Abstract
Campaign success is contingent on adequate exposure; however, exposure opportunities (e.g., ad reach/frequency) are imperfect predictors of message recall. We hypothesized that the exposure-recall relationship would be contingent on message processing. We tested moderation hypotheses using 3 data sets pertinent to “The Real Cost” anti-smoking campaign: past 30-day ad recall from a rolling national survey of adolescents aged 13–17 (n = 5,110); ad-specific target rating points (TRPs), measuring ad reach and frequency; and ad-elicited response in brain regions implicated in social processing and memory encoding, from a separate adolescent sample aged 14–17 (n = 40). Average ad-level brain activation in these regions moderates the relationship between national TRPs and large-scale recall (p < .001), such that the positive exposure-recall relationship is more strongly observed for ads that elicit high levels of social processing and memory encoding in the brain. Findings advance communication theory by demonstrating conditional exposure effects, contingent on social and memory processes in the brain.
Keywords: Health Campaigns; Neuroimaging; Message Effects; Youth, Memory; Mentalizing
Millions of dollars are spent each year on mass media campaigns (Holtgrave, Wunderink, Vallone, & Healton, 2009; MacMonegle et al., 2018), which can influence health-relevant behaviors (Wakefield, Loken, & Hornik, 2010). Yet, identifying which messages are most likely to shift population-level behaviors is difficult (O’Keefe, 2018). The success of a health campaign hinges on its ability to achieve adequate exposure (Hornik, 2002; Randolph & Viswanath, 2004), because exposure is a prerequisite for audience members to process message content, which can, in turn, influence message-consistent beliefs, intentions, and behaviors (Cappella, 2006; Fishbein & Cappella, 2006; Lang, 2000). Indeed, even the most deliberately designed messages are subject to null effects if exposure is insufficient (Hornik, 2002).
Despite evidence that opportunities for message exposure (i.e., measures of ad dissemination) lead to recalled exposure (Cowling, Modayil, & Stevens, 2010; Kranzler, Gibson, & Hornik, 2017; Niederdeppe, 2005; Richardson et al., 2014; Southwell, Barmada, Hornik, & Maklan, 2002), the strength of this relationship varies; not all content to which a person is exposed is equally likely to be recalled (Lang, 2000). For example, though anti-smoking ads may be aired frequently enough for youth to be exposed many times over, ads may not achieve equal effects. Content differs in the amount and quality of processing it engenders, which in turn influences motivation, memory encoding, storage (Henke, 2010; Lang, 2000), and persuasion more broadly (Cappella, 2006; Petty & Cacioppo, 1986). In the example of a youth-targeted campaign, some ads might capture the audience more strongly and, subsequently, be recalled more readily. These intermediate processes are important as well, because greater memory for content is associated with changes in targeted campaign outcomes (Brennan et al., 2012).
Together, past work suggests there are likely conditions under which message exposure and recall are more strongly related. Messages that engage specific mental processes prompt message elaboration more readily (Petty & Cacioppo, 1986), thereby enabling the message to be encoded into memory after fewer exposures (Henke, 2010). By contrast, if a message does not prompt specific processing (e.g., the rapid encoding of associations between prior and new information), it may require considerably more exposures to achieve the same memory outcome (Henke, 2010). This suggests that the relationship between exposure and recall is moderated by the extent to which a message engages certain cognitive processes. However, prior research on the role of exposure has mainly focused on demonstrating the role of exposure itself and has not taken a strong theoretical position about the conditions under which the exposure-recall relationship is stronger or weaker. In parallel, separate research, which we review below, has considered the role of message processing and encoding, primarily independently of the role of exposure.
Message processing and memory encoding
Perhaps the most prominent communication model that accounts for message processing and encoding is the Limited Capacity Model of Motivated Mediated Message Processing (LC4MP). According to this model, messages that are motivationally relevant elicit more resource allocation to message encoding and storage processes, leading to greater recognition and recall (Fisher, Keene, Huskey, & Weber, 2018; Lang, 2000). Indeed, the broader notion of motivation to engage with message content has also played an important role in other persuasion scholarship (Chaiken & Trope, 1999; Petty & Cacioppo, 1986; Petty & Wegener, 1999).
In parallel, in the neuroscience domain, there is a strong, positive link between attention and memory (Cohen & Parra, 2016; Murdock, 1965), and the type of processing that occurs during information encoding critically affects memory formation (Henke, 2010; Schmitz & Johnson, 2007). Classical psychological models, such as the levels-of-processing approach, hold that depth of processing, typically operationalized as the type of cognitive activity carried out during encoding, has a substantial influence on memory, such that deeper information processing is expected to lead to more durable memory traces (Craik & Lockhart, 1972). Although labels differ considerably across domains and different memory subsystems (Squire, 2004), there is agreement that more motivationally relevant messages should be better remembered and recalled if exposure and other factors are held constant (Cohen & Parra, 2016; Lang, 2000).
Social relevance and motivation
Social relevance (e.g., social norms and the conferral of social approval) strongly influences motivation and behavior (Cialdini et al., 2006; Cialdini & Trost, 1998; Fishbein & Ajzen, 2011; Glanz, Rimer, & Viswanath, 2008). Beliefs about others’ behavior and perceptions of social approval, whether influenced through persuasive messages or intuited from others, shape behaviors to be consistent with peers. This can result in beneficial or detrimental outcomes, depending on whether peers are engaging in healthy or unhealthy behaviors (e.g., peers who smoke significantly increase risk of smoking; Liu, Zhao, Chen, Falk, & Albarracín, 2017). Social beliefs are particularly salient for adolescents, a key health campaign audience (Noar, 2006). Adolescence is marked by rapid changes in social and brain development, such that adolescents’ increasing sensitivity to social cues substantially influences their actions (Crone & Dahl, 2012; Steinberg & Monahan, 2007). Moreover, social cognition enhances memory formation in this population (Lieberman, 2012), suggesting that information that engages social thought should facilitate recall. Thus, it is possible that messages that feature social information (e.g., highlight peer behavior) or prompt adolescents to consider the social consequences of their behavior (e.g., what others will think of me if I behave a certain way) are more motivationally relevant and better recalled, given opportunities for message exposure. Given our interest in whether motivational relevance increases the strength of the exposure-recall relationship in the real world, we focused on the degree to which social cognition moderates the exposure-recall relationship as one test of this.
Brain response during message exposure
The brain is where message reception, motivation, and, in turn, memory happen (Henke, 2010; Lang, 2000). Functional magnetic resonance imaging (fMRI) tracks brain response and can be used to examine how messages engage brain activity in different regions. Measuring changes in neural response can elucidate the neurocognitive processes that occur during message exposure without interrupting the viewing experience for introspection (Falk & Scholz, 2018). Thus, a communication neuroscience approach provides a means to tap into those processes engaged during message reception that complement information available from other methods.
Brain responses during message exposure predict message effects. Ad-elicited activity in brain regions tracking message value predicts message-relevant outcomes, such as smoking reduction and cessation (Chua et al., 2011; Cooper, Tompson, O’Donnell, & Falk, 2015; Falk, Berkman, Whalen, & Lieberman, 2011; Wang et al., 2013), and neural responses to health messages in small groups predict large-scale message effects (Falk, Berkman, & Lieberman, 2012; Falk et al., 2016; Scholz et al., 2017; Weber, Huskey, Mangus, Westcott-Baker, & Turner, 2015). A small number of fMRI studies (Huskey, Mangus, Turner, & Weber, 2017; Imhof, Schmälzle, Renner, & Schupp, 2017; Langleben et al., 2009; Seelig et al., 2014; Weber et al., 2015) have incorporated theories relevant to persuasion and media effects, such as the LC4MP (Lang, 2000) and the Elaboration Likelihood Model (Petty & Cacioppo, 1986). Key postulates of these theories converge on the notion that message features impact cognitive resources to process message content, thus affecting key outcomes. Together, these studies suggest that ads vary in the degree to which they engage key neurocognitive processes, which is relevant for ad recall in the scanned participants and for effectiveness at scale. However, no prior studies have examined the conditions under which average, ad-induced brain responses (as inferred from a scanned sample) moderate the link between population-level exposure and recall. Likewise, studies that have linked brain responses in small samples to behaviors at scale have not typically accounted for levels of message exposure. Thus, drawing on and synthesizing theoretical perspectives (including the LC4MP and Elaboration Likelihood Model) suggests that the motivational relevance of a message can influence the strength of the exposure-recall relationship. We next examine the neurocognitive processes most relevant to this argument.
Brain processes of interest
As a case of this argument, we focused on one process that indexes motivational relevance in our target group (i.e., social processing) and one process that more generally indexes the depth of memory processing (the direct antecedent of our target outcome).
Social processing
Social connection is fundamental to human survival and happiness (Baumeister & Leary, 1995; Eisenberger, 2015). In turn, social information (about others’ preferences, behaviors, etc.) is highly salient and affects human judgments (Frith & Singer, 2008). The LC4MP posits that a message must be motivationally relevant to prompt encoding (Lang, 2000); messages that are particularly salient may be more apt to motivate attention and information processing. Given the heightened salience of social cues for adolescents (Crone & Dahl, 2012) and the role of social cognition in memory formation (Lieberman, 2012), messages that feature social information or prompt adolescents to consider the social consequences to themselves and others should be more motivationally relevant and better recalled. In line with this perspective, we first focused on the brain regions implicated in a key social process. “Mentalizing,” or evaluating the mental states of other people (Frith & Frith, 2006), is consistently associated with neural responses in the bilateral temporal parietal junction; dorsal, middle, and ventral regions of the medial prefrontal cortex; precuneus; and the right superior temporal sulcus (Dufour et al., 2013). This type of processing could manifest in several ways relevant to motivation and message effects in adolescents (e.g., considering what peers are likely to think of a message, considering what peers will think of me if I engage in this behavior). Regardless of which message features might induce them, this set of processes can be captured via fMRI, under a common umbrella of activation in regions chosen for their role in helping understand others’ mental states.
Memory encoding
The type and quality of cognitive processing at the time of encoding critically affects memory formation (Henke, 2010). Though numerous studies have demonstrated that hippocampal activation correlates with memory encoding (Frankland & Bontempi, 2005; Schacter & Wagner, 1999), most models posit that memories are initially encoded and stored in the hippocampus and become integrated with preexisting memories in a broadly distributed cortical network (Frankland & Bontempi, 2005). For example, Henke’s (2010) influential model suggests that the “rapid encoding of flexible associations,” (Henke, 2010, p. 526) which roughly corresponds to the type of memory relevant to health messaging, depends on the hippocampus and neocortex. Of note, this model has been proposed as a complement to the LC4MP (Fisher, Huskey, Keene, & Weber, 2018). Following this logic, we focused on brain response during initial message encoding; thus, we focused primarily on the hippocampus/medial temporal lobe system (defined through a meta-analysis of regions involved in memory encoding, as described in the Methods).
Current study
In summary, message recall is contingent on, but not entirely explained by, opportunities for exposure (Cowling et al., 2010; Kranzler et al., 2017; Niederdeppe, 2005; Richardson, McNeill, et al., 2014; Southwell et al., 2002). Beyond this well-established effect, we argue that the extent to which a given message is motivationally relevant and engages deeper memory encoding across people—indexed by neural activation in key brain regions implicated in social processing and memory encoding during message exposure in test groups—should moderate the relationship between opportunities for exposure and recall at the population level. In the current study, we examined these effects in the context of “The Real Cost”: a youth-targeted anti-smoking campaign. We linked 3 unique data sets that captured national opportunities for “The Real Cost” ad exposure, cued recall of “The Real Cost” ads in a national survey of adolescents, and brain response to “The Real Cost” ads in a separate sample. We then examined whether the relationship between national exposure opportunities and message recall differs as a function of message processing, indicated by ad-elicited responses in brain regions associated with social processing and memory encoding, as measured in a small group of adolescents. In line with past work (Southwell et al., 2002), we used national target rating point (TRP) data to measure opportunities for exposure and a nationally representative survey to measure the effects of the campaign on cued recall.
Establishing moderation effects (e.g., the conditional nature of the exposure-recall relationship) provides improved explanations of phenomena. Borrowing the systematization of Holbert & Park (2019), we specifically hypothesized a positive contributory moderation, in which the association between message exposure and recall is typically positive (i.e., more exposure should elicit higher levels of recall), but where the relationship is stronger when motivational relevance is high and encoding is more elaborate (i.e., when messages are not motivationally relevant, large amounts of exposure may produce little more recall than would be produced under low- or no-exposure conditions).
To test this effect, we employed a novel methodological approach, synthesizing 3 distinct sources of data related to a current national health campaign that captured opportunities for ad exposure, cued ad recall, and ad-induced neural processing. We first tested the following hypothesis, concerning the main effects of opportunities for exposure on message recall:
H1: There is a positive relationship between opportunities for message exposure and message recall.
Next, we tested the following positive contributory moderation hypotheses:
H2: Message-induced neural responses in (a) social-processing and (b) memory-encoding regions moderate the relationship between opportunities for message exposure and message recall, such that a greater response in these brain regions is associated with a more positive relationship between message exposure and recall.
Methods
Data sets
This study focused on effects of advertisements from “The Real Cost” anti-smoking campaign, the first nationally funded public education campaign aimed at reducing tobacco use among U.S. youth aged 12 to 17 (Duke et al., 2015). Campaign details are provided on page S1, with descriptions of study ads provided in Supporting Table S1. Study data were drawn from three data sets.
Survey data set
The first data set is a national, observational survey of youth and young adults that was undertaken by the Tobacco Center of Regulatory Science at the University of Pennsylvania. Part of a larger project (see page S2 for details), this 20-minute phone survey measured cued, recalled exposure to specific ads from “The Real Cost” campaign (henceforth abbreviated as “cued recall”), tobacco use risk factors, typical media use patterns, and sociodemographic variables. Data were collected through a rolling, cross-sectional survey from 18 June 2014–20 June 2017, administered to a nationally representative sample of 13- to 25-year-olds (n = 11,847). To align the study sample with the campaign’s target audience (12- to 17-year-old nonsmokers and smoking experimenters), the study sample was limited to 13- to 17-year-olds with lifetime use of <100 cigarettes (n = 5,110).
Target rating points data set
The second data set consists of national television TRPs for “The Real Cost” campaign. TRPs measure the opportunity for exposure to media content in a targeted population (e.g., 12- to 17-year-olds) over a specified period of time, equal to the product of the media content reach and the frequency of exposure (Farris, Bendle, Pfeifer, & Reibstein, 2010).1 TRP data were provided by the Food and Drug Administration’s Center for Tobacco Products, which funds and oversees campaign implementation. National TRPs were provided on a weekly basis for each ad, starting on the Monday of each week after campaign initiation on 10 February 2014, and ending on Sunday, 25 June 2017.
Functional magnetic resonance imaging data set
The third data set is comprised of neural responses to ads from “The Real Cost” campaign (fMRI study) in a sample of 14- to 17-year-old nonsmokers (n = 40), collected 3 December 2015–9 June 2016 in Philadelphia, PA. Participants completed an online survey to assess prior recall of campaign ads and demographics, as well as smoking relevant cognitions and behaviors and individual difference measures not included in this study. At the scanning session, participants viewed ads from “The Real Cost” campaign during an fMRI scan while their brain responses were measured (see Figure 1 for details about the study task), then answered questions about the perceived effectiveness of each ad (see page S4 for details). The data in this study consists of neural responses in (a) social-processing and (b) memory-encoding regions, across fMRI study participants for each of 12 campaign ads (see pages S5-S10 for details). All neuroimaging data were acquired at the University of Pennsylvania using a 3 Tesla Siemens Magnetom MRI scanner equipped with a 32-channel head coil. One functional run consisting of 735 volumes was acquired per participant during exposure to “The Real Cost” campaign ads.
Figure 1.
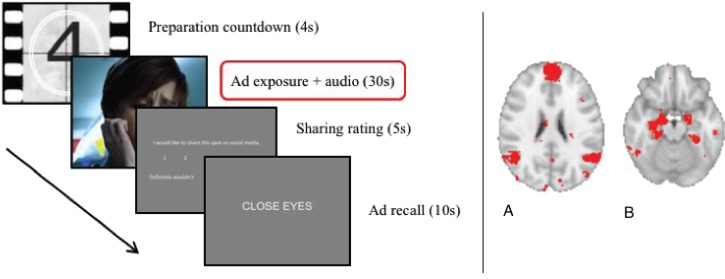
Overview of fMRI study task and brain regions of interest. (Left panel) Participants viewed a 4-second preparation countdown and were then instructed to view one of the 30-second “The Real Cost” ads. Participants then rated their intention to share the ad on social media and were asked to close their eyes and reimagine the ad in their mind’s eye. Each participant completed these tasks in the same order for all 12 ads; however, the order in which the ads were presented was randomized. The current study focused on neural response during the ad exposure period (outlined in red); sharing ratings and neural responses during the sharing and reimagining portions of this task were not assessed in this study. (Right panel) Neural response was measured in (a) social-processing regions and (b) memory-encoding regions. For additional details on brain regions of interest, please see Supplemental Figure S1. FMRI = functional magnetic resonance imaging.
Study design
The dependent variable is self-reported, cued ad recall of 12 ads from “The Real Cost” campaign across 5,110 respondents, as assessed by the Tobacco Center of Regulatory Science survey (Survey data set). To assess cued recall, respondents were asked “about how many times in the past 30 days have you seen or heard of each of the following?” Subsequently, they were read brief descriptions of each ad (Supporting Table S1) and prompted to respond between 0–100 (see pages S2-S3 for more details about survey administration). The independent variable is the total number of national television TRPs attained for each of 12 ads from “The Real Cost” campaign during 8-week intervals prior to (and including) the week during which respondents were interviewed (TRP data set).2 Weekly totals of ad-specific TRPs were aggregated to 8-week measures. We conducted analyses with past 8-week TRPs, because we anticipated lingering reports of cued recall beyond the 30-day period, and past evidence showed increasing ad effects with longer exposure (Richardson, Langley, et al., 2014; White, Durkin, Coomber, & Wakefield, 2015).3
The moderating variables are mean neural response in hypothesized (a) social-processing regions and (b) memory-encoding regions during exposure to each of 12 ads from “The Real Cost” campaign (fMRI data set; see Figure 1). Though potentially related processes, we tested them in isolation for the purpose of this study, and all models that follow treated them separately. Neural response was operationalized as percent signal change in each set of regions during each ad exposure. The social-processing and memory-encoding regions were identified using the Neurosynth database (http://neurosynth.org), which contains neural activation coordinates for a large volume of fMRI studies, based on the occurrence of words or phrases in the text of articles, producing mappings between brain activity and a range of cognitive states. We identified these regions using association test brain maps that correspond with the occurrence of the word “mentalizing” (for social processing) and the phrase “memory encoding” (for memory encoding; see Supporting Figure S1 for more details).4
As the dependent and independent variables were drawn from different data sets, we did not expect any variables to confound the relationship between TRPs and cued recall. However, to reduce noise from individual-level variables that might have been associated with cued recall, analyses controlled for the following potential covariates from the Survey data set: age; sex; race; sensation seeking (Zuckerman, 2007); parental disapproval of smoking, with different response items for users and non-users; household cigarette use; parent education; past 7-day TV watching; and interview week (see page S3 for response options). Parent education and past 7-day TV watching were proxies for socioeconomic status and general TV watching, respectively.
The analytic combined data set
The fMRI values were estimates of neural responses to each ad in (a) social-processing regions and (b) memory-encoding regions during exposure to each of 12 “The Real Cost” ads. We first extracted parameter estimates separately from the social-processing and memory-encoding regions for each ad exposure and each fMRI participant, using the MarsBar toolkit from SPM8 (Brett, Anton, Valabregue, & Poline, 2002), then converted these estimates to percent signal change relative to baseline; this procedure resulted in 12 social-processing and 12 memory-encoding activation values for each fMRI participant. To account for the potential influence of prior ad exposure on neural responses within the fMRI sample, we separately calculated neural response residuals for social-processing and memory-encoding brain regions for each fMRI participant and each ad.5 We averaged these residuals across participants for each ad, resulting in mean neural response values for each set of brain regions across the fMRI sample, creating a measure of the extent to which each ad collectively elicited brain response in social-processing and memory-encoding regions after removing the potential influence of prior ad exposure.6
To control for whole-brain activity exclusive of regions of interest during ad exposure, we extracted parameter estimates capturing whole-brain activity during ad exposure for each fMRI participant, excluding neural activity in social-processing and memory-encoding regions. We converted these estimates to percent signal change relative to baseline, resulting in 12 regressor values for each fMRI participant (one for each ad); we then calculated mean, ad-specific whole-brain responses across fMRI participants. See pages S5-S10 for fMRI methods.
A schematic of the study data sets and data merging procedure is provided in Supporting Figure S2. The analytic combined data set contained cued recall data for each Survey respondent; past 8-week TRPs (based on Survey interview date); mean, ad-level neural responses in social-processing and memory-encoding regions and in the whole brain (exclusive of regions of interest); and Survey respondent covariates. Prior to conducting analyses, variables were centered across respondents.
Analysis plan
We first assessed the main effect of ad-specific TRPs on cued recall (H1). We estimated a mixed-effect multilevel model with the lmerTest package (Kuznetsova, Brockhoff, & Christensen, 2017) in R, regressing past 30-day cued recall on past 8-week TRPs. To assess whether brain responses in (a) social-processing regions and (b) memory-encoding regions during exposure to “The Real Cost” ads moderates the association between TRPs and cued recall (H2), we estimated mixed-effect multilevel models, separately regressing past 30-day cued recall on the interactions between past 8-week TRPs and mean neural response residuals in (a) social-processing regions and (b) memory-encoding regions. We estimated these effects in separate regression models to address distinct (though related) hypotheses, and also because of collinearity between the neural variables (as reported in the Results section). Both models included main effects of TRPs and aggregate neural response derived from the fMRI sample for each ad on cued recall in the national survey. Respondents and ads were treated as random effects, with random intercepts allowed to vary to account for non-independence of repeated measures within respondents and ads. To remove the influence of whole-brain neural response in the fMRI sample during ad exposure and to reduce noise from individual-level variables in the Survey sample that might have been associated with cued recall, analyses controlled for whole-brain neural response and the covariates listed in the Methods section.7
Results
The demographic distributions in the Survey study sample and fMRI sample are presented in Table S2. Survey respondents were evenly distributed by sex and age subgroup (13–15 and 16–17 years), with a mean age of 15.34 (SD = 1.40). Half of respondents (50.2%) were White, a quarter of respondents (24.7%) were Hispanic, and a quarter of respondents were split between Blacks/African Americans (13.2%) and those reporting Other/more than one race (11.8%).
On average, Survey respondents reported cued recall of “The Real Cost” ads approximately 5 times during the previous 30 days (M = 4.92, SD = 11.37). There was variation in cued recall across ads, with ad-specific mean cued recall ranging from 1.14 (SD = 2.91) to 6.80 (SD = 13.78; see Supporting Figure S3 for ad-specific distributions of cued recall). There was also variation in past 8-week TRPs for each ad, ranging from 0–421 TRPs (M = 106.66, SD = 71.59).8 Mean neural response residuals in each set of brain regions, representing percent signal change in blood flow relative to baseline, varied across ads, with a range of -0.034 to 0.034 (M = 0.001, SD = 0.018) in social-processing regions and -0.036 to 0.021 (M = 0.002, SD = 0.015) in memory-encoding regions.9
Main and interaction effects on cued recall
First, we estimated a mixed-effect multilevel model to test the main effect of ad-specific TRPs on cued recall (H1). As hypothesized, results demonstrated a positive relationship between past 30-day cued recall and past 8-week TRPs (β = 0.026, 95% CI 0.006–0.045; p = .011), indicating a positive association between national opportunities for ad exposure and cued ad recall at the individual level.
Next, we estimated mixed-effect multilevel models to examine the moderating effects of aggregate neural responses in (a) social-processing and (b) memory-encoding regions on the association between past 8-week TRPs and cued recall (H2). Results demonstrated significant, positive effects for the interaction between neural response in social-processing regions and past 8-week TRPs on cued recall (β = 0.041, 95% CI 0.023–0.059; p < .001), as well as for the interaction between neural response in memory-encoding regions and past 8-week TRPs on cued recall (β = 0.049, 95% CI 0.027–0.071; p < .001), controlling for global activity in the rest of the brain (see Table 1). Results were robust when models omitted covariates. Thus, ads that elicited greater responses in social-processing regions and in memory-encoding regions showed stronger relationships between TRPs and cued recall, relative to ads that elicited lesser responses, as demonstrated in Figure 2. These effects can be interpreted as follows: for those ads that elicited a lower social-processing brain response, survey respondents reported 0.84 more exposures if their interview date indicated they had the opportunity for exposure to 300 more TRPs, whereas for those ads that elicited a higher social-processing brain response, respondents reported 2.82 more exposures if they had the opportunity for 300 more TRPs. For ads that elicited lower and higher brain responses in memory-encoding regions, the reported exposures associated with 300 TRPs were 0.40 and 2.75, respectively.
Table 1.
Results From Mixed-Effect Multilevel Regression Models
| Social-Processing Brain Regions | Memory-Encoding Brain Regions | |||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | t | p | β | SE | t | p | |
| Past 8-week TRPs | .038** | .013 | 2.87 | .004 | .033* | .013 | 2.56 | .010 |
| Mean neural response in regions | .007 | .040 | 0.18 | .860 | .038 | .042 | 0.90 | .395 |
| Past 8-week TRPs x mean neural response in regions | .041*** | .009 | 4.44 | .000 | .049*** | .011 | 4.49 | .000 |
| Whole-brain neural response | -.039 | .033 | -1.17 | .269 | -.061 | .036 | -1.68 | .125 |
| Age | .028 | .015 | 1.80 | .073 | .027 | .015 | 1.77 | .077 |
| Sex (female = Ref.) | -.035* | .015 | -2.43 | .015 | -.035* | .015 | -2.43 | .015 |
| Race (White = Ref.) | ||||||||
| Hispanic | .033* | .016 | 1.99 | .047 | .032* | .016 | 1.97 | .049 |
| Black/African American | .074*** | .015 | 4.80 | .000 | .074*** | .015 | 4.77 | .000 |
| Other/multiple races | .020 | .015 | 1.30 | .193 | .020 | .015 | 1.29 | .196 |
| Sensation seeking | .058*** | .015 | 3.91 | .000 | .058*** | .015 | 3.93 | .000 |
| Parent disapproval (would/disapprove a lot = Ref.) | ||||||||
| Don’t/wouldn’t mind | -.012 | .015 | -0.78 | .436 | -.012 | .015 | -0.79 | .430 |
| Would/disapprove a little | -.033* | .014 | -2.31 | .021 | -.033* | .014 | -2.32 | .021 |
| Household cigarette use | .046** | .015 | 3.04 | .002 | .046** | .015 | 3.03 | .003 |
| Parental education (HS = Ref.) | ||||||||
| Some college | -.030 | .017 | -1.70 | .089 | -.030 | .017 | -1.70 | .090 |
| College degree | -.035 | .019 | -1.86 | .063 | -.036 | .019 | -1.87 | .062 |
| Graduate degree | -.038* | .019 | -2.01 | .045 | -.039* | .019 | -2.04 | .041 |
| TV watching | .099*** | .015 | 6.76 | .000 | .099*** | .015 | 6.78 | .000 |
| Interview week | -.003 | .018 | -0.17 | .864 | -.004 | .018 | -0.20 | .843 |
Note: Models tested the moderating effect of mean neural response in social-processing and memory-encoding regions on the association between past 8-week TRPs and past 30-day cued recall, controlling for whole-brain neural responses (excluding regions of interest) and potential covariates. Bold data indicates statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001). β = standardized coefficient; HS = high school degree or some high school; Ref. = reference category; TRP = target rating points.
Figure 2.
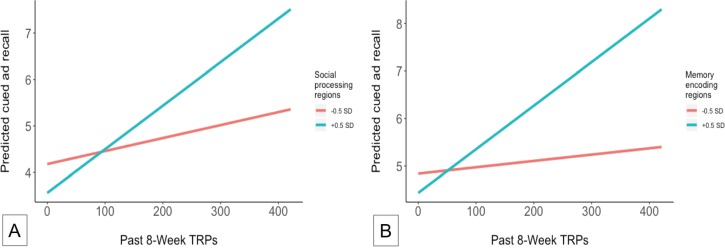
Association between past 8-week TRPs and predicted cued ad recall, at higher and lower levels of ad-elicited neural response in social-processing and memory-encoding regions, controlling for whole-brain neural response and potential covariates. In each figure, the blue line illustrates the relationship between past 8-week TRPs and cued recall, as predicted by (a) the social-processing moderation model and (b) the memory-encoding model (see Table 1), for ads that elicited higher levels (+0.5 SD) of neural response in the corresponding brain regions. The orange line illustrates the relationship between past 8-week TRPs and predicted recall for ads that elicited lower levels (-0.5 SD) of neural response in corresponding brain regions. Results reflect predicted relationships between TRPs, cued recall, and neural response in these regions, holding whole-brain neural response and potential covariates constant. TRP = target rating points. SD = standard deviation.
Lastly, we conducted sensitivity analyses and robustness checks to test (a) the relative fit of regression models with and without fMRI-derived regressors; (b) whether results differed with raw (un-residualized) neural activation values from the fMRI sample, in lieu of residualized values; (c) whether the omission of the whole-brain control variable from models influenced results; (d) whether TRP aggregations over longer and shorter periods (past 4 and 12 weeks) differentially influenced results; (e) whether the observed effects were contingent on respondents’ TV watching (testing a 3-way interaction between TRPs, brain response, and TV watching); (f) whether the results were robust across variation in sensation seeking; and (g) whether results were affected by missing values on the parental education variable. Results were generally consistent across models (full details and exceptions provided on pages S18-S37).
Discussion
In the current study, we first replicated past work (Cowling et al., 2010; Kranzler et al., 2017; Niederdeppe, 2005; Richardson, McNeill, et al., 2014; Southwell et al., 2002) to show a positive and significant relationship between opportunities for exposure (TRPs) and cued recall. We then presented evidence of a positive contributory moderation effect on the exposure-recall relationship. We showed that ads that elicit increased response in brain regions involved in social cognition and memory encoding, measured in a small group of adolescents as an indicator of larger-scale effects, showed stronger positive relationships between national TRPs (opportunities for exposure) and self-reported, cued recall in a large-scale, nationally representative adolescent sample. Together, these results provide new theoretical insight into the message-processing conditions under which exposure and recall are more strongly linked, bringing together theories on message and memory processing across several fields of study and adding evidence for social cognition as a key variable relevant to motivation and subsequent message recall in adolescents.
Under conditions where adolescents had equal opportunity to see “The Real Cost” ads in the preceding weeks (i.e., due to the TV ad flighting schedule), ads that prompted higher memory encoding in the brain (from a separate group of adolescents) were recalled at higher rates, relative to ads that prompted lower memory encoding. Findings suggest that given the opportunity for exposure, ads that evoke enhanced brain activity in memory-encoding regions (primarily the medial temporal lobe, including the hippocampus) in a small group of people are more strongly encoded and, subsequently, better recalled at scale. These findings support theoretical claims across several domains: classical psychological models, which hold that depth of processing leads to more durable memory traces (Craik & Lockhart, 1972); neuroscience literature, which suggests the type of processing that occurs during information encoding critically affects memory formation (Henke, 2010; Schmitz & Johnson, 2007); and established communication theory, which contends that message encoding requires ample cognitive resources to enable the storage and retrieval of messages (Lang, 2000).
Similarly, campaign ads that inspired higher social processing in the brain were recalled more readily, relative to ads that inspired lower social processing. Specifically, ads that elicited stronger activity in brain regions implicated in mentalizing (primarily the dorsal and ventral regions of the medial prefrontal cortex, the bilateral temporal parietal junction, and the precuneus), or in social processing more broadly, were remembered as having been viewed more often, given exposure opportunities. Our interpretation is that, in adolescents exposed to this campaign, social cognition led to stronger motivation to process messages, which in turn facilitated message encoding during exposure. Results are consistent with the notion that adolescents’ enhanced sensitivity to social cues and consequences (Crone & Dahl, 2012) motivates message processing to influence recall (Lang, 2000). This suggests that social processing should be incorporated into theories of message processing, such as the LC4MP, as antecedent or ancillary to motivational relevance. Findings are also consistent with past work showing that messages that communicate social information (e.g., normative beliefs) influence targeted outcomes (Cialdini & Trost, 1998; Rimal & Real, 2005); our findings extend this work by demonstrating that ads that inspire mentalizing produce higher levels of recall, which is a predictor of campaign effects (Kranzler et al., 2017).10 More broadly, incorporating social information, a key component of behavior change models (Fishbein & Ajzen, 2011), into theories of motivated message processing constitutes an opportunity to build more complete models of communication across domains (e.g., health communication, message effects, and neuroscience).
Both moderation models (Table 1) demonstrated robust associations between social-processing and memory-encoding interaction variables (TRPs x neural response) and cued recall, beyond the main effects of TRPs. Moreover, the inclusion of these interaction terms in each model improved model fit, compared to TRP-only models (see pages S18 and S21), highlighting a key advantage of our approach: the brain complements other measures of exposure, as it can be measured concurrently while a message unfolds. This method allowed us to examine multiple hypothesized brain systems at once for a more nuanced understanding of the processes that may co-occur during exposure to memorable messages.
Past fMRI studies have tested theories relevant to persuasion and media effects, showing that a message’s format and features can deplete cognitive resources to process message content (Langleben et al., 2009; Seelig et al., 2014) and that evaluations of persuasive messages can be influenced by affective and executive processing (Huskey et al., 2017). Our results complement prior work by treating ad-induced brain response as a characteristic of messages that affects motivational relevance and memory processing, and by examining the link between these neural processes and population-level campaign outcomes.
To our knowledge, this is the first study to examine the moderating role of neural response on the association between opportunities for exposure and cued recall. Our analyses controlled for whole-brain activity during ad exposure, exclusive of the brain regions of interest, suggesting the findings are not simply driven by global changes in brain activity. Additionally, moderation results were robust after controlling for covariates, and to sensitivity analyses and robustness checks (see pages S18-S37), limiting the scope of alternative explanations.
Although fMRI study recruitment was limited in order to align participants with the campaign’s target audience, the observed moderation effects may not generalize to all members of the target audience. Similarly, the ads assessed in this study were a subset of campaign ads, and may not represent all ads, whether within or beyond this campaign. However, our use of a multilevel model with random intercepts for respondents and ads attempted to address this in part. To our knowledge, the ad flighting schedule and TRPs attained were independent of specific subgroups within the targeted demographic. However, given that we used national, weekly TRP data in aggregate form, we were limited in our assumptions about how it generalized across subgroups (e.g., racial or gender subgroups). Though analyses accounted for a range of potential covariates, findings were limited by the potential influence of unmeasured variables.
Previous research demonstrated that cognitive tasks that inspire social processing and memory encoding prompt brain activations in the regions examined in the current study (Dufour et al., 2013; Frankland & Bontempi, 2005). As with all neuroimaging studies, our psychological interpretations of activity within the brain regions of interest are subject to the constraints of reverse inference (i.e., making inferences about the engagement of specific cognitions, based on the activation of specific brain regions; Poldrack, 2006; Weber, Mangus, & Huskey, 2015). Given the complexity of the human brain, Neurosynth association test maps cannot correspond in a one-to-one manner with the psychological processes of interest. However, these maps, which account for base rates of activation in these regions, offer a meta-analytically defined measure of the general processes we hypothesized to be at play. Furthermore, the hypotheses tested were based on theoretically and empirically supported scholarship, rather than post hoc interpretation of whole-brain results, strengthening our theoretical claim. Although we cannot know the exact nature of mentalizing that might take place when we measure activity in an individual’s brain (e.g., mentalizing about what other teens will think of a given ad, what others will think of me if I engage in this behavior, etc.), we view this as a strength, as the brain-based operationalization has the potential to capture several types of social processing that share a common ingredient (mentalizing). We believe this common ingredient is important to the success of campaigns. More broadly, this approach can inform our understanding of how the brain works, an important scientific goal where communication scientists have increasingly more to contribute.
Implications and future directions
Our results may offer important implications for formative health campaign work, which typically involves the pretesting of potential messages prior to dissemination. Ads from “The Real Cost” campaign were carefully developed and pretested with members of the target audience, according to established procedures, to obtain self-reports of responses (Duke et al., 2015; Zhao et al., 2016). Findings from this study suggest that further exploration of measurement of brain responses to ads at the moment of reception is worthwhile. That information might complement self-report measures to help predict which messages will be more readily encoded and recalled, supporting the dissemination of messages that are ultimately more effective.
We took a communication neuroscience approach to considering which ads produced higher returns for investments in exposure. Other approaches could be taken as well, such as coding the content features of ads (e.g., use of testimonials or, more abstractly, message sensation value [Palmgreen, Donohew, Lorch, Hoyle, & Stephenson, 2001]). Though we acknowledge the utility of measuring content features and establishing their moderating effects for the purpose of producing memorable ads, we contend that brain response offers a distinct path to understanding the conditional relationship between exposure and recall. These analyses may approach understanding the underlying processes more directly, by showing that ads that activate brain regions implicated in social cognition are better recalled. Findings from the current study warrant additional research to examine the relationships between content features (e.g., message sensation value) and message-induced neural response in the regions studied, in order to better understand what message features inspire these types of processing.
Conclusions
Health campaigns hold great promise for influencing health behaviors at scale, but their success is contingent on the ability to achieve not only adequate exposure in their target audience, but also sufficient message engagement and processing. Results provide evidence of a conditional relationship between message exposure and recall, such that ads that are more motivationally relevant and engage greater memory processing (as indexed by social-cognition and memory-encoding brain processes) show stronger relationships between exposure and recall. These findings suggest that capturing ad-specific brain responses in small groups of people may facilitate the selection of campaign messages that are more motivationally relevant, better encoded, and better remembered at large scales. Finally, these findings provide new understanding of the cognitive mechanisms that account for enhanced message processing, complementing existing theory and research and aiding the development of messages that are more readily processed, recalled, and, ultimately, more effective.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Please note: Oxford University Press is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding authors for the article.
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health (NIH; New Innovator Award Number NIH-1 DP2 DA035156-01 to E.B.F.) and the Army Research Laboratory (ARL) Cooperative Agreement Number W911NF-10-2-0022, Subcontract Number APX02-0006, to E.B.F.). Research reported in this publication was also supported by the National Cancer Institute of the NIH and the Food and Drug Administration (FDA) Center for Tobacco Products (Award Number P50CA179546 to R.C.H. and Caryn Lerman). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Notes
1 For example, if a campaign purchased 100 TRPs for a specific ad over a 1-week period, this could reflect 100% of the target population having the opportunity to be exposed to the ad once per week, 1% of the target population having the opportunity to be exposed 100 times per week, or a similar combination of reach and frequency with a product of 100 (Southwell et al., 2002).
2 It is possible that respondents interviewed earlier each week had fewer weekly exposure opportunities than those interviewed later that week. For this reason, 8-week periods receding from each interview week started halfway through the corresponding campaign week.
3 We conducted post hoc sensitivity analyses to examine moderation models, using aggregations of TRPs over shorter (4-week) and longer (12-week) time periods as the independent variable. The results of these analyses are discussed in greater detail on page S19 and pages S26-S29.
4 Brain maps were downloaded from neurosynth.org on 2 February 2018.
5 To estimate neural response residuals, we regressed ad-specific neural responses on fMRI participants’ past 30-day cued recall, controlling for past 8-week TRPs and time since the ad first aired. To account for non-independence of the data (i.e., all fMRI participants completed the scanning task for all ads), regression models included random intercepts for participants and ads.
6 To account for variability across fMRI participants, we tested whether standardizing neural activation values within participants (across ads) prior to averaging these values across participants (within ads) produced similar results. Correlations between original and standardized values were very high (r > .93; p < .001). Details and additional tests are provided on page S8.
7 In the Survey sample, less than 2% of values were missing for covariates, with the exception of parental education (13.3%). To test the influence of missing values, we employed Manski-Horowitz logical bounds (Horowitz & Manski, 2006). Given that the missingness of these items did not affect study results (see Supporting Tables S10 and S11), we omitted missing data rows from analyses.
8 Due to the skewed distributions of cued recalls and TRPs, we log-transformed recall and square root–transformed TRPs to test whether transformations influenced outcomes. As results estimated with transformed variables did not differ substantively from original models, we have reported analyses with untransformed variables to facilitate the interpretation of results.
9 Ad-level neural activation values in social-processing and memory-encoding regions were significantly and positively correlated (r = .66, 95% CI 0.14–0.90; p = .019).
10 Results also extend previous research demonstrating a link between neural responses in social-processing regions and perceptions of ad effectiveness in this fMRI sample (Kranzler, Schmälzle, O’Donnell, Pei, & Falk, 2019).
Supplementary Material
Contributor Information
Elissa C Kranzler, Email: elissak@wharton.upenn.edu.
Ralf Schmälzle, Email: schmaelz@msu.edu.
Rui Pei, Email: rui.pei@asc.upenn.edu.
Robert C Hornik, Email: rhornik@asc.upenn.edu.
Emily B Falk, Email: emily.falk@asc.upenn.edu.
References
- Baumeister R. F., & Leary M. R. (1995). The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117(3), 497– 529 doi: 10.1037/0033-2909.117.3.497. [PubMed] [Google Scholar]
- Brennan E., Momjian A., Jeong M., Naugle D., Parvanta S., & Hornik R. C. (2012). Mass media campaigns to reduce smoking among youth and young adults: Documenting potential campaign targets and reviewing the evidence from previous campaigns (CECCR Working Paper Series.) Retrieved fromhttps://repository.upenn.edu/cgi/viewcontent.cgi?article=1384&context=asc_papers.
- Brett M., Anton J., Valabregue R., & Poline J. (2002). Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Available on CD-ROM in NeuroImage, Vol 16, No 2, abstract 497. [Google Scholar]
- Cappella J. N. (2006). Integrating message effects and behavior change theories: Organizing comments and unanswered questions. Journal of Communication, 56, S265–S279. 10.1111/j.1460-2466.2006.00293.x. [DOI] [Google Scholar]
- Chaiken S., & Trope Y. (Eds.) (1999). Dual-process theories in social psychology. New York: Guilford Press. [Google Scholar]
- Chua H. F., Ho S. S., Jasinska A. J., Polk T. A., Welsh R. C., Liberzon I., & Strecher V. J. (2011). Self-related neural response to tailored smoking-cessation messages predicts quitting. Nature Neuroscience, 14(4), 426–427. 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini R. B., Demaine L. J., Sagarin B. J., Barrett D. W., Rhoads K., & Winter P. L. (2006). Managing social norms for persuasive impact. Social Influence, 1(1), 3–15. 10.1080/15534510500181459. [DOI] [Google Scholar]
- Cialdini R. B., & Trost M. R. (1998). Social influence: Social norms, conformity and compliance In Gilbert D. T., Fiske S. T., & Lindzey G. (Eds.), The handbook of social psychology. (Vols. 1-2, 4th ed., pp. 151–192). New York, NY: McGraw-Hill. [Google Scholar]
- Cohen S. S., & Parra L. C. (2016). Memorable audiovisual narratives synchronize sensory and supramodal neural responses. ENeuro, 3(6), 1–11. 10.1523/ENEURO.0203-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N., Tompson S., O’Donnell M., & Falk E. B. (2015). Brain activity in self- and value-related regions in response to online antismoking messages predicts behavior change. Journal of Media Psychology, 27(3), 93–109. 10.1027/1864-1105/a000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling D. W., Modayil M. V., & Stevens C. (2010). Assessing the relationship between ad volume and awareness of a tobacco education media campaign. Tobacco Control, 19, i37–i42. 10.1136/tc.2009.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik F. I., & Lockhart R. S. (1972). Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior, 11(6), 671–684. 10.1027/1864-1105/a000146. [DOI] [Google Scholar]
- Crone E. A., & Dahl R. E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. 10.1016/S0022-5371(72)80001-X. [DOI] [PubMed] [Google Scholar]
- Dufour N., Redcay E., Young L., Mavros P. L., Moran J. M., Triantafyllou C., Gabrieli J. D. E., & Saxe R. (2013). Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLOS One, 8(9), e75468 10.1371/journal.pone.0075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke J. C., Alexander T. N., Zhao X., Delahanty J. C., Allen J. A., MacMonegle A. J., & Farrelly M. C. (2015). Youth’s awareness of and reactions to The Real Cost national tobacco public education campaign. PLOS One, 10(12), e0144827 10.1371/journal.pone.0144827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N. I. (2015). Social pain and the brain: Controversies, questions, and where to go from here. Annual Review of Psychology, 66(1), 601–629. 10.1146/annurev-psych-010213-115146. [DOI] [PubMed] [Google Scholar]
- Falk E. B., Berkman E. T., & Lieberman M. (2012). From neural responses to population behavior: Neural focus group predicts population-level media effects. Psychological Science, 23(5), 439–445. 10.1177/0956797611434964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E. B., Berkman E. T., Whalen D., & Lieberman M. D. (2011). Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychology, 30(2), 177–185. 10.1037/a0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E. B., O’Donnell M. B., Tompson S., Gonzalez R., Dal Cin S., Strecher V., Cummings K. M., & An L. (2016). Functional brain imaging predicts public health campaign success. Social Cognitive and Affective Neuroscience, 11(2), 204–214. 10.1093/scan/nsv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E. B., & Scholz C. (2018). Persuasion, influence, and value: Perspectives from communication and social neuroscience. Annual Review of Psychology, 69(1), 329–356. 10.1146/annurev-psych-122216-011821. [DOI] [PubMed] [Google Scholar]
- Farris P. W., Bendle N., Pfeifer P., & Reibstein D. (2010). Marketing metrics: The definitive guide to measuring marketing performance. Upper Saddle River, NJ: Pearson Education.
- Fishbein M., & Ajzen I. (2011). Predicting and changing behavior: The reasoned action approach. New York, NY: Taylor & Francis. [Google Scholar]
- Fishbein M., & Cappella J. N. (2006). The role of theory in developing effective health communications. Journal of Communication, 56, S1–S17. 10.1111/j.1460-2466.2006.00280.x. [DOI] [Google Scholar]
- Fisher J. T., Huskey R., Keene J. R., & Weber R. (2018a). The limited capacity model of motivated mediated message processing: Looking to the future. Annals of the International Communication Association, 42(4), 291–315. 10.1080/23808985.2018.1534551 [DOI] [Google Scholar]
- Fisher J. T., Keene J. R., Huskey R., & Weber R. (2018b). The limited capacity model of motivated mediated message processing: Taking stock of the past. Annals of the International Communication Association, 42(4), 270–290. 10.1080/23808985.2018.1534552 [DOI] [Google Scholar]
- Frankland P. W., & Bontempi B. (2005). The organization of recent and remote memories. Nature Reviews Neuroscience, 6(2), 119–130. 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frith C. D., & Frith U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–534. 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith C. D., & Singer T. (2008). The role of social cognition in decision making. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1511), 3875–3886. doi: 10.1098/rstb.2008.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz K., Rimer B. K., & Viswanath K. (2008). Health behavior and health education: Theory, research, and practice. San Francisco, CA: John Wiley & Sons. [Google Scholar]
- Henke K. (2010). A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience, 11(7), 523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Holbert R. L., & Park E. (2019). Conceptualizing, organizing, and positing moderation in communication research. Communication Theory. Advance online publication. doi: 10.1093/ct/qtz006. [DOI] [Google Scholar]
- Holtgrave D. R., Wunderink K. A., Vallone D. M., & Healton C. G. (2009). Cost–utility analysis of the national truth® campaign to prevent youth smoking. American Journal of Preventive Medicine, 36(5), 385–388. 10.1016/j.amepre.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Hornik R. C. (2002). Exposure: Theory and evidence about all the ways it matters. Social Marketing Quarterly, 8(3), 31–37. 10.1080/15245000214135. [DOI] [Google Scholar]
- Huskey R., Mangus J. M., Turner B. O., & Weber R. (2017). The persuasion network is modulated by drug-use risk and predicts anti-drug message effectiveness. Social Cognitive and Affective Neuroscience, 12(12), 1902–1915. 10.1093/scan/nsx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof M. A., Schmälzle R., Renner B., & Schupp H. T. (2017). How real-life health messages engage our brains: Shared processing of effective anti-alcohol videos. Social Cognitive and Affective Neuroscience, 12(7), 1188–1196. 10.1093/scan/nsx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler E. C., Gibson L. A., & Hornik R. (2017). Recall of “The Real Cost” campaign is specifically associated with endorsement of campaign-targeted beliefs. Journal of Health Communication, 22(10), 818–828. doi: 10.1080/10810730.2017.1364311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler E. C., Schmälzle R., O’Donnell M. B., Pei R., & Falk E. B. (2019). Adolescent neural responses to antismoking messages, perceived effectiveness, and sharing intention. Media Psychology, 22(2), 323–349. 10.1080/15213269.2018.1476158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P. B., & Christensen R. H. B. (2017). LmerTest package: Tests in linear mixed effects models. Journal of Statistical Software ,82(13), 1–26. doi: 10.18637/jss.v082.i13 [Google Scholar]
- Lang A. (2000). The limited capacity model of mediated message processing. Journal of Communication, 50(1), 46–70. 10.1111/j.1460-2466.2000.tb02833.x. [DOI] [Google Scholar]
- Langleben D. D., Loughead J. W., Ruparel K., Hakun J. G., Busch-Winokur S., Holloway M. B., Strasser A. A., Cappella J. N., Lerman C. (2009). Reduced prefrontal and temporal processing and recall of high “sensation value” ads. NeuroImage ,46(1), 219–225. 10.1016/j.neuroimage.2008.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. D. (2012). Education and the social brain. Trends in Neuroscience and Education, 1(1), 3–9. 10.1016/j.tine.2012.07.003. [DOI] [Google Scholar]
- Liu J., Zhao S., Chen X., Falk E., & Albarracín D. (2017). The influence of peer behavior as a function of social and cultural closeness: A meta-analysis of normative influence on adolescent smoking initiation and continuation. Psychological Bulletin, 143(10), 1082–1115. doi: 10.1037/bul0000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMonegle A. J., Nonnemaker J., Duke J. C., Farrelly M. C., Zhao X., Delahanty J. C., Smith A. A., Rao P., & Allen J. A. (2018). Cost-effectiveness analysis of The Real Cost campaign’s effect on smoking prevention. American Journal of Preventive Medicine ,55(3), 319–325. 10.1016/j.amepre.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Murdock B. B. (1965). Effects of a subsidiary task on short-term memory. British Journal of Psychology, 56(4), 413–419. 10.1111/j.2044-8295.1965.tb00983.x. [DOI] [Google Scholar]
- Niederdeppe J. (2005). Assessing the validity of confirmed ad recall measures for public health communication campaign evaluation. Journal of Health Communication, 10(7), 635–650. 10.1080/10810730500267662. [DOI] [PubMed] [Google Scholar]
- Noar S. M. (2006). A 10-year retrospective of research in health mass media campaigns: Where do we go from here? Journal of Health Communication, 11(1), 12–42. 10.1080/10810730500461059. [DOI] [PubMed] [Google Scholar]
- O’Keefe D. J. (2018). Message pretesting using assessments of expected or perceived persuasiveness: Evidence about diagnosticity of relative actual persuasiveness. Journal of Communication, 68(1), 120–142. 10.1093/joc/jqx009. [DOI] [Google Scholar]
- Palmgreen P., Donohew L., Lorch E. P., Hoyle R. H., & Stephenson M. T. (2001). Television campaigns and adolescent marijuana use: Tests of sensation seeking targeting. American Journal of Public Health, 91(2), 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty R. E., & Cacioppo J. T. (1986). The elaboration likelihood model of persuasion. Advances in Experimental Social Psychology, 19, 123–205. [Google Scholar]
- Petty R. E., & Wegener D. T. (1999). The elaboration likelihood model: Current status and controversies In Dual-process theories in social psychology (pp. 37–72). New York, NY: Guilford Press. [Google Scholar]
- Poldrack R. A. (2006). Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences, 10(2), 59–63. 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Randolph W., & Viswanath K. (2004). Lessons learned from public health mass media campaigns: Marketing health in a crowded media world. Annual Review of Public Health, 25(1), 419–437. 10.1146/annurev.publhealth.25.101802.123046. [DOI] [PubMed] [Google Scholar]
- Richardson S., Langley T., Szatkowski L., Sims M., Gilmore A., McNeill A., & Lewis S. (2014a). How does the emotive content of televised anti-smoking mass media campaigns influence monthly calls to the NHS Stop Smoking helpline in England? Preventive Medicine, 69, 43–48. 10.1016/j.ypmed.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., McNeill A., Langley T. E., Sims M., Gilmore A., Szatkowski L., Heath R., Fong G. T., & Lewis S. (2014b). The impact of televised tobacco control advertising content on campaign recall: Evidence from the International Tobacco Control (ITC) United Kingdom Survey. BMC Public Health ,14, 432 10.1186/1471-2458-14-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimal R. N., & Real K. (2005). How behaviors are influenced by perceived norms: A test of the theory of normative social behavior. Communication Research, 32(3), 389–414. 10.1177/0093650205275385. [DOI] [Google Scholar]
- Schacter D. L., & Wagner A. D. (1999). Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus, 9(1), 7–24. . [DOI] [PubMed] [Google Scholar]
- Schmitz T. W., & Johnson S. C. (2007). Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews, 31(4), 585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz C., Baek E. C., O’Donnell M. B., Kim H. S., Cappella J. N., & Falk E. B. (2017). A neural model of valuation and information virality. Proceedings of the National Academy of Sciences, 114(11), 2881–2886. 10.1073/pnas.1615259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig D., Wang A.-L., Jaganathan K., Loughead J. W., Blady S. J., Childress A. R., Romer D., & Langleben D. D. (2014). Low message sensation health promotion videos are better remembered and activate areas of the brain associated with memory encoding. PLOS One, 9(11), e113256 10.1371/journal.pone.0113256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell B. G., Barmada C. H., Hornik R. C., & Maklan D. M. (2002). Can we measure encoded exposure? Validation evidence from a national campaign. Journal of Health Communication, 7(5), 445–453. 10.1080/10810730290001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L. R. (2004). Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory, 82(3), 171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L., & Monahan K. C. (2007). Age differences in resistance to peer influence. Developmental Psychology, 43(6), 1531–1543. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield M. A., Loken B., & Hornik R. C. (2010). Use of mass media campaigns to change health behaviour. The Lancet, 376(9748), 1261–1271. 10.1016/S0140-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.-L., Ruparel K., Loughead J. W., Strasser A. A., Blady S. J., Lynch K. G., Romer D., Cappella J. N., Lerman C., & Langleben D. D. (2013). Content matters: Neuroimaging investigation of brain and behavioral impact of televised anti-tobacco public service announcements. The Journal of Neuroscience ,33(17), 7420–7427. 10.1523/JNEUROSCI.3840-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R., Huskey R., Mangus J. M., Westcott-Baker A., & Turner B. O. (2015). Neural predictors of message effectiveness during counterarguing in antidrug campaigns. Communication Monographs, 82(1), 4–30. 10.1080/03637751.2014.971414. [DOI] [Google Scholar]
- White V. M., Durkin S. J., Coomber K., & Wakefield M. A. (2015). What is the role of tobacco control advertising intensity and duration in reducing adolescent smoking prevalence? Findings from 16 years of tobacco control mass media advertising in Australia. Tobacco Control, 24, 198–204. 10.1136/tobaccocontrol-2012-050945. [DOI] [PubMed] [Google Scholar]
- Zhao X., Alexander T. N., Hoffman L., Jones C., Delahanty J., Walker M., Berger A. T., & Talbert E. (2016). Youth receptivity to FDA’s The Real Cost tobacco prevention campaign: Evidence from message pretesting. Journal of Health Communication, 21(11), 1153–1160. 10.1080/10810730.2016.1233307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. (2007). Sensation seeking and risk In Sensation seeking and risky behavior (pp. 51–72). Washington, DC: American Psychological Association. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


