Abstract
Fear memories are critical for survival. Nevertheless, over-generalization of these memories, depicted by a failure to distinguish threats from safe stimuli, is typical in stress-related disorders. Previous studies have supported a protective role of ketamine against stress-induced depressive behavior. However, the effect of ketamine on fear generalization remains unclear. In this study, we investigated the effects of ketamine on fear generalization in a fear-generalized mouse model. The mice were given a single sub-anesthetic dose of ketamine (30 mg/kg, i.p.) 1 h before, 1 week before, immediately after, or 22 h after fear conditioning. The behavioral measure of fear (indicated by freezing level) and synaptic protein expression in the basolateral amygdala (BLA) and inferior-limbic pre-frontal cortex (IL-PFC) of mice were examined. We found that only ketamine administered 22 h after fear conditioning significantly decreased the fear generalization, and the effect was dose-dependent and lasted for at least 2 weeks. The fear-generalized mice showed a lower level of brain-derived neurotrophic factor (BDNF) and a higher level of GluN2B protein in the BLA and IL-PFC, and this was reversed by a single administration of ketamine. Moreover, the GluN2B antagonist ifenprodil decreased the fear generalization when infused into the IL-PFC, but had no effect when infused into the BLA. Infusion of ANA-12 (an antagonist of the BDNF receptor TrkB) into the BLA or IL-PFC blocked the effect of ketamine on fear generalization. These findings support the conclusion that a single dose of ketamine administered 22 h after fear conditioning alleviates the fear memory generalization in mice and the GluN2B-related BDNF signaling pathway plays an important role in the alleviation of fear generalization.
Electronic supplementary material
The online version of this article (10.1007/s12264-019-00422-4) contains supplementary material, which is available to authorized users.
Keywords: Ketamine, Fear generalization, Post-traumatic stress disorder, BDNF, GluN2B, GluN2A
Introduction
Post-traumatic stress disorder (PTSD) is an excessive generalization of fear memory that is characterized not only by a strong response to a previously learned threatening cue but also a debilitating failure to suppress these responses even in the presence of cues that indicate safety [1, 2]. Epidemiological studies have estimated that the incidence of life-time PTSD is ~8% in the general population [3]. Current PTSD treatment includes pharmacotherapy, psychotherapy, or a combination of both. However, there is still a high proportion of PTSD patients without remission after multiple trials of both pharmacotherapy and psychotherapy [4–7].
Ketamine, a non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist, has rapid and sustained antidepressant effects both in humans and in a mouse model of depression [8–10]. Brachman et al. [11] showed that prophylactic ketamine attenuates stress-induced depressive behavior in three mouse models of stress. A previous study found that burned soldiers receiving perioperative ketamine have a lower prevalence of PTSD [12]. However, a follow-up study from the same research group did not support their initial findings [13]. Results from studies in animal models investigating the anxiety-related effect of ketamine are not always consistent, with reports of anxiolytic [14], anxiogenic [15], and null results [16], which raise new questions such as the heterogeneity and translatability of animal models, and how the dosage and timing of ketamine administration influence its affectivity for PTSD treatment.
Clinical studies have reported exaggerated amygdala and medial prefrontal responses in anxiety disorders [17, 18]. Previous studies found that fear generalization increases the overall activity of neurons in the lateral amygdala of mice [19]. Trace fear conditions increase the spiking of projection neurons in the inferior-limbic prefrontal cortex (IL-PFC) and basolateral amygdala (BLA) [20]. NMDARs are required for learning and memory and their subunits undergo dynamic modification following fear conditioning [21–24]. Inhibition of NMDARs prevents the loss of brain-derived neurotrophic factor (BDNF) function [25]. Importantly, BDNF modulates fear generalization in humans [26]. Recently, it was shown that ketamine regulates the expression of BDNF in the medial PFC of the single prolonged stress and electric foot shock (SPS&S) rat model [27]. Protein kinase M zeta (PKMζ) and Ca2+/calmodulin-dependent protein kinase II-alpha (CAMKII-α) are pivotal in learning and memory [28, 29]. However, the roles of NMDAR/BDNF, PKMζ, and CAMKII-α signaling in cued fear memory generalization remain unclear and the effects of ketamine on these molecules during fear generalization remain to be defined.
In the present study, we used a fear generalized mouse model to study the effects of ketamine on fear generalization and the molecular mechanisms underlying the effects.
Material and Methods
Animals
Male C57BL/6 mice weighing 20 g–25 g (6–8 weeks old) were maintained on a 14-h/10-h light/dark cycle with food and water available ad libitum at the Laboratory Animal Center of Sun Yat-sen University. All animal procedures were approved by the Animal Care and Use Committee of Sun Yat-sen University.
Drugs
Ketamine (Gutian Pharma Co., Fujian, China, 30 mg/kg [30, 31]) was administered intraperitoneally, while ifenprodil (MedChemExpress, NJ, 2.0 μg/μL [24]) and ANA-12 (Sigma Aldrich, St. Louis, MO, 1 μg/μL [32]) were infused bilaterally into the BLA or IL-PFC.
Behavioral Protocol
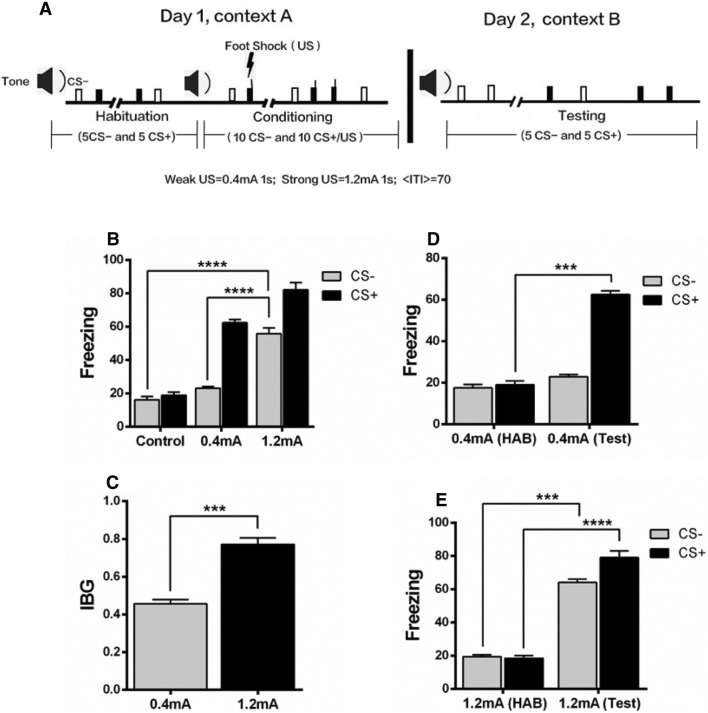
The procedure was a modification of the rat model of fear generalization described previously [19]. All behavioral experiments were performed during the light phase (daytime). Fear conditioning and fear memory recall tests were conducted in different contexts in a sound isolation chamber (Coulbourn Instruments, MA). The freezing behavior of mice was recorded and quantified with a video camera attached to the roof of the sound isolation chamber. Infrared LED cues placed on the wall of the chamber coincided with sound stimuli to check the sound-evoked freezing behavior. The floor, walls, lighting conditions, and odor differed between the conditioning and testing (retrieval) contexts. Before starting experiments, all chambers were cleaned with 70% ethanol. On day 1, the mice were habituated to context A (12 inches wide × 10 inches deep × 12 inches high), receiving 5 presentations of two sounds (duration 10 s, composed of either 5 kHz clicks or a continuous sound at 1 kHz, with a 5-ms rise and fall time, and 70±5 dB sound pressure). Immediately after habituation, fear conditioning was applied, where one of the two sounds (conditioned stimulus, CS+) (10 presentations of each sound at an average interval of 70 s, with a range of 40 s–100 s) was paired with a 1-s foot-shock delivered through metal grids on the floor (weak shock group, 0.4 mA for 1 s; strong shock group, 1.2 mA for 1 s, co-terminated with the CS+). After 24 h (on day 2), the behavioral test was performed in context B with presentations of 5 trials of each CS– (clicks tone, which was not paired with electric shock during training) and CS+ (continous tone, which was paired with electric shock during training) sounds (10 s duration, with an average inter-trail interval of 70 s, in a range of 40 s–100 s), and the freezing levels in response to the two sounds were measured by taking an average freezing level to all CS– tones, and an average freezing levels to all CS+ tones during behavioral test (Fig. 1A). The difference in freezing response to CS–/CS+ was evaluated as the index of behavioral generalization (IBG), which was defined as the ratio of average freezing response to CS– to the average freezing response to CS+ in the testing session.
Fig. 1.
A strong shock causes fear generalization. A Schematic of the behavioral protocol. B Mice with the strong shock (1.2 mA) showed a greater freezing level to the CS– (Clicks tone, which was not paired with shock during training) tone than those with the weak shock (0.4 mA) and controls (0 mA). C The index of behavioral generalization (IBG) was higher in the strong shock group than in the weak shock group. D In the weak shock group, the fear response to a CS+ (Continous tone, which was paired with shock during training, but not paired with shock during behavioral testing) tone during the behavioral test (Test) was significantly higher than that of the habituation session (HAB); while there was no significant difference in the fear response to the CS– tone between HAB and Test. E The strong shock group showed an increased freezing level in response to both the CS– and CS+ tones, indicating fear generalization. n = 8–10 mice/group; mean ± SEM; ***P < 0.001, ****P < 0.0001.
Immunoblotting
Mice were sacrificed immediately, 1 week, or 2 weeks following behavior test. The brain was removed, and IL-PFC and BLA tissues were collected. Samples were homogenized for 1 min in RIPA sample buffer containing a protease inhibitor mixture (1:100; Millipore, Bedford, MA) and 1 mmol/L PMSF. The homogenates were centrifuged at 12000 g for 20 min at 4 °C. Supernatant liquots were assessed for protein concentration using a BCA assay (Pierce, Waltham, MA, Catalog# 23225). Proteins were resolved by SDS-PAGE and transferred to a PVDF membrane (Immobilon-P, Millipore). Blots were incubated overnight at 4 °C with rabbit polyclonal anti-PKCζ (1:500, Catalog# ab59364, Abcam, Cambridge, UK), anti-phospho PKCζ (T560) (1:500, Catalog# ab59412, Abcam), anti-GluN2A (1:1000, Catalog# 07-632, Millipore), anti-GluN2B (1:1000, Catalog# 14544, Cell Signaling Technology, MA), anti-BDNF (1:500, Catalog# 3160-1, Epitomics, CA), or anti-CaMKII-α (1:1000, Catalog# 50049, Cell Signaling Technology), and bands were amplified with HRP-conjugated secondary antibody (1:1000, Catalog# HAF008, R&D, MN). Protein signals were developed using Immobilon Western Chemiluminescent HRP Substrate (Millipore). The target protein immunoreactivity was normalized to GAPDH (1:1000, Catalog# 2118, Cell Signaling Technology) and quantified by densitometry using ImageJ (NIH, MD).
Cannula Infusion Experiment
Mice were anesthetized with sodium pentobarbital, and then positioned into a stereotaxic instrument (RWD Life Science, Shenzhen, China). They were placed on a heating pad, and the temperature was continuously monitored with a rectal thermometer. The body temperature was maintained at 37.5 °C–38.5 °C. Eye cream was applied to both corneas to prevent dehydration. Guide cannulas were implanted bilaterally in the BLA (−1.6 mm posterior, ±3.35 mm lateral, and −4.8 mm dorsal to bregma) or the IL-PFC (+1.78 mm posterior, ±0.4 mm lateral, and −2.5 mm dorsal to bregma), and fixed to the skull with dental cement. The coordinates were based on a mouse brain atlas [33]. Stainless-steel obturators were placed into the guide cannulas, and were changed every day to ensure patency until infusions were completed. Mice were kept in home cages to recover for at least 1 week after surgery.
After recovery, the mice were trained in the fear conditioning protocol, and 22 h later they received bilateral infusions of 0.5 μL ANA-12 (1 μg/μL) or saline into the BLA or IL-PFC through the cannulas, and intraperitoneal injection of 30 mg/kg ketamine or saline, while another group of mice only received 0.5 μL ifenprodil (Ifen, 2.0 μg/μL) or saline in the BLA or IL-PFC bilaterally. Drugs were infused (0.1 μL/min) through tip-sharpened 33-gauge double injector cannulas, which were attached to a micro-syringe. The injector cannulas were kept in place for another 2 min to decrease the spread of the drug. The behavioral tests were performed 2 h after the bilateral infusions.
Statistical Significance
All data are presented as the mean ± SEM. P<0.05 was considered statistically significant. Data were analyzed with the unpaired t-test or one-way ANOVA, using repeated measures where appropriate. Significant ANOVAs were followed by a post-hoc Turkey’s test where appropriate.
Results
Strong Shock Causes Generalization of Conditioned Fear
To determine the effects of weak and strong shocks on fear generalization, mice were divided into control, weak shock (0.4 mA), and strong shock (1.2 mA) groups. The mice with strong shock showed a significantly increased freezing level in response to the CS– tone as compared with the control and weak shock groups (Fig. 1B). The IBG was also higher in the strong shock group than in the weak shock group (Fig. 1C). Furthermore, the mice discriminated between the CS+ and CS– 24 h after weak shock fear conditioning, as evidenced by selectively higher freezing level in response to the CS+ than the CS– (Fig. 1D). However, the mice with strong shock expressed significantly increased freezing behavior in response to both the CS– and CS+ tones during behavioral tests compared to the habituation (Fig. 1E). These results suggest that a strong shock causes generalization of conditioned fear.
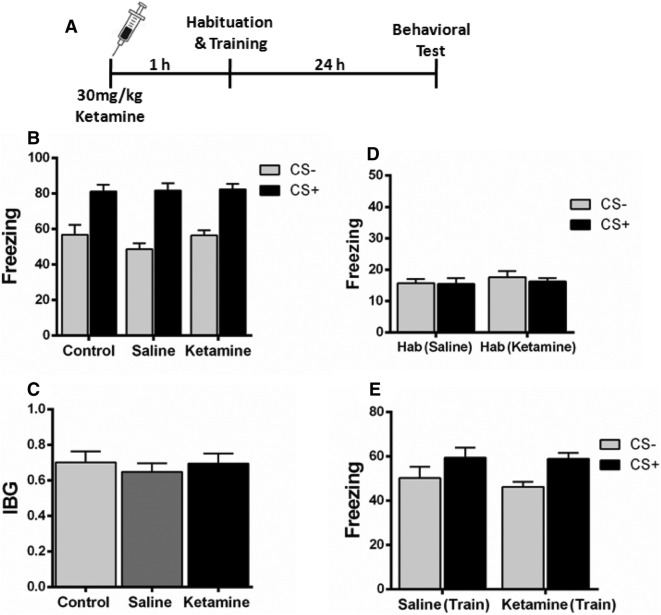
Ketamine Injection 1 h Before Fear Conditioning Does Not Alleviate Fear Generalization
A recent study found that in contextual fear-conditioned mice, a single dose of ketamine (30 mg/kg) attenuates the contextual fear memory only when given a week before as a prophylactic, but had no effect when given immediately before or after a stress-inducing episode [34]. To determine the prophylactic effect of ketamine on fear generalization, we administered a single dose of ketamine (30 mg/kg) or saline intraperitoneally 1 h before fear conditioning (Fig. 2A). These mice expressed comparable freezing levels when tested 24 h after conditioning (Fig. 2B). Also, no significant difference was found in the IBG level (Fig. 2C). Furthermore, the freezing level during habituation and training was similar in the saline and ketamine groups (Fig. 2D, E). These results suggest that ketamine administered 1 h prior to fear conditioning does not alter fear generalization.
Fig. 2.
Effect of ketamine on fear generalization when administered 1 h before fear conditioning. A Experimental design. B Mice injected with saline or ketamine 1 h before fear conditioning expressed comparable levels of freezing behavior. C No significant difference was found in the index of behavioral generalization (IBG) between the saline and ketamine groups. D, E Mice injected with saline or ketamine expressed comparable levels of freezing during habituation (D) and training (E). n = 8 mice/group; mean ± SEM.
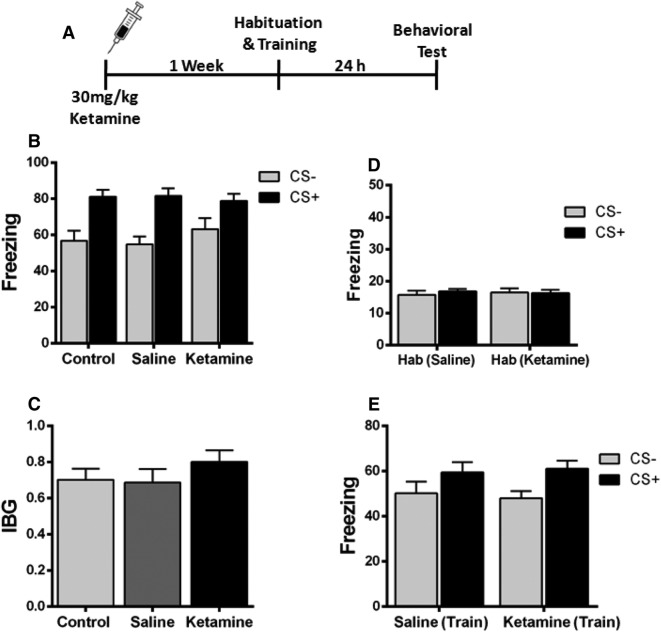
Ketamine Injection 1 Week Before Fear Conditioning Does Not Inhibit Fear Generalization
To further determine the prophylactic effect of ketamine on fear generalization, ketamine (30 mg/kg) was administered intraperitoneally 1 week prior to fear conditioning (Fig. 3A). The results showed that ketamine did not alter the fear response when compared to the saline-injected mice (Fig. 3B). Moreover, the IBG level was similar in ketamine- and saline-injected mice (Fig. 3C). The freezing levels during habituation and training were comparable between the two groups (Fig. 3D, E). These data showed that ketamine injection 1 week before fear conditioning does not change fear generalization in mice.
Fig. 3.
Prophylactic effect of ketamine on fear generalization when injected 1 week before fear conditioning. A Experimental design. B Mice injected with saline or ketamine 1 week before fear conditioning showed comparable levels of freezing during behavioral tests. C The two groups of mice showed comparable values of the index of behavioral generalization (IBG). D, E Mice in both groups showed comparable freezing levels during habituation and training. n = 8 mice/group; mean ± SEM.
Ketamine Injection Immediately After Fear Conditioning Does Not Alleviate Fear Generalization
A single dose of ketamine (30 mg/kg) immediately after fear conditioning did not affect the freezing and IBG levels of mice when compared to the control and saline groups (Fig. 4). These results indicate that ketamine does not affect fear generalization when injected immediately after fear conditioning.
Fig. 4.
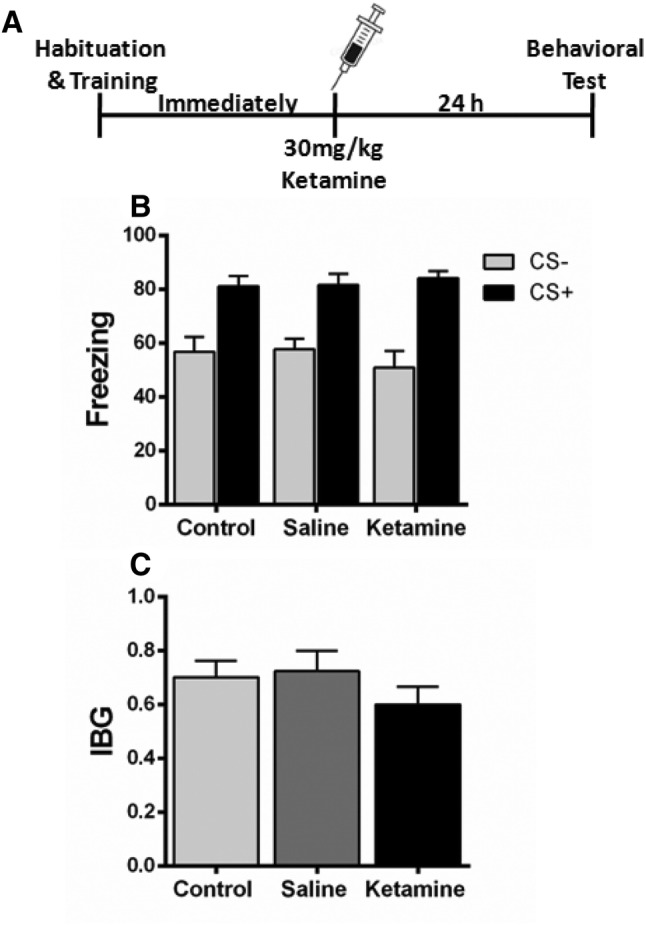
Effect of ketamine when administered immediately after fear conditioning. A Experimental design. B, C Ketamine when administered immediately after fear conditioning did not alter the fear generalization (B) or the index of behavioral generalization (IBG) (C) compared with the saline group. n = 8 mice/group; mean ± SEM.
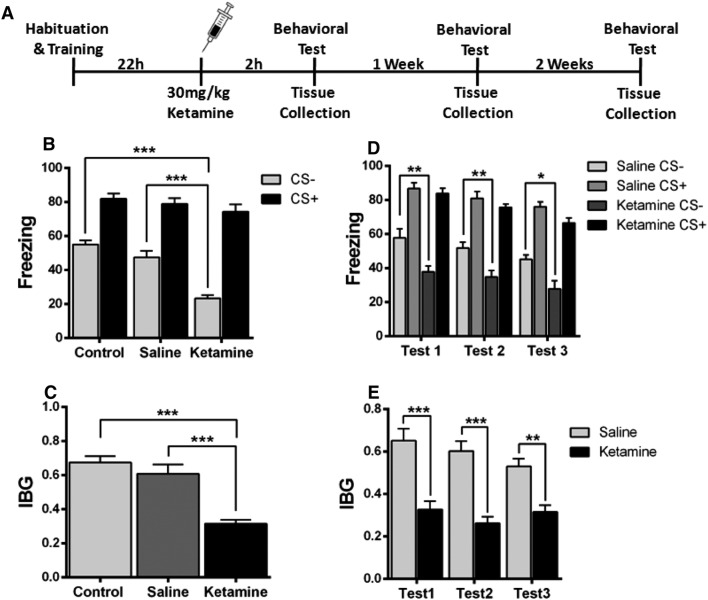
Ketamine Administration 22 h Following Fear Conditioning Alleviates Fear Generalization
Ketamine when administered 22 h after fear conditioning significantly reduced the fear response to the CS– tone (Fig. 5B). There was a significant difference in the IBG between the control, saline, and ketamine groups (Fig. 5C). It has been reported that the antidepressant effect of ketamine remains significant for 1 week [35], so we tested its effects on fear generalization at 2 h (test 1), 1 week (test 2), and 2 weeks (test 3) after ketamine administration. The results showed that the ketamine-induced alleviation of fear generalization remained significant for at least 2 weeks (Fig. 5D, E). In addition, to investigate whether the effect of ketamine was dose-dependent, 7.5 mg/kg, 15 mg/kg, and 30 mg/kg ketamine was applied 22 h after fear conditioning (Fig. S1). Ketamine at 15 mg/kg and 30 mg/kg significantly decreased the fear generalization when compared with the control group, while 7.5 mg/kg did not (Fig. S1).
Fig. 5.
Ketamine administered 22 h after fear conditioning decreases fear generalization. A Experimental design. B Injection of ketamine 22 h after fear conditioning showed a significantly lower fear response to the CS– tone than the saline group. C Ketamine-injected mice expressed lower levels of the index of behavioral generalization (IBG) than the saline group. D, E The effect of ketamine remained significant for at least 2 weeks. n = 8 mice/group; mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. Test 1, 2 h after ketamine injection; Test 2, 1 week after ketamine injection; Test 3, 2 weeks after ketamine injection.
Ketamine Alters the Expression of GluN2B and BDNF in the BLA and IL-PFC
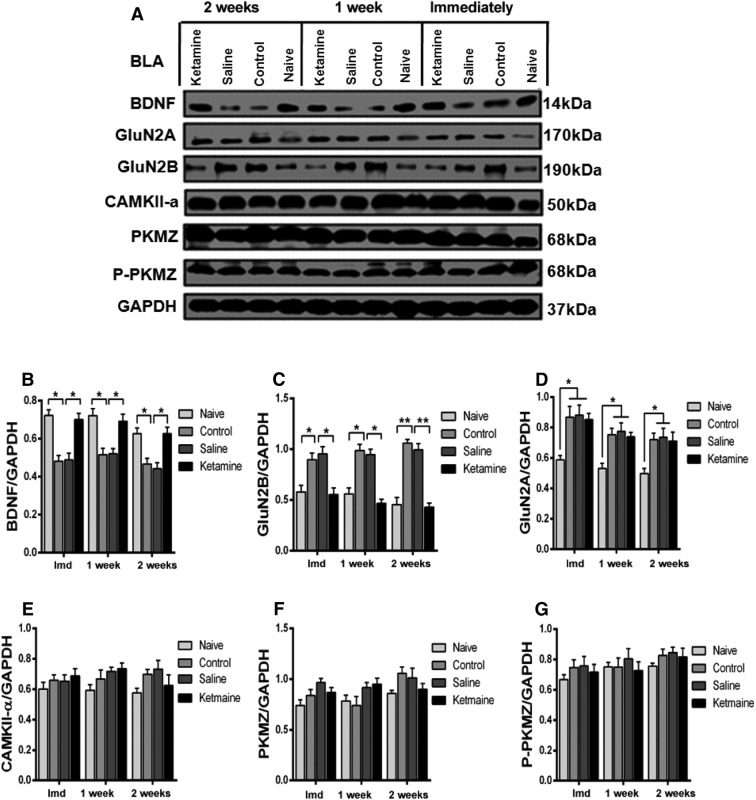
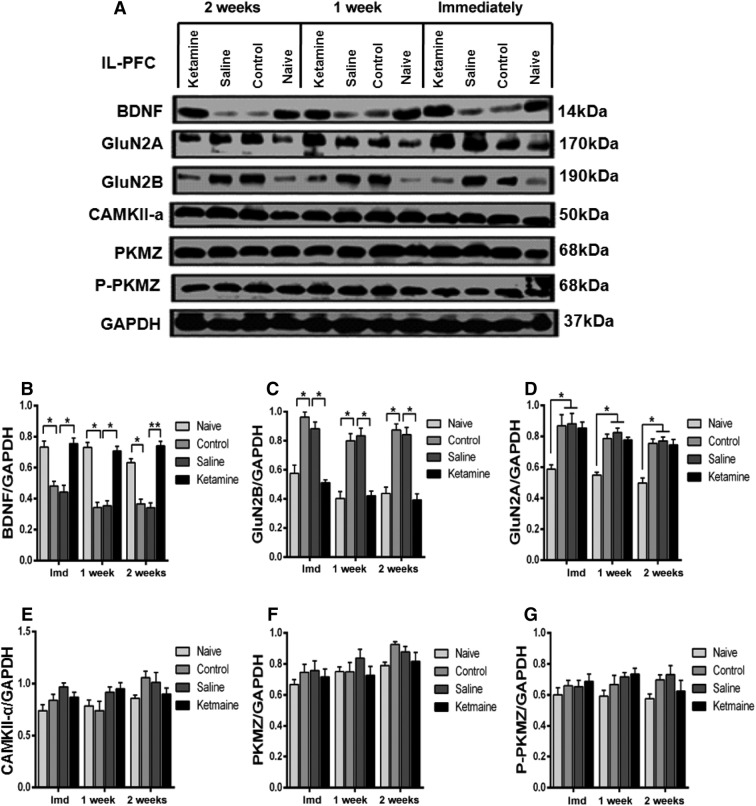
NMDAR subunits undergo modification following training [21–24]. Ketamine is an antagonist of NMDARs, which is critical in learning and memory [36]. We first used Western blot to examine the expression of GluN2A and GluN2B in the BLA and IL-PFC 24 h after fear conditioning, and found that GluN2B was significantly increased after fear memory generalization; this was reversed by a single dose of ketamine (Figs. 6C, 7C). Interestingly, GluN2A was also increased, but the increment was not reversed by ketamine (Figs. 6D, 7D). A previous study reported that inhibition of NMDARs prevented the loss of BDNF function [25]. Therefore, we investigated the expression of BDNF in the BLA and IL-PFC and found that the BDNF level was decreased after formation of generalized fear memory; this was reversed by ketamine (Figs. 6B, 7B). These results highlight an important role of GluN2B-related BDNF signaling in the expression of generalized fear. Previous studies have reported that PKMζ and CAMKII-α are involved in long-term memory formation [28, 29]. To investigate the role of these proteins in fear generalization, the expression of PKMζ, P-PKMζ, and CAMKII-α in the BLA and IL-PFC was examined. We did not find any significant difference in their expression in either region after the formation of generalization of conditioned fear. Moreover, ketamine administration did not affect their expression in these regions of mice exposed to the fear conditioning protocol (Figs. 6, 7E, F, G). These results indicate that PKMζ and CAMKII-α in the BLA and IL-PFC are not necessary for the generalization of conditioned fear.
Fig. 6.
Effects of ketamine on the expression of postsynaptic proteins in the BLA. A Western blots of synaptic protein levels in the BLA. B, C Fear generalization decreased the expression of BDNF and increased the expression of GluN2B, which were reversed by ketamine. D GluN2A was significantly increased in fear-generalized mice but this increase was not reversed by ketamine. E–G The expression of PKMζ, P-PKMζ, and CAMKII-α did not differ among the naïve, control, saline, and ketamine groups. n = 5 mice/group; mean ± SEM. *P < 0.05, **P < 0.001; Imd, immediately; BLA, basolateral amygdala; IL-PFC, inferior-limbic prefrontal cortex.
Fig. 7.
Effect of ketamine on the expression of postsynaptic proteins in the IL-PFC. A Western blots of synaptic protein levels in the IL-PFC. B, C Fear generalization decreased the expression of BDNF and increased the expression of GluN2B, which were reversed by ketamine. D GluN2A was significantly higher in fear-generalized mice but this increase was not reversed by ketamine. E–G The expression of PKMζ, P-PKMζ, and CAMKII-α did not differ among the naïve, control, saline, and ketamine groups. n = 5 mice/group; mean ± SEM; *P < 0.05; Imd, immediately; BLA, basolateral amygdala; IL-PFC, inferior-limbic prefrontal cortex.
Effect of GluN2B and BDNF Receptor Antagonists on Fear Generalization
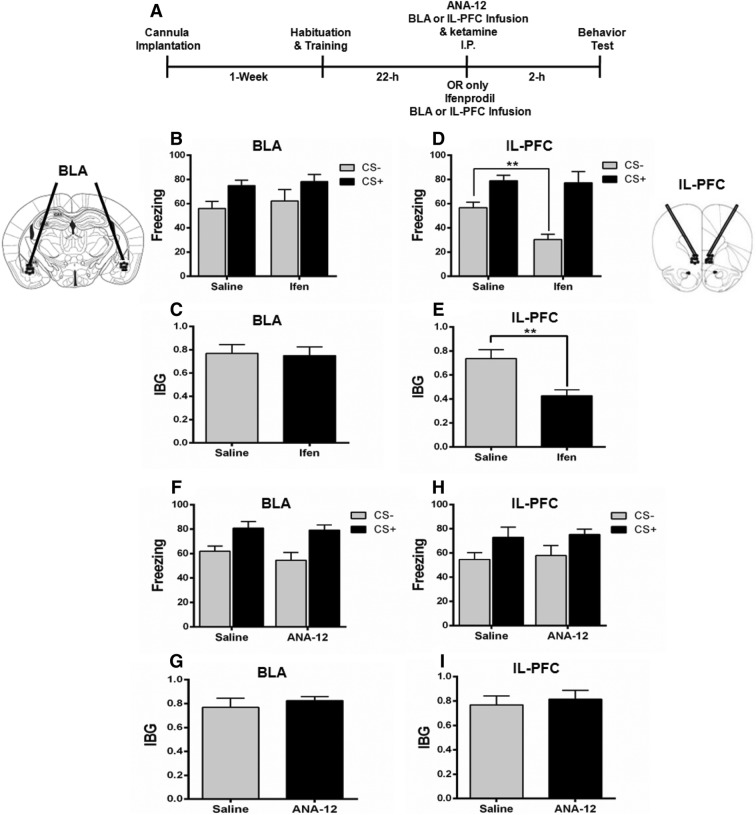
To investigate whether ketamine alleviates fear generalization by regulating GluN2B-related BDNF signaling in the BLA and IL-PFC, mice were either injected with a GluN2B antagonist, or the BDNF receptor TrkB antagonist ANA-12, bilaterally into the BLA or IL-PFC. The GluN2B antagonist ifenprodil did not decrease the fear generalization when infused bilaterally into the BLA (Fig. 8B, C). However, bilateral infusion of ifenprodil into the IL-PFC significantly decreased the fear generalization as compared with the saline group (Fig. 8D, E). Moreover, ANA-12 infused either into the BLA or IL-PFC blocked the effect of ketamine on fear generalization (Fig. 8F–I). These results support an important role of GluN2B-related BDNF signaling in fear generalization and modulation of the GluN2B/BDNF signaling pathway is involved at least in part in the alleviation of fear generalization by ketamine.
Fig. 8.
Effects of GluN2B and BDNF antagonists on fear generalization. A Schematic of the experimental procedure. B, C Infusion of the GluN2B-selective antagonist ifenprodil into the BLA did not decrease the fear generalization as shown by similar freezing levels (B) and IBG (C) in the ifenprodil (Ifen) and saline groups. D, E Ifenprodil infusion into the IL-PFC decreased the fear generalization, as shown by a significantly lower response to the CS– in terms of freezing level (D) and IBG (E) in the Ifen group than in the saline group. F–I The BDNF receptor TrkB antagonist ANA-12 infused either into the BLA (F, G) or the IL-PFC (H, I) blocked the effect of ketamine on fear generalization as shown by comparable freezing levels and IBG in the ANA-12 and saline groups. n=6 mice per group; mean ± SEM; **P < 0.001; BLA, basolateral amygdala; IL-PFC, inferior-limbic prefrontal cortex.
Discussion
We investigated the role of ketamine in fear generalization. A strong stress induced the enduring generalization of conditioned fear. The fear-generalized mice developed PTSD-like behaviors, unable to discriminate dangerous from safe stimuli. A single sub-anesthetic dose of ketamine intraperitoneally administered 22 h after fear conditioning led to significant inhibition of fear generalization and the effect lasted at least 2 weeks. Fear-generalized mice had a lower level of BDNF and a higher level of GluN2B protein in the BLA and IL-PFC, which were reversed by a single administration of ketamine. Moreover, the GluN2B antagonist ifenprodil decreased the fear generalization when infused into the IL-PFC, but had no effect when infused into the BLA. The BDNF receptor TrkB antagonist ANA-12 blocked the effect of ketamine on fear generalization when infused into either the BLA or the IL-PFC. The results support the conclusion that ketamine suppresses fear generalization by modulating GluN2B/BDNF signaling.
According to theoretical models of Pavlovian conditioning, fear learning can also be modulated by changing the unconditioned stimulus itself [37, 38]. A recent study showed that increasing the intensity of electrical shocks strengthens the fear generalization response, which was presented as a novel PTSD model [19]. Fear generalization produces more robust symptoms, including increased freezing in response to a safe cue and impaired safety signal learning, which has been proposed as a biomarker for PTSD patients [1]. Interestingly, clinical studies have reported exaggerated amygdala and medial PFC responses in anxiety disorders [17, 18]. In fear-generalized rats, the overall activity of neurons in the lateral amygdala is significantly enhanced [19], and the freezing level is positively correlated with the excitability of IL-PFC and BLA projection neurons after conditioning [20]. Consistent with these studies, we found that fear-generalized mice had a lower level of BDNF and a higher level of GluN2B protein in the BLA and IL-PFC, which were reversed by a single intraperitoneal dose of ketamine. Recently, it has been shown that blockade of NMDAR-dependent bursting activity in the lateral habenula mediates the antidepressant actions of ketamine in rat and mouse models of depression [39]. It would therefore be interesting to investigate the effects of ketamine on the activity of neurons in the BLA and PFC.
Ketamine increased the BDNF expression in the BLA and IL-PFC in a short period of time (~2 h) after administration, which is consistent with a previous study showing that 1 h–5 h are sufficient for significant upregulation of protein expression [40]. A recent study confirmed that ketamine increases pro-BDNF expression within 40 min after a single dose [41]. These results indicate that ketamine might prevent the degradation of protein or enhance the expression of protein via the cytoplasmic mRNA pool. Acute ketamine administration increased BDNF protein in the BLA and IL-PFC. This is consistent with the antidepressant effects of ketamine, which may depend on rapid activation of the mammalian target of BDNF protein in the PFC [42, 43], and ketamine alleviates the PTSD-like effect by modifying the BDNF and HCN1 expression in the SPS&S animal model [27].
In addition, we found that GluN2B and GluN2A protein expression was increased as a result of fear generalization and ketamine reversed the expression of GluN2B, but interestingly GluN2A expression remained the same. Growing evidence supports the idea that the expression and subunit composition of NMDARs can be dynamically modulated by individual experiences. Interestingly, the expression of membrane GluN2B, not the total amount of GluN1, GluN2A, and GluN2B, is increased in the hippocampal CA1 after contextual fear conditioning in a stress-strength-dependent manner [24], highlighting an important role of NMDAR trafficking in NMDA-dependent synaptic plasticity. Accumulating data indicate that NMDARs dynamically sense and integrate neural signaling in a stimulus-dependent manner, by the regulation of expression, subunit composition, or cellular trafficking. Notably, previous studies revealed that loss of GluN2B function [44] or GluN2B deletion [45] impairs long-term fear memory. Our recent data also supported an important role of GluN2B in long-term fear memory formation [22]. Moreover, inhibition of NMDARs prevents the loss of BDNF function [25]. Our results together with previous data indicate that ketamine up-regulates BDNF signaling by decreasing GluN2B protein to alleviate PTSD-like symptoms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81530061 and 81471829), the Pearl River Nova Program of Guangzhou (201610010154), and the Natural Science Foundation of Guangdong Province China (2017A030313095).
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Muhammad Asim and Bo Hao have contributed equally to this work.
Contributor Information
Yan-Wei Shi, Email: shiyanw@mail.sysu.edu.cn.
Xiao-Guang Wang, Email: wxguang@mail.sysu.edu.cn.
References
- 1.Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Pradhan B, Kluewer D’Amico J, Makani R, Parikh T. Nonconventional interventions for chronic post-traumatic stress disorder: Ketamine, repetitive trans-cranial magnetic stimulation (rTMS), and alternative approaches. J Trauma Dissociation. 2016;17:35–54. doi: 10.1080/15299732.2015.1046101. [DOI] [PubMed] [Google Scholar]
- 5.Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2006: CD002795. [DOI] [PMC free article] [PubMed]
- 6.Golub Y, Mauch CP, Dahlhoff M, Wotjak CT. Consequences of extinction training on associative and non-associative fear in a mouse model of Posttraumatic Stress Disorder (PTSD) Behav Brain Res. 2009;205:544–549. doi: 10.1016/j.bbr.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Bernardini F, Attademo L, Cleary SD, Luther C, Shim RS, Quartesan R, et al. Risk prediction models in psychiatry: toward a new frontier for the prevention of mental illnesses. J Clin Psychiatry. 2017;78:572–583. doi: 10.4088/JCP.15r10003. [DOI] [PubMed] [Google Scholar]
- 8.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 9.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, et al. Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry. 2016;79:776–786. doi: 10.1016/j.biopsych.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGhee Laura L., Maani Christopher V., Garza Thomas H., Gaylord Kathryn M., Black Ian H. The Correlation Between Ketamine and Posttraumatic Stress Disorder in Burned Service Members. The Journal of Trauma: Injury, Infection, and Critical Care. 2008;64(Supplement):S195–S199. doi: 10.1097/TA.0b013e318160ba1d. [DOI] [PubMed] [Google Scholar]
- 13.McGhee Laura L., Maani Christopher V., Garza Thomas H., Slater Terry M., Petz Lawrence N., Fowler Marcie. The Intraoperative Administration of Ketamine to Burned U.S. Service Members Does Not Increase the Incidence of Post-Traumatic Stress Disorder. Military Medicine. 2014;179(8S):41–46. doi: 10.7205/MILMED-D-13-00481. [DOI] [PubMed] [Google Scholar]
- 14.Silvestre JS, Pallares M, Nadal R, Ferre N. Opposite effects of ethanol and ketamine in the elevated plus-maze test in Wistar rats undergoing a chronic oral voluntary consumption procedure. J Psychopharmacol. 2002;16:305–312. doi: 10.1177/026988110201600404. [DOI] [PubMed] [Google Scholar]
- 15.Hayase T, Yamamoto Y, Yamamoto K. Behavioral effects of ketamine and toxic interactions with psychostimulants. BMC Neurosci. 2006;7:25. doi: 10.1186/1471-2202-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: A possible animal model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 17.Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci. 2015;18:112–120. doi: 10.1038/nn.3888. [DOI] [PubMed] [Google Scholar]
- 20.Song C, Ehlers VL, Moyer JR., Jr Trace fear conditioning differentially modulates intrinsic excitability of medial prefrontal cortex-basolateral complex of amygdala projection neurons in infralimbic and prelimbic cortices. J Neurosci. 2015;35:13511–13524. doi: 10.1523/JNEUROSCI.2329-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SH, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009;12:905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- 22.Chen YF, Chen ZX, Wang RH, Shi YW, Xue L, Wang XG, et al. Knockdown of CLC-3 in the hippocampal CA1 impairs contextual fear memory. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:132–145. doi: 10.1016/j.pnpbp.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Holehonnur R, Phensy AJ, Kim LJ, Milivojevic M, Vuong D, Daison DK, et al. Increasing the GluN2A/GluN2B ratio in neurons of the mouse basal and lateral amygdala inhibits the modification of an existing fear memory trace. J Neurosci. 2016;36:9490–9504. doi: 10.1523/JNEUROSCI.1743-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun YY, Cai W, Yu J, Liu SS, Zhuo M, Li BM, et al. Surface expression of hippocampal NMDA GluN2B receptors regulated by fear conditioning determines its contribution to memory consolidation in adult rats. Sci Rep. 2016;6:30743. doi: 10.1038/srep30743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanqueiro SR, Ramalho RM, Rodrigues TM, Lopes LV, Sebastiao AM, Diogenes MJ. Inhibition of NMDA receptors prevents the loss of BDNF function induced by amyloid beta. Front Pharmacol. 2018;9:237. doi: 10.3389/fphar.2018.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muhlberger A, Andreatta M, Ewald H, Glotzbach-Schoon E, Troger C, Baumann C, et al. The BDNF Val66Met polymorphism modulates the generalization of cued fear responses to a novel context. Neuropsychopharmacology. 2014;39:1187–1195. doi: 10.1038/npp.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou L, Qi Y, Sun H, Wang G, Li Q, Wang Y, et al. Applying ketamine to alleviate the PTSD-like effects by regulating the HCN1-related BDNF. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:313–321. doi: 10.1016/j.pnpbp.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Sheng T, Ren S, Tian T, Lu W. Distinct roles of PKCiota/lambda and PKMzeta in the initiation and maintenance of hippocampal long-term potentiation and memory. Cell Rep. 2016;16:1954–1961. doi: 10.1016/j.celrep.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Rossetti T, Banerjee S, Kim C, Leubner M, Lamar C, Gupta P, et al. Memory erasure experiments indicate a critical role of CaMKII in memory storage. Neuron. 2017;96(207–216):e202. doi: 10.1016/j.neuron.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iijima M, Fukumoto K, Chaki S. Acute and sustained effects of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. Behav Brain Res. 2012;235:287–292. doi: 10.1016/j.bbr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 32.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 2014, 18. [DOI] [PMC free article] [PubMed]
- 33.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- 34.McGowan JC, LaGamma CT, Lim SC, Tsitsiklis M, Neria Y, Brachman RA, et al. Prophylactic ketamine attenuates learned fear. Neuropsychopharmacology. 2017;42:1577–1589. doi: 10.1038/npp.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 36.Muir WW. NMDA receptor antagonists and pain: ketamine. Vet Clin North Am Equine Pract. 2010;26:565–578. doi: 10.1016/j.cveq.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Baldi E, Lorenzini CA, Bucherelli C. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem. 2004;81:162–166. doi: 10.1016/j.nlm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Laxmi TR, Stork O, Pape HC. Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Ari Y, Brody Y, Kinor N, Mor A, Tsukamoto T, Spector DL, et al. The life of an mRNA in space and time. J Cell Sci. 2010;123:1761–1774. doi: 10.1242/jcs.062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen L, Lucke-Wold BP, Logsdon AF, Scandinaro AL, Huber JD, Matsumoto RR. Behavioral and biochemical effects of ketamine and dextromethorphan relative to its antidepressant-like effects in Swiss Webster mice. Neuroreport. 2016;27:1004–1011. doi: 10.1097/WNR.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A, et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry. 2010;11:763–773. doi: 10.3109/15622971003611319. [DOI] [PubMed] [Google Scholar]
- 44.Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, et al. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akashi K, Kakizaki T, Kamiya H, Fukaya M, Yamasaki M, Abe M, et al. NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J Neurosci. 2009;29:10869–10882. doi: 10.1523/JNEUROSCI.5531-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.