Abstract
Sympathetic activation and the kidney play critical roles in hypertension and chronic heart failure. The role of the kidney in sympathetic activation is still not well known. In this study, we revealed an excitatory renal reflex (ERR) in rats induced by chemical stimulation of the kidney that regulated sympathetic activity and blood pressure. The ERR was induced by renal infusion of capsaicin, and evaluated by the changes in renal sympathetic outflow, blood pressure, and heart rate. Renal infusion of capsaicin dose-dependently increased the contralateral renal sympathetic nerve activity, mean arterial pressure, and heart rate. Capsaicin in the cortico-medullary border had greater effects than in the cortex or medulla. Intravenous infusion of capsaicin had no significant effects. The effects of renal infusion of capsaicin were abolished by ipsilateral renal denervation, but were not affected by bilateral sinoaortic denervation. Renal infusion of capsaicin increased the ipsilateral renal afferent activity. The ERR was also induced by renal infusion of bradykinin, adenosine, and angiotensin II, but not by ATP. Renal infusion of capsaicin increased c-Fos expression in the paraventricular nucleus (PVN) of hypothalamus. Lesion of neurons in the PVN with kainic acid abolished the capsaicin-induced ERR. These findings indicate that chemical stimulation of kidney causes an excitatory reflex, leading to sympathetic activation, pressor response, and accelerated heart rate. The PVN is an important central nucleus in the pathway of the ERR.
Electronic supplementary material
The online version of this article (10.1007/s12264-019-00417-1) contains supplementary material, which is available to authorized users.
Keywords: Sympathetic activity, Blood pressure, Renal afferents, Renal reflex, Paraventricular nucleus
Introduction
Sympathetic activity is enhanced in hypertension [1, 2], chronic heart failure [3, 4], and chronic kidney disease [5, 6]. Excessive sympathetic activation contributes greatly to the occurrence and development of hypertension and related organ damage [7]. Intervention in sympathetic over-activity has been used as an important strategy for attenuating hypertension and its complications [8, 9]. On the other hand, most patients with chronic kidney disease have high blood pressure and excessive sympathetic activation, which are closely associated with the increases in morbidity and mortality of cardiovascular events [5]. The excessive sympathetic activity not only plays a crucial role in the pathogenesis of hypertension but also contributes greatly to the development of chronic kidney diseases that are independent of hypertension [5]. Accumulating evidence has shown that the kidney plays critical roles in sympathetic activation in hypertension and chronic kidney diseases [10–15]. However, the mechanisms leading to excessive sympathetic activation are complex and not completely understood.
Renal nerves consist of afferent sensory and efferent sympathetic nerves. The afferents are activated by stimulation of mechanoreceptors and chemoreceptors in the kidney [16]. The mechanoreceptors are mainly distributed in the renal pelvis wall, and are activated by increased pelvic pressure. The chemoreceptors respond to the chemical environment in the renal tissue and pelvis, such as hypertonic NaCl, capsaicin, bradykinin, and adenosine. Immunoreactivity for substance P has been found in the interlobar branches of the renal artery, the walls of the renal pelvis, and the proximal ureter, which are involved in chemoreceptor function [17]. It has been shown that the activation of renal afferents reduces renal sympathetic nerve activity (RSNA) in normotensive rats, but increases the RSNA in several pathophysiological conditions such as chronic heart failure, hypertension, and ischemic acute renal failure [10, 18, 19]. The role of renal afferent nerve activity in the reflex control of sympathetic outflow is controversial or opaque [20]. Renal denervation or catheter-based radiofrequency renal denervation which interrupts both the afferent and efferent renal nerves has been used as an interventional approach to treat hypertension [16, 21, 22]. Therefore, it is important to reveal the roles of renal afferents in the reflex regulation of blood pressure and sympathetic activity. The present study was designed to determine the roles of an excitatory renal reflex (ERR) induced by chemical stimulation of the kidney in regulating sympathetic activity and blood pressure. Furthermore, whether the hypothalamic paraventricular nucleus (PVN) is critical for the central neurocircuitry of the ERR was investigated.
Methods
Animals and General Procedures
Experiments were carried out on 132 male Sprague–Dawley rats weighing 280 g–320 g. The protocols were approved by the Experimental Animal Care and Use Committee in Nanjing Medical University. The experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health, NIH publication, 8th edition, 2011). The rats were kept at a controlled humidity and temperature under a 12-h light/dark cycle with free access to lab chow and tap water. Each rat was anesthetized with a mixture of α-chloralose (40 mg/kg) and urethane (800 mg/kg) via intraperitoneal injection. The depth of anesthesia was confirmed before surgery by the absence of both corneal reflexes and paw withdrawal responses to a noxious pinch [23]. The trachea and right carotid artery were exposed by a midline incision in the neck. The trachea was intubated for positive-pressure ventilation with room air using a small-animal ventilator (model 51600, Stoelting, Chicago, IL). The right carotid artery was cannulated to record blood pressure. The arterial blood pressure, mean arterial pressure (MAP), heart rate (HR), and RSNA were simultaneously recorded using a PowerLab data acquisition system (8SP, ADInstruments, Bella Vista, NSW, Australia). The rat was euthanized at the end of the experiment with pentobarbital sodium (100 mg/kg) via intravenous injection.
RSNA Recording
RSNA was recorded as we reported previously [24]. Briefly, the left renal artery and nerves were exposed via a left flank incision. The renal nerve was isolated and cut at its distal end to abolish afferent activity from the kidney. The nerve was then placed on a pair of parallel silver electrodes, and immersed in mineral oil at 37 °C. The nerve activity was amplified with an AC/DC differential amplifier (model DP-304, Warner Instruments, Hamden, CT). The signals were low-pass filtered at 3,000 Hz and high-pass filtered at 100 Hz, and then integrated at a 100-ms time constant. After each experiment, the central end of the renal nerve was cut to record background noise. The RSNA value was obtained by the integrated RSNA value minus the background noise value, and expressed as a percentage change of the control value.
Recording of Renal Afferent Nerve Activity
The right renal nerve was exposed, isolated, and sectioned at its central end to abolish the renal efferent activity. Renal afferent nerve activity was recorded as for RSNA recording.
Chemical Stimulation of Kidney to Induce the ERR
The right kidney was exposed and a stainless-steel tube (0.31 mm outer diameter) was horizontally inserted into the kidney for the infusion of chemicals. The tube was inserted from the right side of the kidney to the left side, except in the protocol used to compare the effects of capsaicin at different sites in the kidney. The tip of the tube was held at the cortico-medullary border or at different depths from the renal surface to compare the effects of capsaicin. The insertion of the tube stopped when a slight resistance was encountered, indicating that the tip reached the cortico-medullary border, which was about 2 mm below the renal surface. The ERR was induced by renal infusion of capsaicin (1 nmol/μL), or bradykinin (0.5 nmol/μL), adenosine (0.5 nmol/μL), ATP (1.5 nmol/μL), or angiotensin II (Ang II, 0.01 nmol/μL). The tube was connected to a microinjector through a PE50 polyethylene catheter. The infusion was carried out using a programmable pressure injector (PM2000B, MicroData Instrument, NJ) at 1.0 μL/min for 20 min. The ERR was evaluated by the RSNA, MAP, and HR responses to the renal infusion of chemicals.
Microinjection into the PVN
Microinjections were performed as we reported previously [25]. Briefly, each rat was fixed in a stereotaxic frame in the prone position (Stoelting, Chicago, IL). The coordinates of the PVN used in the present study were 0.4 mm lateral to the midline, 1.8 mm caudal to bregma, and 7.9 mm below the dorsal surface. The PVN microinjection was performed with a glass micropipette (50 μm tip diameter). The volume of the microinjection for each side was 50 nL, and it was completed in 1 min. After each experiment, the same volume of Evans Blue was microinjected. The injection sites were localized histologically [26]. The visible extent of dye was <300 μm in diameter. The data from rats with microinjection sites outside the PVN were excluded.
Identification of PVN Lesion
Toluidine blue staining of brain sections was used to identify the lesion in the PVN. The nuclei of cells stained blue. The standard for the effectiveness of a PVN lesion was that the number of nuclei within 0.2 mm around the injection site in the bilateral PVN was reduced by >60%.
Sinoaortic Denervation (SAD)
SAD was carried out in only one of the experimental protocols to examine the secondary effect of the baroreflex on the ERR. The bilateral vagal and carotid sinus nerves in the neck were individually identified and sectioned. All other nerve fibers visible in the carotid sinus area were cut. The common carotid arteries and carotid bifurcation were stripped of adventitial tissues 4 mm above and below the bifurcation. Phenol solution (10%) was applied to this area to destroy any remaining nerve fiber. The denervation was confirmed by showing that the HR change was <5 beats/min after intravenous administration of phenylephrine (20 μg/kg) [27].
Immunohistochemistry
Immunohistochemistry for c-Fos expression was examined as we previously described [28, 29]. Mouse monoclonal antibody against c-Fos (sc-271243) was from Santa Cruz (Dallas, TX) and diluted 1:1000 for use. Biotinylated secondary antibody was from Santa Cruz (ABC staining system kit).
Chemicals
Capsaicin was from MedChem Express (Monmouth Junction, NJ); bradykinin, Ang II, adenosine, and ATP were from Sigma Chemical Co. (St Louis, MO); and kainic acid (KA) was from Abcam (Cambridge, MA). Capsaicin was dissolved in ethanol and stored as stock solution. The capsaicin solution was diluted before use; it contained 1% stock solution, 1% Tween 80, and 98% normal saline. The vehicle was used as the control for capsaicin. Other chemicals were dissolved in saline.
Statistics
The changes of RSNA, MAP, and HR were determined by the average values for 1 min when their maximal response was reached. Comparisons between two groups were assessed by Student’s t-test. One-way or two-way ANOVA was used for multiple comparisons followed by post hoc Bonferroni’s test. All data are expressed as the mean ± SEM. A P value <0.05 was considered statistically significant.
Results
Dose- and Time-Effects of Renal Infusion of Capsaicin
Unilateral renal infusion of capsaicin at the cortico-medullary border of the kidney caused immediate increases in the contralateral RSNA, MAP, and HR in a dose-dependent manner (Fig. 1A). The infusion sites in kidneys were confirmed by Evans blue staining (Fig. 1B). The capsaicin had its maximal effects at ~1 nmol/min for 20 min (Fig. 2A), so we used this dose in all subsequent experiments. The maximal effects of capsaicin began at ~15 min after the beginning of infusion (RSNA, +18.3% ± 3.2%; MAP, +8.2 ± 1.3 mmHg; HR, +8.1 ± 2.9 bpm), and lasted 40 min–50 min. Renal infusion of vehicle did not have significant effects on the RSNA, MAP, and HR (Fig. 2B).
Fig. 1.
Capsaicin-induced excitatory renal reflex (ERR). A Representative recordings showing the capsaicin-induced reflex changes of arterial blood pressure (ABP), mean arterial pressure (MAP), heart rate (HR), and contralateral renal sympathetic nerve activity (RSNA). The capsaicin was infused into the cortico-medullary border of the right kidney at 1 nmol/min for 20 min. B Representative section of kidney showing the infusion site.
Fig. 2.
Dose- and time-effects of the capsaicin-induced excitatory renal reflex (ERR). Capsaicin was infused into the cortico-medullary border of the right kidney to induce reflex changes in contralateral renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP) and heart rate (HR). A Dose-response curves for capsaicin (0 nmol/min, 0.01 nmol/min, 0.05 nmol/min, 0.25 nmol/min, 1 nmol/min, and 4 nmol/min for 20 min). B Time-courses for capsaicin (1 nmol/min for 20 min). Values are the mean ± SEM. *P < 0.05 vs 0 nmol or 0 min; †P < 0.05 vs Vehicle. n = 6 per group.
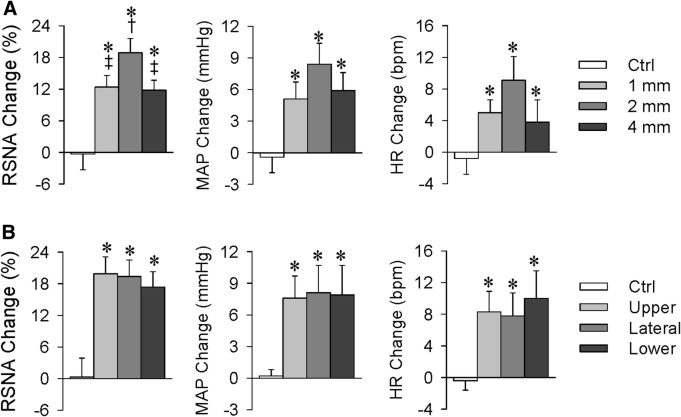
Effects of Capsaicin in Different Sites in the Kidney
Renal infusion of capsaicin 1 mm, 2 mm, or 4 mm below the kidney surface (corresponding to the cortex, cortico-medullary border, and medulla) increased the contralateral RSNA, MAP, and HR. However, the effects of capsaicin at the cortico-medullary border were significantly greater than those in the cortex and medulla (Fig. 3A). Infusion of capsaicin into the cortico-medullary border of the upper, lateral, or lower parts of the kidney showed similar increases in the contralateral RSNA, MAP, and HR (Fig. 3B). There were no significant differences in the baseline RSNA, MAP, and HR among these groups (Tables S1 and S2). The cortico-medullary border in the lateral kidney was selected as the infusion site in all other experiments.
Fig. 3.
Excitatory renal reflex (ERR) induced by capsaicin at different sites in the kidney. Capsaicin (1 nmol/min for 20 min) was infused into the right kidney to induce reflex changes in the contralateral renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), and heart rate (HR). A Effects of capsaicin at different depths in the kidney (1 mm, 2 mm, and 4 mm below the lateral surface). B Effects of capsaicin at different locations in the kidney (upper, lateral, and lower parts, 2 mm below the surface). Values are the mean ± SEM. *P < 0.05 vs Ctrl; †P < 0.05 vs 1 mm; ‡P < 0.05 vs 2 mm. n = 6 per group.
Excluding the Possibility of Extra-Renal Effects Due to Capsaicin Diffusion
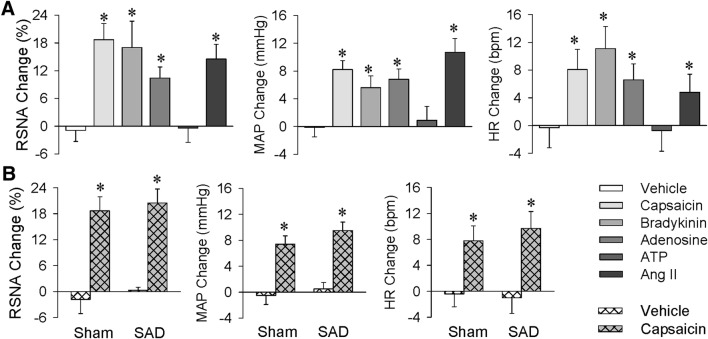
Although renal infusion of capsaicin increased the contralateral RSNA, MAP, and HR, intravenous infusion of same dose failed to have any significant effects on these parameters (Fig. 4A). The results excluded the possibility that the effects of renal infusion might be due to leakage of capsaicin into the circulation. Our previous studies have shown that administration of capsaicin to white adipose tissue induces an adipose afferent reflex that causes sympathetic activation and pressor responses [30–32]. Similar effects were found for capsaicin infusion into the perirenal fat in the present study. However, the effects of capsaicin in the perirenal fat were smaller than those at the same dose in the kidney (RSNA, 11.3% ± 1.5% vs 18.9% ± 2.7%, P < 0.05; MAP, 3.9 ± 1.5 mmHg vs 8.4 ± 2.2 mmHg, P < 0.05; HR, 1.4 ± 2.2 bpm vs 9.1 ± 3.0 bpm, P < 0.05). Furthermore, the effects of capsaicin in perirenal fat were completely abolished by denervation of the perirenal fat (Fig. 4B). More importantly, the effects of renal infusion of capsaicin were completely abolished by ipsilateral renal denervation, but not affected by ipsilateral perirenal lipectomy (Fig. 5A). These results indicate that the effects of renal infusion of capsaicin are caused by stimulation of the kidney rather than perirenal fat.
Fig. 4.
Effects of infusion of capsaicin into the right kidney, jugular vein, and perirenal fat on contralateral renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), and heart rate (HR). A Effects of capsaicin infusion into the cortico-medullary border of the right kidney and the jugular vein. B Effects of capsaicin infusion into intact or denervated perirenal fat. Capsaicin was infused at 1 nmol/min for 20 min. Values are the mean ± SEM. *P < 0.05 vs Vehicle; †P < 0.05 vs Kidney; ‡P < 0.05 vs Intact. n = 6 per group.
Fig. 5.
Role of renal afferents in the capsaicin-induced excitatory renal reflex (ERR). Capsaicin (1 nmol/min for 20 min) was infused into the cortico-medullary border of the right kidney to induce reflex changes in the contralateral renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), and heart rate (HR). A Effects of capsaicin in kidney were not affected by ipsilateral perirenal lipectomy, but were abolished by ipsilateral renal denervation. B Effect of intrarenal infusion of capsaicin on renal afferent nerve activity (RANA). C Representative recordings of RANA. Values are the mean ± SEM. *P < 0.05 vs Intact/Vehicle; †P < 0.05 vs Intact/Capsaicin; ‡P < 0.05 vs Vehicle. n = 6 per group.
Renal Infusion of Capsaicin Increases Ipsilateral Renal Afferent Nerve Activity
Renal infusion of capsaicin increased the ipsilateral renal afferent activity (Fig. 5B). There were no significant differences in baseline afferent activity between the vehicle- and the capsaicin-treated groups (Table S3). Representative recordings showed that capsaicin in the kidney induced an immediate increase in the afferent activity (Fig. 5C). The results showed that capsaicin stimulates renal afferents in the kidney and increases their activity. Taking into account all the above results, we concluded that the effects of capsaicin in the kidney are primarily caused by increased renal afferent nerve activity due to their activation rather than capsaicin-induced release of chemicals or signal molecules into the circulation.
ERR Induced by Different Chemicals
Capsaicin is the main pungent ingredient in hot chili peppers, and selectively activates sensory neurons via acting on a non-selective ligand-gated cation channel, transient receptor potential vanilloid-1. Capsaicin is used to stimulate sensory afferents [33–35]. It was of interest to determine whether ERR can be induced by other chemicals. We found that renal infusion of bradykinin, adenosine, and Ang II had effects similar to capsaicin on the contralateral RSNA, MAP, and HR. However, renal infusion of ATP failed to induce the ERR (Fig. 6A).
Fig. 6.
Excitatory renal reflex (ERR) induced by different chemicals and effects of sinoaortic denervation (SAD) on capsaicin-induced ERR as evaluated by the reflex changes in contralateral renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), and heart rate (HR). A Effects of renal infusion of capsaicin (1 nmol/min), bradykinin (0.5 nmol/min), adenosine (0.5 nmol/min), ATP (1.5 nmol/min), and Ang II (0.01 nmol/min) for 20 min. B Effects of bilateral SAD on the ERR. Renal infusion of vehicle or capsaicin was carried out 1 h after sham operation (Sham) or bilateral SAD. Values are the mean ± SEM. *P < 0.05 vs Vehicle. n = 6 per group.
Effects of SAD on Capsaicin-Induced ERR
The ERR was examined 1 h after SAD surgery to determine whether the baroreflex plays a role in capsaicin-induced ERR. SAD had no significant effects on baseline RSNA, MAP, and HR (Table S4). The SAD rats showed a tendency towards an enhanced ERR, but the difference from sham-operated rats failed to reach significance (Fig. 6B), suggesting that the inhibitory effect of the baroreflex on the ERR is very weak under normal conditions. Therefore, all other experiments were carried out in rats without SAD, better representing the true state of the ERR in regulating sympathetic and cardiovascular activity in intact animals.
The PVN is Important in Mediating the Capsaicin-Induced ERR
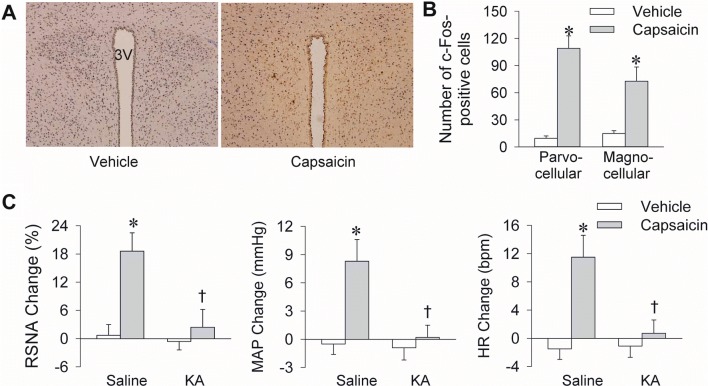
The PVN is critical in the cardiac sympathetic afferent reflex [36] and adipose afferent reflex [30], so we investigated its role in the ERR. Immunohistochemistry showed that unilateral renal infusion of capsaicin increased c-Fos expression in the bilateral PVN (Fig. 7A). The increased c-Fos expression involved both the parvocellular and magnocellular parts of the PVN (Fig. 7B). KA is strongly neurotoxic [37] and is widely used to destroy neurons in brain nuclei without lesioning terminals and axons of passage in the injection site [38]. Neurons immediately fire at a very high rate after KA injection, and no neuronal activity is recorded 1 h later [38]. Bilateral PVN microinjection of KA induces a great and immediate increase in sympathetic outflow, blood pressure, and HR, which recovers within 1 h, and then remains at baseline levels [30, 36]. In the present study, microinjection of KA into the bilateral PVN prevented the capsaicin-induced ERR 2 h later (Fig. 7C). There were no significant differences in baseline RSNA, MAP, and HR before the PVN microinjection of saline or KA (Table S5). The effectiveness of PVN lesioning by KA was confirmed by toluidine blue staining (Fig. S1). Microinjection of KA into anterior hypothalamic areas near the PVN failed to prevent the capsaicin-induced ERR, indicating that the PVN is involved in the central pathway of the ERR.
Fig. 7.
The PVN is involved in the capsaicin-induced excitatory renal reflex (ERR). A Representative immunohistochemical sections showing the c-Fos expression (yellow) in the PVN 30 min after renal infusion of capsaicin. B Numbers of c-Fos-positive cells in the parvocellular and magnocellular parts of the PVN 30 min after renal infusion of capsaicin. C Effects of bilateral PVN lesions with kainic acid (KA) on the capsaicin-induced ERR. Vehicle or capsaicin (1 nmol/min for 20 min) was infused 120 min after the PVN microinjection of saline or KA (2 nmol). Values are the mean ± SEM. *P < 0.05 vs Vehicle; †P < 0.05 vs Saline. n = 6 per group.
Discussion
The kidney is critical in the sympathetic activation in hypertension and chronic kidney diseases [10–15]. Numerous studies have focused on renal sympathetic activity and its underlying mechanism, but the role of renal afferent activity in sympathetic activation is obscure or controversial. In the present study, we established a method to induce an ERR. Chemical stimulation of renal afferents induces the ERR, which leads to an increase in sympathetic activity, blood pressure, and HR. The PVN is an important component of the central neurocircuitry of the ERR.
In a preliminary study, we found that the responses to renal infusion of capsaicin were much more stable than that to a single renal injection of capsaicin, so we used infusion for 20 min to induce the ERR. Activation of renal afferents with capsaicin increased sympathetic activity, blood pressure and HR. That the ERR might involve the activation of renal chemoreceptors was supported by the findings that the infusion of bradykinin, adenosine, or Ang II induced ERR effects similar to capsaicin. Substance P immunoreactivity has been reported in the interlobar branches of the renal artery, the walls of the renal pelvis, and the proximal ureter, which are involved in chemoreceptor function [17]. That the ERR induced by capsaicin at the cortico-medullary border was greater than that in cortex or medulla may be related to the arcuate arteries or interlobar branches of the renal artery containing chemoreceptors near the cortico-medullary border.
Renal infusion of capsaicin increased the ipsilateral renal afferent activity and the capsaicin-induced ERR was not affected by ipsilateral perirenal lipectomy, but was completely abolished by ipsilateral renal denervation. These results indicate that the renal afferent nerves are responsible for the effects of capsaicin in the kidney, and this is supported by the fact that the afferent nerves from the kidney enter the spinal cord through the T6-L2 dorsal root ganglia, and terminate in ipsilateral laminae I–III [39].
Neuroanatomical evidence has shown that renal afferent activity is closely connected with various sites in the brain associated with sympathetic outflow and cardiovascular regulation, including the nucleus of the solitary tract, rostral ventrolateral medulla, PVN, subfornical organ, and preoptic area [10]. The PVN is important in the control of cardiovascular activity and sympathetic outflow via its descending projections to the rostral ventrolateral medulla and intermediolateral column of spinal cord [40–43]. Stimulation of the renal afferent nerve altered the activity of 197 neurons in 407 neurons in the PVN, most of which were excited, but 8% were suppressed [44]. We found that renal infusion of capsaicin increased c-Fos expression in the bilateral PVN. Lesioning of bilateral PVN with KA abolished the effects of capsaicin in the kidney. These findings indicate that the PVN is important in the ERR pathway. It has been reported that afferent activity from one kidney can modulate contralateral efferent renal nerve activity to regulate diuresis and natriuresis to balance overall renal function between the two kidneys [10, 45]. In this study, we found that renal infusion of capsaicin increased c-Fos expression in both the parvocellular and magnocellular parts of the PVN. The latter may be related to its reflex effects in regulating sodium balance.
Sympathetic overactivity contributes greatly to the development and progress of chronic heart failure, hypertension, and chronic kidney disease [16]. Increased renal input may play critical roles in the excessive sympathetic activity in these diseases. Numerous studies have shown that catheter-based radiofrequency renal denervation, which abolishes both afferent and efferent renal activity, has been used as an interventional therapy for chronic heart failure, chronic kidney disease, and hypertension [16, 21, 22]. Selective renal afferent and efferent denervation may be new therapeutic approaches in the treatment of hypertension.
In summary, chemical stimulation of the kidney causes the ERR, which results in increased sympathetic outflow, blood pressure, and HR. The PVN is critical in the central neurocircuitry of the ERR.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31871148, 91639105, 31571167, and 31571168).
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457–466. doi: 10.1016/j.jash.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Mrowka R. Arterial hypertension. Acta Physiol (Oxf) 2017;219:697–699. doi: 10.1111/apha.12855. [DOI] [PubMed] [Google Scholar]
- 3.Zhang DY, Anderson AS. The sympathetic nervous system and heart failure. Cardiol Clin. 2014;32(33–45):vii. doi: 10.1016/j.ccl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–385. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 5.Kaur J, Young BE, Fadel PJ. Sympathetic overactivity in chronic kidney disease: consequences and mechanisms. Int J Mol Sci. 2017;18:E1682. doi: 10.3390/ijms18081682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J. Cardiovascular risk in chronic kidney disease: role of the sympathetic nervous system. Cardiol Res Pract. 2012;2012:319432. doi: 10.1155/2012/319432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esler M. Sympathetic nervous system moves toward center stage in cardiovascular medicine: from Thomas Willis to resistant hypertension. Hypertension. 2014;63:e25–e32. doi: 10.1161/HYPERTENSIONAHA.113.02439. [DOI] [PubMed] [Google Scholar]
- 9.Seravalle G, Mancia G, Grassi G. Role of the sympathetic nervous system in hypertension and hypertension-related cardiovascular disease. High Blood Press Cardiovasc Prev. 2014;21:89–105. doi: 10.1007/s40292-014-0056-1. [DOI] [PubMed] [Google Scholar]
- 10.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–R95. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esler M, Guo L. The future of renal denervation. Auton Neurosci. 2017;204:131–138. doi: 10.1016/j.autneu.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Iliescu R, Lohmeier TE, Tudorancea I, Laffin L, Bakris GL. Renal denervation for the treatment of resistant hypertension: review and clinical perspective. Am J Physiol Renal Physiol. 2015;309:F583–F594. doi: 10.1152/ajprenal.00246.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rettig R, Folberth C, Stauss H, Kopf D, Waldherr R, Unger T. Role of the kidney in primary hypertension: a renal transplantation study in rats. Am J Physiol. 1990;258:F606–F611. doi: 10.1152/ajprenal.1990.258.3.F606. [DOI] [PubMed] [Google Scholar]
- 14.Rettig R, Stauss H, Folberth C, Ganten D, Waldherr B, Unger T. Hypertension transmitted by kidneys from stroke-prone spontaneously hypertensive rats. Am J Physiol. 1989;257:F197–F203. doi: 10.1152/ajprenal.1989.257.2.F197. [DOI] [PubMed] [Google Scholar]
- 15.Bie P, Evans RG. Normotension, hypertension and body fluid regulation: brain and kidney. Acta Physiol (Oxf) 2017;219:288–304. doi: 10.1111/apha.12718. [DOI] [PubMed] [Google Scholar]
- 16.Frame AA, Carmichael CY, Wainford RD. Renal afferents. Curr Hypertens Rep. 2016;18:69. doi: 10.1007/s11906-016-0676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng H, Patel KP. Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton Neurosci. 2017;204:57–64. doi: 10.1016/j.autneu.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1:731–767. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 19.Kalaitzidis RG, Karasavvidou D, Siamopoulos KC. Renal sympathetic denervation and renal physiology. Curr Clin Pharmacol. 2013;8:189–196. doi: 10.2174/1574884711308030004. [DOI] [PubMed] [Google Scholar]
- 20.Abdulla MH, Johns EJ. The innervation of the kidney in renal injury and inflammation: a cause and consequence of deranged cardiovascular control. Acta Physiol (Oxf) 2017;220:404–416. doi: 10.1111/apha.12856. [DOI] [PubMed] [Google Scholar]
- 21.Veelken R, Schmieder RE. Renal denervation–implications for chronic kidney disease. Nat Rev Nephrol. 2014;10:305–313. doi: 10.1038/nrneph.2014.59. [DOI] [PubMed] [Google Scholar]
- 22.Carlstrom M. Therapeutic value of renal denervation in cardiovascular disease? Acta Physiol (Oxf) 2017;220:11–13. doi: 10.1111/apha.12816. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LL, Ding L, Zhang F, Gao R, Chen Q, Li YH, et al. Salusin-beta in rostral ventrolateral medulla increases sympathetic outflow and blood pressure via superoxide anions in hypertensive rats. J Hypertens. 2014;32:1059–1067. doi: 10.1097/HJH.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Shi Z, Zhang F, Yu Y, Zhong MK, Gao XY, et al. Reactive oxygen species in the paraventricular nucleus mediate the cardiac sympathetic afferent reflex in chronic heart failure rats. Eur J Heart Fail. 2007;9:967–973. doi: 10.1016/j.ejheart.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Chen AD, Zhang SJ, Yuan N, Xu Y, De W, Gao XY, et al. AT1 receptors in paraventricular nucleus contribute to sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Exp Physiol. 2011;96:94–103. doi: 10.1113/expphysiol.2010.054353. [DOI] [PubMed] [Google Scholar]
- 26.Geng KW, He T, Wang RR, Li CL, Luo WJ, Wu FF, et al. Ethanol increases mechanical pain sensitivity in rats via activation of GABAA receptors in medial prefrontal cortex. Neurosci Bull. 2016;32:433–444. doi: 10.1007/s12264-016-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreihofer AM, Sved AF. Use of sinoaortic denervation to study the role of baroreceptors in cardiovascular regulation. Am J Physiol. 1994;266:R1705–R1710. doi: 10.1152/ajpregu.1994.266.5.R1705. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Yan Y, Cheng J, Xiao G, Gu J, Zhang L, et al. Toll-like receptor 4 deficiency causes reduced exploratory behavior in mice under approach-avoidance conflict. Neurosci Bull. 2016;32:127–136. doi: 10.1007/s12264-016-0015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He F, Ai H, Wang M, Wang X, Geng X. Altered neuronal activity in the central nucleus of the amygdala induced by restraint water-immersion stress in rats. Neurosci Bull. 2018;34:1067–1076. doi: 10.1007/s12264-018-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z, Chen WW, Xiong XQ, Han Y, Zhou YB, Zhang F, et al. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol. 2012;112:1008–1014. doi: 10.1152/japplphysiol.01164.2011. [DOI] [PubMed] [Google Scholar]
- 31.Xiong XQ, Chen WW, Zhu GQ. Adipose afferent reflex: sympathetic activation and obesity hypertension. Acta Physiol (Oxf) 2014;210:468–478. doi: 10.1111/apha.12182. [DOI] [PubMed] [Google Scholar]
- 32.Xiong XQ, Chen WW, Han Y, Zhou YB, Zhang F, Gao XY, et al. Enhanced adipose afferent reflex contributes to sympathetic activation in diet-induced obesity hypertension. Hypertension. 2012;60:1280–1286. doi: 10.1161/HYPERTENSIONAHA.112.198002. [DOI] [PubMed] [Google Scholar]
- 33.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;179:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa H, Hiura A. Capsaicin, transient receptor potential (TRP) protein subfamilies and the particular relationship between capsaicin receptors and small primary sensory neurons. Anat Sci Int. 2006;81:135–155. doi: 10.1111/j.1447-073X.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 35.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 36.Zhong MK, Duan YC, Chen AD, Xu B, Gao XY, De W, et al. Paraventricular nucleus is involved in the central pathway of cardiac sympathetic afferent reflex in rats. Exp Physiol. 2008;93:746–753. doi: 10.1113/expphysiol.2007.041632. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, You Z, Li M, Pang L, Cheng J, Wang L. Protective effect of resveratrol on the brain in a rat model of epilepsy. Neurosci Bull. 2017;33:273–280. doi: 10.1007/s12264-017-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metzner W, Juranek J. A method to biotinylate and histochemically visualize ibotenic acid for pharmacological inactivation studies. J Neurosci Methods. 1997;76:143–150. doi: 10.1016/s0165-0270(97)00092-7. [DOI] [PubMed] [Google Scholar]
- 39.Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Auton Nerv Syst. 1983;8:273–285. doi: 10.1016/0165-1838(83)90110-8. [DOI] [PubMed] [Google Scholar]
- 40.Badoer E. The role of the hypothalamic PVN in the regulation of renal sympathetic nerve activity and blood flow during hyperthermia and in heart failure. Am J Physiol Renal Physiol. 2010;298:F839–F846. doi: 10.1152/ajprenal.00734.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun J, Ren XS, Kang Y, Dai HB, Ding L, Tong N, et al. Intermedin in paraventricular nucleus attenuates sympathoexcitation and decreases TLR4-mediated sympathetic activation via adrenomedullin receptors in rats with obesity-related hypertension. Neurosci Bull. 2019;35:34–46. doi: 10.1007/s12264-018-0292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu XJ, Miao YW, Li HB, Su Q, Liu KL, Fu LY, et al. Blockade of endogenous angiotensin-(1-7) in hypothalamic paraventricular nucleus attenuates high salt-induced sympathoexcitation and hypertension. Neurosci Bull. 2019;35:47–56. doi: 10.1007/s12264-018-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou JJ, Ma HJ, Shao JY, Pan HL, Li DP. Impaired hypothalamic regulation of sympathetic outflow in primary hypertension. Neurosci Bull. 2019;35:124–132. doi: 10.1007/s12264-018-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst. 1981;3:311–320. doi: 10.1016/0165-1838(81)90072-2. [DOI] [PubMed] [Google Scholar]
- 45.Kopp UC, Grisk O, Cicha MZ, Smith LA, Steinbach A, Schluter T, et al. Dietary sodium modulates the interaction between efferent renal sympathetic nerve activity and afferent renal nerve activity: role of endothelin. Am J Physiol Regul Integr Comp Physiol. 2009;297:R337–R351. doi: 10.1152/ajpregu.91029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.