Abstract
Background
The aim of the present study was to investigate the clinical predictive value of pre-infarction angina (PIA) combined with mean platelet volume to lymphocyte count ratio (MPVLR) for no-reflow phenomenon and short-term mortality in patients with ST-segment elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI).
Material/Methods
A total of 1009 STEMI patients who had undergone PCI were enrolled and subdivided into 4 groups based on the occurrence of PIA and the presence of MPVLR above or below the cutoff value. Analysis of the predictors of the no-reflow phenomenon and 90-day mortality was conducted. Further, evaluation and comparison of the clinical predictive value of PIA, MPVLR, and their combination were done.
Results
Both MPVLR (odds ratio [OR]=1.476, 95% confidence interval [CI]: 1.401 to 1.756, P<0.001; hazard ratio [HR]=1.430, 95% CI: 1.287 to 1.643, P<0.001) and PIA (OR=0.905, 95% CI: 0.783 to 0.986, P<0.001; HR=0.878, 95% CI: 0.796 to 0.948, P<0.001) were independent predictors of no-reflow phenomenon and 90-day mortality. Spearman’s rank correlation test revealed that MPVLR (r=−0.297, P<0.001), monocyte to lymphocyte count ratio (MLR) (r=−0.211, P<0.001) and neutrophil to lymphocyte count ratio (NLR) (r=−0.389, P<0.001) in peripheral blood were significantly negatively correlated with postoperative left ventricular ejection fraction (LVEF). Upon comparing the area under curve (AUC), the MPVLR combined with PIA achieved better performance in differentiating no-reflow phenomenon (AUC=0.847, 95% CI: 0.821 to 0.874) and 90-day mortality (AUC=0.790, 95% CI: 0.725 to 0.855), than the GRACE score, MPVLR and PIA alone, and had similar performance to all other pairwise combinations of the GRACE score, MPVLR and PIA.
Conclusions
High MPVLR and PIA were independent predictors of the no-reflow phenomenon and 90-day mortality in patients with STEMI after PCI. Moreover, Combined application of MPVLR and PIA can effectively predict the occurrence of the no-reflow phenomenon and 90-day mortality.
MeSH Keywords: Angina, Unstable; Mean Platelet Volume; Mortality; Myocardial Infarction; No-Reflow Phenomenon
Background
Acute myocardial infarction (AMI) is one of the most common fatal clinical emergencies [1]. Percutaneous coronary intervention (PCI) is a fast and effective measure used in the opening of infarct-related vessels in ST-segment elevation myocardial infarction (STEMI) patients. PCI has been shown to significantly improve myocardial blood supply, reduce the infarct area and improve prognosis [2,3]. However, even though PCI significantly improve the prognosis, up to 30% of STEMI patients do not attain effective reperfusion after infarct-related artery (IRA) recanalization, a phenomenon known as no-reflow [4,5]. No-reflow is associated with major adverse cardiovascular events (MACE) among STEMI patients, therefore, it is important to accurately predict and identify no-reflow phenomenon during the early stages of admissions to improve on the prognosis [6,7]. Studies have shown that biomarkers, such as mean platelet volume (MPV), hemoglobin, C-reactive protein (CRP), are strong predictors of no-reflow phenomenon and mortality in AMI patients [8–10].
Angina is defined as a clinical syndrome caused by a sharp, transient oxygen supply, and aerobic imbalance of the myocardium due to increased myocardial load on the basis of coronary stenosis [11]. Typical angina pectoris (TA) refers to paroxysmal crush-like pain in the posterior sternum or precordial area that occurs during exertion or agitation. The duration of each episode varies by a few minutes and can be completely relieved after rest or taking nitroglycerin [11]. In contrast, nontypical angina (NTA) may be defined as symptoms ascribed as angina that do not meet criteria for TA. Pre-infarction angina (PIA) refers to the onset of typical angina pectoris within 24 hours prior to the onset of AMI symptoms and lasting less than 30 minutes [12]. Studies have shown that PIA is associated with ischemic preconditioning of occluded coronary arteries, and this has a protective effect on myocardial infarction and can also reduce its size [13,14]. In recent years, the mean platelet volume to lymphocyte count ratio (MPVLR) has been proposed as a new biomarker for inflammatory responses and thrombosis [15]. There are studies indicating that both MPVLR and PIA can be used to predict the burden of thrombosis in patients with STEMI [16]. However, there is no relevant study to explore the relationship between MPVLR combined with PIA and no reflow phenomenon and 90-day mortality in STEMI patients.
In the present study, we aimed at exploring the clinical value of MPVLR, PIA, and their combination in predicting the no-reflow phenomenon and 90-day mortality in patients with STEMI undergoing PCI.
Material and Methods
Study participants
The study was conducted between August 2015 to August 2018, in which 1260 participants were diagnosed with STEMI who underwent primary PCI within 12 hours and were enrolled in this study. The design of the study was prospective. STEMI was defined based on the criteria formulated by the American College of Cardiology [17]. 1) Chest pain symptoms occurring within 24 hours prior to admission and lasting for more than 30 minutes. 2) An electrocardiogram (ECG) showing ST-segment elevation in 2 or more consecutive leads and or an abnormal Q wave and new left bundle-branch block. 3) The serum biochemical marker cardiac troponin T (cTnT) and/or creatinine kinase-myocardial band isoenzyme (CK-MB) is positively elevated within 24 hours after onset of the symptoms. Patients suffering from chronic systemic autoimmune diseases (n=15), malignancies (n=36), congenital heart diseases (n=12), acute and chronic infectious diseases (n=43), severe liver and kidney dysfunction (n=37), were taking steroid drugs within the last 3 months (n=24), and those who had previously undergone PCI (n=28), the medication is not regularly taken and compliance is poor (n=6), the clinical data is incomplete (n=8) were all excluded from participating in the study. The study ensured that all relevant ethical practices were adhered to and reviewal of the study by the ethics committee of Shihezi University was done and all patients received written informed consent.
Study procedures and clinical data
Peripheral venous blood from all participants was collected prior to the PCI. Fresh whole blood/plasma was used for hematological and biochemical analyses which were performed within 30 minutes. The hematological parameters included testing for the highly sensitive c-reactive protein (hsCRP), neutrophil count, lymphocyte count, monocyte count, and MPV which were measured using an XT-4000 automated hematology analyzer (Sysmex, Japan). The biochemical indicators analyzed included blood glucose (Glu), CK-MB, cTnT and triglycerides (TG). cTnT, and CK-MB was determined using a Cobas E601 immunology analyzer (Cobas, Switzerland) while the other biochemical indicators were measured using an Hitachi7180 automatic biochemical analyzer (Hitachi, Japan). The severity of heart failure at admission of all STEMI patients was recorded by Killip classification. The occurrence of PIA was recorded by requesting the patient’s medical history, Patients with chest pain due to dyspnea and psychiatric factors were not accepted. MPVLR was calculated as a ratio of the mean platelet volume to lymphocyte count at admission. The Global Registry of Acute Coronary Events (GRACE) risk score is the preferred risk score in the STEMI clinical practice guidelines and is widely used clinically. A computer program was used to record GRACE risk score of all patients based on their biochemical and hematological indicators on admission. All patients with Philips iE33 transthoracic echocardiography (Philips, Netherlands) to assess left ventricular ejection fraction (LVEF) within 24 hours after PCI.
The application and dosage of aspirin, clopidogrel, and other cardiac drugs in the patients were determined by the clinician based on the clinical guidelines and the patient’s condition prior to PCI and after PCI. The success of PCI was assessed through thrombolysis in myocardial infarction (TIMI) flow grade level 3 after coronary artery therapy and residual stenosis of less than 30%. Coronary angiography, PCI, and reperfusion therapy strategies were all performed by experienced cardiologists.
Primary endpoint and follow-up
The follow-up of the study participants was done through a review of their hospital records, outpatient visits, and telephone contact. However, the endpoint of follow-up was marked either by the end of the follow-up time or the death of the participants, whichever came first. No-reflow was defined as the absence of effective perfusion of myocardial tissue (TIMI flow-grade lower than 3) after coronary artery recanalization without obvious spasm, dissection and residual stenosis [18].
Statistical analysis
The Kolmogorov-Smirnov test was used to determine the normality of each of the random sample. Numerical variables following normal distribution were presented using means and standard deviation; however, those not following normal distribution were presented using interquartile range and median. Mann-Whitney U test, one-way ANOVA test or t-test were used to compare the numerical variables between groups. Nominal variables were described using frequencies and percentages. Fisher exact probability method or χ2 test was used for comparison between groups. The correlation between LVEF, NLR, MLR and MPVLR was analyzed by spearman’s rank correlation. The ROC was used to analyze the value of MPVLR, PIA and their combination in predicting no-reflow phenomenon and 90-day mortality. Delong’s test was used to compare the AUC by MedCalc Statistical Software version 14.8.1 [19]. Kaplan-Meier method was used to compare the cumulative survival rate of each group, and the log-rank test was used for statistical evaluation. Logistic and Cox regression model was used to analyze independent predictors of the no-reflow phenomenon and 90-day mortality. Since no reflow occurred during emergency PCI on the day of admission, the influence of time factor was small, so we chose odds ratio (OR) as the risk measure for no-reflow phenomenon. Since different patients had different time of death during follow-up, and time factors had a great influence, so we chose hazard ratio (HR) as the risk measure for 90-day mortality. A P-value <0.05 was considered as statistically significant.
Results
Baseline clinical characteristics
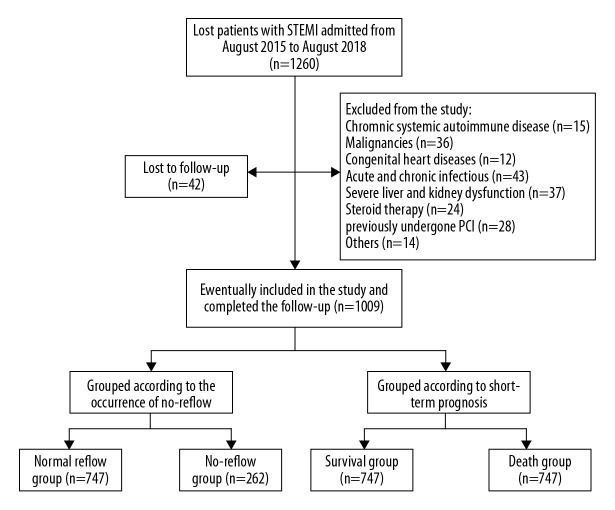
In the present study, 1260 participants were initially included in the study, while 209 patients (16.59%) were excluded because of the exclusion criteria and 42 patients (3.33%) were lost during the follow-up. Eventually, 1009 patients (80.08%) were included in the study for analysis. Figure 1 depicts the flow chart of how the patients were selected to participate in the study. The cutoff value of MPVLR was 5.89 (sensitivity=68.3%, specificity=82.2%, P<0.001) for differentiating angiographic no-reflow. Patients were subdivided into 4 groups based on the occurrence of PIA in combination with the cutoff value of MPVLR. The groups were as follows: PIA and low MPVLR (Group 1, PIA and MPVLR ≤5.89, n=263); PIA and high MPVLR (Group 2, PIA and MPVLR >5.89, n=122); no PIA and low MPVLR (Group 3, no PIA and MPVLR ≤5.89, n=410), no PIA and high MPVLR (Group 4, no PIA and MPVLR >5.38, n=214). Table 1 present the baseline clinical characteristics of the 4 groups. Based on this data, it was evident that patients in Group 4 were older, had a higher admission GRACE score, and with higher levels of the neutrophil count, monocyte count, MPV, MPVLR, neutrophil to lymphocyte count ratio (NLR), monocyte to lymphocyte count ratio (MLR), hsCRP, and peak of cTnT. However, Group 4 patients had lower levels of LVEF and lymphocyte count. The Group 4 patients had a higher proportion of Killip class ≥II compared with the other 3 groups. Overall, there was no statistically significant difference between the 4 groups in terms of culprit vessel, number of stents implanted and postoperative medication.
Figure 1.
Flow diagram of study patient selection.
Table 1.
Baseline clinical characteristics of the patients based on the pre-infarction angina (PIA) and mean platelet volume to lymphocyte ratio (MPVLR).
| Variable | PIA+low MPVLR | PIA+high MPVLR | No PIA+low MPVLR | No PIA+high MPVLR | P |
|---|---|---|---|---|---|
| (Group 1, n=263) | (Group 2, n=122) | (Group 3, n=410) | (Group 4, n=214) | ||
| Baseline characteristics | |||||
| Age (years) | 56.92±7.40 | 58.43±9.24 | 57.63±8.53 | 61.82±9.56*#@ | 0.002 |
| Male [n (%)] | 150 (57.03) | 75 (61.48) | 251 (61.22) | 132 (61.68) | 0.670 |
| Smoking [n (%)] | 90 (34.22) | 45 (36.89) | 128 (31.22) | 86 (40.19) | 0.149 |
| Diabetes mellitus [n (%)] | 108 (41.06) | 51 (41.80) | 158 (38.54) | 90 (42.06) | 0.806 |
| Hypercholesterolemia [n (%)] | 93 (35.36) | 47 (38.52) | 165 (40.24) | 88 (41.12) | 0.543 |
| Hypertension [n (%)] | 179 (68.06) | 81 (66.39) | 267 (65.12) | 155 (72.43) | 0.316 |
| Killip class ≥II [n (%)] | 74 (28.14) | 42 (34.43) | 155 (37.80) | 101 (47.20)*#@ | <0.001 |
| GRACE score | 121.38±13.61 | 136.43±14.72* | 135.93±14.20* | 152.51±16.79*#@ | 0.012 |
| Laboratory data | |||||
| Neutrophil count (×109/l) | 6.52±3.39 | 6.71±3.07 | 6.56±3.54 | 7.35±3.57*#@ | 0.015 |
| Lymphocyte count (×109/l) | 2.67±0.83 | 1.64±0.25* | 2.27±0.33*# | 1.41±0.32*#@ | <0.001 |
| Monocyte count (×109/l) | 0.91±0.38 | 0.85±0.36 | 0.94±0.34 | 1.13±0.89*#@ | 0.001 |
| MPV (fl) | 10.84±0.83 | 10.84±0.84 | 10.66±0.93 | 11.30±1.11*#@ | <0.001 |
| NLR | 3.02±1.83 | 4.37±2.06* | 3.98±1.98* | 5.72±1.83*#@ | <0.001 |
| MPVLR | 4.86 (2.02–5.79) | 6.79 (2.87–8.93)* | 5.68 (2.42–7.88)*# | 8.16 (3.24–10.02)*#@ | <0.001 |
| MLR | 0.41±0.19 | 0.52±0.23* | 0.39±0.20# | 0.65±0.70*#@ | <0.001 |
| hsCRP (mg/l) | 1.90 (0.87–3.43) | 1.92 (1.00–5.63) | 2.10 (1.10–6.35) | 2.60 (1.20–6.95)*#@ | 0.004 |
| Peak CK-MB (U/L) | 107 (55–197) | 104 (38–250) | 117 (61–197) | 112 (62–245) | 0.073 |
| Peak cTnT (ng/ml) | 3.69 (2.37–6.08) | 4.38 (2.51–7.88)* | 4.32 (2.64–7.01)* | 6.16 (3.89–9.95)*#@ | 0.002 |
| Glu (mmol/l) | 8.20±4.25 | 8.08±3.76 | 7.64±3.67 | 7.86±3.77 | 0.294 |
| TC (mmol/l) | 4.09±0.79 | 4.08±0.80 | 4.06±0.98 | 4.16±0.80 | 0.614 |
| TG (mmol/l) | 1.62±0.71 | 1.44±0.61 | 1.63±1.28 | 1.54±0.73 | 0.242 |
| LDL (m l/l) | 2.37±0.58 | 2.39±0.60 | 2.42±0.75 | 2.39±0.61 | 0.428 |
| HDL (mmol/l) | 1.00±0.21 | 0.99±0.20 | 1.02±0.22 | 0.99±0.24 | 0.650 |
| LVEF | 59.07±9.82 | 56.63±9.22* | 56.48±9.91* | 53.41±8.64*#@ | <0.001 |
| Culprit vessel [n (%)] | 0.355 | ||||
| Left main coronary artery | 0 | 1 (0.82) | 3 (0.73) | 2 (0.93) | |
| Left anterior descending | 119 (45.25) | 47 (38.52) | 193 (47.07) | 84 (39.25) | |
| Left circumflex artery | 36 (13.69) | 19 (15.58) | 52 (12.69) | 26 (12.16) | |
| Right coronary artery | 108 (41.06) | 55 (45.08) | 162 (39.51) | 102 (47.66) | |
| Number of implanted stents (n) | 1.19±0.50 | 1.23±0.54 | 1.27±0.51 | 1.28±0.60 | 0.216 |
| Postoperative medication [n (%)] | |||||
| Aspirin | 253 (96.20) | 112 (91.80) | 395 (96.34) | 208 (97.20) | 0.092 |
| Clopidogrel | 242 (92.02) | 114 (93.44) | 384 (93.66) | 203 (94.86) | 0.657 |
| Statin | 218 (82.89) | 101 (82.79) | 329 (80.24) | 182 (85.05) | 0.498 |
| Beta-blocker | 195 (74.14) | 94 (77.05) | 302 (73.66) | 163 (76.17) | 0.831 |
| Calcium channel blocker | 60 (22.81) | 32 (26.23) | 114 (27.80) | 63 (29.44) | 0.373 |
| ACEI or ARB | 114 (43.35) | 56 (45.90) | 193 (47.07) | 94 (43.93) | 0.775 |
PIA – pre-infarction angina; MPVLR – mean platelet volume to lymphocyte ratio; GRACE – global registry of acute coronary events; NLR – neutrophil to lymphocyte ratio; MPV – mean platelet volume; MLR – monocyte to lymphocyte ratio; CK-MB – creatine kinase isoenzyme MB; cTnT – cardia troponin T; Glu – fasting blood sugar; hsCRP – high sensitivity c-reactive protein; TC – total cholesterol; TG – triglycerides; LDL – low-density lipoprotein; HDL – high-density lipoprotein; LVEF – left ventricular ejection fraction; ACEI – angiotensin-converting enzyme inhibitors; ARB – angiotensin type II receptor blockers.
Compared with the PIA+low MPVLR group, P<0.05.
Compared with the PIA+high MPVLR group, P<0.05.
Compared with the no PIA+low MPVLR group, P<0.05.
Baseline clinical characteristics of patients in the no-reflow and normal reflow groups
Table 2 summarizes the baseline characteristics of patients in each group. Compared to the normal reflow group, the no-reflow group patients were older, had higher GRACE score, neutrophil count, monocyte count, MPV, MPVLR, NLR, MLR, hsCRP, peak of cTnT, and also a higher proportion of Killip class ≥II and PIA. However, these patients in the no-reflow group had lower levels of lymphocyte count as well as LVEF. It is important to note that the MPVLR level in the no-reflow group was significantly higher than that in the normal reflow group, and the incidence of PIA was significantly lower in the no-reflow group than in the normal reflow group.
Table 2.
Baseline clinical characteristics of patients in the no-reflow and normal reflow groups.
| Variable | Normal reflow (n=747) | No-reflow (n=262) | P |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 55.54±8.89 | 58.16±10.68 | 0.028 |
| Male [n (%)] | 453 (60.64) | 155 (59.54) | 0.673 |
| Smoking [n (%)] | 269 (36.01) | 80 (30.53) | 0.109 |
| Diabetes mellitus [n (%)] | 302 (40.43) | 105 (40.08) | 0.920 |
| Hypercholesterolemia [n (%)] | 280 (37.48) | 113 (43.13) | 0.107 |
| Hypertension [n (%)] | 496 (66.40) | 186 (70.99) | 0.172 |
| Killip class ≥II [n (%)] | 249 (33.33) | 123 (46.95) | <0.001 |
| Pre-infarction angina [n (%)] | 315 (42.17) | 70 (26.72) | <0.001 |
| GRACE score | 143.73±15.46 | 157.98±14.20 | 0.001 |
| Laboratory data | |||
| Neutrophil count (×109/l) | 6.66±3.44 | 7.30±3.42 | 0.009 |
| Lymphocyte count (×109/l) | 2.35±0.75 | 1.66±0.62 | <0.001 |
| Monocyte count (×109/l) | 0.91±0.47 | 1.08±0.64 | <0.001 |
| MPV (fl) | 10.81±0.90 | 11.40±1.09 | 0.010 |
| NLR | 3.24±2.21 | 4.97±2.92 | <0.001 |
| MPVLR | 5.01 (2.82–7.79) | 7.37 (3.12–9.68) | <0.001 |
| MLR | 0.43±0.33 | 0.56±0.48 | <0.001 |
| hsCRP (mg/l) | 2.20 (1.17–5.40) | 3.50 (2.39–5.00) | <0.001 |
| Peak CK-MB (U/L) | 110 (51–217) | 132 (67–220) | 0.125 |
| Peak cTnT (ng/ml) | 4.25 (2.51–7.08) | 5.82 (3.67–8.49) | <0.001 |
| Glu (mmol/l) | 7.99±3.93 | 7.59±3.65 | 0.135 |
| TC (mmol/l) | 4.09±0.89 | 4.16±0.80 | 0.302 |
| TG (mmol/l) | 1.76±1.08 | 1.84±0.65 | 0.147 |
| LDL (m l/l) | 2.40±0.61 | 2.45±0.68 | 0.326 |
| HDL (mmol/l) | 1.01±0.22 | 1.00±0.23 | 0.345 |
| LVEF | 58.21±10.14 | 53.91±9.70 | <0.001 |
| Culprit vessel [n (%)] | 0.516 | ||
| Left main coronary artery | 4 (0.54) | 2 (0.76) | |
| Left anterior descending | 331 (44.31) | 112 (42.75) | |
| Left circumflex artery | 104 (13.92) | 29 (11.07) | |
| Right coronary artery | 308 (41.23) | 119 (45.42) | |
| Number of implanted stents (n) | 1.19±0.55 | 1.20±0.58 | 0.944 |
| Postoperative medication [n (%)] | |||
| Aspirin | 714 (95.58) | 254 (96.95) | 0.336 |
| Clopidogrel | 697 (93.31) | 246 (93.89) | 0.741 |
| Statin | 622 (83.27) | 208 (79.39) | 0.158 |
| Beta-blocker | 556 (74.43) | 198 (75.57) | 0.715 |
| Calcium channel blocker | 208 (27.84) | 61 (23.28) | 0.151 |
| ACEI or ARB | 332 (44.44) | 125 (47.71) | 0.361 |
PIA – pre-infarction angina; MPVLR – mean platelet volume to lymphocyte ratio; GRACE – global registry of acute coronary events; NLR – neutrophil to lymphocyte ratio; MPV – mean platelet volume; MLR – monocyte to lymphocyte ratio; CK-MB – creatine kinase isoenzyme MB; cTnT – cardia troponin T; Glu – fasting blood sugar; hsCRP – high sensitivity c-reactive protein; TC – total cholesterol; TG – triglycerides; LDL – low-density lipoprotein; HDL – high-density lipoprotein; LVEF – left ventricular ejection fraction; ACEI – angiotensin-converting enzyme inhibitors; ARB – angiotensin type II receptor blockers.
Independent predictors of no-reflow phenomenon
Table 3 shows the independent predictors of no-reflow phenomenon. The univariate analysis revealed that age, Killip class ≥II, PIA, GRACE score, lymphocyte count, MPV, NLR, MPVLR, MLR, hsCRP, peak of cTnT, and LVEF were all related to no-reflow phenomenon. After adjusting the covariates, we found that the potential predictors in univariate analysis are mostly independent predictors of no-reflow phenomenon. Importantly, we also found that high MPVLR (OR=1.476, 95% confidence interval [CI]: 1.401 to 1.756, P<0.001) at admission was an significant adverse predictor, and PIA (OR=0.905, 95% CI: 0.783 to 0.986, P<0.001) was an significant protective predictor.
Table 3.
Univariate and multivariate logistic regression analysis of independent predictors of no-reflow phenomenon.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | 1.048 (1.028–1.075) | 0.042 | 1.020 (0.987–1.085) | 0.172 |
| Killip class ≥II | 2.203 (1.481–2.795) | <0.001 | 1.718 (1.453–2.118) | 0.005 |
| Pre-infarction angina | 0.887 (0.765–0.953) | <0.001 | 0.905 (0.783–0.986) | <0.001 |
| GRACE score | 1.313 (1.028–1.658) | <0.001 | 1.466 (1.251–1.785) | <0.001 |
| NLR | 1.403 (1.135–1.623) | <0.001 | 1.328 (1.131–1.513) | 0.025 |
| MPVLR | 1.637 (1.388–1.802) | <0.001 | 1.476 (1.401–1.756) | <0.001 |
| MLR | 1.432 (1.221–1.632) | <0.001 | 1.335 (1.176–1.558) | 0.018 |
| hsCRP | 1.117 (1.083–1.384) | 0.042 | 1.144 (0.998–1.303) | 0.192 |
| Peak cTnT | 1.588 (1.138–1.887) | 0.015 | 1.300 (1.164–1.765) | 0.027 |
| LVEF | 0.903 (0.880–0.951) | 0.008 | 0.915 (0.883–0.948) | 0.011 |
MPVLR – mean platelet volume to lymphocyte ratio; GRACE – global registry of acute coronary events; NLR – neutrophil to lymphocyte ratio; MPV – mean platelet volume; MLR – monocyte to lymphocyte ratio; hsCRP – high sensitivity c-reactive protein; cTnT – cardiac troponin T; LVEF – left ventricular ejection fraction; HR – odds ratio; CI – confidence interval.
Independent predictors of short-time mortality
We further explored the independent predictors of short-time mortality after PCI in patients with STEMI through Cox regression analysis, as shown in Table 4. After univariate and multivariate Cox regression analysis, we found that Killip class ≥II, PIA, GRACE score, lymphocyte count, NLR, MPVLR, MLR, the peak of cTnT, and LVEF were all independent predictors of 90-day mortality. Interestingly, among the independent predictors of short-time mortality, we found that high MPVLR (HR=1.430, 95% CI: 1.287 to 1.643, P<0.001) at admission was also a significant adverse predictor, and PIA (HR=0.878, 95% CI: 0.796 to 0.948, P<0.001) was a significant protective predictor.
Table 4.
Univariate and multivariate cox regression analysis of independent predictors of 90-day mortality.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.255 (0.952–1.676) | 0.168 | – | – |
| Killip class ≥II | 1.803 (1.448–2.021) | 0.012 | 1.581 (1.330–1.799) | 0.005 |
| Pre-infarction angina | 0.912 (0.838–0.985) | 0.020 | 0.878 (0.796–0.948) | <0.001 |
| GRACE score | 1.553 (1.267–1.874) | <0.001 | 1.408 (1.203–1.689) | <0.001 |
| NLR | 1.477 (1.350–1.689) | <0.001 | 1.337 (1.251–1.607) | <0.001 |
| MPVLR | 1.518 (1.307–1.769) | <0.001 | 1.430 (1.287–1.643) | <0.001 |
| MLR | 1.423 (1.183–1.702) | <0.001 | 1.313 (1.150–1.586) | 0.012 |
| hsCRP | 1.283 (0.977–1.458) | 0.205 | – | – |
| Peak cTnT | 1.328 (1.162–1.562) | 0.016 | 1.206 (1.114–1.405) | <0.001 |
| LVEF | 0.894 (0.836–0.972) | 0.011 | 0.872 (0.817–0.936) | <0.001 |
MPVLR – mean platelet volume to lymphocyte ratio; GRACE – global registry of acute coronary events; NLR – neutrophil to lymphocyte ratio; MPV – mean platelet volume; MLR – monocyte to lymphocyte ratio; hsCRP – high sensitivity c-reactive protein; cTnT – cardiac troponin T; LVEF – left ventricular ejection fraction; HR – hazard ratio; CI – confidence interval.
Comparison of MPVLR, NLR and MLR levels at admission in different risk-stratified groups
Based on GRACE scores, patients were categorized into 3 groups: low-risk group (GRACE score ≤108, n=166); medium-risk group (108 <GRACE score ≤140, n=398); and high-risk group (GRACE score >140, n=445). With the increase of GRACE risk stratification, the MPVLR, NLR, and MLR levels in each group were significantly increased (all P values <0.05) (Figure 2A–2C).
Figure 2.
Comparison of MPVLR, NLR, and MLR levels at admission in different risk-stratified groups. (A) MPVLR levels in different risk-stratified groups; (B) NLR levels in different risk-stratified groups; (C) MLR levels in different risk-stratified groups. * P<0.05 compared with the low risk group. # P<0.05 compared with the medium risk group. MPVLR – mean platelet volume to lymphocyte ratio; MLR – monocyte to lymphocyte ratio; NLR – neutrophil to lymphocyte ratio.
Correlation between MLR, NLR, MPVLR and LVEF
Spearman’s rank correlation test results revealed that the levels of MLR (r=−0.211, P<0.001, Figure 3A) and NLR (r=−0.389, P<0.001, Figure 3B) in peripheral blood were significantly negatively correlated with postoperative LVEF. In addition, this study also found a weak but remarkable negative correlation among peripheral blood MPVLR with postoperative LVEF (r=−0.297, P<0.001, Figure 3C).
Figure 3.
Correlation between MLR, NLR, MPVLR, and LVEF. (A) Correlation between MLR and LVEF, (B) Correlation between NLR and LVEF, (C) Correlation between MPVLR and LVEF. MLR – monocyte to lymphocyte ratio; NLR – neutrophil to lymphocyte ratio; MPVLR – mean platelet volume to lymphocyte ratio; LVEF – left ventricular ejection fraction.
Clinical adverse outcomes
In this study, a total of 262 patients (25.97%) developed no-reflow phenomenon. During the follow-up period, 83 patients (8.23%) died. Generally, the angiographic no-reflow rates and 90-day mortality were remarkably higher in the no PIA combined with high MPVLR group versus all the other 3 groups (Figure 4A, 4B).
Figure 4.
Comparison of the incidence of no-reflow and 90-day mortality in each group. (A) the incidence of no-reflow in each group; (B) 90-day mortality in each group. * P<0.05 compared with the PIA+low MPVLR group. # P<0.05 compared with the PIA+high MPVLR group. $ P<0.05 compared with the no PIA+low MPVLR group. MPVLR – mean platelet volume to lymphocyte ratio; PIA – pre-infarction angina.
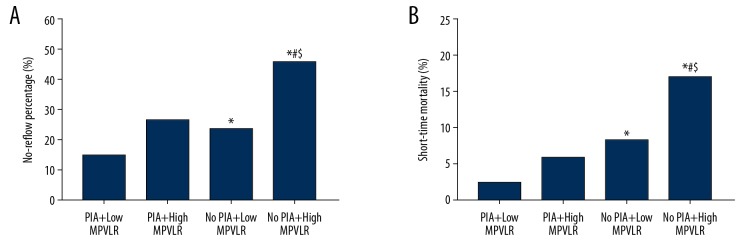
Kaplan-Meier curves based on the cutoff values of MPVLR and the occurrence of PIA are shown in Figure 5A and 5B, respectively. The 90-day mortality in the high MPVLR group (12.80% versus 5.94%, log-rank: P<0.001) and the no PIA group (11.22% versus 3.38%, log-rank: P<0.001) increased significantly than that of the control group during the follow-up period. Similarly, the Kaplan-Meier curves based on the cutoff values of MPVLR combined with the occurrence of PIA are shown in Figure 5C. There was a statistically significant difference in 90-day mortality among the 4 groups and was significantly higher in the no PIA combined with high MPVLR group than in all the other 3 groups (P<0.001, Figure 5C).
Figure 5.
Kaplan-Meier survival curves of 90-day mortality in STEMI patients after PCI (A) according to the cutoff value of MPVLR; (B) according to the occurrence of PIA; (C) according to the cutoff values of MPVLR combined with the occurrence of PIA. STEMI – ST-segment elevation myocardial infarction; PCI – percutaneous coronary intervention; MPVLR – mean platelet volume to lymphocyte ratio; PIA – pre-infarction angina.
Combination of PIA with MPVLR in predicting clinical adverse outcomes
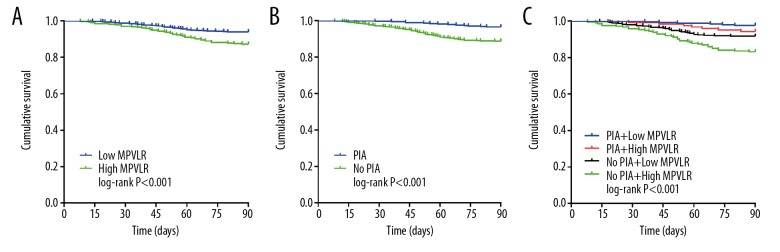
The ROC curve was used to evaluate and compare the predictive efficacy of PIA, MPVLR, and GRACE score and their combination in predicting angiographic no-reflow and short-time mortality after PCI in STEMI patients. Figure 6A shows that PIA together with MPVLR (AUC=0.847, 95% CI: 0.821 to 0.874) had better predictive efficacy for angiographic no-reflow, than the GRACE score (AUC=0.806 95% CI: 0.780 to 0.832), PIA (AUC=0.586, 95% CI: 0.547 to 0.625) and MPVLR (AUC=0.789, 95% CI: 0.754 to 0.825) (all P values <0.05). Figure 6B shows that compared with single prediction by the GRACE score (AUC=0.709, 95% CI: 0.637 to 0.781), PIA (AUC=0.568, 95% CI: 0.515 to 0.645) and MPVLR (AUC=0.716, 95% CI: 0.649 to 0.782), the PIA together with MPVLR (AUC=0.790, 95% CI: 0.725 to 0.855) can significantly improve the prediction efficiency of short-time mortality (all P values <0.05). Figure 7A shows that PIA together with MPVLR (AUC=0.847, 95% CI: 0.821 to 0.874), PIA combined with GRACE score (AUC=0.834, 95% CI: 0.810 to 0.859) and MPVLR combined with GRACE score (AUC=0.823, 95% CI: 0.793 to 0.854) had similar predictive efficacy for angiographic no-reflow (all P values >0.05). Figure 7B shows that PIA together with MPVLR (AUC=0.790, 95% CI: 0.725 to 0.855), PIA combined with GRACE score (AUC=0.782, 95% CI: 0.706 to 0.858) and MPVLR combined with GRACE score (AUC=0.801, 95% CI: 0.727 to 0.874) had similar predictive efficacy for short-time mortality (all P values >0.05).
Figure 6.
ROC curve to evaluate and compare the clinical value of MPVLR, PIA and GRACE score in predicting (A) no-reflow and (B) 90-day mortality. ROC – receiver operating characteristic; MPVLR – mean platelet volume to lymphocyte ratio; PIA – pre-infarction angina; GRACE – Global Registry of Acute Coronary Events.
Figure 7.
ROC curve to evaluate and compare the clinical value of the combination of MPVLR, PIA and GRACE score in predicting (A) no-reflow and (B) 90-day mortality. ROC – receiver operating characteristic; MPVLR – mean platelet volume to lymphocyte ratio; PIA – pre-infarction angina; GRACE – Global Registry of Acute Coronary Events.
Moreover, the combination of PIA, MPVLR, and GRACE score can significantly improve the prediction efficacy of GRACE risk stratification for clinical adverse outcomes. These results indicate that the combination of PIA and MPVLR can be used as predictors to clinical adverse outcomes in patients with STEMI after PCI.
Association between 90-day mortality and no-reflow
The Kaplan-Meier survival curves of the no-reflow group and the normal reflow group are shown in Figure 8. The Figure 8 showed that the 90-day mortality during the follow-up period was significantly higher in the no-reflow group than in the normal reflow group (13.74% versus 6.29%, log-rank: P<0.001). Time period-specific analyses showed that the association between no-reflow and mortality was significant, and the no-reflow group had a stronger 90-day mortality than the normal reflow group (adjusted HR=2.235, 95% CI: 1.365 to 3.661, P<0.001).
Figure 8.
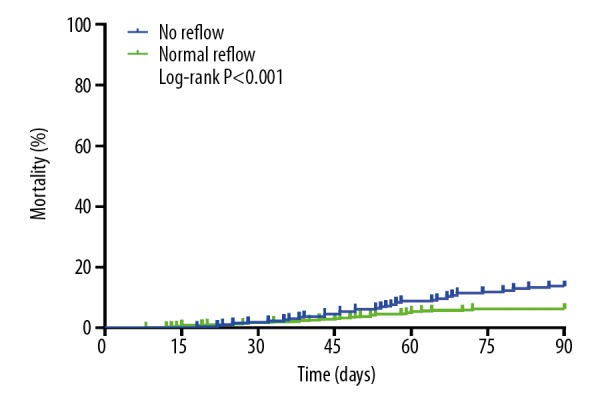
Kaplan-Meier curve for mortality according to no-reflow.
Discussion
The GRACE risk score has been widely used to identify high-risk patients and to adjust treatment decisions and accurately assess patient prognostic information. However, the current GRACE risk scoring system has some defects. It contains only 2 biomarkers and cannot reflect the body’s inflammatory response and thrombosis state. In addition, the GRACE risk scoring system does not involve the presence or absence of protective factors. MPVLR is considered to be a new biomarker of inflammatory response and thrombosis, and PIA is considered a protective factor. So, in the present study, we explored the association among MPVLR, PIA, and the GRACE score with the no-reflow phenomenon and 90-day mortality in STEMI patients undergoing PCI. Meanwhile, this study was the first to evaluate and compare the clinical value of MPVLR, PIA, and GRACE score and their combination in predicting no-reflow and 90-day mortality. The study revealed that both MPVLR and PIA are important independent predictors of no-reflow and 90-day mortality. In addition, the study not only demonstrated for the first time that PIA together with MPVLR, PIA combined with GRACE score, and MPVLR combined with GRACE score had similar predictive efficacy for no-reflow and 90-day mortality, and also proved that PIA, MPVLR, PIA combined with MPVLR can significantly improve the prediction effect of GRACE score for no-reflow and 90-day mortality, and contribute to risk stratification and prognosis assessment in STEMI patients.
In recent years, the no-reflow phenomenon has become a clinical research hot spot, and its pathophysiological mechanism has been associated with platelet aggregation mediated distal vascular microemboli, platelets, or inflammatory cells mediated local inflammation, coronary microvascular spasm, reperfusion injury, coronary microcirculation of damage caused by several factors [20–22]. Related studies have shown that the no-reflow phenomenon after PCI is associated with clinical adverse outcomes during follow-up [23]. However, predicting the occurrence of no-reflow in the clinic is difficult and therefore finding an economical, stable and strong no-reflow predictor is of great importance for timely adjustment of the treatment plan and improved prognosis.
PIA is typical angina pectoris occurring within 24 hours before the onset of AMI patients [12]. Reiter et al. showed that AMI patients with PIA have smaller infarct area and higher ejection fraction compared to AMI patients without PIA, the mechanism of which may be related to PIA activated ischemic preconditioning (IP) [13]. IP can reduce the energy requirements of cardiomyocytes, preserve ATP, slow down the development of osmotic load and acidosis to tolerate ischemia and thus protect the cardiomyocytes [24,25]. Bromage et al. also demonstrated that IP not only reduces infarct size but also protects microcirculation after reperfusion, which may be related to IP improving impaired endothelial function and inhibiting neutrophil activation induced by ischemia-reperfusion [26]. Zhang et al. showed that in the no-reflow group, patients with a lower incidence of PIA and the presence of PIA can significantly reduce the incidence of no-reflow [27]. Kobayashi et al. showed that PIA has a potential link with coronary artery lesion morphology, and patients with AMI who have PIA have a better long-term prognosis [28]. The current study found that patients who did not develop PIA had higher 90-day mortality and no-reflow rate than those who developed PIA, which is consistent with the above studies. In addition, in this study, also it was confirmed that PIA is a protective predictor of 90-day mortality and no-reflow in STEMI patients after PCI.
Celik et al. demonstrated that no-reflow may be associated with increased thrombosis and inflammatory response [29]. Avci et al. showed that an increase in-hospital MPV in STEMI patients treated with PCI was associated with long-term mortality [30]. MPV reflects the activation and aggregation of platelets [31]. The larger the size of platelets, the more active the metabolism is, and the large platelets not only accelerate the formation of coronary artery thrombosis but also exacerbate the body’s inflammatory response [32]. Monocytes can recruit to the artery wall, differentiate into macrophages and stimulate the activation of proinflammatory cytokines. The increase of monocytes reflects the aggravation of the degree of inflammation in the body. A lymphocyte is one of the earliest cells involved in the formation of atherosclerotic plaque, and the reduction of lymphocyte is related to physiological stress and acute inflammatory response of the body. The reduction of lymphocytes contributes to the growth and rupture of plaque and the expansion of the infarct area [33]. Kiris et al. showed that MLR is an indirect marker of inflammation that an increase MLR at 48 hours after admission in STEMI patients treated with PCI was associated with short/long-term mortality [34]. MPVLR is a comprehensive product of MPV and lymphocytes and is a novel biomarker for reaction thrombosis and inflammation [35]. Kurtul et al. demonstrated that MPVLR was a strong independent predictor for no-reflow and 30-day mortality in patients with STEMI [15]. Hudzik et al. showed that elevated MPVLR can predict clinical adverse outcomes in patients with STEMI during follow-up [36]. The current study findings also demonstrated that MPVLR was an independent risk factor for 90-day mortality and no-reflow in STEMI patients after PCI.
Based on the potential association between MPVLR, PIA and no-reflow, this study was the first to demonstrate that the combination of MPVLR and PIA could better predict 90-day mortality and no-reflow in STEMI patients after PCI. In addition, this study was also the first to explore the correlation between MPVLR, NLR, MLR, and LVEF. The results showed that the levels of MPVLR, MLR, and NLR in peripheral blood were significantly negatively correlated with LVEF. This provides a direction for future research on the specific mechanism among these factors. Interestingly, this study also found that the no PIA combined with high MPVLR group had the highest no-reflow rates and 90-day mortality than the other 3 groups. This demonstrated the clinical value of the combination of MPVLR and PIA in predicting no-reflow and 90-day mortality in patients with STEMI after PCI.
This study had several limitations. First, the study was a single-retrospective study with a limited sample size hence there were possibilities of selection bias. Second, the occurrence of PIA depended on the patient’s correct perception of the symptoms, hence this study may have had recall bias. Third, the prognostic value of other biomarkers for STEMI patients was not explored. Fourth, we did not dynamically observe changes in MPVLR and were unable to assess whether MPVLR after treatment had the same predictive value.
Conclusions
Both MPVLR and PIA are independent predictors of no-reflow and 90-day mortality among STEMI patients after PCI. Combined application of MPVLR and PIA could more effectively predict the occurrence of no-reflow and 90-day mortality after PCI for STEMI patients. The combination of MPVLR, a non-invasive, simple, economical and feasible biomarker, and PIA provides a new perspective for risk stratification, treatment, and prognosis of STEMI patients.
Acknowledgements
All authors thank Shihezi University for providing education and support. In addition, Xinsen Chen is grateful to Meng Shao for his understanding and love.
Footnotes
Conflict of interest
None
Source of support: Departmental sources
References
- 1.Sparv D, Hofmann R, Gunnarsson A, et al. The analgesic effect of oxygen in suspected acute myocardial infarction: A substudy of the DETO2X-AMI Trial. JACC Cardiovasc Interv. 2018;11(16):1590–97. doi: 10.1016/j.jcin.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 2.Nallamothu BK, Normand S-LT, Wang Y, et al. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: A retrospective study. Lancet. 2015;85(9973):1114–22. doi: 10.1016/S0140-6736(14)61932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Lancet. 40 years of percutaneous coronary intervention: Where next? Lancet. 2017;390(10096):715. doi: 10.1016/S0140-6736(17)32238-9. [DOI] [PubMed] [Google Scholar]
- 4.Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc Interv. 2017;10(3):215–23. doi: 10.1016/j.jcin.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Mejía-Rentería H, Escaned J. Use of fractional flow reserve in non-infarct-related arteries during ST-segment elevation myocardial infarction: The need to move from experimental studies to real life assessment. JACC Cardiovasc Interv. 2018;11(16):1659–60. doi: 10.1016/j.jcin.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Yesin M, Çağdaş M, Kalçık M, et al. Comparison of syntax score and syntax score II to predict “no reflow phenomenon” in patients with ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging. 2017;33(12):1883–89. doi: 10.1007/s10554-017-1200-5. [DOI] [PubMed] [Google Scholar]
- 7.Papapostolou S, Andrianopoulos N, Duffy SJ, et al. Long-term clinical outcomes of transient and persistent no-reflow following percutaneous coronary intervention (PCI): A multicentre Australian registry. EuroIntervention. 2018;14(2):185–93. doi: 10.4244/EIJ-D-17-00269. [DOI] [PubMed] [Google Scholar]
- 8.Machado GP, Araujo GN, Carpes CK, et al. Comparison of neutrophil-to-lymphocyte ratio and mean platelet volume in the prediction of adverse events after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. Atherosclerosis. 2018;274:212–17. doi: 10.1016/j.atherosclerosis.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Yaylak B, Altıntaş B, Özcan KS, et al. Relation of hemoglobin level to no-reflow in patients with ST-segment elevation myocardial infarction undergoing primary coronary intervention. Postepy Kardiol Interwencyjnej. 2018;14(4):383–90. doi: 10.5114/aic.2018.79868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stankovic S, Obradovic S, Dzudovic B, et al. Lower plasma protein C activity is associated with early myocardial necrosis and no-reflow phenomenon in patients with ST elevation myocardial infarction. Acta Cardiol. 2019;74(4):331–39. doi: 10.1080/00015385.2018.1494116. [DOI] [PubMed] [Google Scholar]
- 11.AlBadri A, Leong D, Bairey Merz CN, et al. Typical angina is associated with greater coronary endothelial dysfunction but not abnormal vasodilatory reserve. Clin Cardiol. 2017;40(10):886–91. doi: 10.1002/clc.22740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gok M, Kundi H, Kiziltunc E, et al. Relationship between prodromal angina pectoris and neutrophil-to lymphocyte ratio in patients with ST elevation myocardial infarction. Heart Lung Circ. 2019;28(6):901–7. doi: 10.1016/j.hlc.2018.04.283. [DOI] [PubMed] [Google Scholar]
- 13.Reiter R, Henry TD, Traverse JH. Preinfarction angina reduces infarct size in ST-elevation myocardial infarction treated with percutaneous coronary intervention. Circ Cardiovasc Interv. 2013;6(1):52–58. doi: 10.1161/CIRCINTERVENTIONS.112.973164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi T, Shiomi H, Toyota T, et al. Effect of preinfarction angina pectoris on long-term survival in patients with ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention. Am J Cardiol. 2014;114(8):1179–86. doi: 10.1016/j.amjcard.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Kurtul A, Acikgoz SK. Usefulness of mean platelet volume-to-lymphocyte ratio for predicting angiographic no-reflow and short-term prognosis after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2017;120(4):534–41. doi: 10.1016/j.amjcard.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed TAN, Sorgdrager BJ, Cannegieter SC, et al. Pre-infarction angina predicts thrombus burden in patients admitted for ST-segment elevation myocardial infarction. Eurointervention. 2012;7(12):1396–405. doi: 10.4244/EIJV7I12A219. [DOI] [PubMed] [Google Scholar]
- 17.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 18.Bayramoğlu A, Taşolar H, Kaya A, et al. Prediction of no-reflow and major adverse cardiovascular events with a new scoring system in STEMI patients. J Interv Cardiol. 2018;31(2):144–49. doi: 10.1111/joic.12463. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RB, Nam BH. Evaluation of the performance of survival analysis models: Discrimination and calibration measures. Handbook of Statistics. 2003;23:1–25. [Google Scholar]
- 20.Kloner RA. No-reflow phenomenon: maintaining vascular integrity. J Cardiovasc Pharmacol Ther. 2011;16(2):244–50. doi: 10.1177/1074248411405990. [DOI] [PubMed] [Google Scholar]
- 21.Oikonomou E, Mourouzis K, Vogiatzi G, et al. Coronary microcirculation and the no-reflow phenomenon. Curr Pharm Des. 2018;24(25):2934–42. doi: 10.2174/1381612824666180911122230. [DOI] [PubMed] [Google Scholar]
- 22.Ng FC, Coulton B, Chambers B, Thijs V. Persistently elevated microvascular resistance postrecanalization. Stroke. 2018;49(10):2912–15. doi: 10.1161/STROKEAHA.118.021631. [DOI] [PubMed] [Google Scholar]
- 23.Ndrepepa G, Tiroch K, Fusaro M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55(21):2983–89. doi: 10.1016/j.jacc.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 24.Bernhagen J. Protective cardiac conditioning by an atypical cytokine. Clin Sci. 2019;133(8):933–37. doi: 10.1042/CS20190036. [DOI] [PubMed] [Google Scholar]
- 25.Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur Heart J. 2017;38(11):774–84. doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 26.Bromage DI, Pickard JMJ, Rossello X, et al. Remote ischaemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: A systematic review and meta-analysis. Cardiovasc Res. 2017;113(3):288–97. doi: 10.1093/cvr/cvw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Qiu B, Zhang Y, et al. The value of pre-infarction angina and plasma D-dimer in predicting no-reflow after primary percutaneous coronary intervention in ST-segment elevation acute myocardial infarction patients. Med Sci Monit. 2018;24:4528–35. doi: 10.12659/MSM.909360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi N, Hata N, Tsurumi M, et al. Relation of coronary culprit lesion morphology determined by optical coherence tomography and cardiac outcomes to preinfarction angina in patients with acute myocardial infarction. Int J Cardiol. 2018;269:356–61. doi: 10.1016/j.ijcard.2018.07.074. [DOI] [PubMed] [Google Scholar]
- 29.Celik T, Balta S, Mikhailidis DP, et al. The relation between no-reflow phenomenon and complete blood count parameters. Angiology. 2017;68(5):381–88. doi: 10.1177/0003319716659193. [DOI] [PubMed] [Google Scholar]
- 30.Avci E, Kiris T, Çelik A, et al. Prognostic value of rising mean platelet volume during hospitalization in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. BMC Cardiovasc Disord. 2018;18(1):226. doi: 10.1186/s12872-018-0970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celik T, Kaya MG, Akpek M, et al. Predictive value of admission platelet volume indices for in-hospital major adverse cardiovascular events in acute ST-segment elevation myocardial infarction. Angiology. 2015;66(2):155–62. doi: 10.1177/0003319713513493. [DOI] [PubMed] [Google Scholar]
- 32.Kırış T, Yazici S, Günaydin ZY, et al. The prognostic impact of in-hospital change in mean platelet volume in patients with non-ST-segment elevation myocardial infarction. Angiology. 2016;67(7):690–96. doi: 10.1177/0003319715627734. [DOI] [PubMed] [Google Scholar]
- 33.Núñez J, Miñana G, Bodí V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18(21):3226–33. doi: 10.2174/092986711796391633. [DOI] [PubMed] [Google Scholar]
- 34.Kiris T, Çelik A, Variş E, et al. Association of lymphocyte-to-monocyte ratio with the mortality in patients with ST-elevation myocardial infarction who underwent primary percutaneous coronary intervention. Angiology. 2017;68(8):707–15. doi: 10.1177/0003319716685480. [DOI] [PubMed] [Google Scholar]
- 35.Moriya J. Critical roles of inflammation in atherosclerosis. J Cardiol. 2019;3(2):22–27. doi: 10.1016/j.jjcc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Hudzik B, Szkodziński J, Lekston A, et al. Mean platelet volume-to-lymphocyte ratio: A novel marker of poor short- and long-term prognosis in patients with diabetes mellitus and acute myocardial infarction. J Diabetes Complicat. 2016;30(6):1097–102. doi: 10.1016/j.jdiacomp.2016.04.010. [DOI] [PubMed] [Google Scholar]