Abstract
Background
Intrauterine adhesion (IUA) is a common reproductive system disease in women, characterized by endometrial stromal cell proliferation, increasing fibroblasts and increasing extracellular matrix secretion. The purpose of this study was to investigate the effect of mitomycin C on reducing endometrial fibrosis for IUA.
Material/Methods
Firstly, a rat IUA model was constructed by intrauterine mechanical injury. The endometrial stromal cells and fibroblasts were isolated and treated with mitomycin C. After that, Cell Counting Kit-8 (CCK-8) assay was used to investigate the endometrial stromal cell viability. Furthermore, cell cycle and apoptosis assays of endometrial stromal cells and fibroblasts were performed, respectively. Finally, the cell viability of human endometrial cells or human uterus adhesion fibroblasts treated with mitomycin C was determined using CCK-8 assay with or without estradiol.
Results
Endometrial stromal cells were isolated from a rat IUA model. Cell cycle assay results showed that mitomycin C inhibited cell viability and promoted G1 cell cycle arrest and apoptosis in rat IUA endometrial stromal cells. Fibroblasts were also isolated from the rat IUA model. We found that mitomycin C inhibited the synthesis and secretion of collagen type I by western blotting analysis. Furthermore, mitomycin C promoted G1 cell cycle arrest and apoptosis in IUA rat uterine fibroblasts. We found that estradiol decreased the inhibitory effects of cell viability of human endometrial cells and human uterus adhesion fibroblasts by mitomycin C.
Conclusions
Our findings revealed that mitomycin C could reduce endometrial fibrosis for intrauterine adhesion.
MeSH Keywords: Cell Cycle, Endometrium, Fibroblasts, Mitomycin
Background
Intrauterine adhesion (IUA), also known as Asherman syndrome, is a common complication of endometrial trauma, producing adhesions within the uterine cavity [1]. IUA is mainly caused by damage to the basal layer of endometrium [2]. IUA is most common in trauma or infection, especially when the level of estradiol is low after pregnancy [3]. During the healing process, the opposing walls of the uterus adhere together, causing occlusion of the uterine cavity [4,5]. Patients with IUA may experience amenorrhea, dysmenorrhea, irregular menstruation, infertility or repeated abortion [6]. Currently, IUA patients usually undergoes cervical adhesion resection through hysteroscopy and postoperative hormone replacement therapy, but the recurrence rate is as high as 62.5% [7]. IUA is a main clinical problem in female infertility, however, there is no ideal treatment.
Mitomycin C has been shown to be an antimetabolite that inhibits fibroblast proliferation after surgery [8,9]. It inhibits fibroblast collagen synthesis by inhibiting DNA-dependent RNA synthesis and reducing fiber adhesion [10]. The clinical application of mitomycin C has proven to be effective and safe in preventing postoperative adhesions [11–13]. Mitomycin C has been widely used to reduce scar adhesion formed by ophthalmology and otolaryngology after surgery [14,15]. However, the effects of mitomycin C on IUA is unknown. Thus, in our study, we hypothesized mitomycin C could reduce endometrial fibrosis for IUA.
Material and Methods
Rat IUA model
We used 24 female Sprague-Dawley (SD) rats, aged 9 to 10 weeks old and weighing approximately 220 to 240 g that were purchased from Shanghai Slack Laboratory Animal Co., Ltd. (SCXK 2012-0002). Rats were anesthetized by intraperitoneal injection of 1 mL/kg 3% pentobarbital sodium. Then, the right uterus was exposed through the midline incision of the abdomen, and a longitudinal incision of 3 mm was cut in the lower part of the uterus. The surgical blade No. 21 was used to scrape the intima of the entire uterus until the walls of the uterus were rough, and then the uterus was thoroughly washed with 300 mL of physiological saline. Finally, the uterus incision was sutured, and the abdomen was closed, followed by incision disinfection. The SD rats were sacrificed on the 12th week after surgery and the right uterus was removed. Our research was approved by the Ethics Committee of Wenzhou People’s Hospital (No. 2018-17).
Isolation of endometrial stromal cells
Firstly, the removed uterus specimens were washed with phosphate-buffered saline (PBS) and antibiotics. After the epithelial and adipose tissues were trimmed, the remaining connective tissues were cut into small pieces of 1×1×1 mm. Next, the connective tissues were digested with type I collagenase at 37°C for 30 minutes, then filtered through a 100 um mesh and centrifuged at 800 g for 5 minutes. After adding an appropriate amount of complete medium, the isolated cells were cultured in an incubator, and the solution was changed once every 2 to 3 days. After the cells were covered with the bottom of the bottle, they were digested with 0.25% trypsin (Gibco) for 2 minutes. Once the cytoplasmic retraction and enhanced refraction were observed under phase contrast microscopy, 2 mL of medium with serum was immediately added to terminate digestion. Finally, the cell suspension was prepared and counted, followed by inoculation culture.
Cell culture
The rat or human endometrial stromal cells and fibroblasts were seeded in Dulbecco’s Modified Eagle Medium (DMEM) containing 20% fetal bovine serum (FBS) and cultured in a saturated humidity incubator (37°C, 5% CO2).
Immunofluorescence
Firstly, the prepared cell slides were washed with PBS. The slides were fixed with 4% paraformaldehyde for 30 minutes at room temperature. After washing with PBS 3 times for 5 minutes, the slide was incubated with 0.1% Triton X-100 solution (sc281181; Santa Cruz, CA, USA) for 10 minutes at room temperature. After that, 1% BSA solution was added to each well and blocked at 37°C for 90 minutes. The slides were incubated with primary antibodies including anti-vimentin (1: 100; 10366-1-AP; Proteintech, USA) and anti-cytokeratin 19 (1: 100; 10712-1-AP; Proteintech, USA) overnight at 4°C in a refrigerator. Next, the cells were incubated with goat anti-rabbit IgG (H+L) 488 fluorescent secondary antibody (1: 800; A11034; Life Technologies, USA) at 37°C for 1 hour in the dark. The nucleus was stained with DAPI (D9452; SIGMA, Canada) for 5 minutes at room temperature. Finally, the slides were observed under a fluorescence microscope.
Cell Counting Kit-8 (CCK-8) assay
The rat endometrial stromal cells were seeded in 96-well plates (1×103/well) and placed in an incubator for 24 hours. After that, the cells were cultured with different concentrations (0, 1.25, 2.5, 5, 10, 20, and 40 ug/mL) of mitomycin C. Then 100 μL of different concentrations of mitomycin C were added to each well. After 24 hours and 48 hours, cell viability was detected by CCK-8 assay. 10 μL of CCK-8 detection solution was added to each well at 37°C for 1 hour in the dark. The optical density (OD) value of each well was read at 450 nm. Cell viability (%)=OD value of mitomycin C group/OD value of control group×100%.
Flow cytometry
The third generation of endometrial stromal cells or the eighth of uterine fibroblast were selected for apoptosis and cell cycle assays. The cells were seeded in 96-well plates (1×103/well) for 24 hours, and then cultured with 4 mL different concentrations (0, 5, 10 ug/mL) of mitomycin C for 48 hours. After adding 300 uL of 1×Binding Buffer to each tube of cells, the apoptotic cells were marked with 5 uL of Annexin V-FITC and incubated for 15 minutes at room temperature in the dark. Then 5 uL of propidium iodide (PI) was then added and incubated for 5 min at room temperature in the dark. Finally, after adding 200 uL of 1×Binding Buffer, apoptotic cells were analyzed by NovoCyte flow cytometry (ACEA, China). For cell cycle, the cells were only stained with PI and analyzed on the flow cytometry.
Western blot analysis
The cells were cultured with 4 mL different concentrations (0, 5, and 10 ug/mL) of mitomycin C for 48 hours. After adding 300 uL of 1×Binding Buffer to each tube of cells, the cells were lysed radioimmunoprecipitation assay (RIPA) buffer (P0013B; Beyotime, China) supplemented with phenylmethylsulfonyl fluoride (PMSF) (ST506; Beyotime, China). After separating on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (P0015L; Beyotime, China), the samples were transferred onto polyvinylidene difluoride (PVDG) membrane. Then, the membrane was blocked by 5% skim milk powder at room temperature for 2 hours. The membrane was incubated with collagen type I primary antibody (10712-1-AP; Proteintech, USA) at 4°C overnight, and then incubated with secondary antibody at room temperature for 2 hours. GAPDH was used as an internal control. Finally, the protein blots were visualized using ECL (Beyotime, China).
Statistical analysis
All statistical analysis was performed using Graphpad Prism 7.0 (San Diego, CA, USA). Data were expressed as the mean±standard deviation (SD). The differences between 2 groups were analyzed using Student’s t-test. P-value <0.05 was considered statistically significant.
Results
Endometrial stromal cells in a rat IUA model
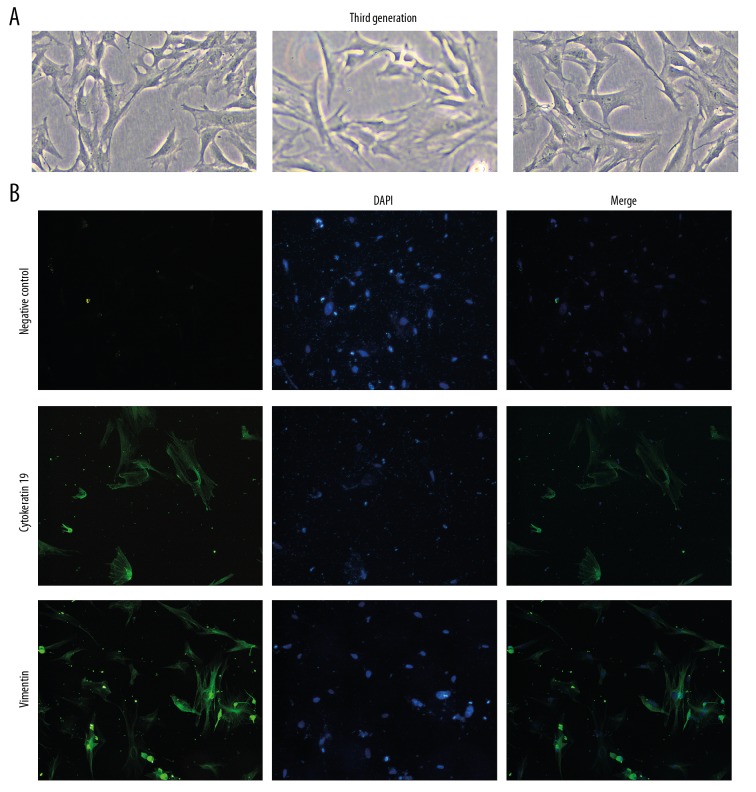
Firstly, a rat IUA model was constructed by endometrial injury and endometrial stromal cells were isolated. We observed the morphology of isolated endometrial stromal cells under a microscope and the growth characteristics of endometrial stromal cells are shown in Figure 1A. To further identify endometrial stromal cells, immunofluorescence staining of vimentin and cytokeratin 19 was performed. Immunofluorescence results showed that the expression of cytokeratin 19 was decreased and the expression of vimentin was elevated (Figure 1B). The aforementioned results showed that the isolated endometrial cells are mainly endometrial stromal cells.
Figure 1.
Identification of endometrial stromal cells in a rat IUA model. (A) Morphology of endometrial stromal cells in a rat IUA model. White bar is 10 μm. (B) Immunofluorescence results showing the expression of vimentin and cytokeratin 19 in endometrial stromal cells of rat IUA model. Representative images are shown.
Mitomycin C inhibited cell viability and promoted G1 cell cycle arrest and apoptosis in rat IUA endometrial stromal cells
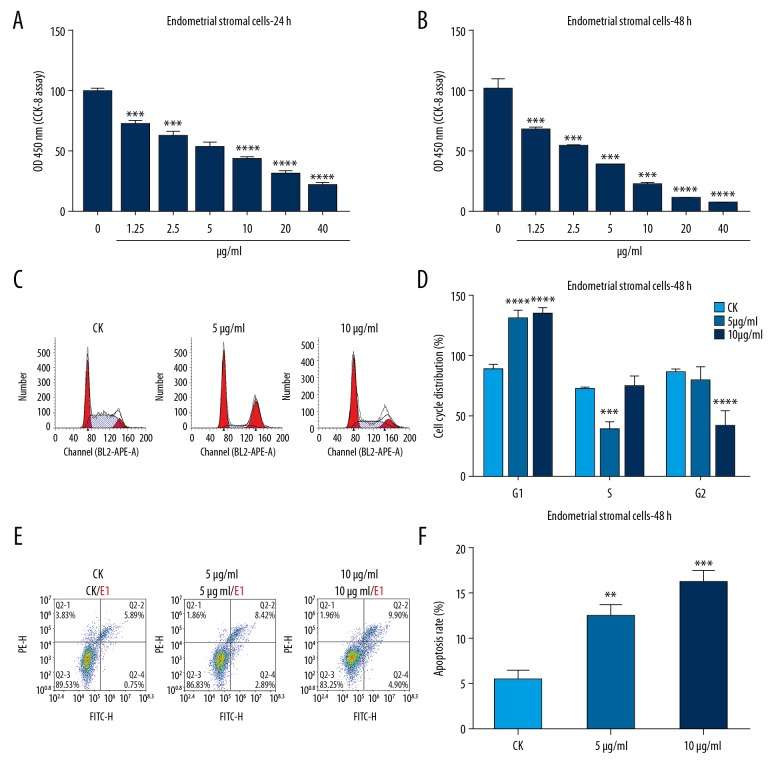
To investigate the effects of mitomycin C on cell viability of rat IUA endometrial stromal cell, CCK-8 assay was performed. The results showed that mitomycin C significantly inhibited cell viability of rat IUA endometrial stromal cell with a concentration-dependent manner after treatment with mitomycin C for 24 hours and 48 hours (Figure 2A, 2B). We also observed cell cycle of rat IUA endometrial stromal cells via flow cytometry. The rat IUA endometrial stromal cells were treated with different concentrations of mitomycin C (CK; 5 μg/mL; 10 μg/mL). We found that rat IUA endometrial stromal cells were arrested at G1 after treatment with 5 μg/mL or 10 μg/mL mitomycin C (Figure 2C, 2D). To examine the effects of mitomycin C on cell apoptosis of rat IUA endometrial stromal cells, apoptotic cells were marked with Annexin V-FITC and analyzed by flow cytometry. We found that mitomycin C promoted cell apoptosis with a concentration-dependent manner after mitomycin C treatment for 48 hours (Figure 2E, 2F). Above results showed that mitomycin C inhibits cell viability and promotes G1 cell cycle arrest and apoptosis in rat IUA endometrial stromal cells.
Figure 2.
Mitomycin C inhibits cell viability and promotes G1 cell cycle arrest and apoptosis in rat intrauterine adhesion (IUA) endometrial stromal cells. (A, B) Cell Counting Kit-8 (CCK-8) assay showing the effects of different concentrations of mitomycin C on cell viability of rat IUA endometrial stromal cell after treatment for 24 hours and 48 hours. (C, D) Cell cycle assay showing the cell cycle distribution of rat IUA endometrial stromal cell after mitomycin C treatment (CK; 5 μg/mL; 10 μg/mL) for 24 hours and 48 hours by flow cytometry. (E, F) Apoptosis assay showing the cell apoptosis of rat IUA endometrial stromal cell treated by (CK; 5 μg/mL; 10 μg/mL) mitomycin C for 48 hours. ** P<0.01; *** P<0.001 and *** P<0.0001.
Mitomycin C inhibited IUA rat uterine fibroblasts to secrete collagen type I
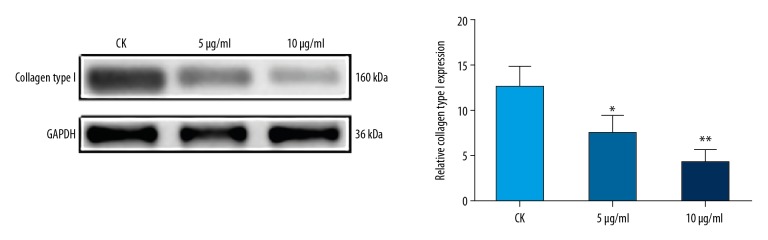
To investigate the ability of IUA rat uterine fibroblasts to secrete type I collagen, fibroblasts were isolated and cultured for 6 to 9 generations. Western blotting analysis was performed, and the results showed that, compared to controls, the expression of collagen type I was significantly inhibited in IUA rat uterine fibroblasts treated with mitomycin C for 48 hours, with a concentration-dependent manner (Figure 3). These results indicated that mitomycin C inhibits IUA rat uterine fibroblast to secrete collagen type I.
Figure 3.
Western blotting analysis showing the expression of collagen type I in intrauterine adhesion (IUA) rat uterine fibroblasts treated with (CK; 5 μg/mL; 10 μg/mL) mitomycin C for 48 hours. GADPH was used as an internal control. * P<0.05 and ** P<0.01.
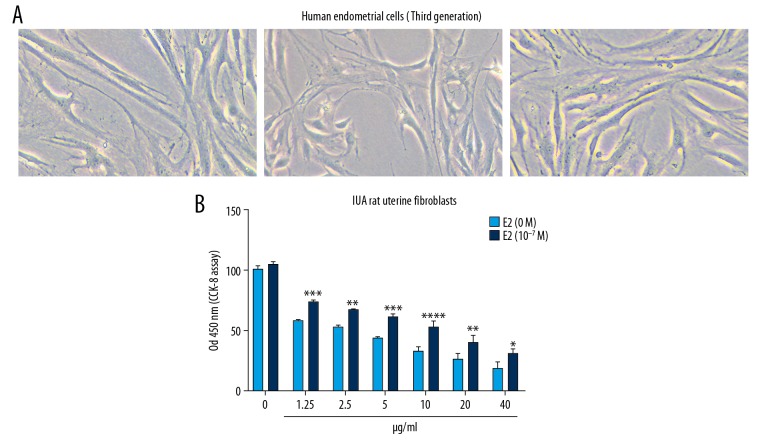
Mitomycin C promoted G1 cell cycle arrest and apoptosis in IUA rat uterine fibroblasts
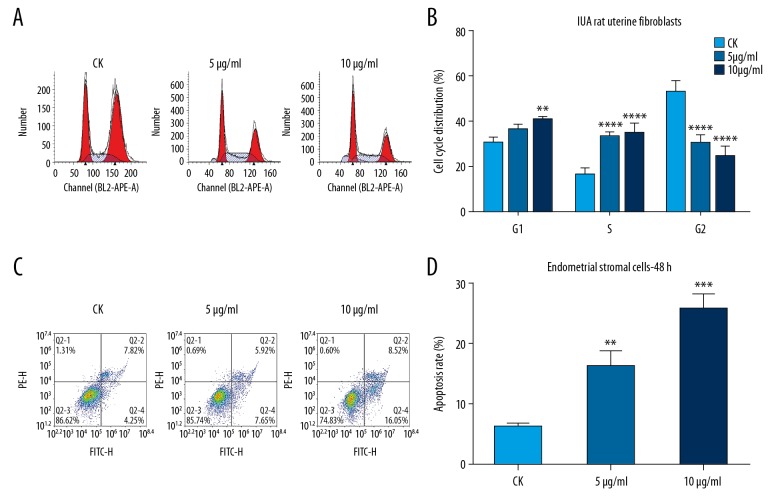
Cell cycle and apoptosis assays were performed using flow cytometry. The cell cycle assay results showed that IUA rat uterine fibroblasts were arrested at G1 phage after treatment with mitomycin C for 48 hours with a concentration-dependent manner (Figure 4A, 4B). Furthermore, the apoptosis assay results suggested that mitomycin C promoted IUA rat uterine fibroblast apoptosis, with a concentration-dependent manner (Figure 4C, 4D). These results revealed that mitomycin C promotes G1 cell cycle arrest and apoptosis in IUA rat uterine fibroblasts.
Figure 4.
Mitomycin C promotes G1 cell cycle arrest and apoptosis in intrauterine adhesion (IUA) rat uterine fibroblasts. (A, B) Cell cycle assay showing the cell cycle distribution of IUA rat uterine fibroblasts after mitomycin C treatment (CK; 5 μg/mL; 10 μg/mL) for 48 hours by flow cytometry. (C, D) Apoptosis assay showing the cell apoptosis of IUA rat uterine fibroblasts treated by (CK; 5 μg/mL; 10 μg/mL) mitomycin C for 48 hours. ** P<0.01; *** P<0.001 and *** P<0.0001.
Estradiol decreased the inhibitory effects of cell viability of human endometrial cells by mitomycin C
We further investigated the effects of mitomycin C on cell viability of human endometrial cells by CCK-8 assay. Firstly, we observed the morphology of human endometrial cells under a microscope (Figure 5A). The CCK-8 results showed that mitomycin C inhibited cell viability of human endometrial cells, with a concentration-dependent manner (Figure 5B). After adding estradiol, we found that the inhibitory effects of human endometrial cell viability were decreased. The aforementioned results indicated that estradiol decreases the inhibitory effects of cell viability of human endometrial cells by mitomycin C.
Figure 5.
Estradiol decreases the inhibitory effects of cell viability of human endometrial cells by mitomycin C. (A) Morphology of human endometrial cells. White bar is 10 μm. (B) Cell Counting Kit-8 (CCK-8) showing the effects of mitomycin C on cell viability of human endometrial cells with or without estradiol (E2). * P<0.05; ** P<0.01; *** P<0.001 and *** P<0.0001.
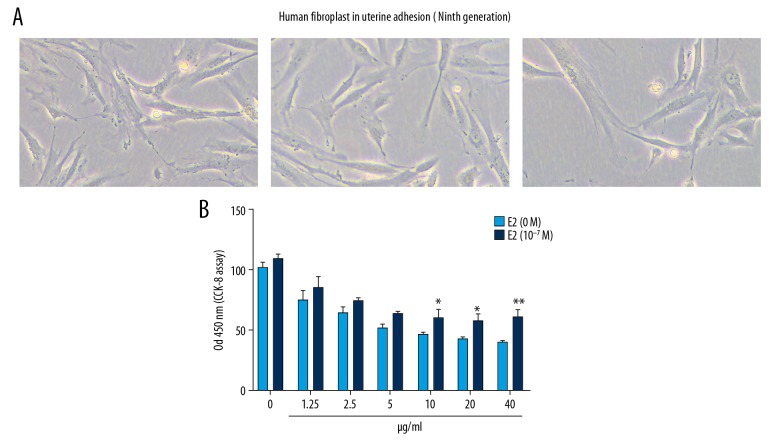
Estradiol decreased the inhibitory effects of cell viability of human uterus adhesion fibroblasts by mitomycin C
We further observed the effects of mitomycin C on cell viability of human uterus adhesion fibroblasts using CCK-8 assay. The morphology of human uterus adhesion fibroblasts is shown in Figure 6A. The CCK-8 results showed that mitomycin C suppressed human uterus adhesion fibroblast viability, with a concentration-dependent manner (Figure 6B). Furthermore, we found that estradiol decreased the inhibitory effects of human uterus adhesion fibroblast viability. These results indicated that estradiol decreases the inhibitory effects of cell viability of human endometrial cells by mitomycin C.
Figure 6.
Estradiol decreases the inhibitory effects of cell viability of human uterus adhesion fibroblasts by mitomycin C. (A) Morphology of human uterus adhesion fibroblasts. White bar is 10 μm. (B) Cell Counting Kit-8 (CCK-8) showing the effects of mitomycin C on cell viability of human uterus adhesion fibroblasts with or without estradiol (E2). * P<0.05 and ** P<0.01.
Discussion
IUA is a major health problem that causes female infertility, irregular menstruation and repeated abortions [16]. Unfortunately, there is currently no effective strategy for treating IUA [17,18]. Mitomycin C has been shown to inhibit fibroblast proliferation, promote fibroblast apoptosis, and ultimately prevent adhesion formation [19]. However, the effect of mitomycin C on IUA remains unclear. Therefore, in our study, we successfully constructed a rat IUA model and our findings revealed the inhibitory effect of mitomycin C on endometrial fibrosis for IUA.
The human endometrium is a highly regenerative tissue. The endometrium is composed of epithelial cells, stromal cells, vascular smooth cells, and vascular endothelial cells [20]. From a functional point of view, the endometrium consists of an outer functional layer and an inner base layer. The matrix is the main part of the inner base layer. The regeneration of the endometrial surface epithelium is due to stromal cell differentiation. However, IUA can cause damage to the endometrial basal layer [4]. When the uterine cavity is anatomically restored, endometrial fibrosis will determine reproductive outcomes [21]. Increased evidence suggests that endometrial fibrosis is associated with the development of IUA, but there has been no satisfactory progress in effective treatment [22]. Therefore, more studies are needed to elucidate the mechanisms of endometrial fibrosis and to develop new prevention and treatment strategies. In this study, we successfully constructed a rat IUA model through endometrial injury. Although it has been recognized that IUA development in human is more complex than in rat models, and the pathophysiology of rat models may be a little different from human, the rat model based on endometrial injury has been considered as a successful NEC model. Furthermore, endometrial stromal cells were isolated from rat IUA model. Our immunofluorescence results showed that the expression of cytokeratin 19 was decreased and the expression of vimentin was elevated, suggesting that the isolated endometrial cells were mainly endometrial stromal cells. We found that mitomycin C inhibited endometrial stromal cell viability with concentration dependent manners. Furthermore, endometrial stromal cells were arrested at G1 cell cycle. We also observed that mitomycin C promoted the apoptosis of endometrial stromal cells. IUA is characterized by the destruction of the basal layer of the endometrium [23]. Histologically, endometrial fibrosis contributes to IUA, which results in impaired endometrial function, uterine cavity deformation and stenosis. In the IUA, the endometrial stroma is primarily replaced by large fibrous tissues [24]. In addition, the IUA destroys the boundary between the basal layer and the functional layer of the endometrium [25]. Our study showed that mitomycin C inhibited cell viability and promoted apoptosis of endometrial stromal cells from a rat IUA model.
Endometrial fibrosis is considered to be a key pathological event in the development of IUA [26,27]. Fibroblasts play a key role in the pathogenesis of fibrous scar formation. Fibrosis may limit the activity of the myometrium, reduce the perfusion of estrogen and progesterone, and eventually lead to atrophy [28]. In our study, we found that IUA rat fibroblasts were arrested at G1 after treatment with mitomycin C. Moreover, mitomycin C promoted IUA rat fibroblast apoptosis. Mitomycin C inhibited IUA rat uterine fibroblasts to secrete collagen type I, a fibrotic marker that provides mechanical stability for tissues and serves as a functional environment for cells [29,30]. Endometrial fibrosis is associated with the development of IUA. Therefore, prevention of endometrial fibrosis is the basis of IUA treatment. Our study suggested that mitomycin C could prevent endometrial fibrosis by inhibiting the proliferation of fibroblasts.
Treatment of IUA includes adhesions to dissolve and prevention of adhesion recurrence [31,32]. The main purpose of treatment is to restore the normal shape and volume of the uterine cavity [33]. High-dose estrogen therapy is often used to prevent recurrence of uterine adhesions and promote endometrial repair [34]. However, the use of estrogen alone is not effective and is often associated with adverse effects, which greatly limits its widespread use. We found that estradiol could decrease the inhibitory effects of cell viability of human endometrial cells or human uterus adhesion fibroblasts by mitomycin C.
Taken together, our study found that mitomycin C could reduce endometrial fibrosis for intrauterine adhesion. However, a limitation of this study should be pointed out. This was a study in an animal model, and the findings require further validation by both in vitro and in vivo studies and human toxicity testing.
Conclusions
In our study, we found that mitomycin C inhibited cell viability and promoted G1 cell cycle arrest and apoptosis in rat IUA endometrial stromal cells. Mitomycin C inhibited the synthesis and secretion of collagen type I. Furthermore, we found that mitomycin C promoted G1 cell cycle arrest and apoptosis in IUA rat uterine fibroblasts. Estradiol decreased the inhibitory effects of cell viability of human endometrial cells and human uterus adhesion fibroblasts by mitomycin C. Our findings revealed that mitomycin C could have an inhibitory effect on endometrial fibrosis for IUA.
Abbreviations
- IUA
intrauterine adhesion
- CCK-8
Cell Counting Kit-8
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Bosteels J, Weyers S, Kasius J, et al. Anti-adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane Database Syst Rev. 2015;(11):CD011110. doi: 10.1002/14651858.CD011110.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Li P, Yuan Z, Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther. 2019;10:61. doi: 10.1186/s13287-019-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao L, Song Y, Huang W, et al. Expression of SOX2, NANOG and OCT4 in a mouse model of lipopolysaccharide-induced acute uterine injury and intrauterine adhesions. Reprod Biol Endocrinol. 2017;15:14. doi: 10.1186/s12958-017-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: Prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262–78. doi: 10.1093/humupd/dmt045. [DOI] [PubMed] [Google Scholar]
- 5.Alizzi AM, Summers P, Boon VH, et al. Reduction of post-surgical pericardial adhesions using a pig model. Heart Lung Circ. 2012;21:22–29. doi: 10.1016/j.hlc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Shi X, Saravelos SH, Zhou Q, et al. Prevention of postoperative adhesion reformation by intermittent intrauterine balloon therapy: A randomised controlled trial. BJOG. 2019;126(10):1259–66. doi: 10.1111/1471-0528.15843. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Liu L, Luo Y, et al. Effects of aspirin and intrauterine balloon on endometrial repair and reproductive prognosis in patients with severe intrauterine adhesion: A prospective cohort study. Biomed Res Int. 2017;2017 doi: 10.1155/2017/8526104. 8526104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Yan L, Wang J, et al. Comparison of the effects of mitomycin C and 10-hydroxycamptothecin on an experimental intraarticular adhesion model in rabbits. Eur J Pharmacol. 2013;703:42–45. doi: 10.1016/j.ejphar.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Su C, Yao C, Lu S, et al. Study on the optimal concentration of topical mitomycin-C in preventing postlaminectomy epidural adhesion. Eur J Pharmacol. 2010;640:63–67. doi: 10.1016/j.ejphar.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 10.Colak N, Nazli Y, Tasoglu I, et al. The effect of mitomycin-C in reducing pericardial adhesion after cardiac surgery in rabbits. Can J Cardiol. 2013;29:712–17. doi: 10.1016/j.cjca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Wang BB, Xie H, Wu T, et al. Controlled-release mitomycin C-polylactic acid film prevents epidural scar hyperplasia after laminectomy by inducing fibroblast autophagy and regulating the expression of miRNAs. Eur Rev Med Pharmacol Sci. 2017;21:2526–37. [PubMed] [Google Scholar]
- 12.Shi R, Huang Y, Zhang J, et al. Effective delivery of mitomycin-C and meloxicam by double-layer electrospun membranes for the prevention of epidural adhesions. J Biomed Mater Res B Appl Biomater. 2019 doi: 10.1002/jbm.b.34394. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Orhan A, Gormus N, Toy H, et al. Prevention of retrosternal pericardial adhesions after cardiac surgery with mitomycin C. Heart Lung Circ. 2014;23:357–62. doi: 10.1016/j.hlc.2013.10.080. [DOI] [PubMed] [Google Scholar]
- 14.Gray SD, Tritle N, Li W. The effect of mitomycin on extracellular matrix proteins in a rat wound model. Laryngoscope. 2003;113:237–42. doi: 10.1097/00005537-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Mendrinos E, Mermoud A, Shaarawy T. Nonpenetrating glaucoma surgery. Surv Ophthalmol. 2008;53:592–630. doi: 10.1016/j.survophthal.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Zhu HY, Ge TX, Pan YB, Zhang SY. Advanced role of Hippo signaling in endometrial fibrosis: Implications for intrauterine adhesion. Chin Med J (Engl) 2017;130:2732–37. doi: 10.4103/0366-6999.218013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooker AB, de Leeuw R, van de Ven PM, et al. Prevalence of intrauterine adhesions after the application of hyaluronic acid gel after dilatation and curettage in women with at least one previous curettage: Short-term outcomes of a multicenter, prospective randomized controlled trial. Fertil Steril. 2017;107:1223–31.e3. doi: 10.1016/j.fertnstert.2017.02.113. [DOI] [PubMed] [Google Scholar]
- 18.Kim T, Ahn KH, Choi DS, et al. A randomized, multi-center, clinical trial to assess the efficacy and safety of alginate carboxymethylcellulose hyaluronic acid compared to carboxymethylcellulose hyaluronic acid to prevent postoperative intrauterine adhesion. J Minim Invasive Gynecol. 2012;19:731–36. doi: 10.1016/j.jmig.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Wang YW, Ren JH, Xia K, et al. Effect of mitomycin on normal dermal fibroblast and HaCat cell: An in vitro study. J Zhejiang Univ Sci B. 2012;13:997–1005. doi: 10.1631/jzus.B1200055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azizi R, Aghebati-Maleki L, Nouri M, et al. Stem cell therapy in Asherman syndrome and thin endometrium: Stem cell- based therapy. Biomed Pharmacother. 2018;102:333–43. doi: 10.1016/j.biopha.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 21.Tu CH, Yang XL, Qin XY, et al. Management of intrauterine adhesions: A novel intrauterine device. Med Hypotheses. 2013;81:394–96. doi: 10.1016/j.mehy.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Liu L, Mou S, et al. Investigation of platelet-rich plasma in increasing proliferation and migration of endometrial mesenchymal stem cells and improving pregnancy outcome of patients with thin endometrium. J Cell Biochem. 2018 doi: 10.1002/jcb.28014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Xiao S, Wan Y, Xue M, et al. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int J Gynaecol Obstet. 2014;125:121–24. doi: 10.1016/j.ijgo.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Myers EM, Hurst BS. Comprehensive management of severe Asherman syndrome and amenorrhea. Fertil Steril. 2012;97:160–64. doi: 10.1016/j.fertnstert.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;17:555–69. doi: 10.1016/j.jmig.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Healy MW, Schexnayder B, Connell MT, et al. Intrauterine adhesion prevention after hysteroscopy: A systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:267–75.e7. doi: 10.1016/j.ajog.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Lin XN, Zhou F, Wei ML, et al. Randomized, controlled trial comparing the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation after hysteroscopic adhesiolysis. Fertil Steril. 2015;104:235–40. doi: 10.1016/j.fertnstert.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Yu D, Wong YM, Cheong Y, et al. Asherman syndrome – one century later. Fertil Steril. 2008;89:759–79. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 29.Ning J, Zhang H, Yang H. MicroRNA326 inhibits endometrial fibrosis by regulating TGFbeta1/Smad3 pathway in intrauterine adhesions. Mol Med Rep. 2018;18:2286–92. doi: 10.3892/mmr.2018.9187. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, Jiang L, Gao Y, et al. Interaction between BMSCs and EPCs promotes IUA angiogenesis via modulating PI3K/Akt/Cox2 axis. Am J Transl Res. 2018;10:4280–89. [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzon I, Favilli A, Cocco P, et al. Does cold loop hysteroscopic myomectomy reduce intrauterine adhesions? A retrospective study. Fertil Steril. 2014;101:294–98.e3. doi: 10.1016/j.fertnstert.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 32.Song D, Liu Y, Xiao Y, et al. A matched cohort study comparing the outcome of intrauterine adhesiolysis for Asherman’s syndrome after uterine artery embolization or surgical trauma. J Minim Invasive Gynecol. 2014;21:1022–28. doi: 10.1016/j.jmig.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Zupi E, Centini G, Lazzeri L. Asherman syndrome: An unsolved clinical definition and management. Fertil Steril. 2015;104:1380–81. doi: 10.1016/j.fertnstert.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 34.Sowter MC, Singla AA, Lethaby A. Pre-operative endometrial thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD001124. doi: 10.1002/14651858.CD001124. [DOI] [PubMed] [Google Scholar]