5×104 cells/mL) using serum-free medium. Cell suspension (400 μL/well) and 600 μL of medium containing 20% FBS were added to the chamber, respectively. After being incubated for 24 hours, the Transwell chamber was subjected to fixation in anhydrous methanol at −20°C for 5 minutes, followed by staining with 0.25% crystal violet for 5 minutes. Excess crystal violet stain was washed off using phosphate-buffered saline prior to visualization and photography under a microscope.
Immunofluorescence
HeLa cells were fixed at 37°C for 20 minutes with 4% paraformaldehyde, permeabilized with 0.25% Triton for 15 minutes, and blocked with 5% bovine serum albumin for 30 minutes, followed by an overnight incubation at 4°C with primary antibodies (E-cadherin, vimentin, MMP-2, MMP-9, Abcam). Next, the cells were incubated with secondary antibodies for 1 hour and rinsed with phosphate-buffered saline (PBS) prior to visualization and photography under a fluorescence microscope. The resulting fluorescence intensity values were analyzed using ImageJ software.
Western blotting
Hela cells were mock-infected or infected with Ad-S100A16 and incubated for 72 hours with LY294002 (dimethyl sulfoxide solution, Abcam) and without LY294002, respectively. The cells were then lysed on ice with radio-immunoprecipitation assay buffer (RIPA, Beyotime) and centrifuged at 13 000 g for 10 minutes to harvest the supernatant. Next, protein samples subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. The protein was transferred onto a membrane, which was then blocked prior to an overnight incubation with primary antibodies at 4°C. After being rinsed with phosphate-buffered saline, the membrane was incubated with secondary antibodies for 1.5 hours. The membrane was then rinsed again with phosphate-buffered saline, and protein bands were detected via enhanced chemiluminescence. Finally, the membrane was photographed, and the resulting D-values were analyzed using ImageJ software.
Gene silence model
HeLa cells (4×106) were washed with 1 mL serum-free medium and added to 6-well plates for 24 hours. Cells was infected with adenovirus expression siRNA S100A16. The transfection mixture was incubated with transfection reagent according to manufacturer’s instruction and added to the wells. The media was changed following 24 hours of incubation. After 72 hours, the stable cell line was treated with puromycin for 96 hours.
Angiogenesis experiments
Matrigel was cooled to 4°C, diluted 1: 4 with serum-free culture medium, and 60 μL/well of this dilution was added to a 96-well plate and incubated at 37°C for 1 hour to allow gel formation. After gel formation, the serum-free culture medium was used to prepare a single-cell suspension at a density of 5×105 cells/mL, and then added to plates. The 96-well plates were incubated for 24 hours and then imaged.
Statistical methods
Data were analyzed using SPSS 21.0 statistical software. The measurement and categorical data were expressed as mean±standard deviations (SD). Comparison between multiple groups was conducted using one-way ANOVA, while pairwise comparisons were assessed via Fisher’s least significant difference test. P-values <0.05 were indicated statistically significant.
Results
S100A16 promotes the proliferation, migration and angiogenesis of HeLa cells
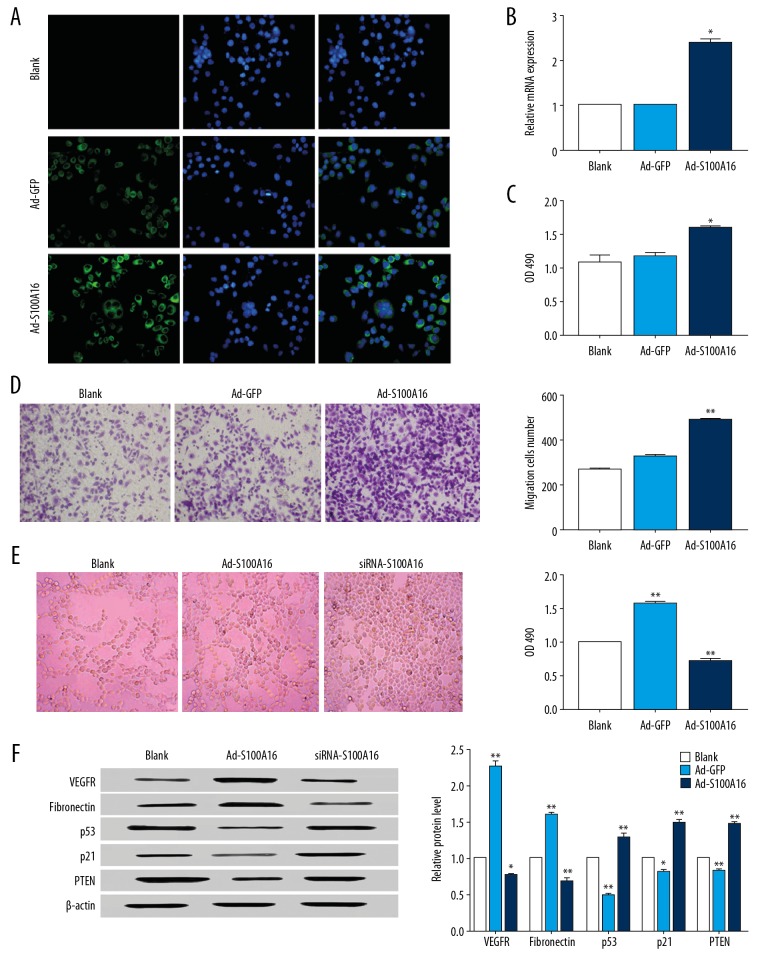
No green fluorescence was detected in the control group, while Ad-GFP and Ad-S100A16-infected cells exhibited relatively intense intracellular green fluorescence. Fluorescence intensity of Ad-S100A16 was higher than Ad-GFP. S100A16 was expressed in HeLa cells, and expression levels was increased after infection of recombinant adenovirus, which yielded a suitable overexpression model for the investigation of S100A16 function (Figure 1A, 1B).
Figure 1.
S100A16 promotes the proliferation of HeLa cells. (A) Determination of the expression of S100A16 after Ad-S100A16 adenovirus. (B) Analysis of the transfection efficiency in Ad-S100A16-transfected HeLa cells. (C) Proliferation capacity of HeLa cells. (D) The migration capacity of HeLa cells. (E) Angiogenesis and cell proliferation assay of HeLa cells. (F) Western blot for vascular endothelial growth factor, fibronectin, p53, p21, and tensin homolog. All results were subjected to statistical analyses and expressed as mean±standard deviation. * P<0.05 and ** P<0.01. All experiments were carried out in triplicates.
After a 72-hour transfection with Ad-S100A16, HeLa cells exhibited a significantly higher proliferative capacity compared to the blank control (P<0.05, Figure 1C), indicating a positive effect of S100A16 on the proliferation of HeLa cells. Transwell migration assay showed a 2-fold increase in cell migration in Ad-S100A16-transfected cells compared to the control (P<0.05), suggesting that S100A16 was able to promote cell migration (Figure 1D).
Compared with the negative control, Ad-S100A16 cells showed better angiogenesis ability, while siRNA-S100A16 cells showed more disorder (Figure 1E). Moreover, cell proliferation decreased after siRNA-S100A16 treatment (P<0.01), expression of epithelial-mesenchymal transition-related vascular endothelial growth factor and fibronectin decreased, the expression of tensin homolog increased (Figure 1F). This suggests that S100A16 is involved in angiogenesis, and that the absence of S100A16 creates significant regulatory obstacles to these processes.
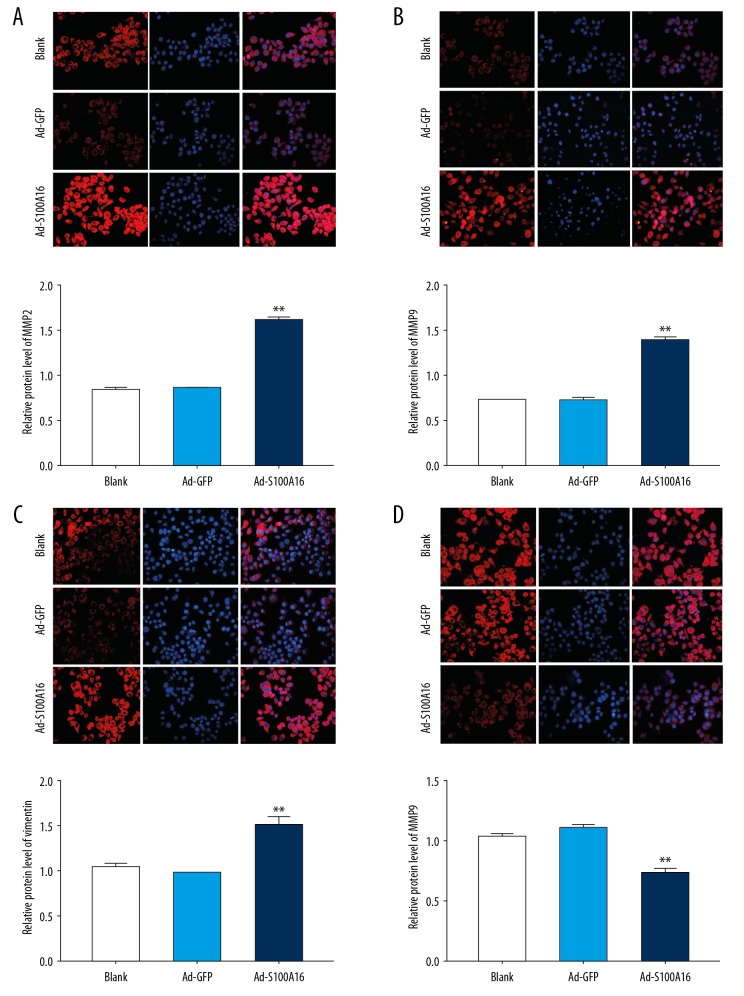
Effects of S100A16 on MMP-2, MMP-9, E-cadherin, and vimentin expression
MMP-2 and MMP-9 expression was analyzed by immunofluorescence. Both proteins showed significantly higher Ad-S100 A16-transfected cells than in the control (Figure 2A, 2B). E-cadherin and vimentin expression was also analyzed in order to verify possible effects of S100A16 on invasion and metastasis. Upregulation of S100A16 increased vimentin expression (Figure 2C) and decreased E-cadherin expression (Figure 2D), suggesting involvement of S100A16 in epithelial-mesenchymal transition induction in HeLa cells.
Figure 2.
S100A16 significantly upregulated in matrix metalloproteinase (MMP)-2, MMP-9, and vimentin expression and significantly downregulated E-cadherin expression in HeLa cells. (A) MMP-2 protein level (40×). (B) MMP-9 protein level (40×). (C) Vimentin protein level (40×). (D) E-cadherin protein level (40×). All results were subjected to statistical analyses and expressed as mean±standard deviation. * P<0.05 and ** P<0.01. All experiments were carried out in triplicates.
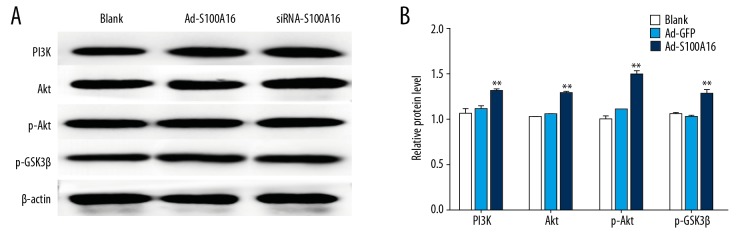
Effect of S100A16 on protein expression of PI3K, Akt, p-protein kinase B, and GSK-3β in HeLa cells
In order to verify the possible involvement of the PI3K/Akt signaling pathway caused by S100A16 on HeLa cell proliferation and migration, a western blot was employed to analyze protein levels of phosphatidylinositol 3 kinase, protein kinase B, phosphorylated protein kinase B, and glycogen synthase kinase 3β. Levels of phosphatidylinositol 3 kinase and protein kinase B, as well as the level of phosphorylated phosphorylated protein kinase B, were significantly higher in Ad-S100A16-infected cells than in control cells (P<0.05). Conversely, glycogen synthase kinase 3β activation was downregulated in Ad-S100A16-transfected cells (P<0.05, Figure 3). The results suggested that S100A16 activates the phosphatidylinositol 3 kinase/protein kinase B signaling pathway.
Figure 3.
(A, B) Expression levels of the PI3K, Akt, p-Akt, and p-GSK-3β in HeLa cells by western blot. All results were subjected to statistical analyses and expressed as mean±standard deviation. * P<0.05 and ** P<0.01. All experiments were carried out in triplicates.
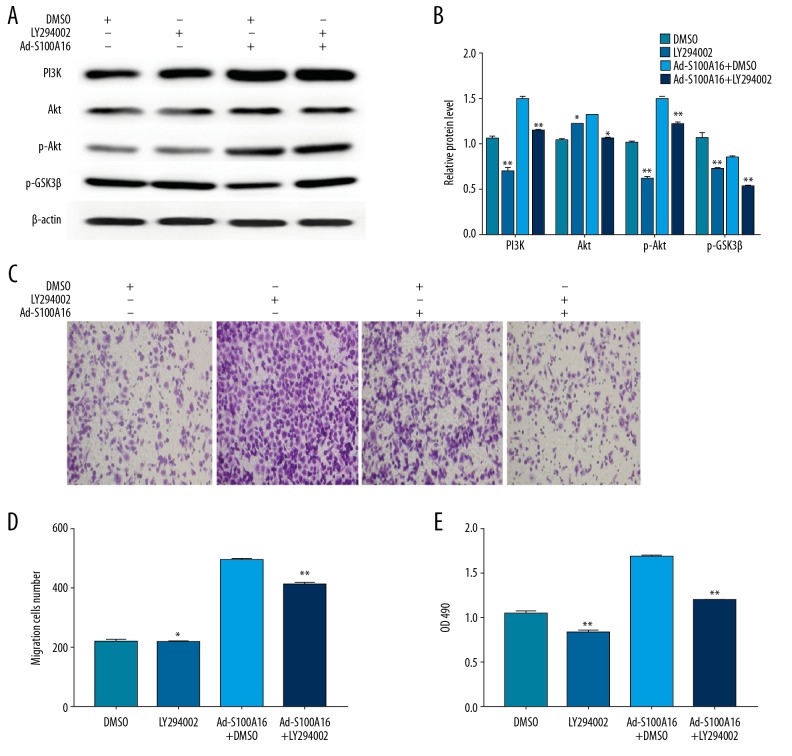
The phosphatidylinositol 3 kinase/protein kinase B pathway mediated the effects of S100A16 overexpression on cell proliferation and migration
In order to definitively establish the involvement of the phosphatidylinositol 3 kinase/protein kinase B signaling pathway and the effects induced by S100A16 on cell proliferation and migration, we treated HeLa cells with LY294002 (phosphatidylinositol 3 kinase inhibitor, 10 μM), alone or in combination with Ad-S100A16 infected cells (10 μg/mL). Inhibition of the phosphatidylinositol 3 kinase/protein kinase B signaling pathway by LY294002 caused a significant decline in phosphatidylinositol 3 kinase expression (P<0.01), p-Akt (P<0.05), and glycogen synthase kinase 3β (P<0.01) proteins (Figure 4A, 4B). In addition, LY294002 significantly attenuated S100A16-mediated proliferation and migration of HeLa cells (P<0.05, Figure 4C–4E). These results indicated that S100A16 promoted proliferation and migration of a cervical cancer-derived cell line by activating the phosphatidylinositol 3 kinase/protein kinase B signaling pathway.
Figure 4.
S100A16 affect HeLa cell proliferation and migration by activating the PI3K/Akt pathway. (A, B) Expression levels of PI3K, Akt, p-Akt, and p-GSK-3β in HeLa cells. (C, D) The migration of HeLa cells. (E) Cell proliferation. All results were subjected to statistical analyses and expressed as mean±standard deviation. * P<0.05 and ** P<0.01. All experiments were carried out in triplicates.
Discussion
The diagnosis and prognosis marker became the main research tendency due to the recurrence rate of cervical cancer. Serum levels of vascular endothelial growth factor [19], C-reactive protein [20], the ratio of serum angiopoietin-1 to angiopoietin-2 [21], and serum protein TKT, human fibrinogen FGA, metabolism-related protein [22], all of these can be a reliable marker for clinical treatment. Similarly, Lon peptidase 2 can be a potential therapeutic target, regulating oxidative damage and peroxidosome homeostasis, changing the proliferation and invasion of cancer cells [23].
S100A16 may be a value therapeutic and prognostic marker by promoting the anti-apoptotic effect mediated by protein kinase B/Bcl-2. Function analysis shown that silencing of S100A16 promoted proliferation, had an anti-apoptotic effect, and reduced chemosensitivity. S100A16 overexpression revealed the opposite trend. S100A16 is increasingly recognized as an oncogene with multiple roles in tumor onset [10,12]. Recently, researchers found that S100A16 can serve as an effective prognostic indicator for prostate cancer and colorectal cancer, as it is abnormal expressed no matter in cell and tissues, and it is related with the onset and progression of this this disease [9,24]. Further, S100A16 is highly expressed in breast cancer and induced epithelial-mesenchymal transition onset via the Notch1/zinc finger E-box binding homeobox (ZEB) pathway [12]. However, the effect of S100A16 on cervical cancer has not been reported. Our results suggested that S100A16 facilitates the metastasis of HeLa cells, it was consistent with previous studies investigating the effect of S100A16 on stomach, prostate, and breast cancer [9,12].
MMPs play key roles in tumor invasion and metastasis, as they are capable of disrupting the extracellular matrix (ECM) and vascular basement membrane of tumor cells, enabling these cells to invade the basement membrane, which leads to the migration, invasion, and spread of tumor cells [25,26]. In cervical cancer, research show the upregulation of MMP-2 and MMP-9 associated with lymphatic infiltration, as well as lymph node metastasis [27]. Overexpression of MMP-2 in cervical cancer promotes the infiltration and metastasis of cervical cancer, while MMP-9 directly induces onset of the epithelial-mesenchymal transition [28]. This is consistent with previous studies, suggesting that overexpression of S100A16 in HeLa cells promote the onset of epithelial-mesenchymal transition, thereby promoting the proliferation and invasion of tumor cells [8].
The phosphatidylinositol 3 kinase/protein kinase B signaling pathway may control various biological activities, such as cell proliferation, apoptosis, invasion, metastasis, and angiogenesis, and is closely associated with the onset, progression, and treatment of tumors [13]. Numerous researchers have found that in cervical cancer cells the phosphatidylinositol 3 kinase/protein kinase B pathway participated in the proliferation, invasion and differentiation [15,29,30]. ARHGAP17 can inhibit the progression of cervical cancer through PI3K/AKT pathway [31]. In the same way, microRNA-433 induced the HeLa apoptosis via PI3K/AKT signal [32]. PI3K/AKT is involved in regulation cisplatin-resistance in cervical cancer [33]. PI3K/AKT showed a huge value in prognosis and treatment. Upon activation by growth factors and hormones, intracellular PI3K triggers the conversion of phosphatidylinositol 4, 5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-trisphosphate and p-Akt to activate its downstream effectors. Such cascades control processes that are crucial for cancer onset, such as cell proliferation, transformation, and apoptosis [34]. Protein kinase B is a key molecule in phosphatidylinositol 3 kinase/protein kinase B signaling pathway, and its expression and activation level may vary in different cancer tissues. Activated protein kinase B exerts its anti-apoptotic effects by phosphorylating various downstream target molecules. GSK3β is a negative regulator of the cell motility via phosphorylation [35]. Recently, researches shown PI3K/AKT-meditated phosphorylation GSK3β and implicated in epidermal-mesenchymal transition. Blocking the PI3K/AKT activation significantly attenuated GSK3β phosphorylation [36]. For instance, protein kinase B-induced phosphorylation inactivates GSK-3β and leads to E-cadherin downregulation, thereby regulated cell motility [37]. Moreover, activated protein kinase B can enhance the transcriptional activity of nuclear factor kappa B and upregulate the expression of MMP-9, which also enables cell motility, facilitating tumor onset and cancer cell invasion [25]. Finally, protein kinase B upregulates MMP-2 via the phosphatidylinositol 3 kinase/protein kinase B/mammalian target of rapamycin pathway, thereby promoting cancer cell invasion and metastasis [30]. Our results showed that the use of LY294002 substantially reversed Ad-S100A16-induced protein kinase B phosphorylation, and proliferation and invasion of HeLa cells. Therefore, we believe that the promoting effects of S100A16 on HeLa cell division and motility are, at least partially, attributable to the activation of the PI3K/Akt signaling pathway.
Conclusions
S100A16 promoted the proliferation and invasion of cervical cancer cells, most likely as a result of epithelial-mesenchymal transition induction via the activation of the PI3K/Akt signaling pathway. Our study identified a potential target of future diagnostic and therapeutic strategies for cervical cancer.
Footnotes
Source of support: This study was supported by National Natural Science Foundation of China (Grant no. 81801419)
Availability of data and materials
The datasets used and analyzed during this study are available from the corresponding author on reasonable request.
Conflict of interest
None.
References
- 1.Ngelangel C, Muñoz N, Bosch FX, et al. Causes of cervical cancer in the Philippines: a case-control study. J Natl Cancer Inst. 1998;90(1):43–49. doi: 10.1093/jnci/90.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Ahn WS, Bae SM, Lee JM, et al. Searching for pathogenic gene functions to cervical cancer. Gynecol Oncol. 2004;93(1):41–48. doi: 10.1016/j.ygyno.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Panjković M, Ivković-Kapicl T. [Etiology and pathogenesis of precancerous lesions and invasive cervical carcinoma]. Med Pregl. 2008;61:364–68. doi: 10.2298/mpns0808364p. [in Serbian] [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Xu C, Jin Q, Liu Z. S100 protein family in human cancer. Am J Cancer Res. 2014;4(2):89–115. [PMC free article] [PubMed] [Google Scholar]
- 5.Katono K, Sato Y, Kobayashi M, et al. S100A16, a promising candidate as a prognostic marker for platinum-based adjuvant chemotherapy in resected lung adenocarcinoma. OncoTargets Ther. 2017;10:5273–79. doi: 10.2147/OTT.S145072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin F, Song Y, Li Z, et al. S100A8/A9 induces apoptosis and inhibits metastasis of CasKi human cervical cancer cells. Pathol Oncol Res. 2010;16(3):353–60. doi: 10.1007/s12253-009-9225-2. [DOI] [PubMed] [Google Scholar]
- 7.Sturchler E, Cox JA, Durussel I, et al. S100A16, a novel calcium-binding protein of the EF-hand superfamily. J Biol Chem. 2006;281(50):38905–17. doi: 10.1074/jbc.M605798200. [DOI] [PubMed] [Google Scholar]
- 8.Saito K, Kobayashi M, Nagashio R, et al. S100A16 is a prognostic marker for lung adenocarcinomas. Asian Pac J Cancer Prev. 2015;16(16):7039–44. doi: 10.7314/apjcp.2015.16.16.7039. [DOI] [PubMed] [Google Scholar]
- 9.Zhu W, Zhang R, Xue Y, et al. P0065 S100A16 promotes cell migration and invasion in prostate cancer in vitro via AKT and ERK cell signalling pathways. Eur J Cancer. 2014;50(4):e26–27. [Google Scholar]
- 10.Sapkota D, Bruland O, Parajuli H, et al. S100A16 promotes differentiation and contributes to a less aggressive tumor phenotype in oral squamous cell carcinoma. BMC Cancer. 2015;15:631. doi: 10.1186/s12885-015-1622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W, Xue Y, Liang C, et al. S100A16 promotes cell proliferation and metastasis via AKT and ERK cell signaling pathways in human prostate cancer. Tumour Biol. 2016;37(9):12241–50. doi: 10.1007/s13277-016-5096-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W, Pan H, Xia T, et al. Up-regulation of S100A16 expression promotes epithelial-mesenchymal transition via Notch1 pathway in breast cancer. J Biomed Sci. 2014;21:97. doi: 10.1186/s12929-014-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu X, Yang Y, Li Y, et al. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3K/Akt pathway. Int J Biochem Cell Biol. 2018;94:107–18. doi: 10.1016/j.biocel.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: Its role in tumour progression and response to therapy. Cancer Lett. 2015;356:321–31. doi: 10.1016/j.canlet.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clinical Cancer Res. 2008;14(15):4743–50. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Han L, Zhou C, et al. LTGF-β1-induced CK17 enhances cancer stem cell-like properties rather than EMT in promoting cervical cancer metastasis via the ERK1/2-MZF1 signaling pathway. FEBS J. 2017;284(18):3000–17. doi: 10.1111/febs.14162. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Wang T, Wang W, Wu Z. 4HPV16 E6/E7 induces EMT via Twist and promotes carcinogenesis and metastasis of cervical cancer. Gynecol Oncol. 2013;130(1):e52–53. [Google Scholar]
- 19.Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: A review of the literature. Crit Rev Oncol Hematol. 2008;66(1):10–20. doi: 10.1016/j.critrevonc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Bodner-Adler B, Kimberger O, Schneidinger C, et al. Prognostic significance of pre-treatment serum c-reactive protein level in patients with adenocarcinoma of the uterine cervix. Anticancer Res. 2016;36(9):4691–96. doi: 10.21873/anticanres.11022. [DOI] [PubMed] [Google Scholar]
- 21.Yang P, Chen N, Yang D, et al. The ratio of serum angiopoietin-1 to angiopoietin-2 in patients with cervical cancer is a valuable diagnostic and prognostic biomarker. Peer J. 2017;5:e3387. doi: 10.7717/peerj.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Xiong X, Wang Y, et al. Proteomic screening for serum biomarkers for cervical cancer and their clinical significance. Med Sci Monit. 2019;25:288–97. doi: 10.12659/MSM.911478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu W, Liu F, Wu K, et al. Lon peptidase 2, peroxisomal (LONP2) contributes to cervical carcinogenesis via oxidative stress. Med Sci Monit. 2018;24:1310–20. doi: 10.12659/MSM.908966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, Wang T, Zhang C, et al. S100A16 is a prognostic marker for colorectal cancer. J Surg Oncol. 2018;117(2):275–83. doi: 10.1002/jso.24822. [DOI] [PubMed] [Google Scholar]
- 25.Abou-Fayçal C, Hatat AS, Gazzeri S, Eymin B. Splice variants of the RTK family: Their role in tumour progression and response to targeted therapy. Int J Mol Sci. 2017;18(2) doi: 10.3390/ijms18020383. pii: E383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Z, Song Q, Zeng R, et al. MicroRNA-218 inhibits EMT, migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical cancer. Oncotarget. 2016;7(29):45622–36. doi: 10.18632/oncotarget.9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang C, Xu R, Li XX, et al. p53R2 overexpression in cervical cancer promotes AKT signaling and EMT, and is correlated with tumor progression, metastasis and poor prognosis. Cell Cycle. 2017;16(18):1673–82. doi: 10.1080/15384101.2017.1320629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Wang J, Li J, et al. MicroRNA-150 promotes cell proliferation, migration, and invasion of cervical cancer through targeting PDCD4. Biomed Pharmacother. 2018;97:511–17. doi: 10.1016/j.biopha.2017.09.143. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Sun Z, Qi M, et al. INPP4B restrains cell proliferation and metastasis via regulation of the PI3K/AKT/SGK pathway. J Cell Mol Med. 2018;22(5):2935–43. doi: 10.1111/jcmm.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Tan WH, Liu W, et al. Effects of miR-214 on cervical cancer cell proliferation, apoptosis and invasion via modulating PI3K/AKT/mTOR signal pathway. Eur Rev Med Pharmacol Sci. 2018;22(7):1891–98. doi: 10.26355/eurrev_201804_14711. [DOI] [PubMed] [Google Scholar]
- 31.Guo Q, Xiong Y, Song Y, et al. ARHGAP17 suppresses tumor progression and up-regulates P21 and P27 expression via inhibiting PI3K/AKT signaling pathway in cervical cancer. Gene. 2019;692:9–16. doi: 10.1016/j.gene.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Zhu W, Chen L, Liu L. MicroRNA-433 inhibits cell growth and induces apoptosis in human cervical cancer through PI3K/AKT signaling by targeting FAK. Oncol Rep. 2018;40(6):3469–78. doi: 10.3892/or.2018.6718. [DOI] [PubMed] [Google Scholar]
- 33.Shu NXR, Wu J, Sun H, et al. PACE confers the malignance of cervical cancers and contributes to the cisplatin-resistance in cervical cancer cells via PIKE/AKT pathway. Diagn Pathol. 2015;10:177. doi: 10.1186/s13000-015-0404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prasad SB, Yadav SS, Das M, et al. PI3K/AKT pathway-mediated regulation of p27(Kip1) is associated with cell cycle arrest and apoptosis in cervical cancer. Cell Oncol. 2015;38(3):215–25. doi: 10.1007/s13402-015-0224-x. [DOI] [PubMed] [Google Scholar]
- 35.Fultang N, Illendula A, Chen B, et al. Strictinin, a novel ROR1-inhibitor, represses triple negative breast cancer survival and migration via modulation of PI3K/AKT/GSK3β activity. PLoS One. 2019;14(5):e0217789. doi: 10.1371/journal.pone.0217789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karrasch T, Spaeth T, Allard B, Jobin C. PI3K-dependent GSK3β(Ser9)-phosphorylation is implicated in the intestinal epithelial cell wound-healing response. PLoS One. 2011;6(10):e26340. doi: 10.1371/journal.pone.0026340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge H, Liang C, Li Z, et al. DcR3 induces proliferation, migration, invasion, and EMT in gastric cancer cells via the PI3K/AKT/GSK-3β/β-catenin signaling pathway. Onco Targets Ther. 2018;11:4177–87. doi: 10.2147/OTT.S172713. [DOI] [PMC free article] [PubMed] [Google Scholar]