Abstract
Endothelial cells line all blood vessels in vertebrates. These cells contribute to whole-body nutrient distribution in a variety of ways, including regulation of local blood flow, regulation of trans-endothelial nutrient transport, and paracrine effects. Obesity elicits dramatic whole-body nutrient redistribution, in particular of fat. We briefly review here recent progress on understanding endothelial fat transport; the impact of obesity on the endothelium; and, conversely, how endothelial function can modulate obesity.
Introduction
Endothelial cells (ECs) comprise the largest proportion of non-blood cells in the body (Bianconi, et al., 2013). ECs form the endothelium, a thin layer of simple squamous cells that lines both circulatory and lymphatic networks, carriers of blood and lymph, respectively. The endothelium can be leaky to nutrients, by either being discontinuous, as found in liver sinusoids, or by containing fenestrae, large channels that permit bulk trans-endothelial transport of solutes, as typically found in endocrine organs. In most tissues, however, the endothelium forms a continuous barrier, separating circulating nutrients from the underlying parenchyma. Transport of nutrients, and in particular of fat, across that barrier remains poorly understood. Obesity is marked by dramatic accumulation of fat stores. This process has profound effects on endothelial function, both in adipose tissue and systemically. Conversely, endothelial dysfunction can, surprisingly, contribute to obesity. Here we provide a brief review of recent progress in our understanding of trans-endothelial fat transport and of the reciprocal relationship between endothelial cell function and obesity.
Endothelium and the physiology of fat intake
The endothelial response to food intake is carefully regulated to meet the energetic demands of the digestive system, deliver necessary nutrients to tissues, and prevent the accumulation of toxic metabolites. After food intake, blood flow increases to digestive organs within 5 minutes (Granger, et al., 1980; Norryd, et al., 1975), and within 10–15 minutes insulin secretion increases capillary blood flow (Clerk, et al., 2004). This increase in blood flow, and consequently shear stress, results in vasodilation, which is regulated by increased levels of endothelial-specific nitric oxide (NO). Endothelial NO, first identified as “endothelium derived relaxing factor” (Ignarro, et al., 1987; Palmer, et al., 1987), is synthesized by endothelial-specific nitric oxide synthase (eNOS) and signals to the surrounding smooth muscle cells to elicit relaxation. Vasodilatation increases blood flow and increases the surface-area available for nutrients to be transported in and out of the vasculature.
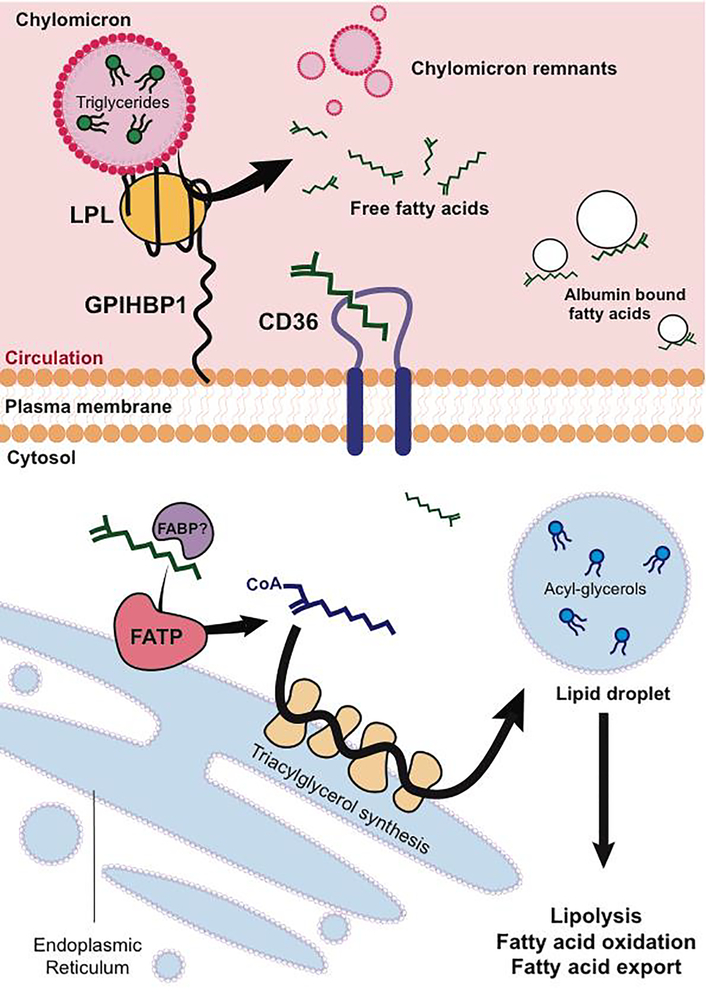
Dietary fats are first processed in the intestinal lumen by lipases and bile acids secreted from the pancreas and liver, respectively. Free fatty acids are then esterified by intestinal epithelia, and packaged into lipoprotein particles called chylomicrons. Chylomicrons enter circulation through specialized intestinal lymphatic vessels called lacteals, which transport chylomicrons through the lymphatic system and into circulation (Cifarelli, et al., 2019). Fatty acids are then once again liberated from chylomicrons by the action of lipoprotein lipase (LPL) in target tissues (Figure 1). Even though LPL is synthesized by underlying parenchyma, it acts on the luminal side of the endothelium. Endothelial GPIHBP1 transports and anchors lipoprotein lipase to the luminal membrane (Sonnenburg, et al., 2009), facilitating hydrolysis of triacylglycerides (TAGs) and release of free fatty acids (Beigneux, et al., 2007; Fong, et al., 2016).
Figure 1:
Transport of fatty acids across the endothelium. Free Fatty acids (FFAs) packaged as triglycerides in lipoprotein particles are liberated via lipoprotein lipase (LPL) on the luminal side of the endothelium, anchored by the protein GPIHBP1. FFAs uptake into endothelium is facilitated by fatty acid translocase CD36, on the endoplasmic reticulum, and fatty acid binding proteins (FABPs). fatty acid transport proteins (FATPs) catalyze formation of Acyl-CoAs, which are further processed into the triglyceride pool of lipid droplets. Lipolysis once again liberates FFAs, to be used for fatty acid oxidation or transported to the underlying parenchyma.
While the process described above has been characterized for some time, the mechanisms surrounding the subsequent transport of free fatty acids across the endothelium are less well understood. Unlike hydrophilic molecules like sugars and amino acids, fatty acids are unlikely to be transported through canonical solute transporters. Early debates that focused on whether fatty acids were transported via paracellular or transcellular routes, or whether the import of fats is regulated or diffusion limited (McArthur, et al., 1999) have not been entirely resolved. However, the discovery over the past 25 years of a number of proteins that can modulate FFA transport has proven that FFA transport can be transcellular, and can be regulated. Transport is in part mediated by the fatty acid translocase CD36 (Son, et al., 2018), which binds to long chain fatty acids and transfers them to the plasma membrane. EC-specific deletion of CD36 blunts delivery of fatty acids to tissues with continuous endothelium, such as the heart {Goldberg, 2018}. The nuclear receptor peroxisome proliferator activated receptor gamma (PPARg) regulates the expression of CD36, as well as the most abundant fatty acid binding protein in ECs, fatty-acid binding protein (FABP4) (Kanda, et al., 2009), which has been shown to deliver ligands from the cytosol to PPARg in the nucleus, enhancing fatty acid uptake. Conversely, knockout of endothelial PPARg results in decreased fatty acid uptake into underlying tissue, as well as decreased adipocyte size. Interestingly, endothelial cells have recently been suggested to also produce PPARg ligands to the underlying adipose tissue, although the exact nature of these ligands remains unresolved (Gogg, et al., 2019). The transcription factor Notch, induced in quiescent cells and a well-established inhibitor of migration and angiogenesis (Blanco, et al., 2013) also positively regulates CD36, endothelial LPL, and fatty acid binding protein (FABP4) expression (Jabs, et al., 2018).
Fatty acid transport into cells has also been proposed to occur by “vectorial” transport (Figure 1), whereby fatty acids are “trapped” in the cytosol by conversion to acyl-CoAs, a process akin to trapping glucose in cells by phosphorylation. This reaction is carried out by so-called fatty-acid transport proteins, of which FATP3 and FATP4 are expressed in ECs. These molecules are single-pass membrane proteins with cytoplasmic acyl-CoA synthetase activities (Hagberg, et al., 2013; Jia, et al., 2007; Milger, et al., 2006). Although frequently schematized as plasma membrane proteins, FATP3 and 4 are likely in fact localized to the endoplasmic reticulum (ER) (Jia, et al., 2007; Milger, et al., 2006). siRNA-mediated suppression of FATP3 and 4 blocks fatty acid uptake and transport in ECs (Hagberg, et al., 2013; Jang, et al., 2016). How these molecules perform this task from the ER is not clear.
The observation that the formation of acyl-CoA in ECs may be required for fatty acid transport suggested the possibility that these species may in turn cycle through the triglyceride pool, which Kuo et al. recently demonstrated to be the case (Kuo, et al., 2017). After oil gavage, significant amounts of lipid droplets can transiently be seen in the endothelium of mice, and cycling through the TG pool appears to require canonical pathways of TG formation and degradation, including DGAT and ATGL. The physiological consequence of this transient accumulation is not clear, but may serve to delay and buffer entry of potentially toxic fatty acid species into the underlying parenchyma (Ibrahim, et al., 2017). Evidence of increased EC lipid droplets has also been found in obesity models (Guyton, et al.; Sima, 2002), although again, the functional consequence of these droplets is not clear.
Finally, recent evidence has shown that the underlying parenchyma can signal to the endothelium to increase fatty acid transport. In skeletal muscle, expression of PPARg coactivator-1alpha (PGC-1alpha) promotes the secretion of the branched chain amino acid (BCAA) metabolite 3-hydroxyisobutyrate (3-HIB), which acts on endothelial cells to increase lipid transport (Jang, et al., 2016), thus mechanistically linking BCAA and fat catabolism. PGC-1alpha in myocytes also promotes the expression of VEGFB. Despite being a member of the VEGF family, VEGFB is poorly angiogenic; instead VEGFB, like 3HIB, appears to promote trans-endothelial fatty acid transport, in a manner dependent on FATP3 and 4 (Hagberg, et al., 2010). In contrast, adipose tissue can secrete the peptide Apelin to reduce fatty acid uptake, in large part via the endothelial apelin receptor (APLNR), leading to inactivating phosphorylation of the transcription factor FOXO1, and reduced expression of FABP4 (Hwangbo, et al., 2017). In summary, endothelial cells and the underlying tissues reciprocally interact to regulate and hone fatty acid processing and transport across the endothelium and delivery to the parenchyma. These recent observations are likely only beginning to uncover this complex regulatory system.
How obesity affects the endothelium
Many classic studies showed that fats acutely (but temporarily) induce endothelial dysfunction in healthy subjects. For example, a single high fat meal decreases flow-mediated vasodilation, inversely proportional to the levels of serum triglycerides (Vogel, et al., 1997). Similar impairment of vasoreactivity follows infusion or oral administration of lipids in healthy subjects (Lundman, et al., 1997; Wilmink, et al., 1999). Saturated fatty acids also induce an acute inflammatory reaction (Kim, 2007; Harvey, 2010; Kroggman, 2011; Li, 2013}, resulting in increased expression of the pro-inflammatory adhesion molecules ICAM1 and VCAM1. Furthermore, long-chain saturated fatty acids have been found to inhibit endothelial cell growth by inducing apoptosis and necrosis (Harvey, et al., 2010).
The chronic effects of obesity on the endothelium, on the other hand, are different. The marked increase in adipose tissue that accompanies obesity necessarily requires expansion of the adipose tissue vascular bed. Activation of angiogenesis, an otherwise relatively rare process in normal adult physiology, is thus a sine qua non for increased adiposity in obesity. Angiogenesis is likely triggered by hypoxia in the setting of expanding fat mass (Pasarica, et al., 2009), resulting in decreased capillary density, local hypoxia, and activation of hypoxia-inducible factor 1 (Hif-1alpha) that drives expression of VEGF (Sun, et al., 2013; Trayhurn, 2013). Hif-1alpha KO animals exposed to high fat diet have less fat mass and reduced adipocyte size in addition to lower expression of endothelial growth factors, consistent with insufficient vascular support for adipose tissue expansion (Jiang, et al., 2011). Pathologically, this angiogenic expansion can often be insufficient to match the increasing size of adipose tissue, leading to local hypoxia and inflammation, and contributing to systemic metabolic disease (Cao, 2013).Adipose tissue from obese subjects also show markers of senescence, also resulting in an increased inflammatory profile (Villaret, et al., 2010). Short term weight loss ameliorates some endothelial dysfunction (Csipo, et al., 2018) suggesting that while chronic obesity alters homeostasis of the endothelium, changes are reversible.
In addition to triggering angiogenesis in adipose tissue, obesity can impair the physiological and molecular function of the endothelium systemically. Obese but normotensive, normoglycemic human subjects show decreased endothelium-dependent vasodilation capacity (Van Guilder, et al., 2006) and increased aortic stiffness (Brunner Eric, et al., 2015) compared to non-obese controls. Non-diabetic, obese subjects also have decreased vascular capillary density not only in adipose tissue (Gealekman, et al., 2011) but also in skeletal muscle (Gavin, et al., 2005), and have decreased capillary recruitment following vascular occlusion in skin (Csipo, et al., 2018; de Jongh Renate, et al., 2004), and insulin-mediated capillary recruitment in skeletal muscle (Clerk, et al., 2006; Wallis, et al., 2002). On a molecular level, these phenotypes have been generally attributed to decreased expression of eNOS (Georgescu, et al., 2011; Sansbury, et al., 2012; Valerio, et al., 2006), but how this occurs remains incompletely understood. One prominent mechanism is likely the impact of adipose-derived hormones, including adiponectin and leptin. In obesity, adiponectin is decreased (Arita, et al., 1999), while leptin is increased (Considine, et al., 1996). Adiponectin, which is negatively correlated with BMI and insulin levels, stimulates glucose utilization and fatty-acid oxidation, as well as NO production (Ouchi, et al., 2003; Yamauchi, et al., 2002), and inhibits inflammatory gene expression response to TNFa in endothelial cells {Ouchi, 2000}. Conversely, adiponectin deficiency increases pro-inflammatory adhesion molecules, resulting in increased leukocyte-endothelium interaction (Ouedraogo, et al., 2007). In contrast, leptin is pro-inflammatory, and increases both vascular permeability and angiogenesis, resulting in increased delivery of nutrients to adipose tissue (Cao, et al.; Sierra-Honigmann, et al., 1998).
How the endothelium can affect obesity
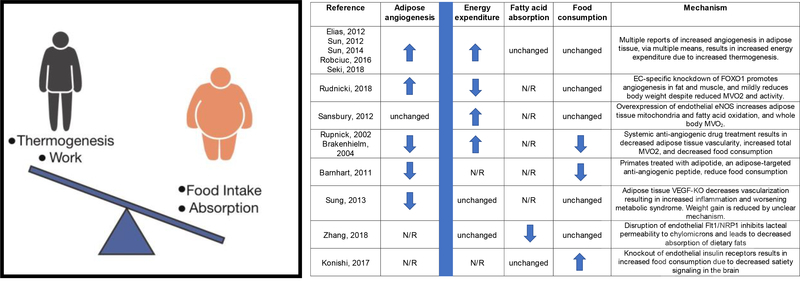
Thermodynamically, changes in body weight can only occur in one of two ways: (1) by altering intake of calories, either via modulating food intake or absorption of nutrients; or (2) by altering consumption of calories, either via thermogenesis or work (Figure 2). Surprisingly, recent research has indicated that the endothelium can impact these systemic processes in obesity.
Figure 2:
How the endothelium can affect obesity. Left, schematic of thermodynamic contributors to body weight regulation. Right, table outlining recent publications in which endothelial biology is altered, leading to changes in body weight. Effects on adipose tissue angiogenesis, energy expenditure (calories out), fatty acid absorption and food consumption (calories in) highlighted for each study.
Calorie consumption
The bulk of these studies have focused on the role of adipose tissue endothelial cells (Graupera, et al.). Enhanced angiogenesis in both brown or white fat can activate thermogenesis, leading to reduced body weight via increased energy expenditure (Elias, et al., 2012; Robciuc, et al., 2016; Seki, et al., 2018; Sun, et al.; Sun, et al.; Sung, et al., 2013). These studies highlight the role of endothelial growth factors (VEGF’s) and their receptors (VEGFR) in these processes. Secretion of VEGF-A by tissues, including adipose tissue, activates the endothelial cell VEGFR2 receptor to stimulate angiogenesis. A number of early studies demonstrated that promoting this process in adipose tissue was sufficient to activate a thermogenic program (Elias, et al., 2012; Sun, et al., 2012; Sung, et al., 2013), although the precise details of how angiogenesis promotes mitochondrial uncoupling and thermogenesis remain unclear. More recent work has leveraged VEGFR1, which on ECs acts as a decoy receptor by binding to VEGFA and preventing it from signaling through VEGFR2 to promote angiogenesis. Overexpression of VEGF-B, which binds even more avidly to VEGFR1, displaces VEGFA, allowing it to signal through VEGFR2 and promote angiogenesis. Robciuc, et al recently demonstrated that overexpression of VEGFB augments angiogenesis and increases thermogenesis by enhancing activation of VEGFR2. Similarly, Seki, et al showed that genetic ablation or blockade of VEGFR1 increased adipose tissue angiogenesis and thermogenesis. These vascular effects of VEGFB vs VEGFA have also been demonstrated in other tissues, highlighting the complex regulation of angiogenesis. It is not clear, however, why thermogenesis and body weight are affected by manipulations of the VEGFB/VEGFR1 axis in these studies, while they were not in the studies by Hagberg et al (Hagberg, et al., 2010; Hagberg, et al., 2012). Recently, EC-specific knockout of FOXO1 was also shown to increase adipose vascular density and decrease adiposity, but in this case the thermogenesis was not activated, and the explanation for reduced body weight is not clear (Rudnicki, et al., 2018). EC-specific overexpression of eNOS also reduces diet-induced obesity by increasing energy expenditure, likely via the adipose tissue (Sansbury, et al., 2012).
Paradoxically, inhibition, rather than stimulation, of adipose tissue angiogenesis has also been suggested to decrease obesity. Pharmacological or genetic inhibition of angiogenesis has resulted in weight loss and reduced adiposity in both rodent (Bråkenhielm, et al., 2004; Rupnick, et al., 2002; Sung, et al., 2013) and non-human primate (Barnhart, et al., 2011) models. In many of these cases, however, the effect appears to be on food intake, rather than thermogenesis, via unclear mechanisms (Barnhart, et al., 2011; Bråkenhielm, et al., 2004; Rupnick, et al., 2002). Loss of adipose vasculature in normal animals also results in increased inflammation due to hypoxia, and worsening metabolic defects despite reduced fat mass (Sung, et al., 2013). In contrast, blocking adipose tissue angiogenesis in a different model of obesity (ob/ob mice) appears to have beneficial effects on both body weight and metabolism, suggesting that the consequences of modulating adipose angiogenic activity are context dependent (Sun, et al., 2012), which may explain some of the paradoxical observations made above.
Calorie intake
Can endothelial function also affect calorie intake? Recent studies have suggested that it can (Zhang, et al., 2018). Zhang et al. showed that ablation of VEGFR1 and its co-receptor, Neuropilin 1 (NRP1) renders mice resistant to obesity by decreasing chylomicron uptake. Reduced VEGFA activity during the formation of gut lacteals is critical to render them permeable to chylomicrons. Deletion of the decoy VEGFR1/NRP1 receptor in adjacent ECs results in high VEGFA activity, and these animals thus lose calories in their feces due to lipid malabsorption. Interestingly, in these studies, deletion of VEFGR1 had little impact on adipose thermogenesis, in contrast to the studies above. In addition to absorption, endothelial function may also affect food intake. As noted above, some anti-angiogenic agents may suppress food intake. Konishi et al also recently showed that the insulin receptor (IR) on endothelial cells can affect food consumption (Konishi, et al., 2017). Endothelial IR is required for trans-endothelial transport of insulin, and thus affects the kinetics of insulin signaling to tissues with tight endothelial junctions, including the brain. Knockout of endothelial IR delayed insulin signaling in the brain, disrupting the satiety response and delaying reduction of food intake, leading to mild obesity. Overall, these studies show that endothelial cells can modulate obesity at multiple levels, including lipid storage in the white adipose tissue, energy expenditure, fat processing in the intestine, and food consumption.
Conclusion
The endothelium is intricately involved in whole-body nutrient transport and distribution. Obesity is a disease of nutrient excess, and in particular of excess fat distribution. It is thus not surprising that a reciprocal relationship exists between endothelial function and obesity. The details of that relationship are only beginning to emerge. The hope is that deeper understanding of that relationship may lead to novel approaches to treating obesity.
Acknowledgement
Z.A. is supported by NIDDK DK119565 and DK114103
Footnotes
Conflict of interest statement
The authors declare no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J. i., Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. (1999). Paradoxical Decrease of an Adipose-Specific Protein, Adiponectin, in Obesity. Biochemical and Biophysical Research Communications 257, 79–83. [DOI] [PubMed] [Google Scholar]
- 2.Barnhart KF, Christianson DR, Hanley PW, Driessen WHP, Bernacky BJ, Baze WB, Wen S, Tian M, Ma J, Kolonin MG, et al. (2011). A peptidomimetic targeting white fat causes weight loss and improved insulin resistance in obese monkeys. Science translational medicine 3, 108ra112–108ra112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigneux AP, Davies BSJ, Gin P, Weinstein MM, Farber E, Qiao X, Peale F, Bunting S, Walzem RL, Wong JS, et al. (2007). Glycosylphosphatidylinositol-Anchored High-Density Lipoprotein-Binding Protein 1 Plays a Critical Role in the Lipolytic Processing of Chylomicrons. Cell Metabolism 5, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, et al. (2013). An estimation of the number of cells in the human body. Annals of Human Biology 40, 463–471. [DOI] [PubMed] [Google Scholar]
- 5.Blanco R, and Gerhardt H (2013). VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med 3, a006569–a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bråkenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, and Cao Y (2004). Angiogenesis Inhibitor, TNP-470, Prevents Diet-Induced and Genetic Obesity in Mice. Circulation Research 94, 1579–1588. [DOI] [PubMed] [Google Scholar]
- 7.Brunner Eric J, Shipley Martin J, Ahmadi-Abhari S, Tabak Adam G, McEniery Carmel M, Wilkinson Ian B, Marmot Michael G, Singh-Manoux A, and Kivimaki M (2015). Adiposity, Obesity, and Arterial Aging. Hypertension 66, 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, and Cao Y (2001). Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proceedings of the National Academy of Sciences of the United States of America 98, 6390–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y (2013). Angiogenesis and Vascular Functions in Modulation of Obesity, Adipose Metabolism, and Insulin Sensitivity. Cell Metabolism 18, 478–489. [DOI] [PubMed] [Google Scholar]
- 10.Cifarelli V, and Eichmann A (2019). The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell Mol Gastroenterol Hepatol 7, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, and Barrett EJ (2006). Obesity Blunts Insulin-Mediated Microvascular Recruitment in Human Forearm Muscle. Diabetes 55, 1436. [DOI] [PubMed] [Google Scholar]
- 12.Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, and Barrett EJ (2004). The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes/Metabolism Research and Reviews 20, 3–12. [DOI] [PubMed] [Google Scholar]
- 13.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. (1996). Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. New England Journal of Medicine 334, 292–295. [DOI] [PubMed] [Google Scholar]
- 14.Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, and Yabluchanskiy A (2018). Short-term weight loss reverses obesityinduced microvascular endothelial dysfunction. Geroscience 40, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jongh Renate T, Serné Erik H, G, I.J.R., de Vries G, and Stehouwer Coen DA (2004). Impaired Microvascular Function in Obesity. Circulation 109, 2529–2535. [DOI] [PubMed] [Google Scholar]
- 16.Elias I, Franckhauser S, Ferré T, Vilà L, Tafuro S, Muñoz S, Roca C, Ramos D, Pujol A, Riu E, et al. (2012). Adipose Tissue Overexpression of Vascular Endothelial Growth Factor Protects Against Diet-Induced Obesity and Insulin Resistance. Diabetes 61, 1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong LG, Young SG, Beigneux AP, Bensadoun A, Oberer M, Jiang H, and Ploug M (2016). GPIHBP1 and Plasma Triglyceride Metabolism. Trends Endocrinol Metab 27, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavin TP, Stallings HW, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, Pofahl WE, and Hickner RC (2005). Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. Journal of Applied Physiology 98, 315–321. [DOI] [PubMed] [Google Scholar]
- 19.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran K-V, Straubhaar J, Nicoloro S, Czech Michael P, et al. (2011). Depot-Specific Differences and Insufficient Subcutaneous Adipose Tissue Angiogenesis in Human Obesity. Circulation 123, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgescu A, Popov D, Constantin A, Nemecz M, Alexandru N, Cochior D, and Tudor A (2011). Dysfunction of human subcutaneous fat arterioles in obesity alone or obesity associated with Type 2 diabetes. Clinical Science 120, 463. [DOI] [PubMed] [Google Scholar]
- 21.Gogg S, Nerstedt A, Boren J, and Smith U (2019). Human adipose tissue microvascular endothelial cells secrete PPARγ ligands and regulate adipose tissue lipid uptake. JCI Insight 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granger DN, Richardson PDI, Kvietys PR, and Mortillaro NA (1980). Intestinal blood flow. Gastroenterology 78, 837–863. [PubMed] [Google Scholar]
- 23.Graupera M, and Claret M (2018). Endothelial Cells: New Players in Obesity and Related Metabolic Disorders. Trends Endocrinol Metab 29, 781–794. [DOI] [PubMed] [Google Scholar]
- 24.Guyton JR, and Klemp KF (1992). Early extracellular and cellular lipid deposits in aorta of cholesterol-fed rabbits. The American journal of pathology 141, 925–936. [PMC free article] [PubMed] [Google Scholar]
- 25.Hagberg C, Mehlem A, Falkevall A, Muhl L, and Eriksson U (2013). Endothelial fatty acid transport: role of vascular endothelial growth factor B. Physiology (Bethesda, Md) 28, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, et al. (2010). Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464, 917–921. [DOI] [PubMed] [Google Scholar]
- 27.Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsäter H, Scotney P, Nyqvist D, Samén E, Lu L, et al. (2012). Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 490, 426. [DOI] [PubMed] [Google Scholar]
- 28.Harvey KA, Walker CL, Pavlina TM, Xu Z, Zaloga GP, and Siddiqui RA (2010). Long-chain saturated fatty acids induce pro-inflammatory responses and impact endothelial cell growth. Clinical Nutrition 29, 492–500. [DOI] [PubMed] [Google Scholar]
- 29.Hwangbo C, Wu J, Papangeli I, Adachi T, Sharma B, Park S, Zhao L, Ju H, Go G. w., Cui G, et al. (2017). Endothelial APLNR regulates tissue fatty acid uptake and is essential for apelin’s glucose-lowering effects. Science Translational Medicine 9, eaad4000.* This manuscript identifies Apelin as a novel adipose paracrine regulator of endothelial fatty acid uptake and transport, and links it to effects on the metabolic syndrome.
- 30.Ibrahim A, and Arany Z (2017). Does Endothelium Buffer Fat? Circulation Research 120, 1219–1221. [DOI] [PubMed] [Google Scholar]
- 31.Ignarro LJ, Buga GM, Wood KS, Byrns RE, and Chaudhuri G (1987). Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences 84, 9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jabs M, Rose Adam J, Lehmann Lorenz H, Taylor J, Moll I, Sijmonsma Tjeerd P, Herberich Stefanie E, Sauer Sven W, Poschet G, Federico G, et al. (2018). Inhibition of Endothelial Notch Signaling Impairs Fatty Acid Transport and Leads to Metabolic and Vascular Remodeling of the Adult Heart. Circulation 137, 2592–2608.** The authors demonstrate that the Notch pathway, a well-established inhibitor of endothelial migration and angiogenesis, also regulates a number of key mediators of fatty acid handling and transport in endothelial cells.
- 33.Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, et al. (2016). A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nature medicine 22, 421–426.** This manuscript identifies a novel metabolite, derived from branched chain amino acids, and secreted from muscle fibers, that regulates in a paracrine fashion trans-endothelial delivery of fatty acids to the underlying parenchyma
- 34.Jia Z, Pei Z, Maiguel D, Toomer CJ, and Watkins PA (2007). The Fatty Acid Transport Protein (FATP) Family: Very Long Chain Acyl-CoA Synthetases or Solute Carriers? Journal of Molecular Neuroscience 33, 25–31. [DOI] [PubMed] [Google Scholar]
- 35.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, and Gonzalez FJ (2011). Disruption of Hypoxia-Inducible Factor 1 in Adipocytes Improves Insulin Sensitivity and Decreases Adiposity in High-Fat Diet–Fed Mice. Diabetes 60, 2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, and Plutzky J (2009). PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. The Journal of clinical investigation 119, 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konishi M, Sakaguchi M, Lockhart SM, Cai W, Li ME, Homan EP, Rask-Madsen C, and Kahn CR (2017). Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proceedings of the National Academy of Sciences of the United States of America 114, E8478–E8487.* Here, the authors show that modulating insulin receptor in endothelial cells can impact satiety, demonstrating a link between endothelium and food intake.
- 38.Kuo A, Lee MY, and Sessa WC (2017). Lipid Droplet Biogenesis and Function in the Endothelium. Circulation research 120, 1289–1297.** This manuscript demonstrates the surprising observation that fatty acids transported across the endothelium can flow through the endothelial triglyceride pool, and do so quite extensively after a fat-rich meal.
- 39.Lundman P, Eriksson M, Schenck-Gustafsson K, Karpe F, and Tornvall P (1997). Transient Triglyceridemia Decreases Vascular Reactivity in Young, Healthy Men Without Risk Factors for Coronary Heart Disease. Circulation 96, 3266–3268. [DOI] [PubMed] [Google Scholar]
- 40.McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, and Schroeder F (1999). Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res 40, 1371–1383. [PubMed] [Google Scholar]
- 41.Milger K, Herrmann T, Becker C, Gotthardt D, Zickwolf J, Ehehalt R, Watkins PA, Stremmel W, and Füllekrug J (2006). Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. Journal of Cell Science 119, 4678. [DOI] [PubMed] [Google Scholar]
- 42.Norryd C, Denker H, Lunderquist A, Olin T, and Tylen U (1975). Superior mesenteric blood flow during digestion in man. Acta chirurgica Scandinavica 141, 197–202. [PubMed] [Google Scholar]
- 43.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, et al. (2003). Association of Hypoadiponectinemia With Impaired Vasoreactivity. Hypertension 42, 231–234. [DOI] [PubMed] [Google Scholar]
- 44.Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, and Scalia R (2007). Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. The Journal of clinical investigation 117, 1718–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer RMJ, Ferrige AG, and Moncada S (1987). Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526. [DOI] [PubMed] [Google Scholar]
- 46.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, and Smith SR (2009). Reduced Adipose Tissue Oxygenation in Human Obesity. Diabetes 58, 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robciuc MR, Kivelä R, Williams IM, de Boer JF, van Dijk TH, Elamaa H, Tigistu-Sahle F, Molotkov D, Leppänen V-M, Käkelä R, et al. (2016). VEGFB/VEGFR1Induced Expansion of Adipose Vasculature Counteracts Obesity and Related Metabolic Complications. Cell metabolism 23, 712–724.* This recent paper continues to provide support for the notion that activation of angiogenesis in adipose tissue can promote beiging of the tissue, and obesity-protecting thermogenesis
- 48.Rudnicki M, Abdifarkosh G, Nwadozi E, Ramos SV, Makki A, Sepa-Kishi DM, Ceddia RB, Perry CG, Roudier E, and Haas TL (2018). Endothelial-specific FoxO1 depletion prevents obesity-related disorders by increasing vascular metabolism and growth. eLife 7, e24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rupnick MA, Panigrahy D, Zhang C-Y, Dallabrida SM, Lowell BB, Langer R, and Folkman MJ (2002). Adipose tissue mass can be regulated through the vasculature. Proceedings of the National Academy of Sciences of the United States of America 99, 10730–10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sansbury BE, Cummins TD, Tang Y, Hellmann J, Holden CR, Harbeson MA, Chen Y, Patel RP, Spite M, Bhatnagar A, et al. (2012). Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circulation research 111, 1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seki T, Hosaka K, Fischer C, Lim S, Andersson P, Abe M, Iwamoto H, Gao Y, Wang X, Fong G-H, et al. (2018). Ablation of endothelial VEGFR1 improves metabolic dysfunction by inducing adipose tissue browning. The Journal of experimental medicine 215, 611–626.* This recent paper continues to provide support for the notion that activation of angiogenesis in adipose tissue can promote beiging of the tissue, and obesity-protecting thermogenesis
- 52.Sierra-Honigmann M.R.o., Nath AK, Murakami C, García -Cardeña G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, et al. (1998). Biological Action of Leptin as an Angiogenic Factor. Science 281, 1683. [DOI] [PubMed] [Google Scholar]
- 53.Sima A (2002). Experimental atheroma formation in the coronary artery of the hyperlipemic hamster. Journal of Cellular and Molecular Medicine 6, 276–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Son N-H, Basu D, Samovski D, Pietka TA, Peche VS, Willecke F, Fang X, Yu S-Q, Scerbo D, Chang HR, et al. (2018). Endothelial cell CD36 optimizes tissue fatty acid uptake. The Journal of Clinical Investigation 128, 4329–4342.** This manuscript provides conclusive evidence that CD36 in endothelium is absolutely required for efficient delivery of fatty acids to organs, in particular the heart
- 55.Sonnenburg WK, Yu D, Lee EC, Xiong W, Gololobov G, Key B, Gay J, Wilganowski N, Hu Y, Zhao S, et al. (2009). GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4. J Lipid Res 50, 2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun K, Asterholm IW, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, and Scherer PE (2012). Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proceedings of the National Academy of Sciences 109, 5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun K, Halberg N, Khan M, Magalang UJ, and Scherer PE (2013). Selective Inhibition of Hypoxia-Inducible Factor 1α Ameliorates Adipose Tissue Dysfunction. Mol Cell Biol 33, 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun K, Kusminski CM, Luby-Phelps K, Spurgin SB, An YA, Wang QA, Holland WL, and Scherer PE (2014). Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Molecular Metabolism 3, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sung H-K, Doh K-O, Son JE, Park JG, Bae Y, Choi S, Nelson SML, Cowling R, Nagy K, Michael IP, et al. (2013). Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell metabolism 17, 61–72. [DOI] [PubMed] [Google Scholar]
- 60.Trayhurn P (2013). Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiological Reviews 93, 1–21. [DOI] [PubMed] [Google Scholar]
- 61.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, et al. (2006). TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. The Journal of clinical investigation 116, 2791–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Guilder GP, Hoetzer Gl Fau - Dengel DR, Dengel Dr Fau - Stauffer BL, Stauffer Bl Fau - DeSouza CA, and DeSouza CA (2006). Impaired endothelium-dependent vasodilation in normotensive and normoglycemic obese adult humans. J Cardiovasc Pharmacol 47, 0160–2446 (Print). [DOI] [PubMed] [Google Scholar]
- 63.Villaret A, Galitzky J, Decaunes P, Estève D, Marques M-A, Sengenès C, Chiotasso P, Tchkonia T, Lafontan M, Kirkland JL, et al. (2010). Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes 59, 2755–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogel RA, Corretti MC, and Plotnick GD (1997). Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 79, 350–354. [DOI] [PubMed] [Google Scholar]
- 65.Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark ADH, and Clark MG (2002). Insulin-Mediated Hemodynamic Changes Are Impaired in Muscle of Zucker Obese Rats. Diabetes 51, 3492. [DOI] [PubMed] [Google Scholar]
- 66.Wilmink HW, Banga JD, Hijmering M, Erkelens WD, Stroes ESG, and Rabelink TJ (1999). Effect of angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor antagonism on postprandial endothelial function. Journal of the American College of Cardiology 34, 140–145. [DOI] [PubMed] [Google Scholar]
- 67.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. (2002). Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine 8, 1288–1295. [DOI] [PubMed] [Google Scholar]
- 68.Zhang F, Zarkada G, Han J, Li J, Dubrac A, Ola R, Genet G, Boyé K, Michon P, Künzel SE, et al. (2018). Lacteal junction zippering protects against diet-induced obesity. Science 361, 599.** This manuscript demonstrates the surprising observation that endothelium in the gut regulates lacteal homeostasis and uptake of fatty acids via chylomicrons