Abstract
Background
Controversies exist in imaging modalities for predicting adenoma consistency. In this study, we proposed a method of predicting consistency by magnetic resonance T2-sequence imaging based on adenoma to cerebellar peduncle signal (TCTI) ratio.
Material/Methods
Between January 2013 and May 2017, 191 consecutive patients with pituitary adenoma diagnosed at our institution were retrospectively studied. The consistency grade for each lesion was assigned. And the TCTI ratio based on preoperative and postoperative T2-weighted imaging was calculated.
Results
The median TCTI ratio was 1.55, 1.28, and 1.25 for soft, fibrous, and hard adenomas, respectively. The differences were significant for all groups (p<0.001). A cutoff value of 1.38 for soft adenomas was found to be 80.2% sensitive and 88.7% specific. The median ratio of the outermost layer of residual tumor was 1.25 (SD±0.408, 95% CI 1.27–1.42). It was less than that ratio of the upper, lower quarter, and middle region of adenoma, respectively, and the inter-group differences were all statistically significant with p≤0.001. The extent of resection for the soft group was significantly greater than that of the hard group (85.3% vs. 70.6%, p=0.011). Analysis of Variance (ANOVA) revealed that the consistency grade was the influencing factor of degree of resection. p=0.003.
Conclusions
The TCTI ratio showed a good correlation with pituitary adenoma consistency. We also determined the optimal ratio of the residual adenoma.
MeSH Keywords: Decision Support Techniques, Magnetic Resonance Imaging, Pituitary Neoplasms
Background
Pituitary adenomas usually have a soft texture [1–5], and this type of adenoma is easily resected with curettage and suction [6–8]. Approximately 5–13% of pituitary adenomas have a firm consistency [6–9]. Compared with the easy suctioning of soft tumors, firm tumors can pose a great challenge to neurosurgeons because they are more difficult to dissect from critical structures [9–11]. If the texture of a pituitary adenoma is known preoperatively, patients can be informed about the real target, surgical expectations, the risk for second surgeries from above, and any need for post-surgical adjuvant radiotherapy/radiosurgery. Furthermore, it may help surgeons to plan a better operative strategy and to reduce the risk of surgery.
There have been many studies on distinguishing tissues or predicting tumor consistency, including development of a variety of imaging modalities [1–4,6–8,10–18]. However, it is unclear whether the signal intensity of a pituitary adenoma in radiologic imaging correlates with its consistency. Some studies suggest that diffusion-weighted imaging and magnetic resonance T2-sequence intensity help to predict the texture of pituitary adenomas [5,7,14,16], while other research did not yield statistically significant results [6,8,13,19]. Ma et al. concluded that the consistency of a pituitary adenoma could be predicted using MR T1-spin echo imaging [15]. Yamamoto et al. obtained a similar conclusion on contrast-enhanced fast imaging employing steady-state acquisition [3]. Although there are controversies, it seems that T2-weighted sequences strongly indicate tumor consistency.
The consistency of an adenoma affects its removal [20]. To the best of our knowledge, there has been no research investigating the relationship between pituitary adenoma consistency and degree of resection based on the tumor/cerebellar peduncle T2-weighted imaging intensity (TCTI) ratio of the point on preoperative MR image corresponding to the residual point on postoperative MR image. We hypothesized that the signal value in T2-weighted sequences predicted the consistency of adenomas. Thus, the signal of the residual adenoma should be different from that of its surrounding tumor measured before surgery. We conducted this study to determine whether TCTI ratio allows prediction of tumor consistency and whether the signal ratio of the residual tumor is different from its surrounding area [16,18].
Material and Methods
Patients and study outline
Between January 2013 and May 2017, 191 consecutive patients who met the inclusion and exclusion criteria with pituitary adenoma diagnosed at the First Affiliated Hospital of Fujian Medical University were retrospectively included in our study. Patient data, including clinical assessment (at admission, during the immediate postoperative period), and preoperative and postoperative images were collected. The inclusion criteria included the MRI consistent with pituitary adenoma, which was done within 1 month before surgery. The exclusion criteria included previous surgery, history of radiotherapy, and history of bromocriptine or other drugs therapy.
Clinical setting
All patients who underwent microscopic transsphenoidal surgery or neuroendoscopic surgery were operated on by a senior surgeon and his assistant at our institution. Utilizing the most widely used classification method for pituitary adenomas, a consistency grade was assigned based on the surgical instruments used and surgeons’ unanimous identification [8]. Based on their consistency, all tumors included in our study were categorized as soft (easily removed by suction), fibrous (removed with difficulty by suction), or hard (not removable by suction, and excised en bloc), as determined by the primary neurosurgeon during surgery.
Image analysis
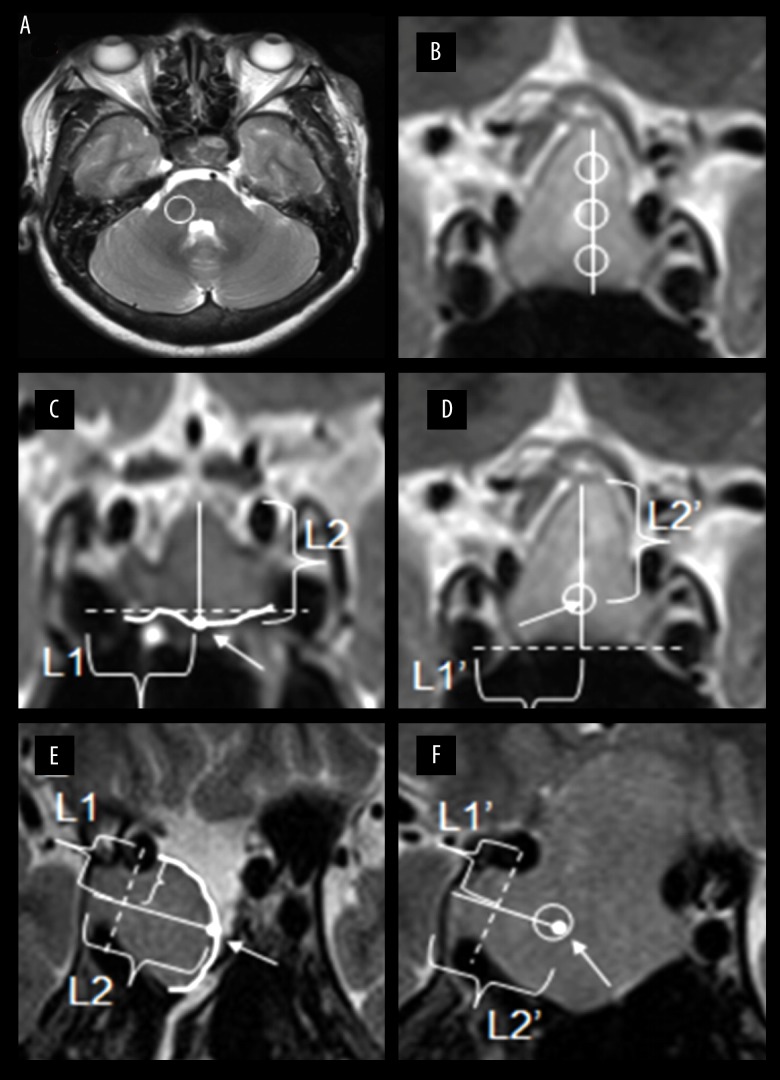
All imaging evaluations were conducted by a senior neurosurgical expert who was blind to patient information and has focused on the comprehensive treatment of pituitary adenoma for over 10 years (C.Z.J.). The postoperative 1.5T MR images of these patients were all scanned 3 to 7 days after surgery. A region of interest (ROI) was selected from the cerebellar peduncle in axial sequence [16,18] (Figure 1A). This peduncle ROI, which was circular and of fixed size, was selected by the neurosurgeon blinded to patient information. Three ROIs was selected in a circular shape within the adenoma for representative samplings in coronal sequencing of homogenous tumors [14,16,18] (Figure 1B). Alternatively, for heterogeneous adenomas, multiple ROIs were selected and a representative mean ratio was calculated for the lesion. Microhemorrhagic, necrotic, and microcystic areas were excluded from the ROIs [14]. Utilizing preoperative T2-weighted MR images, TCTI ratios were calculated with the upper and lower quarter regions and middle region of the adenoma. The residual tumor descended or moved in the horizontal line. Thus, the distance from the residual stump to the unresected margin was relatively fixed. In our study, we localized the residual tumor stump in pre-/post-operative MR images with the help of the cavernous segment of the internal carotid artery and the relative distance from the residual stump to the opposite margin. Using postoperative MR images, the distance between the incisal margin and the opposite margin of the residual adenoma was calculated (Figure 1C, 1E). Then, the point of the residual tumor stump was found on the preoperative MR image (Figure 1D, 1F). Similarly, the TCTI ratio using this point was calculated. In this manner, we reduced sampling error. Using MR T2-weighted sequence ROIs, adenomas of different consistencies had corresponding signal ratios. Tumor size was defined as the maximum diameter of the pituitary adenoma in 3 relative planes (axial, coronal, and sagittal). Then, consistency categories and ratios were analyzed.
Figure 1.
Schematic diagrams for 2 cases in different residual forms. (A) ROI in the cerebellar peduncle was drawn on axial T2-weighted image. (B) The solid line represents the maximum length of tumor. Three points divided the solid line into 4 equal parts. Based on the points, tumor ROIs in 3 regions were selected on coronal T2-weighted image. (C, D) Case 1 shows the residual tumor mainly in the upper part. A dotted line was made connecting the central points of the cavernous segment of internal carotid artery on both sides. The white dot was the midpoint of the curve which represented incisal margin (white arrow). The distance between the incisal margin and the opposite margin based on white dot perpendicular to the dotted line was drawn and named L2. The length from the intersection of 2 lines to the central point of right internal carotid artery was drawn and named L1. According to line L1’ and line L2’, which was equal to line L1 and line L2, the point we found on preoperative MR was equal to that which was drawn on postoperative MR both in length and location (white arrow). ROI for the residual point was selected on the preoperative T2-weighted image. (E, F) Case 2 shows the residual tumor mainly in the invasive part of cavernous sinus segment of internal carotid artery. A dotted line was made connecting the central points of the 2 segments of internal carotid artery on the right side. The white dot was the midpoint of the curve which represented incisal margin (white arrow). The diameter of the residual adenoma based on white dot perpendicular to the dotted line was drawn and named L2. The length from the intersection of 2 lines to the central point of right internal carotid artery was drawn and named L1. According to line L1’ and line L2’, which was equal to line L1 and line L2, the point we found on preoperative MR was equal to that which was drawn on postoperative MR both in length and location (white arrow). The ROI for the residual point was selected on the preoperative T2-weighted image.
Statistical analysis
The independent-samples t test was used to compare mean ratios of pituitary adenomas to brainstem signal intensity on MR T2-weighted sequences. Receiver operating characteristic analysis was performed to determine an optimal ratio of pituitary adenoma to brainstem T2-weighted signal intensity threshold, for which the Youden index is theoretically the maximum. Using 1-way ANOVA, we compared TCTI ratios and consistency grades. Dunnett’s test was used to analyze variance (degree of resection) on the 3 consistency groups. For all statistics of patient’s characteristics related to consistency grade, 1-way ANOVA and chi-square test were used to assess statistically significant differences. Statistical analysis was performed with SPSS version 19.0. P values less than 0.05 were considered as statistically significant.
Results
Demographic and clinical data
Patient characteristics are displayed in Table 1. There were 161 (85.3%) patients with adenoma size of 1 to 4 cm. Symptoms of 118 (62.1%) patients were associated with compressed surrounding structure; 17 (8.9%) patients had complications and 2 (1.0%) of them had reoperation; 64 (38.3%) patients had residual adenoma invading the cavernous sinus, and the other 103 did not. Immunohistochemical subtypes in the 3 groups were significantly different (p=0.001). The preoperative and postoperative hematological indexes are shown in detail in Table 1.
Table 1.
Summary of characteristics of 191 patients.
| Characteristic | Value | Soft | Fibrous | Hard | p |
|---|---|---|---|---|---|
| Mean age±SD, years | 47.9±14 | 47.5±14 | 49.8±14 | 47.2±14 | 0.641 |
| Sex | 0.772 | ||||
| Male | 91 (47.6%) | 59 (53.2%) | 19 (47.5%) | 22 (55%) | |
| Female | 100 (52.4%) | 52 (46.8%) | 21 (52.5%) | 18 (45%) | |
| Size | 0.255 | ||||
| Micro ≤1 cm | 4 (2.1%) | 2 (1.8%) | 0 (0.0%) | 2 (5.0%) | |
| Large 1–4 cm | 163 (85.3%) | 91 (82.0%) | 37 (92.5%) | 35 (87.5%) | |
| Giant >4 cm | 24 (12.6%) | 18 (16.2%) | 3 (7.5%) | 3 (7.5%) | |
| Knosp grade | 0.369 | ||||
| 0 | 11 (5.8%) | 5 (4.5%) | 4 (10.0%) | 2 (5.0%) | |
| 1 | 38 (19.9%) | 22 (19.8%) | 9 (22.5%) | 7 (17.5%) | |
| 2 | 44 (23.0%) | 21 (18.9%) | 9 (22.5%) | 14 (35.0%) | |
| 3 | 33 (17.3%) | 18 (16.2%) | 8 (20.0%) | 7 (17.5%) | |
| 4 | 65 (34.0%) | 45 (40.5%) | 10 (25.0%) | 10 (25.0%) | |
| Preoperative hematological parameters | |||||
| ACTH 8am pg/mL | 37.96±32.23 | 36.90±34.78 | 38.39±27.56 | 40.70±29.51 | 0.858 |
| ACTH 4pm pg/mL | 22.03±22.13 | 20.95±17.43 | 26.13±30.25 | 20.63±24.31 | 0.497 |
| PRL mIU/L | 416.90 (204.60–830.00) | 385.80 (197.10–803.00) | 497.70 (296.35–967.65) | 328.30 (204.50–844.00) | 0.387 |
| GH ug/L | 92.37±1058 | 147.71±1378 | 7.41±17.09 | 18.35±52.12 | 0.721 |
| TSH mIU/L | 2.34±3.90 | 2.03±2.71 | 3.39±6.92 | 2.21±2.31 | 0.198 |
| FSH IU/L | 15.64±26.87 | 16.56±30.65 | 12.81±16.43 | 15.96±24.24 | 0.768 |
| LH IU/L | 5.26±7.66 | 5.52±8.94 | 4.76±5.72 | 5.01±5.1 | 0.857 |
| Main complain | |||||
| Excessive hormone secretion | 25 (13.2%) | 13 (11.8%) | 6 (15.0%) | 6 (15.0%) | |
| Insufficient hormone secretion | 26 (13.7%) | 15 (13.6%) | 4 (10.0%) | 7 (17.5%) | |
| Compression of surrounding structure | 118 (62.1%) | 75 (68.2%) | 22 (55.0%) | 21 (52.5%) | |
| Tumor stroke | 5 (2.6%) | 1 (0.9%) | 2 (5.0%) | 2 (5.0%) | |
| Absence of symptoms | 16 (8.4%) | 6 (5.5%) | 6 (15.0%) | 4 (10.0%) | |
| Hematological index after surgery (Day 1) | |||||
| ACTH 8 am pg/mL | 41.33±77.64 | 35.72±52.61 | 62.02±131.05 | 28.00±18.93 | 0.329 |
| ACTH 4 pm pg/mL | 59.86±101.11 | 75.71±114.78 | 55.38±77.99 | 68.09±57.28 | 0.739 |
| PRL mIU/L | 207.80 (91.90–524.80) | 191.15 (78.09–531.08) | 312.85 (187.65–570.50) | 181.40 (45.94–305.40) | 0.604 |
| GH ug/L | 5.95±10.89 | 5.74±11.26 | 6.54±12.08 | 5.96±8.08 | 0.962 |
| TSH mIU/L | 5.39±40.63 | 7.94±52.17 | 1.83±3.42 | 1.02±1.00 | 0.702 |
| FSH IU/L | 10.40±11.98 | 10.37±12.98 | 9.78±9.75 | 11.66±11.87 | 0.896 |
| LH IU/L | 4.84±6.32 | 4.37±6.15 | 4.60±5.79 | 7.50±7.87 | 0.263 |
| Immunohistochemical subtypes | 0.001* | ||||
| ACTH positive | 6 (3.1%) | 5 (4.5%) | 1 (2.5%) | 0 (0.0%) | |
| FSH positive | 30 (15.7%) | 8 (7.2%) | 14 (35.0%) | 8 (20.0%) | |
| GH positive | 4 (2.1%) | 3 (2.7%) | 0 (0.0%) | 1 (2.5%) | |
| LH positive | 1 (0.5%) | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | |
| PRL positive | 18 (9.4%) | 11 (9.9%) | 5 (12.5%) | 2 (5.0%) | |
| TSH positive | 1 (0.5%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) | |
| Multiple items positive | 17 (8.9%) | 9 (8.1%) | 4 (10.0%) | 4 (10.0%) | |
| Negative | 110 (57.6%) | 71 (64.0%) | 15 (37.5%) | 24 (60.0%) | |
| Residual adenoma invading cavernous sinus | 0.912 | ||||
| No | 104 (61.5%) | 60 (60.6%) | 22 (61.1%) | 22 (64.7%) | |
| Yes | 65 (38.5%) | 39 (39.4%) | 14 (38.9%) | 12 (35.3%) | |
| Complication | 0.333 | ||||
| No | 174 (91.1%) | 103 (92.8%) | 34 (85.0%) | 37 (92.5%) | |
| Yes | 17 (8.9%) | 8 (7.2%) | 6 (15.0%) | 3 (7.5%) | |
Relationship between preoperative images and adenoma consistency
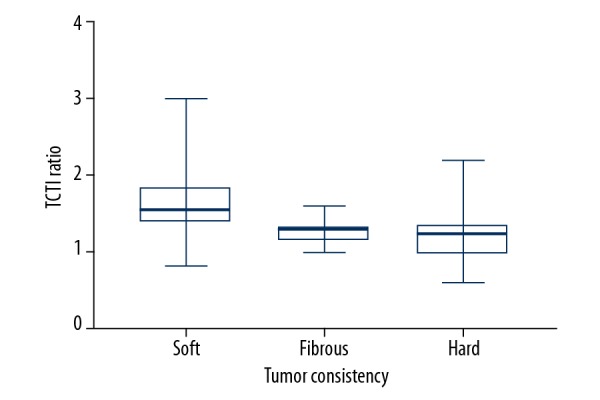
Data were collected for 191 consecutive patients (Table 1); 111 tumors were categorized as soft, 40 as fibrous, and 40 as hard. Figure 2 illustrates the frequency distribution of tumor consistency groups. The TCTI ratio for all pituitary adenoma was from 0.6 to 3.01, with a mean ratio of 1.46. For soft adenomas ratios, the mean value was 1.64 (SD±0.400, 95% CI 1.56–1.71); for fibrous adenomas, the mean ratio was 1.25 (SD±0.128, 95% CI 1.21–1.29); and for hard adenomas, the mean ratio was 1.20 (SD±0.388, 95% CI 1.13–1.29).
Figure 2.
Frequency distribution for TCTI ratio by consistency.
The consistency grades of pituitary adenomas and TCTI ratios were compared with 1-way ANOVA. The mean TCTI ratio of soft adenomas was significantly different from that of the other groups (p<0.001) (Table 2). A correlation was determined between consistency ratings and TCTI ratios.
Table 2.
Pituitary adenoma consistency groups statistics.
| Category | Overall TCTI Ratio | Pituitary adenoma Consistency Grade | ||
|---|---|---|---|---|
| Soft | Fibrous | Hard | ||
| Mean | 1.46 | 1.64 | 1.25 | 1.21 |
| Median | 1.39 | 1.55 | 1.28 | 1.23 |
| Minimum | 0.60 | 0.83 | 1.00 | 0.60 |
| Maximum | 3.01 | 3.01 | 1.60 | 2.2 |
| SD | 0.40 | 0.13 | 0.26 | |
| 95% CI | 1.56–1.71 | 1.21–1.29 | 1.23–1.29 | |
ROC curve analysis was done to determine an exact number that could quickly verify the consistency of the adenoma. The optimal cutoff value of TCTI ratios was determined to be 1.38, with soft adenomas ≥1.38. The value of area under the curve (AUC) was 0.862. This ratio cutoff is 80.2% sensitive and 88.7% specific.L2 The point of residual adenoma on the preoperative image and its TICI ratio on the preoperative image
The preoperative and postoperative imaging data were obtained for 157 consecutive patients, with 49 patients received total resection. The residual tumor volume for the other 108 patients was calculated. Two patients, whose MR image files were not loaded, were eliminated as well. Thus, the distance of the residual adenoma from stump to top was calculated for 106 patients, as well as the TICI ratio, using the point where the residual tumor stump was in found in the preoperative MR image as a reference.
The mean residual tumor volume was 4.03±7.803 cm≥ (95% CI 2.73–5.67). The mean degree of resection was 79.9±25.9% (95% CI 75.8–83.6%), and the mean distance between the incisal margin and the opposite margin of the residual adenoma was 1.54 cm (SD±0.76, 95% CI 1.40–1.69 cm). The mean ratio for the residual point was 1.34 (SD±0.408, 95% CI 1.27–1.42). The ratio was less than the ratio of the upper quarter, lower quarter, and middle regions of adenomas, and the differences were all statistically significant (p≤0.001).
According to the Knosp E’s classification system, the degrees of resection for grade 0–4 adenomas were 84.0%, 81.0%, 86.6%, 78.2%, and 74.2%, respectively, but there was no significant difference between them. When they were divided into 2 groups (grade 0–2 and grade 3–4), degree of resection of the former group was greater than that of the latter group (83.8% vs. 75.6%, p=0.045). The degree of resection for adenomas in which there was a residual tumor invading the cavernous sinus after resection was less than in the ones without such invasion, at 69.9% vs. 85.6%, respectively (p<0.001). The mean preoperative adenoma volume of the total resection group was less than that of the partial resection group (4.23 cm3 vs. 13.10 cm3) (p<0.001). The degree of resection for the soft group was significantly greater than that of the hard group, at 85.3% vs. 70.6% (p=0.011). There were no significant differences in degree of resection between the soft group and the fibrous group (85.3% vs. 72.5%) (p=0.032); similarly, for the fibrous group and hard group, the degrees of resection were 72.5% vs. 70.6% (p=0.991). ANOVA results showed that the consistency rating was the factor influencing the degree of resection (p=0.003). After dividing the patients into higher Knosp grades (3–4) and lower Knosp grades (0–2) for subgroup analysis, the degree of resection for the soft group was significantly greater than that of the hard group in the lower Knosp grades group (90.7% vs. 69.3%, p=0.003). In the higher Knosp grades group, there was no significant difference between thew soft group and hard group (80.0% vs. 66.6%, p=0.17).
Discussion
Prediction of consistency carries implications for the neurosurgeon. However, it is clinically valuable to reliably evaluate consistency as it can lead to better counseling of surgical risks.
Several research studies had shown that pituitary adenoma texture is one of the factors affecting resection rate [10,14]. We also found that the consistency rate was correlated with degree of resection. In the subgroups analysis, we divided the patients into higher Knosp grades (3–4) and lower Knosp grades (0–2), and we got the same result in the lower Knosp grades group. However, the different consistency groups did not have a statistically significant difference with extent of resection in the higher Knosp grades group. Compared to the lower Knosp grades group, tumors with higher Knosp grades are more difficult to remove. Besides consistency, there are many factors affecting tumor resection in the higher Knosp grades group. For pituitary adenomas with lower Knosp grades, tumor consistency is the main factor determining the surgical difficulty, which may why the 2 subgroups had different results. Detailed preoperative evaluation is helpful to increase the degree of resection. By means of postoperative images, we analyzed the TCTI ratio of residual adenomas for 106 patients. The ratio of the outermost layer of residual tumor was less than that of the 3 regions we picked in preoperative imaging. The upper limit of the 95% confidence interval was less than 1.38, which means the TCTI ratio of the outermost layer a residual tumor is less than its surrounding region. As the consistency affected degree of tumor resection, the outer layer was believed to be harder than its surrounding region. These results further strengthen the evidence linking TCTI ratio with adenoma consistency. Compared with other studies focusing on predicting consistency of pituitary adenomas, this is the first study utilizing the postoperative image to test a prior hypothesis. In consideration of tumor transposition and descending of the sellar septum, we took advantage of the cavernous segment of the internal carotid artery to measure the relative distance and locate the residual point. There were 2 measurement methods because of the 2 main residual conditions. The comparison between preoperative and postoperative images showed the residual adenoma on a preoperative image by calculating the distance from the residual top to the stump. We did not calculate TCTI ratio on postoperative image because it could be caused by fillers used during the surgery, rather than from the residual adenoma. This also reduced errors in our study. Our results confirmed that TCTI ratio is strongly correlated with consistency of pituitary adenomas, and the consistency rating was the factor influencing the degree of resection. We also found that the outermost layer of residual adenomas was harder than the unitary texture, illustrating that use of a single region for evaluating tumor consistency is insufficient, and consistency can vary in different regions of adenomas. En bloc evaluation on preoperative image is necessary to reflecting its overall consistency.
In this study, we investigated the relationship between pituitary adenoma consistency and extent of resection based on TCTI ratio of the point on the preoperative MR images corresponding to the residual point on the postoperative MR image. TCTI ratios of fibrous and hard adenomas were significantly lower than that of soft adenomas. A TCTI of 1.38 was 80.2% sensitive and 88.7% specific for soft adenomas (≥1.38). Pathological studies suggest that collagen content in firm adenomas is greater than that of soft ones [3,7,10,11,14]. It has been proven that the percentage of collagen content is reversely associated with T2-sequence intensity of adenomas and other relevant tissues [7,9,21–24]. Furthermore, fibrous tumors with low water content would have lower T2-weighted signal intensity than soft resectable tumors. Other studies also showed that increased vascularity and expression of TGFβ1 play an important role in adenoma consistency [11,25]. For our study, the lower ratio of hard adenomas was a reasonable result. Prediction of adenoma consistency helps plan better operative strategies and reduces the risk of surgery.
There were also limitations regarding our consistency analysis. Different researchers graded tumor consistency differently. Our consistency categories were classified into 3 groups, while some authors rate into only soft and firm groups. In addition, there is a continuum of consistency in clinical practice. Although we used standard criteria to assess texture by the same surgeon, this method for assessment is subjective and can cause bias. Although we utilized the postoperative MRI scan performed 3 to 7 days after surgery, results may be affected by artifacts or other effects. Intraoperative MRI, which precisely assesses the degree of tumor removal, could minimize the bias.
Conclusions
The TCTI ratio on MR T2-sequence imaging showed good correlation with adenoma consistency. A cutoff ratio of 1.38 showed good sensitivity and specificity for predicting soft consistency. Also, the residual adenoma had a signal ratio lower than that of its surrounding region, which meant hard consistency in our study. The consistency rate was correlated with degree of resection, especially in the patients with lower Knosp grades. Further prospective and large-scale studies should be conducted to validate our conclusions.
Abbreviations
- MRI
magnetic resonance imaging
- ANOVA
analysis of variance
- FIESTA
fast imaging employing steady-state acquisition
- TCTI
tumor/cerebellar peduncle T2-weighted imaging intensity
- ROI
region of interest
- AUC
area under curve
- DWI
diffusion-weighted imaging
- ADC
apparent diffusion coefficient
Footnotes
Conflict of interest
None.
Source of support: This study was supported by grants from the Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C., the Major Project of Fujian Provincial Department of Science and Technology (No. 2014YZ0003) and the Startup Fund for Scientific Research, Fujian Medical University (No. 2017XQ1084)
References
- 1.Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–91. doi: 10.1148/radiol.2353031338. [DOI] [PubMed] [Google Scholar]
- 2.Heck A, Ringstad G, Fougner SL, et al. Intensity of pituitary adenoma on T2-weighted magnetic resonance imaging predicts the response to octreotide treatment in newly diagnosed acromegaly. Clin Endocrinol. 2012;77:72–78. doi: 10.1111/j.1365-2265.2011.04286.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto J, Kakeda S, Shimajiri S, et al. Tumor consistency of pituitary macroadenomas: predictive analysis on the basis of imaging features with contrast-enhanced 3D FIESTA at 3T. Am J Neuroradiol. 2013;35:297–303. doi: 10.3174/ajnr.A3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potorac I, Petrossians P, Daly AF, et al. Pituitary MRI characteristics in 297 acromegaly patients based on T2-weighted sequences. Endocr Relat Cancer. 2015;22:169–77. doi: 10.1530/ERC-14-0305. [DOI] [PubMed] [Google Scholar]
- 5.Wei L, Lin SA, Fan K, et al. Relationship between pituitary adenoma texture and collagen content revealed by comparative study of MRI and pathology analysis. Int J Clin Exp Med. 2015;8:12898–905. [PMC free article] [PubMed] [Google Scholar]
- 6.Bahuleyan B, Raghuram L, Rajshekhar V, et al. To assess the ability of MRI to predict consistency of pituitary macroadenomas. Br J Neurosurg. 2006;20:324–26. doi: 10.1080/02688690601000717. [DOI] [PubMed] [Google Scholar]
- 7.Pierallini A, Caramia F, Falcone C, et al. Pituitary macroadenomas: preoperative evaluation of consistency with diffusion-weighted MR imaging – initial experience. Radiology. 2006;239:223–31. doi: 10.1148/radiol.2383042204. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoud OM, Tominaga A, Amatya VJ, et al. Role of PROPELLER diffusion-weighted imaging and apparent diffusion coefficient in the evaluation of pituitary adenomas. Eur J Radiol. 2011;80:412–17. doi: 10.1016/j.ejrad.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Naganuma H, Satoh E, Nukui H. Technical considerations of transsphenoidal removal of fibrous pituitary adenomas and evaluation of collagen content and subtype in the adenomas. Neurol Med Chir. 2002;42:202–12. doi: 10.2176/nmc.42.202. discussion 213. [DOI] [PubMed] [Google Scholar]
- 10.Alimohamadi M, Sanjari R, Mortazavi A, et al. Predictive value of diffusion-weighted MRI for tumor consistency and resection rate of nonfunctional pituitary macroadenomas. Acta Neurochirur. 2014;156:2245–52. doi: 10.1007/s00701-014-2259-6. discussion 2252. [DOI] [PubMed] [Google Scholar]
- 11.Romano A, Coppola V, Lombardi M, et al. Predictive role of dynamic contrast enhanced T1-weighted MR sequences in pre-surgical evaluation of macroadenomas consistency. Pituitary. 2017;20:201–9. doi: 10.1007/s11102-016-0760-z. [DOI] [PubMed] [Google Scholar]
- 12.Madabhushi A, Udupa JK, Moonis G. Comparing MR image intensity standardization against tissue characterizability of magnetization transfer ratio imaging. J Magn Reson Imaging. 2006;24:667–75. doi: 10.1002/jmri.20658. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki C, Maeda M, Hori K, et al. Apparent diffusion coefficient of pituitary macroadenoma evaluated with line-scan diffusion-weighted imaging. J Neuroradiol. 2007;34:228–35. doi: 10.1016/j.neurad.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Boxerman JL, Rogg JM, Donahue JE, et al. Preoperative MRI evaluation of pituitary macroadenoma: Imaging features predictive of successful transsphenoidal surgery. Am Roentgenol. 2010;195:720–28. doi: 10.2214/AJR.09.4128. [DOI] [PubMed] [Google Scholar]
- 15.Zengyi M, Wenqiang H, Yao Z, et al. Predictive value of PWI for blood supply and T1-spin echo MRI for consistency of pituitary adenoma. Neuroradiology. 2015;58:51–57. doi: 10.1007/s00234-015-1591-8. [DOI] [PubMed] [Google Scholar]
- 16.Smith KA, Leever JD, Chamoun RB. Prediction of consistency of pituitary adenomas by magnetic resonance imaging. J Neurol Surg B Skull Base. 2015;76:340–43. doi: 10.1055/s-0035-1549005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes JD, Fattahi N, Van Gompel J, et al. Magnetic resonance elastography detects tumoral consistency in pituitary macroadenomas. Pituitary. 2016;19:286–92. doi: 10.1007/s11102-016-0706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith KA, Leever JD, Hylton D, et al. Meningioma consistency prediction utilizing tumor to cerebellar peduncle intensity on T2-weighted magnetic resonance imaging sequences: TCTI ratio. J Neurosurg. 2017;126:242–48. doi: 10.3171/2016.1.JNS152329. [DOI] [PubMed] [Google Scholar]
- 19.Chakrabortty S, Oi S, Yamaguchi M, et al. Growth hormone-producing pituitary adenomas: MR characteristics and pre- and postoperative evaluation. Neurol Medi Chir. 1993;33:81–85. doi: 10.2176/nmc.33.81. [DOI] [PubMed] [Google Scholar]
- 20.Azab WA, Nasim K, Abdelnabi EA, et al. Endoscopic endonasal excision of large and giant pituitary adenomas: Radiological and intraoperative correlates of the extent of resection. World Neurosurg. 2019;126:e793–802. doi: 10.1016/j.wneu.2019.02.151. [DOI] [PubMed] [Google Scholar]
- 21.Mastorakos P, Mehta GU, Chatrath A, et al. Tumor to cerebellar peduncle T2-weighted imaging intensity ratio fails to predict pituitary adenoma consistency. J Neurol Surg B Skull Base. 2019;80:252–57. doi: 10.1055/s-0038-1668516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soyama N, Kuratsu J, Ushio Y. Correlation between magnetic resonance images and histology in meningiomas: T2-weighted images indicate collagen contents in tissues. Neurol Med Chir. 1995;35:438–41. doi: 10.2176/nmc.35.438. [DOI] [PubMed] [Google Scholar]
- 23.Yao W, Qu N, Lu Z, et al. The application of T1 and T2 relaxation time and magnetization transfer ratios to the early diagnosis of patellar cartilage osteoarthritis. Skeletal Radiol. 2009;38:1055–62. doi: 10.1007/s00256-009-0769-8. [DOI] [PubMed] [Google Scholar]
- 24.Iuchi T, Saeki N, Tanaka M, et al. MRI prediction of fibrous pituitary adenomas. Acta Neurochir. 1998;140:779–86. doi: 10.1007/s007010050179. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Li W, Shi D, et al. Expression of TGFbeta1 and pituitary adenoma fibrosis. Br J Neurosurg. 2009;23:293–96. doi: 10.1080/02688690802617046. [DOI] [PubMed] [Google Scholar]