Abstract
DIAPH1 is a formin protein involved in actin polymerization with important roles in vascular remodeling and thrombosis. To investigate potential associations of DIAPH1 single-nucleotide polymorphisms (SNPs) with hypertension and stroke, 2,012 patients with hypertension and 2,210 controls, 2,966 stroke cases [2,212 ischemic stroke (IS), 754 hemorrhagic stroke (HS)] and 2,590 controls were enrolled respectively in the case-control study. A total of 4,098 individual were included in the cohort study. DIAPH1 mRNA expression was compared between 66 IS [43 small artery occlusion (SAO) and 23 large-artery atherosclerosis (LAA)] and 58 controls. Odds ratio (OR), hazard ratio (HR) and 95% confidence interval (CI) were calculated by logistic and cox regression analysis. Rs7703688 T>C variation was significantly associated with an increased risk of IS [OR (95% CI) was 1.721 (1.486-1.993), P=4.139×10-12]. Association of rs7703688 with stroke risk was further validated in the cohort study [adjusted HRs (95% CIs) for additive and recessive models were 1.385 (1.001-1.918), P=0.049, and 2.882 (1.038-8.004), P=0.042, respectively)]. DIAPH1 mRNA expression was significantly downregulated in IS. In SAO stroke subtype, DIAPH1 expression has an increased trend among rs251019 genotypes (Ptrend=0.048). These novel findings suggest that DIAPH1 variation contributes to genetic susceptibility to stroke risk, especially the SAO subtype of IS.
Keywords: DIAPH1, polymorphisms, stroke, mRNA
INTRODUCTION
Stroke ranks as the first leading cause of death in China, with bearing the highest stroke burden in the world [1]. Hypertension is a major risk factor for cerebrovascular disease, including ischemic stroke (IS) and hemorrhagic stroke (HS) [2]. However, characterization of the mechanisms underlying stroke is still incomplete.
Chronic hypertension induces vascular remodeling of cerebral arteries, an essential risk factor for stroke. At play in this phenomenon is activation of the Rho/Rho-kinase pathway, a crucial modulator of proliferation, motility, and contractility of smooth muscle cells (SMCs) [3–5]. Supporting a key association between Rho/Rho-kinase pathway and cardiovascular diseases via vascular remodeling, our previous studies showed that genetic variations in Rho kinases (ROCK1 and ROCK2) modulate susceptibility to hypertension and stroke [6].
Mammalian homolog of Drosophila diaphanous 1 (DIAPH1), a formin protein, is a canonical effector for Rho signaling in humans [7, 8]. After being activated by GTP-bound RhoA, the formin homology-2 domain of DIAPH1 stimulates actin filament assembly at the barbed ends [9]. DIAPH1 mediates vascular remodeling via integration of oxidative stress and signal transduction pathways in SMCs [10]. Besides, DIAPH1 induces pro-platelet formation in megakaryocytes by coordinating the actin and microtubule cytoskeleton [11], which critically impacts blood clotting and thrombogenic processes.
Previous studies have focused on the association of DIAPH1 polymorphisms and macrothrombocytopenia, hearing loss, blindness, and cancer [12–14]. Importantly, animal experiments have demonstrated that genetic deletion of DIAPH1 led to infarct size reduction and improved contractile function after myocardial ischemia/reperfusion [15]. However, whether changes in DIAPH1 expression or function may contribute to stroke incidence has not been established. On account of the important role of DIAPH1 on vascular remodeling and thrombosis, i.e. two key aspects in the pathophysiology of stroke, we decided to investigate potential associations between DIAPH1 gene variations and stroke risk.
To this end, we performed case-control and cohort studies to evaluate the association of single-nucleotide polymorphisms (SNPs) in the human DIAPH1 gene with susceptibility to hypertension and stroke. In addition, the distribution of DIAPH1 SNP genotypes was typified by measuring DIAPH1 mRNA expression in peripheral blood mononuclear cells (PBMCs) from IS and hypertensive controls. The present findings provide novel insights about the potential contribution of DIAPH1 polymorphisms to the pathogenesis of hypertension and stroke.
RESULTS
Demographic and clinical characteristics of the study population
Clinic-demographic characteristics of participants in the hypertension case-control study are summarized in Supplementary Table 1. Although study subjects were matched for age (5 year-group), hypertensive cases were on average 3.42 years older than controls (P < 0.001). Participants with hypertension had higher BMI, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), glucose (GLU), and a higher rate of type 2 diabetes mellitus (T2DM) than controls (P < 0.001). No significant differences in gender, high-density lipoprotein cholesterol (HDL-C), or smoking and drinking statuses were observed (P > 0.05).
The characteristics of individuals in the stroke case-control study are summarized in Supplementary Table 2. Significant differences were observed among IS, HS, and controls for age, gender, smoking and drinking habits, hypertension, lipid profiles, and T2DM (P < 0.05). Post-hoc multiple comparisons showed that total TC, HDL-C, and LDL-C levels were significantly higher in IS cases than in controls. Compared to controls, HS cases were older, had higher levels of TC and HDL-C, and lower TG levels. These characteristics were adjusted as confounding factors when evaluating the association of DIAPH1 with stroke.
Association analysis of DIAPH1 variants in the case-control study of hypertension
In the case-control study of hypertension, the allele frequencies of the DIAPH1 rs3805691, rs251019, and rs11954998 SNPs in controls were consistent with Hardy-Weinberg equilibrium (HWE), whereas the allele frequencies of rs251018 and rs7703688 were not (HWE P = 0.004 and P < 0.001, respectively). Compared with CC/CT carriers, the rs251019 TT genotype was significantly associated with decreased risk of hypertension, after adjusting for covariates including age, gender, BMI, TC, TG, HDL-C, LDL-C, GLU, smoking status, and drinking status [adjusted odds ratio (OR), 0.791; 95% confidence interval (CI), 0.636-0.984; P=0.035] (Table 1).
Table 1. Association analyses of DIAPH1 SNPs with hypertension in the case-control study.
| SNP | Group | WT/HT/MT | OR(95% CI)a | |||
| Additive model | Dominant model | Recessive model | P-valuesb | |||
| rs3805691 | CC/CT/TT | |||||
| Case | 1136/749/126 | 1.000(0.902-1.109) | 0.994(0.875-1.13) | 1.031(0.793-1.341) | 0.805 | |
| Control | 1253/825/132 | P=0.996 | P=0.931 | P=0.820 | ||
| rs251018 | TT/TG/GG | |||||
| Case | 1421/551/40 | 0.953(0.845-1.075) | 1.001(0.878-1.158) | 0.572(0.384-0.812) | 0.004 | |
| Control | 1556/574/79 | P=0.435 | P=0.906 | P=0.006 | ||
| rs251019 | CC/CT/TT | |||||
| Case | 980/866/166 | 0.946(0.859-1.042) | 0.985(0.868-1.117) | 0.791(0.636-0.984) | 0.249 | |
| Control | 1055/927/228 | P=0.261 | P=0.813 | P=0.035 | ||
| rs7703688 | TT/TC/CC | |||||
| Case | 1672/330/10 | 0.893(0.766-1.040) | 0.942(0.797-1.113) | 0.315(0.152-0.650) | <0.001 | |
| Control | 1818/356/35 | P=0.146 | P=0.485 | P=0.002 | ||
| rs11954998 | TT/TC/CC | |||||
| Case | 1482/488/42 | 0.943(0.830-1.071) | 0.920(0.798-1.060) | 1.112(0.710-1.742) | 0.205 | |
| Control | 1590/578/42 | P=0.366 | P=0.248 | P=0.642 | ||
WT wild type, HT heterozygote, MT mutant type;
Rs251018 and rs7703688 were not consistent with HWE, with P values of 0.004 and 0.001.
a: Adjusted for age, gender, BMI, GLU, HDL-C, LDL-C, TC, TG, smoking status and drinking status.
b: The P-values of HWE test in controls.
Association analysis of DIAPH1 variants in the case-control study of IS
In the case-control study of stroke, the frequencies of all DIAPH1 SNPs in controls were consistent with HWE. Table 2 shows the results of the association analyses after adjusting for age, gender, smoking status, drinking status, TC, TG, LDL-C, HDL-C, T2DM, and hypertension. The additive model (CC vs CT vs TT) suggested that the rs3805691 variant was associated with decreased risk of IS (adjusted OR=0.782, 95% CI=0.700–0.874, P=1.3×10-5). Compared with CC carriers, CT/TT carriers of the rs3805691 SNP were also at lower risk of IS (adjusted OR=0.712, 95% CI=0.622–0.815, P=7.986×10-7). Comparable protective effects against stroke were observed for the rs251019 variant in both additive and dominant models. In contrast, rs7703688 and rs11954998 C allele carriers were associated with increased risk of IS [ORs (95% CIs) for the additive model were 1.721 (1.486-1.993) and 1.537 (1.356-1.743), P=4.139×10-12 and P=2.058×10-11, respectively]. These associations were still significant after Bonferroni correction. No significant association with IS was found for rs251018, nor between the five DIAPH1 SNPs and HS.
Table 2. Association analyses of DIAPH1 SNPs with stroke sub-types in the case-control study.
| Stroke subtypes | SNP | Group | WT/HT/MT | OR (95% CI)a | ||
| Additive model | Dominant model | Recessive model | ||||
| IS | rs3805691 | CC/CT/TT | ||||
| Case | 1429/623/109 | 0.782(0.700-0.874) | 0.712(0.622-0.815) | 0.883(0.666-1.171) | ||
| Control | 1434/990/166 | P=1.300×10-5 | P=7.986×10-7 | P=0.388 | ||
| rs251018 | TT/TG/GG | |||||
| Case | 1533/619/46 | 1.041(0.916-1.182) | 1.066(0.923-1.232) | 0.893(0.579-1.378) | ||
| Control | 1857/663/70 | P=0.539 | P=0.386 | P=0.609 | ||
| rs251019 | CC/CT/TT | |||||
| Case | 1322/681/206 | 0.813(0.734-0.900) | 0.685(0.600-0.782) | 1.084(0.862-1.362) | ||
| Control | 1277/1082/231 | P=6.200×10-5 | P=1.899×10-8 | P=0.491 | ||
| rs7703688 | TT/TC/CC | |||||
| Case | 1638/509/61 | 1.721(1.486-1.993) | 1.771(1.504-2.086) | 3.076(1.790-5.287) | ||
| Control | 2164/401/25 | P=4.139×10-12 | P=6.923×10-12 | P=4.800×10-5 | ||
| rs11954998 | TT/TC/CC | |||||
| Case | 1336/763/60 | 1.537(1.356-1.743) | 1.626(1.410-1.874) | 1.752(1.159-2.650) | ||
| Control | 1893/644/53 | P=2.058×10-11 | P=2.275×10-11 | P=0.008 | ||
| HS | rs3805691 | CC/CT/TT | ||||
| Case | 411/296/41 | 0.979(0.809-1.185) | 1.023(0.808-1.295) | 0.771(0.449-1.322) | ||
| Control | 1434/990/166 | P=0.830 | P=0.850 | P=0.344 | ||
| rs251018 | TT/TG/GG | |||||
| Case | 548/176/24 | 0.941(0.745-1.189) | 0.945(0.726-1.231) | 0.831(0.372-1.855) | ||
| Control | 1857/663/70 | P=0.978 | P=0.677 | P=0.652 | ||
| rs251019 | CC/CT/TT | |||||
| Case | 392/284/67 | 0.972(0.814-1.160) | 0.940(0.743-1.188) | 1.042(0.691-1.569) | ||
| Control | 1277/1082/231 | P=0.754 | P=0.604 | P=0.845 | ||
| rs7703688 | TT/TC/CC | |||||
| Case | 610/126/15 | 1.103(0.832-1.463) | 1.078(0.787-1.476) | 1.633(0.605-4.411) | ||
| Control | 2164/401/25 | P=0.494 | P=0.640 | P=0.333 | ||
| rs11954998 | TT/TC/CC | |||||
| Case | 547/181/20 | 1.101(0.870-1.393) | 1.107(0.853-1.438) | 1.238(0.549-2.791) | ||
| Control | 1893/644/53 | P=0.424 | P=0.445 | P=0.606 | ||
IS: ischemic stroke; HS: hemorrhagic stroke. The allele frequencies of all SNPs in controls were consistent with HWE; HWE P-values for rs3805691, rs251018, rs251019, rs7703688, and rs11954998 were 0.780, 0.244, 0.933, 0.184, and 0.837 respectively.
a: Adjusted for age, gender, smoking status, drinking status, TC, TG, LDL-C, HDL-C, T2DM, and hypertension.
Furthermore, we conducted SNP association analysis stratified by TOAST subtypes (Supplementary Table 8). After covariates adjustment, both rs3805691 and rs251019 were negatively associated with small artery occlusion (SAO) and large-artery atherosclerosis (LAA) under the additive and dominant models. Meanwhile, the rs11954998 and rs7703688 variants were instead associated with increased risks of SAO and LAA under all three genetic models, while no association was detected between rs251018 and either SAO or LAA. Analysis for HS subtypes showed that compared to the TT/TC genotypes, the CC genotype of rs7703688 conferred higher risk for subarachnoid hemorrhage (SAH), whereas none of the DIAPH1 SNPs studied showed association with intracerebral hemorrhage (ICH) (Supplementary Table 9).
Association analysis of DIAPH1 variants in the cohort study of hypertension and stroke
The clinic-demographic characteristics of participants in the cohort study of hypertension and stroke are shown in Supplementary Table 3. No significant associations between selected DIAPH1 gene variants and hypertension were observed (Table 3). Regarding stroke, rs251018 GG genotype carriers showed significantly higher incidence rate than TT/TG carriers after adjusting for age, gender, TC, TG, HDL-C, LDL-C, smoking, drinking, BMI, T2DM, and hypertension. Increased risk for stroke was also found for rs7703688 genotypes in the additive and recessive models (P=0.049 and P=0.042, respectively; Table 3).
Table 3. Association analyses of DIAPH1 SNPs and hypertension and stroke in the cohort study.
| End point | SNP | Genotype | N | Person-years | Incidence density (/104) | HR (95% CI) | ||
| Additive model | Dominant model | Recessive model | ||||||
| Hypertension | rs3805691 | CC | 347 | 5059.10 | 685.89 | 1.019 (0.896-1.166) | 1.022 (0.874-1.206) | 1.031 (0.734-1.447) |
| CT | 230 | 3383.53 | 682.72 | P=0.744a | P=0.748a | P=0.862a | ||
| TT | 36 | 551.32 | 652.98 | |||||
| rs251018 | TT | 435 | 6315.13 | 690.41 | 0.964 (0.831-1.118) | 0.960 (0.806-1.144) | 0.937 (0.605-1.453) | |
| TG | 157 | 2352.28 | 667.44 | P=0.630a | P=0.650a | P=0.772a | ||
| GG | 21 | 326.53 | 643.13 | |||||
| rs251019 | CC | 306 | 4311.25 | 712.09 | 0.910 (0.808-1.026) | 0.872 (0.744-1.022) | 0.920 (0.712-1.19) | |
| CT | 241 | 3724.56 | 647.06 | P=0.123a | P=0.092a | P=0.525a | ||
| TT | 66 | 958.13 | 688.84 | |||||
| rs7703688 | TT | 503 | 7373.11 | 683.57 | 0.877 (0.728-1.056) | 0.902 (0.733-1.111) | 0.489 (0.218-1.095) | |
| TC | 104 | 1470.03 | 707.47 | P=0.166a | P=0.333a | P=0.082a | ||
| CC | 6 | 148.27 | 404.67 | |||||
| rs11954998 | TT | 446 | 6488.01 | 688.96 | 0.877 (0.745-1.032) | 0.865 (0.723-1.035) | 0.837 (0.46-1.524) | |
| TC | 156 | 2335.90 | 667.84 | P=0.113a | P=0.113a | P=0.561a | ||
| CC | 11 | 170.04 | 646.91 | |||||
| Stroke | rs3805691 | CC | 104 | 11943.44 | 87.07 | 1.025 (0.796-1.319) | 0.887 (0.663-1.186) | 1.166 (0.655-2.075) |
| CT | 64 | 7919.26 | 80.82 | P=0.851b | P=0.419b | P=0.601b | ||
| TT | 15 | 1281.25 | 117.07 | |||||
| rs251018 | TT | 133 | 14859.96 | 89.50 | 1.239 (0.943-1.627) | 1.178 (0.858-1.615) | 2.224 (1.081-4.574) | |
| TG | 44 | 5689.45 | 77.34 | P=0.124b | P=0.311b | P=0.030b | ||
| GG | 6 | 598.16 | 100.31 | |||||
| rs251019 | CC | 90 | 10196.63 | 88.26 | 1.111 (0.894-1.381) | 1.103 (0.829-1.469) | 1.264 (0.791-2.018) | |
| CT | 74 | 8973.51 | 82.46 | P=0.343b | P=0.501b | P=0.327b | ||
| TT | 19 | 1977.42 | 96.08 | |||||
| rs7703688 | TT | 149 | 17468.61 | 85.29 | 1.385 (1.001-1.918) | 1.345 (0.936-1.935) | 2.882 (1.038-8.004) | |
| TC | 31 | 3451.09 | 89.83 | P=0.049b | P=0.109b | P=0.042b | ||
| CC | 3 | 222.77 | 134.67 | |||||
| rs11954998 | TT | 129 | 15450.27 | 83.49 | 0.880 (0.661-1.171) | 1.004 (0.731-1.379) | 0.215 (0.03-1.541) | |
| TC | 53 | 5283.27 | 100.32 | P=0.631b | P=0.982b | P=0.126b | ||
| CC | 1 | 414.02 | 24.15 | |||||
a: Adjusted for age, gender, TC, TG, HDL-C, LDL-C, smoking, drinking, BMI and GLU.
b: Adjusted for age, gender, TC, TG, HDL-C, LDL-C, smoking, drinking, BMI, T2DM and hypertension
Comparison of DIAPH1 mRNA expression between IS and controls
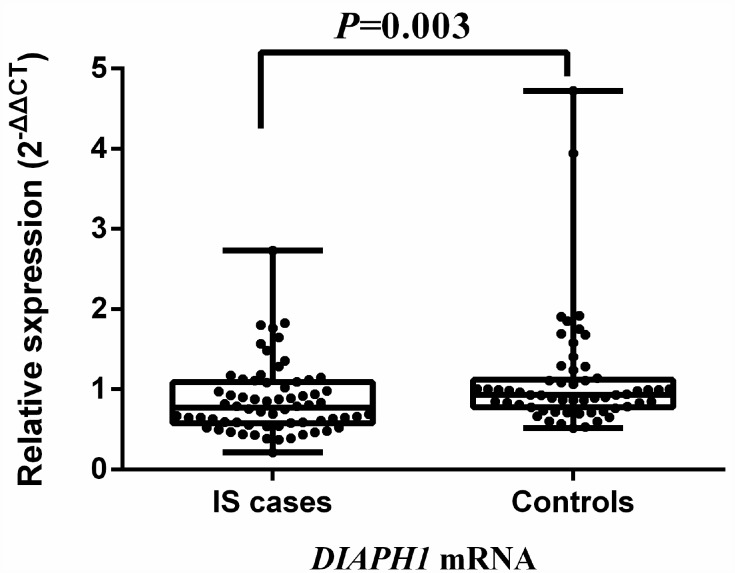
Comparative analysis of mRNA expression for the selected SNPs was further conducted in 58 controls and 66 IS cases (43 SAO and 23 LAA). Compared with hypertensive controls, the expression of DIAPH1 mRNA was significantly downregulated in IS [0.773 (0.575, 1.088) vs 0.933 (0.775, 1.117), P = 0.003]. Results are depicted in Figure 1. The expression of DIAPH1 mRNA among the genotypes of rs3805691, rs251018, rs251019, rs11954998, and rs7703688 did not differ significantly, neither in IS cases nor in controls (Supplementary Figure 2). However, the DIAPH1 mRNA level in SAO was upregulated with rs251019 genotypes, especially in homozygous CC carriers (mean expression levels in TT, TC, and CC carriers were 0.742, 0.889, and 1.765, respectively, Ptrend = 0.048; Supplementary Figure 3).
Figure 1.
Comparison of DIAPH1 mRNA expression between ischemic stroke cases and controls. The expression of DIAPH1 mRNA (2-∆∆CT) in PBMCs was significantly downregulated in IS compared with controls [0.773 (0.575, 1.088) vs 0.933 (0.775, 1.117); P = 0.003]. IS, ischemic stroke.
DISCUSSION
The current study conducted case-control and cohort studies to investigate the associations of DIAPH1 polymorphisms with hypertension and stroke. The key findings showed indicated that rs7703688 was significantly associated with risk of stroke, especially with IS. The significant association between rs7703688 and stroke was further validated in the cohort study. Our study also noted, for the first time, that DIAPH1 mRNA expression is downregulated in IS, implying that DIAPH1 might affect its pathogenesis.
Previous studies have identified an essential role for DIAPH1 in actin cytoskeletal remodeling, arterial SMC cell migration, and as a mediator of myocardial ischemia/reperfusion injury and vascular and neuroinflammatory dysfunction [16]. Whether DIAPH1 polymorphisms affect stroke susceptibility had not been so far determined in GWAS. Rs7703688 was associated with an increased risk of IS, and modestly, but still significantly, it correlated with higher incidence of stroke in the cohort study. This suggests that rs7703688 may constitute a positive locus for stroke diagnosis and treatment. Besides, we also observed that DIAPH1 rs3805691, rs251019 and rs11954998 were associated with IS and with two IS TOAST subtypes, i.e. SAO and LAA. However, we noticed that these three SNPs in IS cases were deviated from HWE (P<0.001), thus, the associations of the later three SNPs with IS were still need further validation.
Bioinformatics analysis for rs7703688 showed that it overlaps with a Hidden Markov Model-predicted enhancer in 15 issues, including brain, a finding that may help elucidate its involvement in stroke. The position weight matrix (PWM)-scanning process showed that the variation at rs7703688 changes the match to the AP-4 and Spz1 motifs (Supplementary Table 4). Of note, our analysis revealed that s7703688 is related to 4 eQTLs, reported by a single experiment, for RELL2, ARAP3, FCHSD1, and PCDHGA6 expression. ARAP3 encodes a phosphoinositide binding protein containing ARF-GAP and RHO-GAP, which cooperate in cell cytoskeleton remodelling and determining cell shape. Therefore, motifs coupled with eQTL data suggest that functional studies looking at whether AP-4 and Spz1 bind differentially to rs7703688 are warranted. Moreover, the GTEx Portal indicates that the rs7703688 variant is linked to lower ARAP3 expression in whole blood. Thus, investigating whether ARAP3 variation contributes to IS risk is also of great interest.
Accumulation of soluble forms of the receptor for advanced glycation end products (RAGEs, also known as AGER) in serum/plasma has been implicated in multiple physiological and pathological processes, including aging, diabetes, neurodegeneration, ischemia/reperfusion injury, among others [17, 18]. DIAPH1 is a key intracellular signaling effector of RAGE [19]. Mutation in the cytoplasmic domain of RAGE involving alanine substitution of R5/Q6 residues inhibits physical interaction with DIAPH1 (FH1 domain) and RAGE ligand-stimulated signal transduction. Our previous study focused on the relationship between RAGE genetic variations and hypertension [20]. Positive associations between RAGE variations and IS have been observed as well [21].
Our expression analyses of DIAPH1 mRNA in PBMCs from IS cases and hypertensive controls showed that DIAPH1 mRNA expression was significantly downregulated in IS. We speculate that DIAPH1 downregulation would lead to RAGEs accumulation, increasing IS risk. Besides, since DIAPH1 is also involved in the platelet release process [11], its downregulation might enhance platelet production and promote thrombosis. On the other hand, DIAPH1 silencing improved intracellular calcium homeostasis in cardiomyocytes following I/R injury [15, 22], which suggests that DIAPH1 downregulation may be beneficial in ischemic contexts.
Since DIAPH1 mRNA expression has an increased trend across rs251019 genotypes in SAO, especially in homozygous carriers, which might be considered as a novel eQTL for this IS subtype. Of note, a correlation between rs251019 and both HDAC3 and TAF7 expression has been reported [23], while another study showed that the HDAC3 rs2530223 SNP was associated with CpG site cg24137543, which is close to the transcription start site of DIAPH1 [24]. Although rs251019 showed a big deviation from HWE in our study, the significant result of rs251019 in SAO patients would still inspire us to conduct further function studies to explore its role in stroke.
The distinct advantages of the current study are reflected in the following aspects. First, the positive DIAPH1 loci associated with stroke could be mutually validated by the case-control and cohort study design. Second, this genetic association study contains a relatively large number of IS and HS cases from south China, further classified by clinical sub-phenotype (SAO and LAA; SAH and ICH). The novel associations detected between DIAPH1 rs7703688 and stroke may provide further insight about molecular differences between etiological stroke subtypes, especially in the Asian population.
Several limitations are also apparent in our study. First, by selecting candidate SNPs with the criterion MAF ≥ 0.05 we may have missed the chance of evaluating rare variants in DIAPH1 also associated to stroke or hypertension. Second, all participants were from the south China Han population, so the present findings might not be representative of other demographic groups. Third, rs7703688 was not in HWE neither in controls within the case-control study of hypertension. Although the association of rs7703688 with stroke was further validated in the cohort study, the P values were not significant after multiple testing correction. Indeed, we should be cautious about the association of rs7703688 with stroke, until larger scale population studies validate these findings.
In conclusion, the current study reports original evidence for the association of genetic variation in the DIAPH1 gene with stroke risk, especially the SAO subtype of IS. In parallel, down regulation of DIAPH1 expression was observed in IS, suggesting that DIAPH1 mRNA level might be a potential biomarker for IS diagnosis. Further work to elucidate the specific influence of DIAPH1 gene variation on cerebrovascular conditions may help discover new pharmacological targets and design better therapies against stroke.
MATERIALS AND METHODS
Study population
Case-control and cohort studies were conducted to investigate the association of polymorphisms in the DIAPH1 gene with hypertension and stroke. A total of 4,128 participants from the community hypertension survey were recruited between 2009 and 2010 from Guanlin and Xushe towns in Yixing city (Jiangsu, China). In the case-control study of hypertension, 2,012 patients with hypertension and 2,116 controls were recruited. Hypertension was determined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg, or currently receiving anti-hypertensive medication. Subjects who had a clinical history of secondary hypertension were excluded. As the average age was higher in hypertension cases than in controls, 94 elder controls were selected from local communities for age-matching with hypertension cases. The demographic characteristics of the sample population are listed in Supplementary Table 1, and have been previously reported [25].
In the case-control study of stroke, 2,212 IS and 754 HS cases were recruited between 2013 and 2017 in the Peopleʼs Hospital of Yixing City. All stroke cases were admitted with first-time acute stroke within 72 hours. Individuals older than 85 years were excluded from the study. 2,590 age (± 5 years)- and gender-matched controls were selected from community members. The demographic and clinical characteristics of the studied population are summarized in Supplementary Table 2.
IS and HS sub-types were confirmed by a neurologist according to medical records of computed tomography (CT) and/or magnetic resonance imaging (MRI). As per TOAST criteria [26], 2,212 IS cases were classified into 1,199 SAO, 882 LAA, 108 cardiogenic cerebral embolisms (CE), 12 strokes of undetermined etiology (SUE), and 11 strokes of other determined etiology (SOE). In turn, 754 HS cases were classified into 103 SAH and 651 intracerebral hemorrhages (ICH).
For cohort studies, 2,116 participants with normal blood pressure were enrolled for the hypertension study, while 4,098 subjects were enrolled for the study assessing stroke. The clinic-demographic characteristics of these populations are listed in Supplementary Table 3. During a median follow-up time of 5.01 years, 613 cases of hypertension and 183 strokes (171 IS and 12 HS) were recorded. The flow chart of the study design is outlined in Supplementary Figure 1.
Interviews, physical examinations, and laboratory tests were conducted for all participants. Demographic characteristics including age, gender, smoking status and drinking status were obtained by trained research staff though a standard questionnaire. Weight, height, and blood pressure measurements were obtained by trained assistants according to standard protocols.
Drinking habit was defined as self-reported drinking frequency (current or past consumption of an alcoholic beverage at least 2 times per week for at least 6 months per year). Smoking habit was defined as current or past consumption of at least 20 cigarettes per week for at least 3 months per year.
The research protocol was approved by the ethics committee of Nanjing Medical University. All participants were informed in detail about the investigation and voluntarily signed the informed consent form.
SNP selection
We selected SNPs covering the DIAPH1 gene within 5 kb upstream and 2 kb downstream of the 5' and 3’ ends of the transcript, respectively, according to the International Hap MAP Project database (HapMap Data Rel 24/phase II Nov08, on NCBI B36 assembly, dbSNPb126). All SNPs were selected with the criteria of minor allele frequency (MAF) ≥0.05 and linkage disequilibrium (LD) r2 ≥0.8. SNPs with predictive biological effects and functions were obtained from HaploReg v4.1 [27] and rerun as tagSNPs. Finally, five tagSNPs, rs3805691, rs251018, rs251019, rs7703688, and rs11954998 were selected. Corresponding biological information, primers, and probes are summarized in Supplementary Tables 4, 5.
Blood sampling and SNP genotyping
Blood samples were collected in EDTA-containing receptacles after overnight fasting (>10 h), and TC, TG, HDL-C, LDL-C, and GLU were measured. Stroke cases consented to donate 5 ml venous blood to the Department of Neurology after admission. Anticoagulated samples were stored at −20°C within 24 h after separating serum and plasma.
Genomic DNA was isolated applying a standard phenol-chloroform method. Genotyping was performed using the TaqMan allelic discrimination assay on a 7900HT Real-time PCR System (Applied Biosystems, Foster City, CA). Each plate included blank samples as negative controls to verify genotyping quality. Call rates for each SNP were > 99.9%.
DIAPH1 mRNA measurement
To explore whether DIAPH1 was differentially expressed between IS cases and controls, 66 inpatients with newly diagnosed IS were recruited from Yixing People's Hospital (from Jan to Nov. 2017). Considering that hypertension is a dominant characteristic of IS, we selected 58 age- and gender- matched hypertensive controls to compare the expression of DIAPH1 at the mRNA level. To this end, PBMCs were isolated within 4 h after blood extraction and DIAPH1 mRNA was isolated and quantified using a standard protocol (Supplementary Methods). Primer sequences for DIAPH1 and control GAPDH mRNAs are listed in Supplementary Table 6. In addition, DNA genotyping was conducted in both groups to investigate potential association of DIAPH1 polymorphisms with mRNA level variation. Clinic-demographic characteristics of IS cases and hypertensive controls are summarized in Supplementary Table 7.
Statistical analysis
Unpaired Student’s t-tests were used to assess inter-group differences for quantitative variables, presented as means ± SD. HWE for genotype frequencies was estimated with a Fisher's exact test in controls. For comparisons between not normally distributed independent samples, the Mann–Whitney U test was applied. Unconditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) as well as to adjust for covariates. Cox regression was applied to estimate hazard ratios (HRs) and 95% CIs in the cohort study. A two-tailed P value of 0.05 was defined as the cutoff for statistical significance. All statistical analyses were performed with SPSS version 18.0 (SPSS, Inc., Chicago, IL).
Supplementary Material
Footnotes
AUTHOR CONTRIBUTIONS: Chong Shen designed the research. Zhuanyun Ren, Xiaotian Chen, and Chunlan Liu performed the majority of the experiments. Yanchun Chen, Xianghai Zhao, Huihua Zong, Wuzhuang Tang, and Song Yang provided clinical specimens and performed clinical observations. Xiaotian Chen and Chunlan Liu and analyzed data. Zhanyun Ren, Xiaotian Chen, and Jie Li wrote and Chong Shen revised the manuscript.
CONFLICTS OF INTEREST: We declare that the authors do not have financial disclosures and conflicts of interest.
FUNDING: This work was supported by the National Natural Science Foundation of China (Grant No. 81573232, No. 81673266, No. 81390543, No. 91439202, and No. 81773537), Jiangsu Provincial Fourth “333 Project”, the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine) and the Flagship Major Development of Jiangsu Higher Education Institutions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, et al. , and NESS-China Investigators. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation. 2017; 135:759–71. 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G, Study HO, and Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000; 342:145–53. 10.1056/NEJM200001203420301 [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011; 32:167–73. 10.1016/j.tips.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gien J, Tseng N, Seedorf G, Roe G, Abman SH. Peroxisome proliferator activated receptor-γ-Rho-kinase interactions contribute to vascular remodeling after chronic intrauterine pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014; 306:L299–308. 10.1152/ajplung.00271.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001; 38:1307–10. 10.1161/hy1201.096541 [DOI] [PubMed] [Google Scholar]
- 6.Yang S, Zhao Y, Tian Y, Chen Y, Zhao X, Li Y, Zhao H, Chen X, Zhu L, Fang Z, Yao Y, Hu Z, Shen C. Common variants of ROCKs and the risk of hypertension, and stroke: two case-control studies and a follow-up study in Chinese Han population. Biochim Biophys Acta Mol Basis Dis. 2018; 1864:778–83. 10.1016/j.bbadis.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 7.Young KG, Copeland JW. Formins in cell signaling. Biochim Biophys Acta. 2010; 1803:183–90. 10.1016/j.bbamcr.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 8.Shinohara R, Thumkeo D, Kamijo H, Kaneko N, Sawamoto K, Watanabe K, Takebayashi H, Kiyonari H, Ishizaki T, Furuyashiki T, Narumiya S. A role for mDia, a Rho-regulated actin nucleator, in tangential migration of interneuron precursors. Nat Neurosci. 2012; 15:373–80, S1–2. 10.1038/nn.3020 [DOI] [PubMed] [Google Scholar]
- 9.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007; 76:593–627. 10.1146/annurev.biochem.75.103004.142647 [DOI] [PubMed] [Google Scholar]
- 10.Touré F, Fritz G, Li Q, Rai V, Daffu G, Zou YS, Rosario R, Ramasamy R, Alberts AS, Yan SF, Schmidt AM. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res. 2012; 110:1279–93. 10.1161/CIRCRESAHA.111.262519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan J, Lordier L, Meyran D, Rameau P, Lecluse Y, Kitchen-Goosen S, Badirou I, Mokrani H, Narumiya S, Alberts AS, Vainchenker W, Chang Y. The formin DIAPH1 (mDia1) regulates megakaryocyte proplatelet formation by remodeling the actin and microtubule cytoskeletons. Blood. 2014; 124:3967–77. 10.1182/blood-2013-12-544924 [DOI] [PubMed] [Google Scholar]
- 12.Stritt S, Nurden P, Turro E, Greene D, Jansen SB, Westbury SK, Petersen R, Astle WJ, Marlin S, Bariana TK, Kostadima M, Lentaigne C, Maiwald S, et al. , and BRIDGE-BPD Consortium. A gain-of-function variant in DIAPH1 causes dominant macrothrombocytopenia and hearing loss. Blood. 2016; 127:2903–14. 10.1182/blood-2015-10-675629 [DOI] [PubMed] [Google Scholar]
- 13.Al-Maawali A, Barry BJ, Rajab A, El-Quessny M, Seman A, Coury SN, Barkovich AJ, Yang E, Walsh CA, Mochida GH, Stoler JM. Novel loss-of-function variants in DIAPH1 associated with syndromic microcephaly, blindness, and early onset seizures. Am J Med Genet A. 2016; 170A:435–40. 10.1002/ajmg.a.37422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YN, Izbicki JR, König A, Habermann JK, Blechner C, Lange T, Schumacher U, Windhorst S. Expression of DIAPH1 is up-regulated in colorectal cancer and its down-regulation strongly reduces the metastatic capacity of colon carcinoma cells. Int J Cancer. 2014; 134:1571–82. 10.1002/ijc.28486 [DOI] [PubMed] [Google Scholar]
- 15.O’Shea KM, Ananthakrishnan R, Li Q, Quadri N, Thiagarajan D, Sreejit G, Wang L, Zirpoli H, Aranda JF, Alberts AS, Schmidt AM, Ramasamy R. The Formin, DIAPH1, is a Key Modulator of Myocardial Ischemia/Reperfusion Injury. EBioMedicine. 2017; 26:165–74. 10.1016/j.ebiom.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLean M, Derk J, Ruiz HH, Juranek JK, Ramasamy R, Schmidt AM. The Receptor for Advanced Glycation End Products (RAGE) and DIAPH1: implications for vascular and neuroinflammatory dysfunction in disorders of the central nervous system. Neurochem Int. 2019; 126:154–64. 10.1016/j.neuint.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reichert S, Triebert U, Santos AN, Hofmann B, Schaller HG, Schlitt A, Schulz S. Soluble form of receptor for advanced glycation end products and incidence of new cardiovascular events among patients with cardiovascular disease. Atherosclerosis. 2017; 266:234–39. 10.1016/j.atherosclerosis.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 18.Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, Lerner S, Bakr S, Li Q, Lu Y, Song F, Qu W, Gomez T, et al. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation. 2006; 113:1226–34. 10.1161/CIRCULATIONAHA.105.575993 [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy R, Shekhtman A, Schmidt AM. The multiple faces of RAGE—opportunities for therapeutic intervention in aging and chronic disease. Expert Opin Ther Targets. 2016; 20:431–46. 10.1517/14728222.2016.1111873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, Wang H, Yang Y, Wang W, Jiang J, Zhao X, Du Q, Wang X, Yao Y, Shen H, Shen C, Zhao Y. Association study of AGER gene polymorphism and hypertension in Han Chinese population. Gene. 2012; 498:311–16. 10.1016/j.gene.2012.01.080 [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Zhu J, Chen L, Hu W, Wang M, Li S, Gu X, Tao H, Zhao B, Ma G, Li K. Genetic predisposition to ischaemic stroke by RAGE and HMGB1 gene variants in Chinese Han population. Oncotarget. 2017; 8:100150–64. 10.18632/oncotarget.22112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999; 79:763–854. 10.1152/physrev.1999.79.3.763 [DOI] [PubMed] [Google Scholar]
- 23.Westra HJ, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, Christiansen MW, Fairfax BP, Schramm K, Powell JE, Zhernakova A, Zhernakova DV, Veldink JH, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013; 45:1238–43. 10.1038/ng.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciuculete DM, Boström AE, Voisin S, Philipps H, Titova OE, Bandstein M, Nikontovic L, Williams MJ, Mwinyi J, Schiöth HB. A methylome-wide mQTL analysis reveals associations of methylation sites with GAD1 and HDAC3 SNPs and a general psychiatric risk score. Transl Psychiatry. 2017; 7:e1002. 10.1038/tp.2016.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Zhao X, Wang H, Chen Y, Wang W, Zhou W, Wang X, Tang J, Zhao Y, Lu X, Chen S, Wang L, Shen C, Yang S. Common variants in TGFBR2 and miR-518 genes are associated with hypertension in the Chinese population. Am J Hypertens. 2014; 27:1268–76. 10.1093/ajh/hpu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 27.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012; 40:D930–34. 10.1093/nar/gkr917 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.