Abstract
Background
Ustekinumab, a human-derived monoclonal antibody that targets the p40 subunit of interleukin (IL)-12 and IL-23, has excellent clinical efficacy and safety in treating psoriasis, with a long half-life. However, no reports have described the use of human skin/serum samples to elucidate its molecular mechanisms.
Material/Methods
Twenty-four psoriasis patients were enrolled in our double-blind study and randomly divided into placebo and ustekinumab-administered groups. Dynamic changes in psoriasis area-severity index scores, and mRNA and protein levels of p35 and p40 were analyzed at 3 time points (before treatment and during the 12th and 24th weeks of treatment).
Results
Ustekinumab initially increased and then decreased p35 mRNA expression, but increased p40 mRNA levels throughout the study. The p35 protein levels were not significantly altered, while p40 protein levels were increased after the first 2 injections but decreased after the third injection.
Conclusions
We concluded that 2 equilibria influence the efficacy of ustekinumab against psoriasis. First, because of the dual roles of p35 in psoriasis pathogenesis, homeostasis occurs between p35 and p40 expression levels. The second balance lies between the upregulation of p40 mRNA levels and the ability of ustekinumab to neutralize the function of the elevated p40 protein.
MeSH Keywords: Antibodies, Monoclonal; Interleukin-12 Subunit p35; Interleukin-12 Subunit p40; Psoriasis
Background
Psoriasis is an inflammatory skin disorder with a global prevalence rate of approximately 2%. Its chronicity and recurrence impose physiological and mental hardships on affected patients. The costly treatment expenses negatively impact personal and social-financial conditions. Psoriasis is characterized by impaired differentiation of epidermal cells into keratinocytes, epidermal keratinocyte hyperproliferation, and keratinization dysfunction. It is generally acknowledged that immunologic dysfunction mediated by different subsets of T lymphocytes is critical to the immunologic mechanism of psoriasis. Helper T cell (Th) 1 and Th17 are 2 relevant subsets [1]. The immunologic mechanism of psoriasis involves a complicated pro-inflammatory cytokine network, including the interleukin (IL)-12/Th1 and IL-23/Th17 axes [1,2]. Dendritic cells and macrophages are primary sources of IL-12 and IL-23. IL-12 is a cytokine that acts upstream of Th1 responses [2]. IL-23 activates the proliferation and survival of both Th17 cells and keratinocytes.
Ustekinumab (USTK), a recently emerging biological agent against psoriasis, is a fully human monoclonal antibody. USTK has been demonstrated to stimulate peripheral blood monocytes (PBMCs) to modulate cytokine secretion [3,4]. The antibody targets the subunit p40 shared by IL-12 and IL-23, neutralizing their biological activities and attenuating immune cell activation. Data from multiple clinical trials have verified the remarkable clinical outcomes of the treatment of psoriasis and psoriatic arthritis with USTK, without many adverse effects [5–8] and with a lasting clinical effect after 3 years of treatment [9].
It has neither been revealed how p35 and p40 expression levels change in the patients’ peripheral blood after USTK administration nor whether a feedback loop exists between the 2 subunits mRNA and protein expression levels. Besides, it is unclear how USTK works effectively and why it is distinct from other biologic agents. Hence, in this study, we investigated the effects of USTK on IL-12/23 p40 and IL-12 p35 expression, at both the mRNA and protein levels.
Material and Methods
Patient recruitment criteria
To be eligible for enrollment in the study, only patients meeting all the following criteria received p40 mono-antibody treatment: 1) age between 18–65 years old at the time of consent; 2) diagnosis of plaque type psoriasis for over 6 months; 3) moderate to severe plaque type psoriasis, with at least 10% total body surface area involvement and a psoriasis area-severity index (PASI) score ≥12 at screening and at the time of the first administration of treatment; 5) no history of latent or active tuberculosis; and 6) no prior exposure to biological agents.
Patient exclusion criteria
Patients with any of the following criteria were not enrolled in the study: 1) diagnosis of any other type of psoriasis, including psoriatic arthritis, erythrodermic psoriasis, psoriasis guttate, and psoriasis pustulosa; 2) severe and uncontrollable active or potential infection, either local or systemic; 3) asthma history; 4) cancer history; 5) other severe systemic diseases; 6) prior exposure to investigational drugs or biological agents; 7) administration of an immunosuppressor within the previous month; 8) undergoing systemic treatment for psoriasis or phototherapy within the previous month; and 9) topical psoriasis treatment within the previous 2 weeks.
Psoriasis
Study design
Twenty-four qualified psoriasis patients were recruited (population composition between age 18–60; 18 males and 6 females). PASI scores ranged from 12.2 to 54 (mean PASI score±standard error of the mean [SEM]=21.61±1.86). The patients were randomly and double-blind distributed into a placebo group (n=13, PASI=21.35±3.19 (mean±SEM)) and an USTK-administered group (USTK group, n=11, PASI=21.91±1.71 (mean±SEM)). The project was approved by the ethical review board of the Second Affiliated Hospital, Zhejiang University School of Medicine. The registration number was 2009L01542. All patients read the study protocol and the risks associated with the study and signed informed consent forms before being enrolled in the study.
We conducted a double-blind, placebo-controlled randomized trial. Patients in the placebo group received subcutaneous injections of 0.9% sodium chloride solution (NS; 45 mg) in week 0 and week 4. The USTK group received subcutaneous injections of USTK (45 mg) in week 0 and week 4. In week 12, patients in the placebo group were administered 45 mg of USTK, and patients in the USTK group received NS. All patients were administered 45 mg of USTK in week 16 (Figure 1). We treated the placebo group in the latter half of the trial because the patients’ symptoms would worsen without medication for 26 to 28 weeks, considering the 8th and 9th terms of the exclusion criteria. Such aggravation would manifest in physical and mental health issues, which we considered inhumane. Throughout the whole trial, the USTK group received one more injection than the placebo group. We compared the results based on dosage frequencies.
Figure 1.
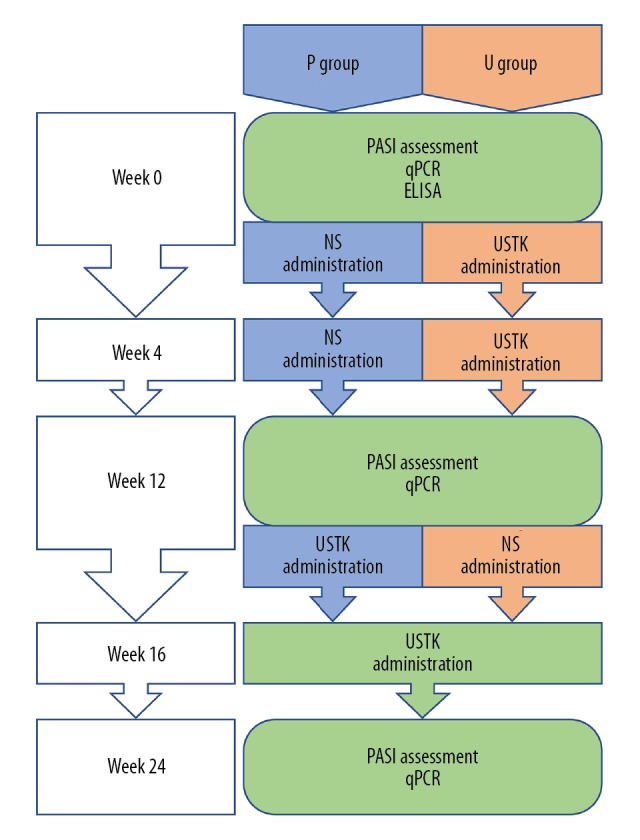
Study flowchart. NS – 0.9% sodium chloride solution; USTK – ustekinumab; P group – placebo group; U group – psoriasis area-severity index group.
Efficacy assessment
Efficacy was determined by measuring the PASI scores. One author (Liu) performed the PASI assessments throughout the trial. Lesioned areas were measured based on the size of the patient’s palm, in which the area of 1 palm equaled approximately 1% of the total body surface area. The PASI of each patient was assessed in week 0, week 12, and week 24. PASI scores at weeks 0 and 12 were assessed immediately before the placebo or USTK administration. All the PASI scores are provided in Supplementary Table 1. The PASI scores at week 0 demonstrated each patient’s initial condition. Treatment efficacy was confirmed in cases where the PASI-improvement rate was ≥75% (PASI-75). See the statistical analysis subsection for the calculation method.
Sample collection
We collected 8 mL of whole blood from each patient at each time point: weeks 0, 12, and 24. PMBCs were extracted from 4 mL of blood, and 4 mL were used for enzyme-linked immunosorbent assay (ELISA) analysis. Blood samplings were synchronized with PASI assessments (Figure 1). Blood sampling from week 0 provided baseline values. Blood sampling from week 12 was conducted before medication, when the USTK group had been administered 2 injections of USTK and the placebo group had been administered 2 injections of NS. A comparison between the placebo and USTK groups revealed how 2 injections of USTK affected the target cytokines. Blood sampling from week 24 was done 8 weeks after the medications had finished, when the USTK group had been administered 3 injections of USTK and 1 injection of NS and the placebo group had been administered 2 injections of NS and 2 injections of USTK. A comparison between the placebo and USTK groups indicated different effects caused by dosage differences.
Whole blood sampling, centrifugation, ELISA, RNA preparation, and reverse transcription of RNA were conducted on the same day. Before centrifugation, whole blood samples were refrigerated at 4°C. Subsequently, serum, PBMCs, RNA extracted from PBMCs, and cDNA reverse-transcribed from RNA were stored at −80°C until later experiments were performed.
RNA preparation and real-time quantitative PCR (qPCR)
Whole blood samples were lysed in cell lysis buffer. The latter was comprised of 8.99 g NH4Cl, 1.00 g KHCO3, and 37.00 mg tetrasodium EDTA dissolved in 1 L of distilled water and adjusted to pH 7.3. Total RNA was extracted from peripheral leukocytes with the TRIzol reagent (Invitrogen). The quality of RNA was evaluated using a NanoDrop 2000C spectrophotometer (Thermo Fisher Scientific). cDNA was reverse transcribed with Superscript II Reverse Transcriptase (Invitrogen). We used SYBR Green PCR Master Mix (Roche) to determine gene expression using 10 ng cDNA template. Quantitative PCR (qPCR) analysis was performed with an ABI Step One Plus instrument (Life Technologies), with the following amplification protocol:
For p35: 5 seconds at 95°C, followed by 45 cycles of 10 seconds at 56°C, and 10 seconds at 72°C;
For p40: 5 seconds at 95°C, followed by 45 cycles of 15 seconds at 60°C.
Primers were designed by Beacon Designer (Premier Software):
p35, 5′-TGGCAGTTATTGATGAGC-3′ and
5′-TTAGGAAGCATTCAGATAGC-3′;
p40, 5′-CAGAGCAGTGAGGTCTTAGGC-3′ and
5′-AAGCAGCAGGAGCGAATGG-3′.
Transcript expression was normalized to that of the reference gene GAPDH and was represented as 2−ΔΔCT, where 2−ΔΔCT=2−ΔCT (gene of interest/average [2−ΔCT reference gene]). Technical triplicates were performed for each assay.
ELISA experiments
ELISA kits were used to measure serum p35 (SEA059Hu 96 Test, Cloud-Clone Corp) and p40 (Catalog #ELH-IL12P40, RayBiotech, Inc.) levels.
Statistical analysis
Independent-samples t-tests were used to evaluate the differences between the 2 groups. PASI-75 rates (the percentage of patients with a 75% or greater reduction in the PASI score) were compared between the placebo and USTK groups using the kappa test. PASI-improvement rates were calculated as follows: PASI-improvement rates (week 12 versus baseline or week 24 versus baseline)=(PASI [week 12 or week 24]–PASI [baseline])/PASI (baseline).The qPCR results were compared horizontally between groups and longitudinally within groups. Due to sample shortage (for reasons explained in the results section), only samples from 7 patients in the placebo group and from 5 patients in the USTK group at baseline and week 24 were reserved for ELISA. Thus, we could only adopt a longitudinal comparison within groups to reduce bias. Data were analyzed with GraphPad Prism software (GraphPad Software, Inc.).
Results
Maximum efficacy of USTK was achieved after 3 administrations of USTK (45 mg)
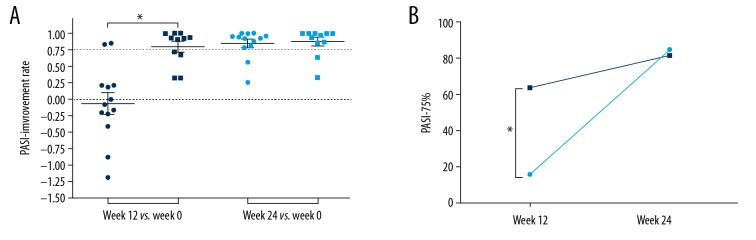
To demonstrate the clinical response to USTK (Figure 2), PASI-improvement rates (week 12 or week 24 versus baseline) were compared between placebo and USTK groups (Figure 2A). When comparing week 12 versus week 0, significant PASI reduction was found in the treatment group (USTK group versus placebo group, P<0.05). However, no significant difference was observed at week 24. Similarly, the kappa test demonstrated that PASI-75% was higher in the USTK group at week 12, but not at week 24 (Figure 2B). Additionally, PASI-75% was >60% for the USTK group at week 12 and the placebo group at week 24 (USTK group at week 12: 63.6%; USTK group at week 24: 81.8%; placebo group at week 24: 84.6%). It was concluded from the data that the maximum efficacy of USTK could be obtained after 3 injections for most patients. Subsequent injections exert more of a maintenance effect.
Figure 2.
PASI-improvement rates of the placebo and USTK groups compared between weeks 0, 12, and 24 (A). The PASI-75 rate (PASI-75%) was compared between the placebo and USTK groups at week 12 and week 24 (B). ● Denotes placebo group; ■ denotes USTK group; * p<0.05. PSAI – psoriasis area-severity index; USTK – ustekinumab.
USTK induced p40 expression but suppressed p35 expression in the long term
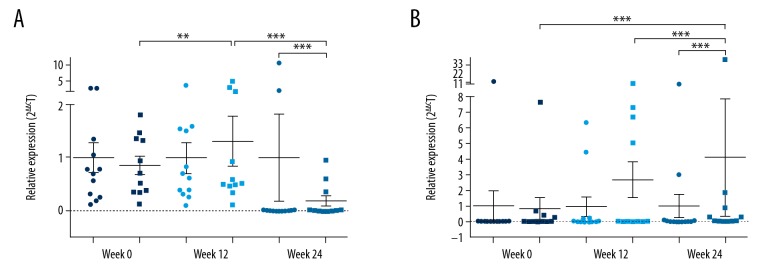
To reveal the molecular mechanisms underlying the efficacy of USTK, we compared p35 and p40 expression levels between the placebo and USTK groups by qPCR (Figure 3). The initial p35 and p40 mRNA expression levels between both groups were not statistically different. Significant differences were observed between the 2 groups at week 12 and week 24. For the USTK group, p35 expression levels at week 12 were higher than those at weeks 0 and 24. At week 24, p35 expression levels in the USTK group were lower compared to those in the placebo group.
Figure 3.
Scatter plots of qPCR results of p35 (A) and p40 (B) expression in the placebo group (●) and USTK group (■) at weeks 0, 12, and 24. ** P<0.005, *** P<0.0001. qPCR – quantitative polymerase chain reaction; USTK – ustekinumab.
The p40 expression in the USTK group was steady between weeks 0 and 12 but increased at week 24 (internal comparisons of the USTK group of week 24 versus week 0 and week 24 versus week 12; comparison between the placebo and USTK groups at week 24: P<0.0001). Intriguingly, the results seemed to contrast with USTK’s property of blocking p40 protein expression. However, USTK is a specific antibody against the p40 protein and does not interact with mRNA. We hypothesize that negative feedback occurs between the p40 protein and mRNA expression levels. ELISA was performed to measure variations in p40 protein expression.
Periodic application of USTK was essential for neutralizing excessive p40
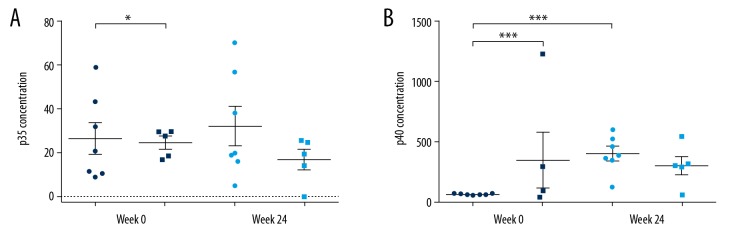
Changes in the serum levels of p35 and p40 protein expression were determined by ELISA (Figure 4). However, the samples were inadequate for 2 reasons. Technically, hemolysis during centrifugation made some samples unsuitable for analysis. Because ELISA requires at least 3 replicates, the number of samples was relatively insufficient. Patients were reluctant to contribute additional blood samples. Finally, serum samples from only 7 patients in the placebo group and 5 patients in the USTK group at weeks 0 and 24 were analyzed. At week 0, both p35 and p40 protein levels were different between the placebo and USTK groups, so we performed self-control longitudinal comparison within groups to eliminate the bias. The p35 concentration in the placebo group was higher than that in the USTK group at week 0 (P<0.05). No difference in p35 levels was found between the 2 groups at week 24, nor did they change at week 24 when compared internally with the baseline level. The p40 levels at week 0 were higher in the USTK group (P<0.0001). The p40 levels of the placebo group at week 24 were higher than those at week 0 (P<0.0001). No difference was identified for the USTK group between weeks 0 and 24. Regarding the change in p40 expression in the placebo and USTK groups as a result of 2 and 3 USTK injections, respectively, we deduced that, after the first 2 injections of USTK, serum p40 protein levels were upregulated as p40 mRNA elevated, while the third USTK injection suppressed further increase in serum p40 protein levels. These data are summarized in Table 1.
Figure 4.
Scatter plots of p35 (A) and p40 (B) serum levels, as determined by ELISA. ● Denotes placebo group; ■ denotes USTK group; * P<0.05; *** P<0.0001. ELISA, enzyme-linked immunosorbent assay; USTK, ustekinumab.
Table 1.
Summary of qPCR and ELISA results.
| USTK injections | p35 | p40 | ||
|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | |
| 2 s.c. vs. pre | 2 s.c. >pre | n.c. | n.c. | 2 s.c. >pre |
| 3 s.c. vs. 2 s.c. | 3 s.c. <2 s.c. | n.d. | 3 s.c. >2 s.c. | n.c. |
| 3 s.c. vs. pre | n.d. | n.c. | 3 s.c. >pre | n.c. |
qPCR – quantitative polymerase chain reaction; ELISA – enzyme-linked immunosorbent assay; s.c. – subcutaneous injections; pre – pretreatment; n.c. – no change; n.d. – not determined.
Discussion
IL-12 and IL-23 are both heterodimeric cytokines. IL-12 is composed of the p35 and p40 subunits, whereas IL-23 is composed of p19 and p40 [10]. Similarly, the IL-12 receptor is a heterodimer consisting of IL-12RB1 and IL-12RB2, whereas the IL-23 receptor consists of IL-12RB1 and IL-23R. IL-12RB1 interacts with the p40 subunit, IL-12RB2 interacts with p35/IL-12, and IL-23R interacts with p19/IL-23.
IN psoriasis development, p35/IL-12 is a double-edged sword. Besides its ability to promote interferon-γ activity [11,12], p35 stimulates Janus kinase 2 and tyrosine kinase 2, leading to the activation of signal transducer and activator (STAT) family members, particularly STAT4 homodimers [13,14]. STAT4 activation can enhance Th1 cell differentiation [15] to aggravate psoriasis. However, IL-12 was later found to serve a protective role against psoriasis pathogenesis, as IL-12−/− mice showed psoriasiform skin lesions that were more severe than those of wild-type mice. IL-12RB2 and IL-12 p35 deficiency results in the upregulation of IL-17A, IL-17F, C-C motif chemokine ligand 20 (a marker of dermatotropic type 17 effector T cells) [16], granulocyte-macrophage colony-stimulating factor-2, C-X-C motif chemokine ligand 1 (CXCL)-1, CXCL-5, transforming growth factor-β, and tumor necrosis factor [17,18].
It is known that p40 is a cytokine that acts as a regulator of a variety of hematopoietic cells. It can induce IL-8 [19,20], which promotes angiogenesis and keratinocyte proliferation [21].
In our study, we found a significant improvement in PASI scores in the USTK group at week 12 in contrast to week 0, which confirmed the efficacy of USTK in treating psoriasis. However, the improvement levels between the 2 groups were similar at week 24. PASI-75% was significantly higher in the USTK group at week 12 compared to week 0 but was similar in both groups at week 24. Furthermore, PASI-75% was over 60% in the USTK group at week 12 and the placebo group at week 24. These findings indicate that 2 injections of USTK were adequate in achieving the maximum efficacy.
The recommended dosage of USTK for active psoriatic arthritis and moderate-to-severe plaque type psoriasis is 45 mg at weeks 0 and 4, then every 12 weeks afterward. Alternatively, 90 mg may be used for patients with a body weight greater than 100 kg. Therefore, we hypothesized that the subsequent injections intensified the effect of USTK and maintained its efficacy.
In our study, compared to the placebo group, the USTK group’s p35 mRNA levels increased at week 12 but decreased at week 24. No statistical difference in p35 protein levels was found when comparing week 24 to week 0. This outcome was understandable considering that mRNA serves as the template for protein translation.
Regarding p40, the USTK group showed a constant increase in its mRNA levels. p40 protein levels increased in the placebo group but did not change in the USTK group. USTK neutralized the p40 protein but did not directly interact with p40 mRNA. Thus, we think that the increase in mRNA led to an upregulated protein synthesis of p40. Sustained administrations of USTK eliminated the corresponding excessive production levels of p40 protein, which potentially explains why long-term USTK therapy is necessary. The course of treatment should be extended, and the number of samples should be increased to validate this possibility.
Although USTK has been approved by U.S. Food and Drug Administration for years, its clinic data in China has limited due to economic reasons. As is known, psoriasis patients suffer from the frequent relapses of the disease under the traditional treatment, which made them depressed. Meanwhile, based on the small cohort, we observed interactions between USTK and gene expressions, suggesting that USTK cured psoriasis by regulating disease-associated genes, the upstream factors of cytokine networks. So, it may explain why USTK possesses a rapid and long-lasting effect, compared with traditional management [6,7]. From this perspective, the mechanism of USTK may light up new hopes for patients and the dermatologists.
Unfortunately, the sample size of our research was not adequate to be convincing. However, the result provides an initial investigation in the field of pharmacogenomics, implying the necessity of exploring USTK’s interactions with genes. With the rising acceptance of biologic agents, USTK included, we hope to be able to continue accumulating data from Chinese patients to validate the interplays between biologics and genes in the future. Further studies may include 1) determine if additional injections of USTK can neutralize excessive p40 protein, 2) quantify mRNA and protein levels of the p19 subunit of IL-23, and 3) investigate larger cohorts with longer study durations to decipher the underlying molecular mechanisms.
Conclusions
We demonstrated that 2 equilibria are involved in USTK efficacy against psoriasis (Figure 5). Firstly, IL-12 plays a dual role in psoriasis pathogenesis. While the suppression of p40 levels by USTK is helpful for patients to recover from psoriasis, the same effect on p35 levels may bring about negative effects. Subtle homeostasis exists between p35 and p40 expression levels. Secondly, although USTK is a neutralizing antibody against p40, mRNA and protein expression of p40 did not demonstrate a tendency to be reduced at all points in the study period. During long-term treatment with USTK, an equilibrium may be reached between the upregulation of p40 mRNA levels and the neutralization of the elevated p40 protein by USTK. We cannot predict which one predominates.
Figure 5.
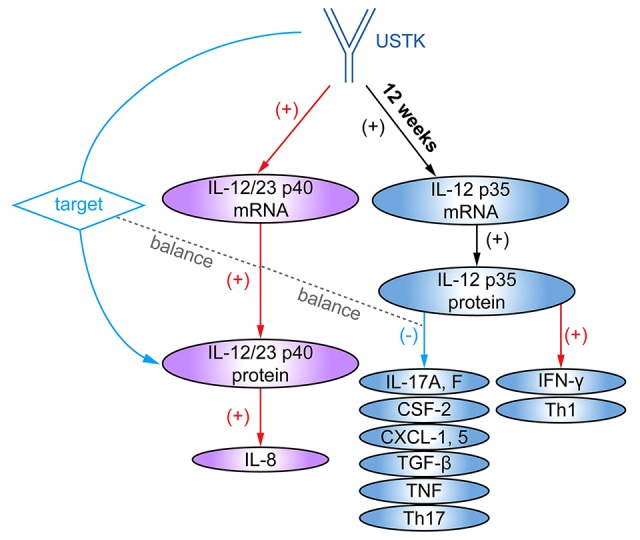
Equilibrium p35 and p40 levels involved in USTK efficacy in treating psoriasis. The blue arrows denote positive effects in the treatment of psoriasis. The red arrows denote adverse effects. The 2 gray dashed lines between the red and blue arrows indicate the balance between positive and negative effects. USTK – ustekinumab.
Although our result seemed to be preliminary, we noticed it may be worthy in treatment selection. To optimize the management of moderate-to-severe psoriasis, dermatologists should weigh the pros and cons of the therapy. In other word, we should identify patients appropriate for USTK before treatment initiation. A more personalized dosage may be predicted in the further study of dose-response relationship associated with pharmacogenomics. Although the clinical efficacy of USTK has been confirmed, its exact molecular mechanism needs to be deciphered.
Supplementary Data
Supplementary Table 1.
PASI scores of all patients throughout the study.
| Patient No. | Group | PASI | ||
|---|---|---|---|---|
| W0 | W12 | W24 | ||
| 1 | P | 28.2 | 22.2 | 0.4 |
| 2 | P | 27.4 | 4.2 | 2.2 |
| 3 | P | 19.8 | 23.1 | 1.0 |
| 4 | P | 54.0 | 54.0 | 6.9 |
| 5 | P | 15.6 | 12.8 | 0.0 |
| 6 | P | 12.2 | 13.2 | 0.7 |
| 7 | P | 17.6 | 33.1 | 1.6 |
| 8 | P | 28.2 | 4.8 | 1.4 |
| 9 | P | 14.6 | 17.8 | 6.4 |
| 10 | P | 12.2 | 9.6 | 2.7 |
| 11 | P | 21.4 | 30.2 | 4.2 |
| 12 | P | 13.2 | 15.9 | 0.0 |
| 13 | P | 13.2 | 28.8 | 9.8 |
| 14 | U | 23.2 | 15.8 | 15.6 |
| 15 | U | 22.9 | 15.6 | 3.0 |
| 16 | U | 22.5 | 6.1 | 3.3 |
| 17 | U | 20.6 | 1.6 | 0.5 |
| 18 | U | 26.0 | 1.8 | 9.3 |
| 19 | U | 14.9 | 4.8 | 0.0 |
| 20 | U | 34.8 | 2.4 | 0.0 |
| 21 | U | 16.2 | 0.0 | 0.0 |
| 22 | U | 20.0 | 0.0 | 1.4 |
| 23 | U | 24.6 | 2.3 | 0.1 |
| 24 | U | 15.4 | 0.1 | 0.0 |
P – placebo; U – ustekinumab. W0 – week 0; W12 – week 12, W24 – week 24.
Footnotes
Conflict of interest
None.
Source of support: The National Natural Science Foundation of China (NSFC) (grant number 81402594); the Zhejiang Provincial Health Technology Planning Project (grant number 2018KY088); and the A 2018 CMA-L’OREAL China Skin/Hair Grant (grant number S2018-012)
References
- 1.Deng Y, Chang C, Lu Q. The inflammatory response in psoriasis: A comprehensive review. Clin Rev Allergy Immunol. 2016;50:377–89. doi: 10.1007/s12016-016-8535-x. [DOI] [PubMed] [Google Scholar]
- 2.Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: A comprehensive review. J Autoimmun. 2015;64:66–73. doi: 10.1016/j.jaut.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy M, Torres G, McCormick T, et al. Positive treatment effects of ustekinumab in psoriasis: Analysis of lesional and systemic parameters. J Dermatol. 2010;37:413–25. doi: 10.1111/j.1346-8138.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 4.Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: A phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL) J Dermatol Sci. 2011;63:154–63. doi: 10.1016/j.jdermsci.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: A systematic review and meta-analysis. J Invest Dermatol. 2015;135:2641–48. doi: 10.1038/jid.2015.206. [DOI] [PubMed] [Google Scholar]
- 7.Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: Results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSORIASISLAR]) J Am Acad Dermatol. 2016;74:851–61.e4. doi: 10.1016/j.jaad.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000–6. doi: 10.1136/annrheumdis-2013-204741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimball AB, Gordon KB, Fakharzadeh S, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: Results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol. 2012;166:861–72. doi: 10.1111/j.1365-2133.2012.10901.x. [DOI] [PubMed] [Google Scholar]
- 10.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Vignali DA, Kuchroo VK. IL-12 family cytokines: Immunological playmakers. Nat Immunol. 2012;13:722–28. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasconcellos R, Carter NA, Rosser EC, Mauri C. IL-12p35 subunit contributes to autoimmunity by limiting IL-27-driven regulatory responses. J Immunol. 2011;187:3402–12. doi: 10.4049/jimmunol.1100224. [DOI] [PubMed] [Google Scholar]
- 13.Watford WT, Hissong BD, Bream JH, et al. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–56. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 15.Letimier FA, Passini N, Gasparian S, et al. Chromatin remodeling by the SWI/SNF-like BAF complex and STAT4 activation synergistically induce IL-12Rbeta2 expression during human Th1 cell differentiation. EMBO J. 2007;26:1292–302. doi: 10.1038/sj.emboj.7601586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulig P, Musiol S, Freiberger SN, et al. IL-12 protects from psoriasiform skin inflammation. Nat Commun. 2016;7:13466. doi: 10.1038/ncomms13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Held J, Preusse C, Doser A, et al. Enhanced acute immune response in IL-12p35−/− mice is followed by accelerated distinct repair mechanisms in Staphylococcus aureus-induced murine brain abscess. J Infect Dis. 2013;208:749–60. doi: 10.1093/infdis/jit126. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Zhang C, Wu Y, et al. Interleukin-12p35 deletion promotes CD4 T-cell-dependent macrophage differentiation and enhances angiotensin II-Induced cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32:1662–74. doi: 10.1161/ATVBAHA.112.249706. [DOI] [PubMed] [Google Scholar]
- 19.Nalleweg N, Chiriac MT, Podstawa E, et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut. 2015;64:743–55. doi: 10.1136/gutjnl-2013-305947. [DOI] [PubMed] [Google Scholar]
- 20.Hong CH, Chang KL, Wang HJ, et al. IL-9 induces IL-8 production via STIM1 activation and ERK phosphorylation in epidermal keratinocytes: A plausible mechanism of IL-9R in atopic dermatitis. J Dermatol Sci. 2015;78:206–14. doi: 10.1016/j.jdermsci.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Coimbra S, Oliveira H, Reis F, et al. Interleukin (IL)-22, IL-17, IL-23, IL-8, vascular endothelial growth factor and tumor necrosis factor-alpha levels in patients with psoriasis before, during and after psoralen-ultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol. 2010;163:1282–90. doi: 10.1111/j.1365-2133.2010.09992.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
PASI scores of all patients throughout the study.
| Patient No. | Group | PASI | ||
|---|---|---|---|---|
| W0 | W12 | W24 | ||
| 1 | P | 28.2 | 22.2 | 0.4 |
| 2 | P | 27.4 | 4.2 | 2.2 |
| 3 | P | 19.8 | 23.1 | 1.0 |
| 4 | P | 54.0 | 54.0 | 6.9 |
| 5 | P | 15.6 | 12.8 | 0.0 |
| 6 | P | 12.2 | 13.2 | 0.7 |
| 7 | P | 17.6 | 33.1 | 1.6 |
| 8 | P | 28.2 | 4.8 | 1.4 |
| 9 | P | 14.6 | 17.8 | 6.4 |
| 10 | P | 12.2 | 9.6 | 2.7 |
| 11 | P | 21.4 | 30.2 | 4.2 |
| 12 | P | 13.2 | 15.9 | 0.0 |
| 13 | P | 13.2 | 28.8 | 9.8 |
| 14 | U | 23.2 | 15.8 | 15.6 |
| 15 | U | 22.9 | 15.6 | 3.0 |
| 16 | U | 22.5 | 6.1 | 3.3 |
| 17 | U | 20.6 | 1.6 | 0.5 |
| 18 | U | 26.0 | 1.8 | 9.3 |
| 19 | U | 14.9 | 4.8 | 0.0 |
| 20 | U | 34.8 | 2.4 | 0.0 |
| 21 | U | 16.2 | 0.0 | 0.0 |
| 22 | U | 20.0 | 0.0 | 1.4 |
| 23 | U | 24.6 | 2.3 | 0.1 |
| 24 | U | 15.4 | 0.1 | 0.0 |
P – placebo; U – ustekinumab. W0 – week 0; W12 – week 12, W24 – week 24.