Abstract
Study objective:
Regional, coordinated care for time-sensitive and high-risk medical conditions is a priority in the United States. A necessary precursor to coordinated regional care is regions that are actionable from clinical and policy standpoints. The Dartmouth Atlas of Health Care, the major health care referral construct in the United States, uses regions that cross state and county boundaries, limiting fiscal or political ownership by key governmental stakeholders in positions to create incentive and regulate regional care coordination. Our objective is to develop and evaluate referral regions that define care patterns for patients with acute myocardial infraction, acute stroke, or trauma, yet also preserve essential political boundaries.
Methods:
We developed a novel set of acute care referral regions using Medicare data in the United States from 2011. For acute myocardial infraction, acute stroke, or trauma, we iteratively aggregated counties according to patient home location and treating hospital address, using a spatial algorithm. We evaluated referral political boundary preservation and spatial accuracy for each set of referral regions.
Results:
The new set of referral regions, the Pittsburgh Atlas, had 326 distinct regions. These referral regions did not cross any county or state borders, whereas 43.1% and 98.1% of all Dartmouth Atlas hospital referral regions crossed county and state borders. The Pittsburgh Atlas was comparable to the Dartmouth Atlas in measures of spatial accuracy and identified larger at-risk populations for all 3 conditions.
Conclusion:
A novel and straightforward spatial algorithm generated referral regions that were politically actionable and accountable for time-sensitive medical emergencies.
INTRODUCTION
Regionalized care is a system-level public health strategy in which patients are routed to specific hospitals according to diagnosis, illness severity, and hospital capability.1,2 For time-sensitive conditions such as acute myocardial infarction,3,4 acute stroke,5 and major trauma,6,7 regionalization has the potential to save lives by concentrating complex care in high-volume, high-quality centers. More than 10 years ago, a National Academy of Medicine report endorsed coordinated, regional, accountable systems as an approach to improve health care quality for severe acute conditions related to trauma and emergency care.8 Despite this recommendation, implementation efforts face barriers, including a lack of accountable geographic referral regions to organize or evaluate these systems of care.
The Dartmouth Atlas of Health Care, a set of geographic regions based on Medicare and Medicaid hospital discharge claims, is used epidemiologically for comparing the cost,9 quality,10 and consumption11–13 of health care in different parts of the country. Unfortunately, the hospital referral regions of the Dartmouth Atlas are not actionable from either fiscal or political standpoints, with almost all regions crossing county and state borders,14 and therefore lack accountability. Consequently, Dartmouth Atlas regions do not engender ownership, do not have clear stakeholders, and are not well suited for organizing or evaluating regionalized care.
We sought to develop actionable referral regions for 3 time-sensitive acute care emergencies—acute myocardial infarction, acute stroke, and trauma—and then compare these regions with the Dartmouth Atlas. We hypothesized that a set of accountable referral regions for time-sensitive medical emergencies could be developed with an iterative data-driven spatial algorithm.
MATERIALS AND METHODS
Data Collection and Processing and Selection of Participants
We performed a geospatial analysis using data from the Medicare Provider Analysis and Review (MedPAR) file from 2011. MedPAR contains inpatient hospital records for all fee-for-service Medicare beneficiaries. We identified patients with acute myocardial infarction, acute stroke, and trauma, using previously validated International Classification of Diseases, Ninth Revision, Clinical Modification codes (see Appendix E1, available online at http://www.annemergmed.com).15–17 We chose these conditions because they are time-sensitive acute care emergencies that require coordination between out-of-hospital providers, emergency departments, specialist teams, and special hospital resources. These conditions are also subject to ongoing regionalization efforts in which patients are transported to specific centers according to patient condition and hospital capabilities. We elected to pool all 3 conditions, rather than create separate referral regions for each condition, because condition-specific referral regions would be more difficult to implement and would emphasize silos of care delivery rather than coordination. To construct regions, we used maps of US ZIP codes, counties, and state boundaries for 2011, obtained from the United States Census Bureau,18 and a map of Dartmouth Atlas hospital referral regions14 for comparison.
Details about the development of candidate sets of referral regions are provided in Appendix E1, available online at http://www.annemergmed.com. Briefly, we developed candidate sets of referral regions by aggregating counties in the United States, using patient home locations and treating hospital locations. We used counties as unit of aggregation because they are accountable political and fiscal units (unlike individual or groups of ZIP codes), and they frequently coordinate local public health activities. The process involved 3 sequential steps. First, we calculated the frequency and location of hospital referrals (ie, where patients were treated) for each residential county (ie, where patients live) in the United States. Second, we joined each residential county to the most frequent hospital referral county, allowing for multiple residential counties to join to a single hospital referral county. Third, in accordance with a decreasing threshold of relatedness, we combined the aggregated groups of counties from the second step. This process resulted in progressively smaller overall numbers of referral regions. We performed these steps twice: with and without a restriction that regions could cross state lines. This process resulted in 6 total candidate sets of regions.
Primary Data Analysis
We compared our 6 candidate sets of regions to one another and the Dartmouth Atlas Hospital Referral Regions, using a conceptual framework, which holds that useful hospital referral regions should delineate where patients live and receive their health care, and that valid hospital referral regions should foster collaboration between hospitals, physician organizations, and public health agencies.8 To evaluate the potential utility of each set of regions and the Dartmouth Atlas, we used separate measures of accountability and accuracy. For a region to engender accountability from stakeholders, it should not intersect political boundaries because this creates fragmented constituencies with potentially unclaimed political ownership. As a fundamental construct, a region must also accurately define where patients live and receive care.
To assess accountability, we summarized the number of referral regions that intersected county or state boundaries for each set of referral region builds and for the Dartmouth Atlas.
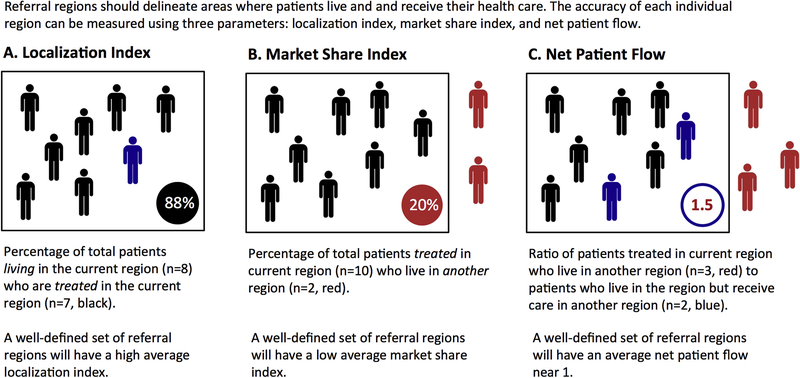
To assess accuracy, we evaluated each set of referral region builds using 3 spatial statistics measures: localization index, market share index, and net patient flow (Figure 1). The localization index is the percentage of total patients living in a region who are also treated in that region. The market share index is the percentage of patients treated in a region who live in another region. The net patient flow is the ratio of patients who are treated in the current region and live in another region to patients who live in the current region but receive care in another region. A highly accurate set of regions would have high localization indices, low market share indices, and net patient flows close to 1. We performed these calculations at the region level both overall and for each time-sensitive emergency separately. We performed quantile regression testing to compare the median accuracy between the Pittsburgh Atlas and the Dartmouth Atlas. We reported magnitude differences and 95% confidence intervals.
Figure 1.
Measures of spatial accuracy. Geographic accuracy measures: localization index (A), market share index (B), and net patient flow (C). These measures evaluate the accuracy of region constructions, as they relate to where patients live and receive their acute care.
From the 6 candidate sets of regions, we selected the best build based on location accuracy, political boundary preservation, and infrastructure. Then, in a final step, we evaluated the selected build for sparsely populated regions, and then aggregated those regions with adjacent referral regions, based on a distance-location rule. The completion of the selection process resulted in a final set of acute care referral regions, designated the Pittsburgh Atlas.
We did not prespecify a target number of referral regions for the final Pittsburgh Atlas. We expected that creating actionable regions could require a trade-off between epidemiologic accuracy and actionable political utility.
We performed one common application of referral regions through an accessibility analysis. This type of analysis is used to highlight geographic gaps in access, a particularly important application for time-sensitive emergencies.
We identified individual referral regions that did not have referral centers, for both the Pittsburgh Atlas and the Dartmouth Atlas. We defined potential referral centers as high-volume hospitals, using 2011 MedPAR discharge volume for acute myocardial infarction and acute stroke, and as Level I or Level II trauma centers, using the American College of Surgeons hospital designations. We calculated thresholds for acute myocardial infarction19 and acute stroke20 based on reported volume-outcome relationships, adjusted for the age mix of patients included in MedPAR. We classified hospitals as high volume for acute myocardial infarction if they reported greater than 109 annual cases and high volume for acute stroke if they reported more than 198 annual cases. We compared the frequencies of regions without candidate referral centers between the Pittsburgh Atlas and the Dartmouth Atlas by using χ2 statistics. We reported the magnitude of difference and 95% confidence intervals.
The University of Pittsburgh Institutional Review Board classified this study as having exempt status.
RESULTS
We identified and geographically located 730,708 total patients from 2011 MedPAR records: 311,997 with acute myocardial infarction, 316,109 with acute stroke, and 106,797 with moderate or major trauma (Table 1). There were 4,195 patients with more than one reference condition. Using these patient records, we successfully built 6 sets of candidate acute care referral regions, using an iterative spatial algorithm (Table E1A and B, available online at http://www.annemergmed.com). From these candidate region sets, we selected the best-performing set (see Appendix E1, available online at http://www.annemergmed.com) and then further improved it to account for sparsely populated areas and spatial contiguousness. After we performed these steps, the final region build had 326 regions. We designated this set of regions the Pittsburgh Atlas.
Table 1.
Characteristics of Medicare admissions for acute myocardial infarction, stroke and trauma in 2011.
| Characteristic | Acute Myocardial Infarction (n=311,997) |
Stroke (n=316,109) |
Trauma (n=106,797) |
|---|---|---|---|
| Age, median (25%–75% IQR), y | 76 (68–84) | 79 (71–86) | 80 (71–86) |
| Female, No. (%) | 147,463 (47.3) | 181,189 (57.3) | 55,294 (51.8) |
| Race, No. (%) | |||
| White | 265,404 (85.1) | 253,684 (80.3) | 93,699 (87.7) |
| Black | 29,986 (9.6) | 44,246 (14.0) | 6,466 (6.1) |
| Hispanic | 6,009 (1.9) | 6,430 (2.0) | 2,178 (2.0) |
| Other | 5,818 (1.9) | 5,792 (1.8) | 2,088 (2.0) |
| Asian | 3,777 (1.2) | 5,048 (1.6) | 2,023 (1.9) |
| Unknown | 1,003 (0.3) | 909 (0.3) | 343 (0.3) |
| Select comorbidities, No. (%) | |||
| Congestive heart failure | 114,337 (36.6) | 45,989 (14.5) | 12,739 (11.9) |
| Diabetes | 95,231 (30.5) | 89,745 (28.4) | 20,996 (19.7) |
| Renal disease | 66,632 (21.4) | 41,086 (13.0) | 10,272 (9.6) |
| Chronic pulmonary disease | 59,208 (19.0) | 39,534 (12.5) | 12,412 (11.6) |
| Peripheral vascular disease | 20,432 (6.5) | 14,143 (4.5) | 3,197 (3.0) |
| Cancer | 12,035 (3.9) | 13,293 (4.2) | 4,471 (4.2) |
| Region, No. (%) | |||
| South | 124,769 (40.0) | 62,038 (19.6) | 22,289 (20.9) |
| Midwest | 75,408 (24.2) | 73,267 (23.2) | 24,717 (23.1) |
| Northeast | 63,669 (20.4) | 128,714 (40.7) | 40,646 (38.1) |
| West | 48,151 (15.4) | 52,090 (16.5) | 19,145 (17.9) |
IQR, Interquartile range.
Pittsburgh Atlas referral regions did not cross any state or county borders (Figure 2 and Table E2, available online at http://www.annemergmed.com), whereas 132 (43.1%) of the Dartmouth Atlas hospital referral regions crossed state borders and 302 (98.7%) crossed county boundaries.
Figure 2.
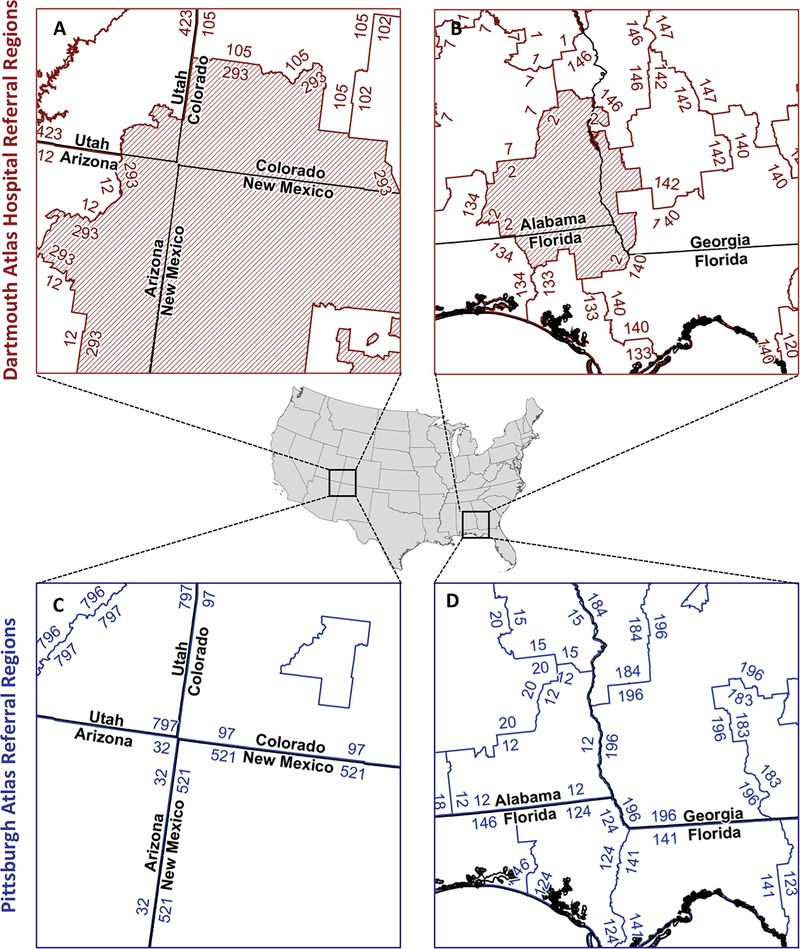
Political boundary preservation by referral region type. A and B, Lack of state boundary preservation in Dartmouth Atlas hospital referral regions. A, Referral region 293 (red hash marks) is shown crossing into Utah, Arizona, Colorado, and New Mexico. B, Referral region 2 (red hash marks) is shown crossing into Alabama, Florida, and Georgia. Overall, state boundaries are crossed by 43.1% (n=132) of Dartmouth Atlas hospital referral regions. Black lines show state borders and red lines show referral region boundaries. C and D, Corresponding areas for the Pittsburgh Atlas, which preserves state boundaries. Black lines show state borders and blue lines show referral region boundaries. All panels are shown at 1:2,000,000 scale in Equal Area Albers projection.
Both the Pittsburgh Atlas and Dartmouth Atlas had good overall spatial accuracy, as measured by localization index (83.6% and 85.6%), market share index (14.3% and 13.4%), and net patient flow (0.88 and 0.97) (Table 2).Localization accuracy was statistically higher for the Dartmouth Atlas overall and for stroke, although the magnitude of difference was small (1.9% overall and 2.1% for stroke). Results were generally similar for each separate time-sensitive condition comparison.
Table 2.
Spatial accuracy.
| Pittsburgh Atlas (n=326) |
Dartmouth Atlas (n=306) |
Median Difference (95% CI) |
|
|---|---|---|---|
| Localization index, % | |||
| Overall percentage | |||
| Median | 83.6 | 85.5 | −1.9 (−3.5 to −0.3)* |
| 25%–75% IQR | 75.3 to 89.0 | 80.7 to 88.9 | |
| Range | 27.3 to 96.2 | 53.2 to 96.0 | |
| Acute myocardial infarction | |||
| Median | 84.7 | 85.9 | −1.2 (−2.7 to 0.2) |
| 25%–75% IQR | 77.6 to 89.6 | 81.4 to 89.9 | |
| Range | 7.8 to 97.3 | 51.9 to 96.1 | |
| Stroke | |||
| Median | 84.9 | 86.9 | −2.1 (−3.7 to −0.4)* |
| 25%–75% IQR | 76.3 to 89.9 | 81.0 to 90.0 | |
| Range | 39.6 to 97.2 | 48.3 to 96.2 | |
| Trauma | |||
| Median | 79.6 | 82.2 | −2.6 (−5.4 to 0.15) |
| 25%–75% IQR | 62.5 to 87.1 | 72.6 to 87.4 | |
| Range | 11.3 to 95.8 | 18.6 to 95.7 | |
| Market share index | |||
| Overall | |||
| Median | 14.3 | 13.4 | 0.83 (–0.7 to 2.3) |
| 25%–75% IQR | 10.2 to 20.4 | 9.9 to 20.1 | |
| Range | 2.8 to 57.0 | 4.6 to 53.4 | |
| Acute myocardial infarction | |||
| Median | 14.1 | 13.3 | 0.7 (–0.7 to 2.2) |
| 25%–75% IQR | 9.6 to 21.1 | 9.2 to 20.7 | |
| Range | 2.8 to 64.5 | 3.5 to 62.5 | |
| Stroke | |||
| Median | 12.6 | 12.0 | 0.6 (−0.8 to 2.0) |
| 25%–75% IQR | 9.0 to 18.3 | 8.7 to 17.9 | |
| Range | 1.8 to 56.1 | 3.3 to 51.8 | |
| Trauma | |||
| Median | 16.7 | 17.5 | −0.8 (−2.6 to 1.00) |
| 25%–75% IQR | 12.0 to 25.9 | 12.7 to 24.7 | |
| Range | 0.0 to 64.4 | 2.1 to 69.8 | |
| Net patient flow | |||
| Overall | |||
| Median | 0.88 | 0.97 | −0.08 (−0.21 to 0.05) |
| 25%–75% IQR | 0.52 to 1.48 | 0.61 to 1.45 | |
| Range | 0.02 to 10.35 | 0.20 to 7.83 | |
| Acute myocardial infarction | |||
| Median | 0.97 | 0.97 | 0 (−0.14 to 0.14) |
| 25%–75% IQR | 0.52 to 1.65 | 0.62 to 1.62 | |
| Range | 0.02 to 13.36 | 0.10 to 15.72 | |
| Stroke | |||
| Median | 0.86 | 0.92 | −0.06 (−0.18 to 0.05) |
| 25%–75% IQR | 0.51 to 1.45 | 0.63 to 1.37 | |
| Range | 0.02 to 11.29 | 0.14 to 10.77 | |
| Trauma | |||
| Median | 0.81 | 0.95 | −0.13 (−0.28 to 0.02) |
| 25%–75% IQR | 0.33 to 1.56 | 0.52 to 1.57 | |
| Range | 0 to 10.86 | 0.02 to 9.17 |
CI, Confidence interval.
P<.05.
The Pittsburgh Atlas identified more individual regions without a high-volume acute myocardial infarction hospital (6.4% versus 2.0%), high-volume stroke hospital (51.5% versus 40.9%), and a Level I or II trauma center (41.1% versus 27.1%) compared with the Dartmouth Atlas (Table 3). The Pittsburgh Atlas also identified larger potentially at-risk populations for acute myocardial infarction (3,632,673 versus 1,451,098), stroke (60,955,347 versus 57,116,929), and trauma (42,542,356 versus 17,729,481), defined as persons living in a referral region that did not contain a referral center (Figures E1 to E3, available online at http://www.annemergmed.com).
Table 3.
Referral hospital accessibility analysis.
| Pittsburgh Atlas (n=326) |
Dartmouth Atlas (n=306) |
Difference (95% CI) |
|
|---|---|---|---|
| No high-volume acute myocardial infarction hospitals | |||
| Number of regions (%) | 21 (6.4) | 6 (2.0) | 4.4 (7.6–0)* |
| Population at risk, n | 3,632,673 | 1,451,098 | |
| Localization index, median (IQR) | 73.4 (39.4–79.0) | 75.0 (57.5–89.4) | |
| No high-volume stroke hospitals | |||
| Number of regions (%) | 168 (51.5) | 125 (40.8) | 10.7 (18.4–0)* |
| Population at risk, n | 60,955,347 | 57,116,929 | |
| Localization index, median (IQR) | 78.7 (72.2–86.0) | 83.9 (79.3–88.1) | |
| No Level I or II trauma centers | |||
| Number of regions (%) | 134 (41.1) | 83 (27.1) | 14.0 (21.3–0.1)* |
| Population at risk, n | 42,542,356 | 17,729,481 | |
| Localization index, median (IQR) | 63.8 (45.3–77.0) | 70.1 (56.2–84.3) |
P<.05.
The Pittsburgh Atlas referral regions are available for download as a geographic information system shapefile or crosswalk from http://ccm.pitt.edu/files/pittsburgh_atlas.zip.
LIMITATIONS
Our analysis has several limitations. First, we used fee-for-service Medicare to develop the acute care referral regions. Although this is the largest collective source of discharges for acute myocardial infarction, acute stroke, and major trauma in the United States, the database is primarily limited to patients aged 65 years and older, and therefore may not represent referral patterns for all patients. However, because acute care for these conditions is frequently initiated in the out-of-hospital setting and routed by emergency providers,21 we do not expect that patterns of referral would vary substantially for younger and non-Medicare-insured patients. Second, we developed the regions by using data from 2011. In the intercurrent years, referral patterns may have changed. We propose a process in which the Pittsburgh Atlas would be updated at regular intervals, possibly every 4 to 5 years. This consideration was a part of our final spatial algorithm, which stressed mechanistic simplicity over black-box calculations or more complex aggregation rules. Third, our nontrauma definition of referral center was based on annual discharge volumes for acute myocardial infarction and acute stroke rather than absolute quality. Although we expect that there are many high-performing low-volume hospitals in the United States, we intentionally used a simple definition of high-volume center for these conditions to highlight variation across regions.
DISCUSSION
Using national Medicare hospitalizations for acute myocardial infarction, acute stroke, and major trauma, we successfully developed and evaluated novel referral regions for acute care in the United States. The Pittsburgh Atlas preserved state and county boundaries and performed similarly to the Dartmouth Atlas in terms of location accuracy. Calls for regionalized care of time-sensitive emergencies consistently state the importance of approaches that foster local health department collaborations,22 a core component that cannot be attained with collections of fragmented counties and states. Relatedly, these systems must be designed with accountable regional performance measures in mind—again, a feature that is not possible when referral regions extensively cross state and county lines. Last, the Pittsburgh Atlas, although developed with advanced geospatial tools, is itself quite simple: it is a collection of groups of counties where people live and receive their care for 3 representative time-sensitive medical conditions. The Pittsburgh Atlas therefore succeeds in being usable, meaningful, and understandable.
The Pittsburgh Atlas conceptually builds on an existing local public health organization at the county level, which has defined constituencies and can be held accountable for quality through in-place fiscal and policy levers. Relatedly, these regions are capable of performance improvement, linking measures of quality with efforts to improve outcomes. It is through these complementary mechanisms (ie, having regions that are accountable and regions that can engage in performance improvement based on quality measures) that the Pittsburgh Atlas represents a substantial advancement over the Dartmouth Atlas and a direct response to calls for accountable geographic regions from the National Quality Forum22 and the National Academy of Sciences.8 We were surprised that our regions had spatial accuracy similar to that of the Dartmouth Atlas because we expected there would be a greater trade-off from our applied geographic constraints.
The 2007 Institute of Medicine report Emergency Medical Services: At the Crossroads23 emphasized accountability as a component essential to successful care coordination and regionalization. Similarly, in their 2013 report Regionalized Emergency Medical Care Services: Emergency Department Crowding and Boarding, Healthcare System Preparedness and Surge Capacity—Performance Measurement Gap Analysis and Topic Prioritization, the National Quality Forum22 highlighted the importance of region-level performance measures that “promot[e] timely care for all patients at the population-level, ensuring that patients with time-critical illness receive the highest quality care, and holding hospitals accountable for system-wide performance during a disaster. Holding both hospitals and regions accountable for acute care quality, population health, and emergency management through performance measurement is vital to promoting the cooperation necessary to achieve these goals.” There is precedent for quality measures that hold hospitals accountable for outcomes such as readmission or 30-day mortality, which created incentives for cooperation between facilities and between providers at different stages along the acute care continuum. Geographic regions with shared hospital accountability should promote efforts to coordinate care, move us away from fragmented delivery systems, and improve population outcomes. Strategically defined referral regions that maintain county-level public health structures will facilitate improved outcomes through population health measurement, innovation, and delivery.
The Pittsburgh Atlas has specific relevance for states as well. Recently proposed changes in health care policy at the national level could significantly increase the role of states in providing and evaluating the quality of health care.24 One example of this shift may be through a block grant system for Medicaid payments. States would have more flexibility in determining who qualifies for Medicaid and what services are provided, with coverage and benefits varying from state to state. To the degree that coverage for emergency medical services might vary across states, it is essential that the unit of evaluation for regional care coordination align with state boundaries rather than cross them.
Our analysis also shows that there are many areas of the country without a high-volume referral center for time-sensitive medical conditions, suggesting an important quality gap. Whereas previous work used the hospital as the starting point for measures of geographic access,24–28 our approach offers a complementary alternative perspective, based on patterns of where patients actually seek care (rather than developed from estimates of coverage based on the drive time to a hospital). A more constrained definition that included hospital services and capacity would have likely reduced the number of referral centers even farther. Similarly, the percentage of regions without a Level I or II trauma center was also high, at 41%. Future work should use referral regions to evaluate outcomes as they relate to the presence of referral centers, the organization of hospitals within regions, and measures of region-level market competition. Pittsburgh Atlas referral regions not only facilitate the answer to these and other questions but also provide actionable targets where quality differences are identified.
Using a novel approach, we developed the Pittsburgh Atlas, a set of referral regions for 3 time-sensitive acute care condition in the United States. We developed the atlas with the intention of producing geographic areas with logical stakeholders. The regions have both local fiscal and political relevance, essential components of regions that can be held accountable for quality. Additionally, Pittsburgh Atlas referral regions are logical targets of performance improvement efforts based on quality measures. The regions are an important tool for evaluating the effects of health care policy on regional public health, and will help identify preferred organizational strategies for acute care conditions.
Supplementary Material
Editor’s Capsule Summary.
What is already known on this topic
To improve the quality of emergency and trauma care provided in the United States, we need a regional system.
What question this study addressed
The authors used 2011 Medicare fee-for-service inpatient claims for 3 time-sensitive conditions to identify distinct geographic regions based on where the majority of residents in each county received their care and the preservation of county and state boundaries.
What this study adds to our knowledge
The Pittsburgh Atlas consists of 326 emergency and trauma referral regions for emergency and trauma care that uphold political boundaries.
How this is relevant to clinical practice
The Pittsburgh Atlas provides a framework for the development of an integrated and coordinated emergency and trauma care system in the United States.
Acknowledgments
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. All phases of this study were supported by National Institutes of Health grants K08HL122478 (Dr. Wallace), R35GM119519 (Dr. Seymour), DP2 LM012339 (Dr. Mohan), and K24HL133444 (Dr. Kahn).
The funding source was not involved in the design of the study or the decision to submit the article for publication.
Footnotes
All authors attest to meeting the four ICMJE.org authorship criteria: (1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- 1.Nguyen Y-L, Wunsch H, Angus DC. Critical care: the impact of organization and management on outcomes. Curr Opin Crit Care. 2010;16:487–492. [DOI] [PubMed] [Google Scholar]
- 2.Kahn JM, Linde-Zwirble WT, Wunsch H, et al. Potential value of regionalized intensive care for mechanically ventilated medical patients. Am J Respir Crit Care Med. 2008;177:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuchihashi M, Tsutsui H, Tada H, et al. Volume-outcome relation for hospitals performing angioplasty for acute myocardial infarction: results from the Nationwide Japanese Registry. Circ J. 2004;68:887–891. [DOI] [PubMed] [Google Scholar]
- 4.Vakili BA, Kaplan R, Brown DL. Volume-outcome relation for physicians and hospitals performing angioplasty for acute myocardial infarction in New York state. Circulation. 2001;104:2171–2176. [DOI] [PubMed] [Google Scholar]
- 5.Votruba ME, Cebul RD. Redirecting patients to improve stroke outcomes: implications of a volume-based approach in one urban market. Med Care. 2006;44:1129–1136. [DOI] [PubMed] [Google Scholar]
- 6.Nathens AB, Jurkovich GJ, Maier RV, et al. Relationship between trauma center volume and outcomes. JAMA. 2001;285:1164–1171. [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe D, Bouamra O, Parsons NR, et al. Effect of regional trauma centralization on volume, injury severity and outcomes of injured patients admitted to trauma centres. Br J Surg. 2014;101:959–964. [DOI] [PubMed] [Google Scholar]
- 8.National Academy of Sciences. Hospital-Based Emergency Care: At the Breaking Point. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 9.Robinson JC. Hospital market concentration, pricing, and profitability in orthopedic surgery and interventional cardiology. Am J Manag Care. 2011;17:e241–e248. [PubMed] [Google Scholar]
- 10.Alruwaily AF, Dauw CA, Bierlein MJ, et al. Geographic variation in the quality of secondary prevention for nephrolithiasis. Urology. 2015;86:454–458. [DOI] [PubMed] [Google Scholar]
- 11.Joynt KE, Gawande AA, Orav EJ, et al. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309:2572–2578. [DOI] [PubMed] [Google Scholar]
- 12.Nicholas LH, Langa KM, Iwashyna TJ, et al. Regional variation in the association between advance directives and end-of-life Medicare expenditures. JAMA. 2011;306:1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34:1351–1362. [PMC free article] [PubMed] [Google Scholar]
- 14.Center for the Evaluative Clinical Sciences. The Dartmouth Atlas of Health Care. Chicago, IL: American Hospital Publishing; 1998. [PubMed] [Google Scholar]
- 15.Metcalfe A, Neudam A, Forde S, et al. Case definitions for acute myocardial infarction in administrative databases and their impact on in-hospital mortality rates. Health Serv Res. 2012;48:290–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21:100–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan D, Barnato AE, Rosengart MR, et al. Trauma triage in the emergency departments of nontrauma centers. J Trauma Acute Care Surg. 2013;74:1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Census Bureau. TIGER/Line® with demographic data—geography—US Census Bureau. Available at: http://www.census.gov/geo/maps-data/data/tiger-data.html. Accessed March 6, 2013.
- 19.Tung Y-C, Chang G-M, Chien K-L, et al. The relationships among physician and hospital volume, processes, and outcomes of care for acute myocardial infarction. Med Care. 2014;52:519–527. [DOI] [PubMed] [Google Scholar]
- 20.Hall RE, Fang J, Hodwitz K, et al. Does the volume of ischemic stroke admissions relate to clinical outcomes in the Ontario Stroke System? Circ Cardiovasc Qual Outcomes. 2015;8(6 suppl 3):S141–S147. [DOI] [PubMed] [Google Scholar]
- 21.Tataris K, Kivlehan S, Govindarajan P. National trends in the utilization of emergency medical services for acute myocardial infarction and stroke. West J Emerg Med. 2014;15:744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Quality Forum. Regionalized Emergency Medical Care Services: Emergency Department Crowding and Boarding, Healthcare System Preparedness and Surge Capacity—Performance Measurement Gap Analysis and Topic Prioritization. Washington, DC: National Quality Forum; 2013:1–89. [Google Scholar]
- 23.Institute of Medicine of the National Academies. Emergency Medical Services at the Crossroads. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 24.House of Representatives Committee on Rules. HR-1628. Available at: https://rules.house.gov/bill/115/hr-1628. Accessed July 13, 2017.
- 25.Albright KC, Branas CC, Meyer BC, et al. ACCESS: acute cerebrovascular care in emergency stroke systems. Arch Neurol. 2010;67:1210–1218. [DOI] [PubMed] [Google Scholar]
- 26.Branas CC, MacKenzie EJ, Williams JC, et al. Access to trauma centers in the United States. JAMA. 2005;293:2626–2633. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DJ, Angus DC, Seymour CW, et al. Geographic access to high capability severe acute respiratory failure centers in the United States. PLoS One. 2014;9:e94057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr BG, Branas CC, Metlay JP, et al. Access to emergency care in the United States. Ann Emerg Med. 2009;54:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.