Abstract
Cognitive reserve is inherently a dynamic construct; however, traditional methods of estimating reserve have focused on static proxy variables. A recently proposed psychometric approach entails modeling reserve as residual cognition not explained by demographic and brain variables. In this study, we extended this approach to longitudinal measurement and examined how change in reserve relates to clinical outcomes in late life and influences the effect of brain atrophy on cognitive decline. Results indicated that cognitive reserve changes were associated with progression of clinical diagnosis. More rapid depletion of cognitive reserve was associated with faster decline in non-memory cognitive functions, even after accounting for longitudinal brain atrophy. The effect of longitudinal brain atrophy on cognitive decline differed based on the extent to which an individual’s reserve changed. Whereas depletion of reserve appeared to unmask the effects of brain atrophy on cognitive decline, maintenance of reserve buffered against the negative effects of brain atrophy. Study results highlight that changes in reserve may have important implications for individual differences in cognitive aging trajectories.
Keywords: Aging, cognitive reserve, cognitive decline, MRI, gray matter change, hippocampus
1. Introduction
A core feature of aging is the heterogeneity of cognitive trajectories. Clinically normal older adults and symptomatic older adults with memory impairment both show considerable variability in their cognitive courses, rendering it challenging to identify a single cause or etiology of individual differences in trajectories (Knopman et al. 2015; Nettiksimmons et al. 2014). The notion that cognition is influenced by multiple determinants originates from several lines of research, including recent evidence that canonical measures of brain disease (e.g., total gray matter volume; white matter hyperintensities; hippocampal volume) account for less than 50% of variance in memory performance (Boyle et al. 2013; Reed et al. 2010). In turn, accumulating evidence indicates that 20–40% of non-demented older adults meet neuropathological criteria for Alzheimer’s disease (AD) upon death (O’Brien et al. 2009; Price et al. 2009), suggesting that the presence of AD-related brain pathology alone does not fully account for individual differences in cognitive decline. The concept of ‘cognitive reserve’ has been used to explain this well-documented mismatch between pathology and cognitive performance and has stirred considerable interest in the dynamic interplay between measurable brain pathology and cognitive outcomes over time (Stern 2009).
Cognitive reserve is theorized to be an active process of cognitive adaptation to pathology. Within this framework, people display differential cognitive vulnerability to brain disease at a given time point as a function of their ability to adapt to neurological insults (Stern et al. 2018). Although cognitive reserve is inherently a dynamic construct, traditional methods of inferring reserve have focused on static proxy variables that represent a retrospective summation of early life experiences (e.g., years of education). Numerous conceptual and methodological concerns accompany this approach, including the correlation between demographic proxies and other confounding historical variables (e.g., socioeconomic status), the oversimplification of individual differences in brain disease susceptibility, and the inability of these proxies to change during late life (Jones et al. 2011; Satz et al. 2011; Zahodne et al. 2015). More recently, a psychometric approach to the measurement of reserve has been proposed, wherein cognitive reserve is operationalized as the difference between observed and expected cognitive function as predicted by brain and demographic variables (Reed et al. 2010). Utilizing this psychometric approach results in a residualized reserve index for each individual, with a higher residual reserve index indicating that the individual’s cognitive performance is better than expected when accounting for brain pathology and demographic characteristics.
The residual index approach circumvents many prior methodological concerns, as it provides a more direct estimate of cognitive reserve while minimizing the inherent confounds of historical proxy variables. Perhaps most importantly, the residual index methodology allows for the longitudinal estimation of cognitive reserve. To understand individual variability in aging trajectories and person-specific susceptibility to brain pathology, longitudinal appraisal of cognitive reserve in both asymptomatic and clinical populations is critically needed; however, to our knowledge, application of the residual index approach beyond a cross-sectional framework has been reported by only one study (Zahodne et al. 2015). In the context of their two-time point study design, Zahodne and colleagues reported that dynamic changes in reserve might be a better predictor of future clinical status in initially non-demented older adults than reserve measured at a single time point; however, it remains unclear whether longitudinal change in cognitive reserve across more than two time points moderates or influences the association between brain atrophy and rate of change in cognitive test scores (i.e., slopes).
To extend this important, foundational work, the current study examined dynamic cognitive reserve in a fully longitudinal design using a residual reserve index approach. The overarching goal of the study was to develop longitudinal models of dynamic cognitive reserve in a diverse clinical aging cohort to examine how changes in reserve: a) relate to changes in clinical diagnostic status; b) relate to cognitive decline; and c) interact with changing brain status to influence cognitive decline. To minimize circularity in the definition of our reserve index, measures used to build the cognitive reserve index did not overlap with measures used to define clinical status. To accomplish our aims, we developed a latent variable model predicated on the assumption that changes in reserve, as defined by a memory residual index, influence the relation between changing brain structure and cognitive decline in late life.
2. Materials and Methods
2.1. Participants:
Study participants were from the UC Davis Diversity Cohort, a longitudinal study that includes high numbers of Hispanic (e.g., Latina/o and other Spanish origin), African American, and non-Hispanic White older adults. This cohort is also heterogenous in educational attainment, and their clinical severity levels span a spectrum of cognitive function from normal to mildly demented at baseline assessment. Cohort composition and recruitment methods are described in Hinton et al. (2010). In brief, participants were identified through a community screening program designed to recruit individuals with cognitive functioning representative of the community-dwelling population in a six-county catchment area in the central Sacramento/San Joaquin Valley and east San Francisco Bay Area of Northern California or are referred for research following a clinical evaluation at a university memory/dementia clinic.
Participants in this study were evaluated and followed within the research program of the UC Davis Alzheimer’s Disease Center. A rolling enrollment design, initiated in 2002, was used to build and maintain the cohort. Inclusion criteria for the larger cohort included age 60 or older at their first examination and ability to speak English or Spanish. Exclusion criteria included unstable major medical illness, major primary psychiatric disorder, or substance abuse or dependence in the last five years. All participants signed informed consent, and all human subject involvement was overseen by institutional review boards at UC Davis, the Veterans Administration Northern California Health Care System and San Joaquin General Hospital in Stockton, California.
The current study included 338 participants who had received at least two cognitive evaluations (median=6, range=2–14) and at least two MRI brain scans (median=2, range=2–6). There were 160 non-Hispanic Whites, 85 Hispanics, 76 African Americans, and 17 individuals from other racial and ethnic groups; 41 Hispanics were tested in Spanish, and all others were tested in English. The majority of the sample (91 Whites, 80 Hispanics, 69 African Americans, 15 Other) was recruited through the community screening program. The remaining 82 (68 Whites, 5 Hispanics, 7 African Americans, 2 Other) were recruited from the clinic.
2.2. Clinical Diagnosis:
All participants received multidisciplinary diagnostic evaluations at baseline and at approximately annual intervals following the baseline evaluation. Baseline and follow-up evaluations followed the same protocol with a detailed medical history, physical and neurological exam, and clinical neuropsychological assessment. A physician fluent in Spanish examined subjects who spoke only Spanish. A family member or other informant was interviewed to obtain information about cognitive and independent functioning. Clinical neuropsychological tests were different from the cognitive measures used in analyses in this study to estimate reserve and longitudinal cognitive trajectories. Routine dementia work-up laboratory tests were obtained at the baseline evaluation and when clinically indicated at the time of follow-up evaluations.
Diagnosis of cognitive syndrome (Normal, mild cognitive impairment (MCI), Dementia) and, for individuals with dementia, underlying etiology, was made in a multidisciplinary consensus conference following standardized criteria and methods. Dementia was diagnosed using DSM-III-R (Association 1987) criteria for dementia modified such that dementia could be diagnosed in the absence of memory impairment if there was significant impairment of two or more other cognitive domains. MCI was diagnosed according to standard clinical criteria and was further sub-typed according to current Alzheimer’s Disease Centers Uniform Data Set guidelines (Morris et al. 2006). Normal cognitive function was diagnosed if there was no clinically significant cognitive impairment. All diagnoses were made blind to the neuropsychological tests that were analyzed in this study.
Change of diagnosis from the first to last evaluation was the independent variable in one of our primary analyses. This change variable included the following mutually exclusive groups: normal to normal (Stable Normal, N=126), normal progressing to MCI (Normal to MCI, N=46), normal progressing to dementia (Normal to Dementia, N=31), MCI to MCI (Stable MCI, N=29), MCI progressing to dementia (MCI to Dementia, N=76), and dementia to dementia (Dementia, N=22).
2.3. Cognitive Assessment:
The cognitive outcomes in this study were measures of episodic memory, semantic memory, executive function, and spatial ability derived from the Spanish and English Neuropsychological Assessment Scales (SENAS). The SENAS has undergone extensive development as a battery of cognitive tests relevant to cognitive aging that allow for valid comparisons across racial, ethnic, and linguistic groups (Mungas et al. 2004; Mungas, Reed, Haan, et al. 2005; Mungas et al. 2000; Mungas, Reed, Tomaszewski Farias, et al. 2005; Mungas et al. 2011). See Supplementary Materials for additional details.
2.4. MRI Measures
2.4.1. MRI Volume Measurements:
Brain image acquisition was performed under a standard protocol at the UC Davis Imaging Research Center or at the Veterans Administration Northern California Health System Medical Center in Martinez, CA. MRI baseline measurements were derived using an in-house processing pipeline described previously (Fletcher et al. 2014; Lee et al. 2012). White matter hyperintensities (WMH) were computed by an in-house method combining native FLAIR with structural MRI as described previously (DeCarli et al., 2005).
2.4.2. Gray Matter Volume Change:
We computed longitudinal structural change between the two most widely separated time points. We used a tensor-based morphometry (TBM) method designed to enhance sensitivity and specificity for biological change by incorporating estimates of likely tissue boundaries (Fletcher 2014; Fletcher et al. 2013). TBM generates deformation fields by registering brain scans at differing time points and using these to estimate local volume changes between the scans (Ashburner and Friston 2000). This processing was done via an in-house processing pipeline that has been previously described (Fletcher et al. 2016). Gray matter (GM) volume was computed over a cortical GM region of interest (ROI) that averaged volume change over frontal, parietal, temporal (excluding hippocampus), and occipital lobar regions. These were the same ROIs used for measuring baseline cortical gray matter. Log-Jacobians from these ROIs from both hemispheres were averaged to constitute a global cortical gray matter change measure. Longitudinal change over these regions was computed as the mean log-Jacobian over the ROI intersected with the segmented GM. Cortical gray matter change defined in this manner had the strongest effect on cognitive decline in a previous study based on this cohort (Fletcher et al. 2018). Change in a hippocampus ROI was separately measured using these same methods.
2.5. Data Analysis
2.5.1. Measures and Data Processing:
SENAS measures of episodic memory, semantic memory, executive function, and spatial ability were the primary dependent variables. Independent variables included: MRI gray matter volume change (average of frontal, temporal, parietal, and occipital ROIs); hippocampal volume change (average of left and right); MRI baseline cortical gray matter, hippocampus, white matter hyperintensity (WMH), and intracranial volumes; and the demographic variables race/ethnicity, education, gender, and language of test administration. We applied a rank-based inverse normal transformation (Blom 1958) to normalize the variables and establish a common standardized scale (M = 0, SD = 1). Education was centered at 12 years. Gender, race/ethnicity, and language of test administration were categorical covariates coded using indicator variables. Race/ethnicity was coded using three indicator variables: African American (1=yes, 0 =no), Hispanic (1=yes, 0=no), and Other minority (1=yes, 0=no); non-Hispanic White was the reference group. Gender (male=1, female=0) and language of test administration (Spanish=1, English=0) were represented by single indicator variables. This coding establishes a White female, with 12 years of education, tested in English, as a reference.
Change of diagnosis from the first to last evaluation was the independent variable in one of our primary analyses. The six groups of interest were represented by five indicator variables. The Stable Normal group was the reference group. There were a small number of individuals who reverted from MCI to Normal (N=8) who were not included in this analysis.
2.5.2. Longitudinal Modeling of Cognitive Trajectories:
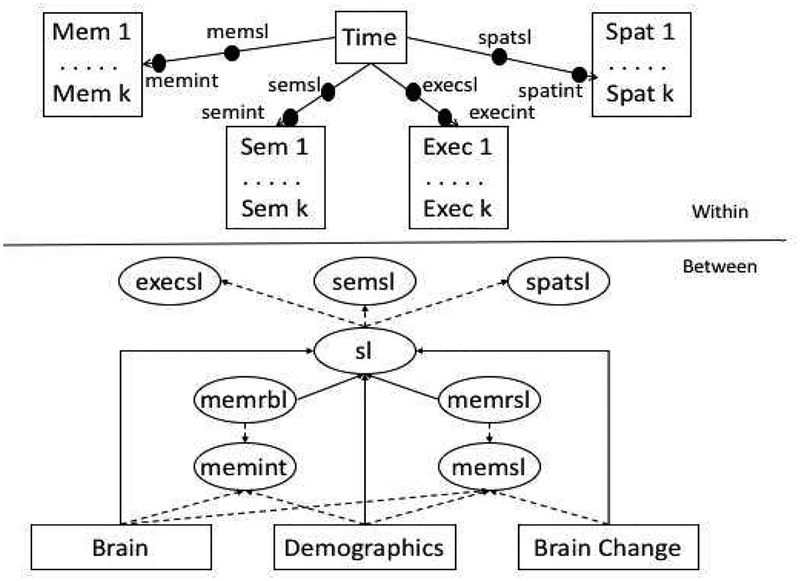
Mixed-effects, parallel process longitudinal analyses were performed using Mplus version 8.1 multilevel modeling platform (Muthén and Muthén 1998). Figure 1 shows a schematic of the basic modeling approach. Within each person’s longitudinal observations, each of the four cognitive outcomes was regressed on time in years since the first MRI scan. The Within-Subjects part of the model included terms to account for practice effects and a practice effect by Spanish test administration interaction that has been identified in previous studies with this sample (Early et al. 2013; Brewster et al. 2014; Melrose et al. 2015). Random intercepts and slopes estimated in the Within-Subjects part of the model served as dependent variables in the Between-Subjects part of the model. All parameters in the model, including Within and Between components, were estimated simultaneously. The multilevel modeling platform allows for heterogeneity in the number of assessment time points and in the lags between assessments across persons.
Figure 1.
Longitudinal Reserve Model.
Episodic Memory intercept (memint) and slope (memsl) random effects were used to measure baseline cognitive reserve and longitudinal change in reserve. Baseline reserve (memrbl) was estimated as a latent variable that captured residual variance in memint that was not explained by demographic variables (race/ethnicity, education, gender, language) and baseline brain variables (cortical gray matter and hippocampus volumes, residualized in the model for intracranial volume, and white matter hyperintensity volume). Reserve change (memrsl) was a latent variable that captured residual variance in memsl that was not explained by demographic variables, baseline brain variables, and brain change variables (cortical gray matter and hippocampus volume change). Baseline reserve measured how each individual’s observed memory intercept differed from what would be expected given that person’s observed demographic and baseline brain characteristics. Reserve change measured how each individual’s observed memory slope deviated from what would be expected given that person’s observed demographic characteristics, baseline brain, and brain change measurements.
Our three primary analytic aims were: 1) To examine how baseline reserve and reserve change differed across groups that were defined by clinical diagnosis at the first and last assessments, 2) To examine how baseline reserve and reserve change predicted change in non-memory cognitive abilities, and specifically, the incremental effects of these reserve measures above and beyond effects of brain and demographic variables, and 3) To examine whether reserve measures interacted with and therefore modified effects of brain change variables on cognitive decline
For Aim 1, baseline reserve and reserve change were the primary dependent variables, and diagnosis change indicator variables were the primary independent variables. The model depicted in Figure 1 was modified by removing non-memory cognitive variables from the Within and Between models and regressing baseline reserve and reserve change on diagnosis change indicator variables in the Between part of the model. The Stable Normal group was the reference for group comparisons for both reserve variables. For Aims 2 and 3, semantic memory (Semantic), executive function (Executive), and spatial ability (Spatial) slope random effects (semsl, execsl, spatsl) were used as indicators for a second-order global cognitive slope factor. We used a model that had a global slope second-order factor but individual intercept random effects. Slopes were highly correlated, and this model provided optimal fit (see Supplementary materials).
Aim 3 added interactions of baseline reserve and reserve change with brain variables. One model included all of the effects from the Aim 2 model plus a Baseline Reserve by Cortical Gray Matter Change interaction effect, a second tested the Reserve Change interaction with Gray Matter Change, and two models tested the two reserve interactions with Hippocampus Change. Models for Aims 1 and 2 simultaneously estimated all model parameters and effects. The Aim 3 analyses involved interactions with latent variables. While latent variable interactions are possible within Mplus, estimation is computationally intensive, and we were not able to successfully estimate the latent variable interactions required for Aim 3 within a single model estimation. We addressed this limitation by estimating a model in Aim 2 that did not include interaction effects and saved the baseline reserve and reserve change factor scores from that analysis. These factor scores were entered as observed variables into analyses that included the brain by baseline reserve and brain by reserve change interactions. Because this approach problematically treats the reserve factor scores as observed variables without accounting for the error in their measurement (which could influence inferences by negatively biasing standard errors for effects involving the reserve factors), we further estimated the reliability of these reserve variables and their interactions with brain variables and modeled these empirical reliabilities in the models that included interaction effects. To estimate reliabilities, we used bootstrap resampling to estimate the reliability of the reserve factor scores and products of these factor scores with brain variables across 500 bootstrap samples. Each bootstrap draw could include duplicated records from the same participant, fewer records for a given participant than in the original dataset, or no records for an individual. Cases that had only one unique record for a given participant were dropped from the draw. Reliability was estimated by calculating the intraclass correlation (ICC) of the relevant scores across the 500 bootstrap samples using the R ICC module (ICCest with THD confidence intervals). We then fixed error variances in the models with interaction effects to the estimated amount of error variance in these measures.
2.6. Data availability
The raw data that support the findings of this study are available from the corresponding author upon request subject to establishing a data use agreement.
3. Results
3.1. Sample Characteristics:
Sample characteristics are presented in Table 1. Results are stratified by baseline clinical diagnosis to clarify the range of clinical expression of cognitive impairment covered in this study. Sixty percent of the sample was normal, 33% had a baseline diagnosis of MCI, and 7% were diagnosed with dementia. About 60% were females. Sex differed across diagnosis groups (χ2[2]=5.734, p=0.057); Normals and dementia cases were more likely to be female, but MCI cases were evenly divided among males and females. About 22% were African Americans, 25% were Hispanics, 47% were non-Hispanic Whites, and 5% were Other races or ethnicities. Race/ethnicity differed by diagnosis (χ2[6]=36.532, p=0.001) with Whites more likely to have a diagnosis of MCI. Approximately two-thirds of the sample were recruited from the community (76%). Recruitment source differed by diagnosis (χ2[2]=30.040, p=0.001), with MCI cases more likely to be clinic referrals. Average age was about 75 years, and this differed across groups (F[2,335]=3.902, p=0.021) with Dementia older than MCI who were older than Normals. Average education was 13.4 and differed across diagnosis groups (F[2,335]=5.968, p=0.003), with highest education in MCI and lowest in dementia. Gray matter volume change, baseline gray matter volume, and baseline cognitive test scores all differed across diagnostic groups (p’s < 0.001), with a consistent pattern of normal > MCI > dementia.
Table 1.
Sample characteristics.
| Demented | MCI | Normal | Total | |
|---|---|---|---|---|
| Gender - Female | 11 (50.0%) | 60 (53.1%) | 133 (65.5%) | 204 (60.4%) |
| Gender - Male | 11 (50.0%) | 53 (46.9%) | 70 (34.5%) | 134 (39.6%) |
| Age_IA - Mean (SD) | 78.5 (±7.7) | 75.2 (±7.3) | 74.2 (±7.0) | 74.8 (±7.2) |
| Education - Mean (SD) | 11.9 (±5.2) | 14.5 (±3.9) | 13.0 (±4.6) | 13.4 (±4.4) |
| Recruitment Source - Clinic | 9 (40.9%) | 45 (39.8%) | 28 (13.8%) | 82 (24.3%) |
| Recruitment Source - Community | 13 (59.1%) | 68 (60.2%) | 174 (85.7%) | 255 (75.4%) |
| Recruitment Source - Missing | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 1 (0.3%) |
| Race/Ethnicity - African American (N=76) | 3 (13.6%) | 23 (20.4%) | 50 (24.6%) | 76 (22.5%) |
| Race/Ethnicity - Hispanic (N=85) | 6 (27.3%) | 10 (8.8%) | 69 (34.0%) | 85 (25.1%) |
| Race/Ethnicity - Other (N=17) | 0 (0.0%) | 5 (4.4%) | 12 (5.9%) | 17 (5.0%) |
| Race/Ethnicity - White (N=160) | 13 (59.1%) | 75 (66.4%) | 72 (35.5%) | 160 (47.3%) |
| Follow-up Time (years) - Mean (SD) | 4.8 (±2.4) | 5.5 (±3.1) | 8.2 (±3.6) | 7.1 (±3.6) |
| N Cognitive Assessments - Mean (SD) | 4.8 (±2.3) | 5.6 (±2.6) | 7.6 (±3.0) | 6.7 (±3.0) |
| N MRI - Mean (SD) | 2.1 (±0.3) | 2.4 (±0.7) | 2.7 (±0.8) | 2.5 (±0.8) |
| Global Gray Change (standardized) - Mean (SD) | −0.0 (±0.0) | −0.0 (±0.0) | −0.0 (±0.0) | −0.0 (±0.0) |
| Global Gray Baseline (standardized) - Mean (SD) | −0.1 (±1.0) | 0.1 (±1.2) | −0.1 (±0.9) | −0.0 (±1.0) |
| Hippocampus Change (standardized) - Mean (SD) | −0.7 (±1.2) | −0.3 (±1.0) | 0.3 (±0.9) | −0.0 (±1.0) |
| Hippocampus Baseline (standardized) - Mean (SD) | −0.8 (±0.8) | −0.1 (±1.0) | 0.5 (±0.9) | 0.2 (±1.0) |
| White Matter Hyperintensity Baseline (standardized) - Mean (SD) | 0.2 (±0.7) | 0.1 (±0.9) | −0.4 (±1.0) | −0.2 (±1.0) |
| Semantic Memory BL - Mean (SD) | −0.5 (±0.9) | 0.1 (±0.7) | 0.1 (±0.9) | 0.1 (±0.8) |
| Executive Function BL - Mean (SD) | −0.5 (±0.8) | 0.0 (±0.7) | 0.4 (±0.8) | 0.2 (±0.8) |
| Spatial BL - Mean (SD) | −0.4 (±1.1) | 0.1 (±0.9) | 0.2 (±1.0) | 0.1 (±1.0) |
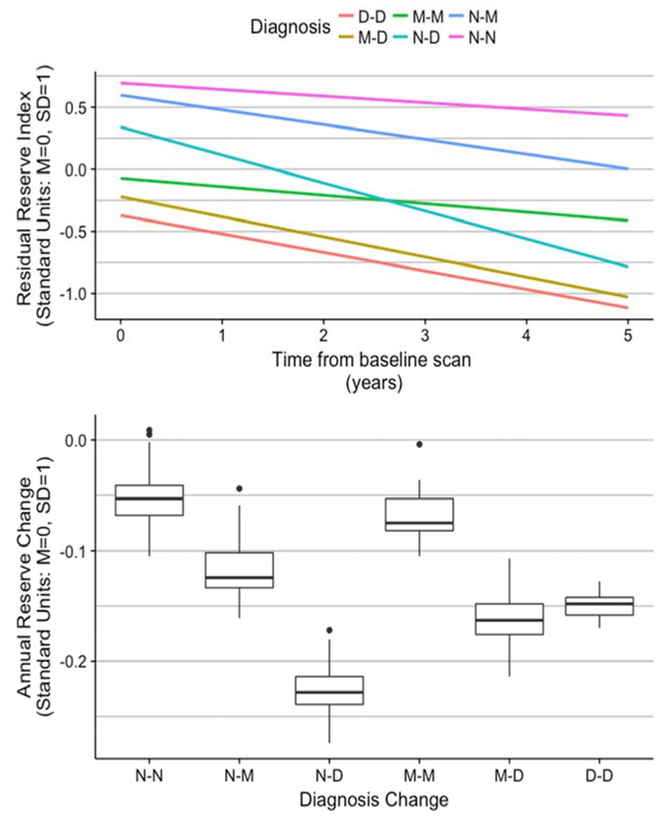
3.2. Reserve Trajectories by Diagnosis Change:
We first examined how the baseline reserve index and reserve change were related to change in diagnosis over the follow-up period. The average amount of follow-up time differed across the diagnosis change groups (F[5,324]=12.213, p=0.001; mean Stable Normal =8.3 (SD=3.6) years, Normal to MCI =8.4 (3.8), Normal to Dementia = 7.7 (3), Stable MCI = 5.4 (3.1), MCI to Dementia = 5.4 (3), Dementia = 4.8 (3.6)). Length of follow-up was longer for those who started as Normal and was shortest for those who were demented at the baseline assessment.
Table 2 presents estimated diagnosis change group effects on Baseline Reserve and Reserve Change. Estimates in Table 2 are average values for the Stable Normal Group and are average differences from Stable Normals for the other groups. As would be expected, Stable Normals had the highest Baseline Reserve and the Dementia group the lowest, with progressive gradations across the other Normal and MCI groups. Average Reserve Index Change was significantly negative in the Stable Normal Group (−0.053 SD per year). Rate of decline in reserve in the Stable MCI group did not differ from that of Stable Normals, but all other groups showed more rapid decline. Clear group differences in average Baseline Reserve could be observed, as well as substantial group differences in rate of Reserve Change (see Figure 2). It is noteworthy that the Normal to Dementia group had the fastest rate of decline (−0.225 SD per year). By contrast, Reserve Change in the MCI to Dementia and Stable Dementia groups were similar, (−0.162 & −0.149 SD per year), while Reserve Change in the Normal to MCI group was less dramatic (−0.119 SD per year) but still differed significantly from that of Stable Normals.
Table 2.
Diagnosis Change Effects on Residual Reserve Index. The estimate represents the mean for the reference group; estimates for non-reference groups represent average differences from the reference group. (Stable Normal=Normal at first and last assessments (N=126); Normal to MCI=Normal at first assessment, MCI at last (N=46); Normal to Dementia=Normal at first assessment, Dementia at last (N=31); MCI to MCI=MCI at first and last assessments (N=29); MCI to Dementia=MCI at first assessment, Dementia at last (N=76); Dementia=Dementia at first and last assessments (N =22))
| Reserve_Type | Diagnosis_Change | estimate | se | p |
|---|---|---|---|---|
| Baseline | Stable Normal (reference) | 0.696 | 0.082 | 0.000 |
| Baseline | Normal to MCI | −0.098 | 0.086 | 0.252 |
| Baseline | Normal to Dementia | −0.356 | 0.125 | 0.004 |
| Baseline | Stable MCI | −0.768 | 0.102 | 0.000 |
| Baseline | MCI to Dementia | −0.915 | 0.099 | 0.000 |
| Baseline | Dementia | −1.067 | 0.147 | 0.000 |
| Change | Stable Normal (reference) | −0.053 | 0.012 | 0.000 |
| Change | Normal to MCI | −0.066 | 0.013 | 0.000 |
| Change | Normal to Dementia | −0.172 | 0.017 | 0.000 |
| Change | Stable MCI | −0.015 | 0.021 | 0.472 |
| Change | MCI to Dementia | −0.109 | 0.018 | 0.000 |
| Change | Dementia | −0.096 | 0.026 | 0.000 |
Figure 2.
Residual reserve index by diagnosis change. The upper panel shows average trajectories over time of reserve across groups defined by first and last clinical diagnosis. The lower panel shows distributions of reserve change by diagnosis change groups. Abbreviations: MCI, mild cognitive impairment; D-D, dementia at first and last assessments; M-D, MCI at first assessment, dementia at last; M-M, MCI at first and last assessments; N-N, normal at first and last assessments; N-M, normal at first and MCI at last; N-D, normal at first and dementia at last.
3.3. Incremental Effects on Non-Memory Cognitive Trajectories of Reserve, Demographic, and Brain Variables:
To address Aim 2, we estimated Baseline Reserve and Reserve Change using the latent variable model depicted in Figure 1 and evaluated how these reserve indices, baseline brain variables, brain change variables, and demographic variables influenced cognitive decline. Results showing independent effects of the various classes of variables on global cognitive slope are presented in Table 3.
Table 3.
Brain, Demographic, and Reserve Effects on Global Cognitive Slope. The Intercept estimate represents the mean for the reference individual for group indicator variables and average values for continuous variables. Estimates for non-reference group indicator variables represent average difference from the reference value for that variable. Estimates for continuous values indicate the effect of a 1 SD difference in that variable.
| Independent Variable | estimate | se | p |
|---|---|---|---|
| Intercept (reference) | −0.108 | 0.011 | 0.000 |
| Male | −0.013 | 0.009 | 0.129 |
| Spanish | −0.009 | 0.013 | 0.487 |
| Education (centered at 12 years) | 0.001 | 0.001 | 0.379 |
| African American | 0.071 | 0.011 | 0.000 |
| Hispanic | 0.049 | 0.012 | 0.000 |
| Other non-White Race/Ethnicity | 0.024 | 0.019 | 0.209 |
| Cortical Gray (baseline) | −0.001 | 0.005 | 0.792 |
| Hippocampus (baseline) | 0.022 | 0.005 | 0.000 |
| White Matter Hyperintensity (baseline) | −0.006 | 0.004 | 0.147 |
| Cortical Gray Matter (change) | 0.047 | 0.009 | 0.000 |
| Hippocampus (change) | 0.017 | 0.006 | 0.004 |
| Residual Reserve Index (baseline) | 0.003 | 0.004 | 0.363 |
The average cognitive decline of a reference case (female, non-Hispanic white, 12 years of education, English speaking, average brain and brain change values in the sample) was −0.108 SD per year. African Americans (average decline rate =−0.037 SD/year) and Hispanics (−0.059 SD/year) declined at significantly slower rates on average. Of the brain variables, cortical gray matter change had the largest effect on cognitive decline (0.047 SD/year per SD of gray matter change), followed by baseline hippocampal volume (0.022 SD/Year/SD) and hippocampal change (0.017 SD/Year/SD). Reserve change was related to cognitive decline independent of all other predictors in the model (0.052 SD/Year/SD) and had an independent effect that was about equal to that of cortical gray matter change. Baseline reserve was not independently related to cognitive change (p=0.363).
3.4. Interaction of Reserve and Brain Change:
Aim 3 examined whether the reserve indices modified the effects of brain change on cognitive decline. In terms of estimated reliabilities of the reserve indices and their cross-products with brain change variables, Baseline Reserve was more reliable (ICC=0.845, 95% CI=0.825 to 0.865) than Reserve Change (ICC=0.727; 95%CI=0.697 to 0.757), and the reliabilities of the cross-products were generally about the same as that of the involved reserve variables (Baseline Reserve by Cortical Gray Matter Change: ICC=0.865; 95%CI=0.847 to 0.882; Reserve Change by Cortical Gray Matter Change: ICC=0.722; 95% CI=0.692 to 0.752; Baseline Reserve by Hippocampus Change: ICC=0.831; 95% CI=0.801 to 0.861; Reserve Change by Hippocampus Change: ICC=0.725; 95%CI=0.682 to 0.767). Table 4 summarizes results from the four analyses that tested reserve by brain change interactions. Significant interaction effects were observed for Baseline Reserve by Gray Matter Change (estimate=−0.021, s.e.=0.005, p=0.001), Reserve Change by Gray Matter Change (estimate=−0.015, s.e.=0.006, p=0.008), Reserve Change by Hippocampus Change (estimate=−0.013, s.e.=0.004, p=0.004), and Baseline Reserve by Hippocampus Change (estimate=−0.014, s.e.=0.005, p=0.007). These results can be interpreted to mean that the brain change effect is smaller in individuals with higher reserve indices. For example, the cortical gray matter change effect is 0.032 SD/year/SD gray matter change in those with Reserve Change 1 SD above average, 0.047 SD/year/SD in those with average Reserve Change, and 0.062 SD/year/SD in those with Reserve Change 1 SD below average.
Table 4.
Reserve by Cortical Gray Matter (GM) and Hippocampus (HC) Interaction Effects (standard errors in parentheses). Separate models were used to test each of the four interactions. Main effects show average rate of global cognitive change for reference individuals with average values on other variables in the model. The interaction effects show how reserve variables modify the effects of brain change variables. (+ p<0.05, ++ p <0.01, +++ p<0.001)
| Model | Reserve Baseline | Reserve Change | Brain Change | Reserve by Brain Change |
|---|---|---|---|---|
| Reserve Baseline by GM Change | 0.005 (0.004) | 0.034 (0.004)+++ | 0.048 (0.007)+++ | −0.021 (0.005)+++ |
| Reserve Change by GM Change | −0.001 (0.004) | 0.036 (0.004)+++ | 0.047 (0.007)+++ | −0.015 (0.006)++ |
| Reserve Baseline by HC Change | 0.002 (0.004) | 0.036 (0.004)+++ | 0.019 (0.005)+++ | −0.014 (0.005)++ |
| Reserve Change by HC Change | −0.001 (0.004) | 0.037 (0.004)+++ | 0.020 (0.005)+++ | −0.013 (0.004)++ |
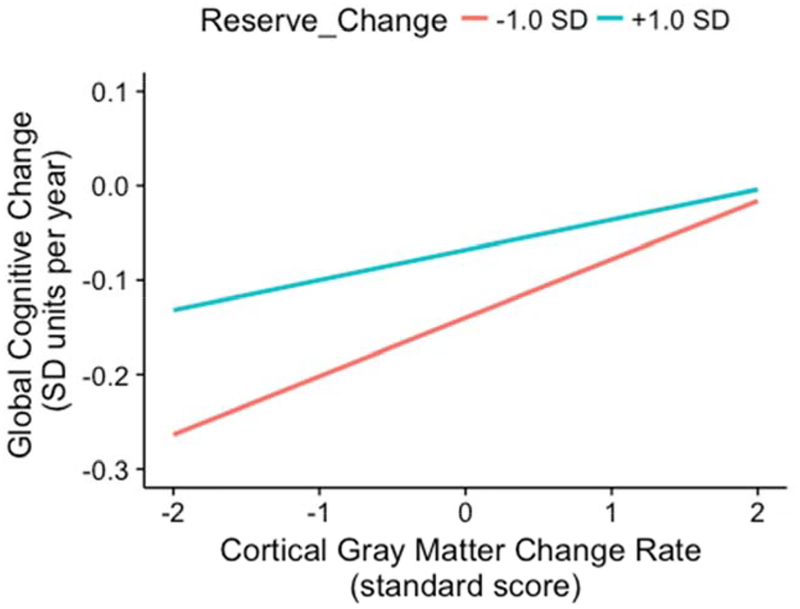
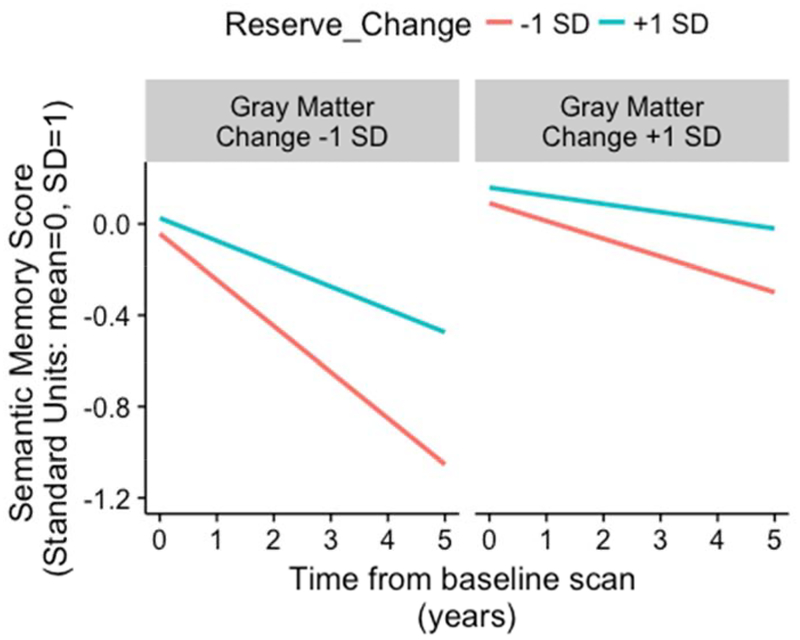
The Reserve Change by Cortical Gray Matter Change interaction effect is presented graphically in Figures 3 and 4. Figure 3 shows annualized rate of non-memory change on the y-axis and shows how this relates to Gray Matter Change rate (x-axis) for two levels of Reserve Change. Rate of cognitive decline was more strongly related to Gray Matter Change in those with more rapidly declining reserve (Reserve Change of - 1 SD). Rate of cognitive decline was near 0 when there was little gray matter atrophy regardless of Reserve Change level. In contrast, if an individual displayed rapidly declining reserve in the context of faster gray matter atrophy over time, then their annual rate of cognitive decline doubled (compared to those whose reserve decline less rapidly than average). Figure 4 presents these results in terms of predicted cognitive trajectories for one outcome (Semantic Memory). The effect of Gray Matter Change on cognitive trajectories is stronger among participants with more rapidly declining reserve, but reserve that is declining less rapidly than average protects against the effects of declining gray matter.
Figure 3.
Reserve Change by Cortical Gray Matter Change interaction effect on global cognitive slope. The graph shows the association between annual change in non-memory cognitive function and annual change in cortical gray matter volume as a function of reserve status. Reserve status was based on model predicted trajectories that were faster than average (−1 SD, shown in red; rapidly declining reserve status over time) or slower than average (+1 SD, shown in teal; slowly declining reserve status over time).
Figure 4.
Expected 5-y longitudinal cognitive trajectory of semantic memory for specific reserve change levels (−1 and +1 SD) and specific cortical gray matter atrophy rates (−1 and +1 SD).
4. Discussion:
In a diverse cohort of aging adults with clinical severity levels ranging from normal to mildly demented, we developed longitudinal, latent variable models of cognitive reserve to examine how changes in reserve influence the effect of brain atrophy on cognitive decline. We measured the effects of reserve as residual memory performance not explained by brain and demographic variables. Results showed that this measure of cognitive reserve changed in tandem with progression of clinical diagnosis, with more rapid depletion of reserve observed in those who transitioned to a more impaired clinical state. Depletion of our indicator of cognitive reserve was related to faster decline in non-memory cognitive domains, even after accounting for longitudinal brain atrophy. Finally, in a stringent test of the construct validity of the residual-defined dynamic measure of cognitive reserve, we found that maintaining reserve buffered the negative effect of brain atrophy on cognitive decline. Results from the study underscore the dynamic nature of reserve in late life, and highlight that changes in reserve may have meaningful clinical implications for individual aging trajectories.
A primary aim of the study was to model changes in cognitive reserve and determine how these changes relate to longitudinal changes in clinical status (i.e., normal, MCI, dementia) adjudicated independently of the memory measure used to compute reserve. We extended cross-sectional (Hohman et al. 2016; Reed et al. 2010; Zahodne et al. 2013) and two-time point (Zahodne et al. 2015) residual approaches through our use of an extended, longitudinal framework, and we used latent variable modeling to appraise change in cognitive reserve over an average of 7 assessments. In our study, all diagnosis groups experienced, on average, a decrement in this measure of reserve over time, although significant differences in rates of change in cognitive reserve were noted across most clinical status groups relative to individuals who remained in the Stable Normal category. While change in reserve in the Stable MCI group did not significantly differ from that of the Stable Normal group, more robust declines in reserve were associated with worsening diagnostic classification over time (particularly Normal to Dementia status) and were also evident in individuals who entered the study with, and maintained, a dementia diagnosis.
The idea that cognitive reserve may be depleted over time is not a new concept, and has been proffered as an explanation for the widely reported finding that older adults with higher reserve show a more precipitous decline in functioning after they cross a dementia severity threshold (Scarmeas et al. 2006; Stern et al. 1999) and/or as brain degeneration progresses (Mungas et al. 2018). These studies have utilized historical variables (e.g., education) as proxies for cognitive reserve, rendering it difficult to quantify the degree to which cognitive reserve changes over time and to measure how this change maps onto evolving clinical presentation. Change in cognitive reserve over time and its relation to clinical diagnostic status has previously been characterized by only one study (Zahodne et al. 2015), which reported that in non-demented, community-dwelling adults, change in reserve was a better predictor of a future dementia diagnosis than cognitive reserve measured at a single time point.
Our study further examined the association between dynamic cognitive reserve and longitudinal cognitive change and demonstrated that more rapid decline in our measure of reserve was associated with faster decline in non-memory cognitive domains (i.e., global cognitive slopes, excluding memory function). These results were consistent with our hypotheses and intuitive in many respects. Our dynamic measure of reserve was defined by longitudinal memory performance, and it is reasonable to expect that declines in memory would be associated with non-memory cognitive decline. Indeed, previous studies with this cohort have shown that rates of decline of the cognitive domains measured in this study are highly correlated (Fletcher et al. 2018; Mungas et al. 2018). However, widespread volumetric brain changes are often presumed to be the underlying cause of diffuse cognitive decline. Results from this study, however, indicated that declines in our measure of reserve (which, by definition, is memory performance not explained by brain atrophy) were associated with faster rates of global cognitive decline, independent of both baseline and longitudinal brain atrophy (i.e., cortical gray matter; hippocampal gray matter) and white matter hyperintensities. To rephrase, dynamic change in our indicator of reserve was an independent predictor of cognitive decline, even after accounting for canonical measures of brain health. This is an important point regarding correlations between memory and other cognitive domains; if correlations between cognitive domains are strictly due to shared, underlying brain morphology, then we would not expect this outcome. Instead, our findings highlight a critical and independent role for reserve in global cognitive trajectories.
A primary tenet of the cognitive reserve construct is that it moderates the impact of brain insults on clinical presentation. Thus, a critical appraisal of the reserve change index is to assess how it influences the effect of brain change on cognitive decline. Consistent with our hypotheses, the present study demonstrated that maintenance of reserve reduces the impact of brain atrophy (both global cortical atrophy and hippocampal atrophy) on non-memory cognitive decline. The effects sizes were notably strong, such that for a decline in reserve that was 1 standard deviation (SD) faster than the average (i.e., depleting reserve), the effect of a 1 SD loss of hippocampal volume on non-memory cognitive decline increased by 65% (from 0.020 to 0.033 SD/year). By contrast, if residual reserve change was 1 SD above average (i.e., maintaining reserve), the effect of longitudinal hippocampal atrophy on non-memory cognitive decline was minimized (0.007 SD/year). Similar moderating effects of the residual reserve index were noted for the association between cortical gray matter change and non-memory cognitive decline. Overall, these findings suggest that the effect of longitudinal brain atrophy on cognitive decline is markedly different based on the extent to which an individual’s residual-defined measure of reserve changes. Thus, whereas depletion of reserve may reveal the effects of brain atrophy on cognitive decline, maintenance of reserve may exert a buffer against the effects of brain atrophy on cognitive decline. Our results also indicate that change in an individual’s residual-defined measure of reserve is a much stronger predictor of cognitive change than is baseline cognitive reserve, which did not exert a meaningful effect on non-memory cognitive slope independent of reserve change. This finding suggests that establishing a high level of cognitive reserve in and of itself may not confer protection against cognitive decline if that level of cognitive reserve cannot be maintained over time.
Our study design and hypotheses were rooted in the idea that heterogeneity in cognitive trajectories may be driven –in part– by individual differences in the ability to cognitively adapt to or stave off impending brain disease (Stern 2009). The measurement of cognitive reserve has presented many methodological challenges and has raised questions regarding the utility of this construct in aging and AD research. Consistent with the consensus definitions and guidelines provided by the recent whitepaper on cognitive reserve (Stern 2018), one of the advantages of the residual reserve approach is that it provides an objective estimate of reserve that is not reliant on retrospective proxy variables. While our approach cannot be considered a direct measure of reserve, through the parameterization of residual variance in memory performance, we were nonetheless able to operationalize reserve and dynamically track its changes over time, which is critical for understanding how this construct relates to symptom onset and salient clinical features of AD and dementia more broadly.
Although our results suggest that applying a psychometric approach to the longitudinal measurement of cognitive reserve is not only feasible but also clinically meaningful, there are theoretical implications of this approach that warrant further consideration. A residual represents the variance in the outcome measure (in this case, memory performance) that is not explained by the predictors (in this case, demographics, baseline brain volumes and white matter hyperintensities, longitudinal brain volumes). As such, within the residual reserve framework, cognitive reserve can be viewed as an index of what we do not know and what we may not currently be able to measure. This is also reflected in the broader literature, as cognitive reserve is frequently used to encapsulate the mismatch between pathology and measured cognition; however, it is more directly parameterized in the residual reserve index approach. With methodological advancements in the in-vivo measurement of brain pathology, improved accuracy in the diagnosis of complex syndromes, and increased knowledge of functional brain mechanisms (e.g., neuroplasticity) that underlie cognitive reserve, the residual memory variance should become smaller. Taking this one step further, a long-term implication of this work is that residual memory variance should ultimately lose some of its utility as we gain empirical knowledge of the mechanisms of cognitive decline. This is not to undermine the importance of the construct, however, as delineating the mechanisms for cognitive reserve is pivotal to our understanding of how the brain compensates for or adapts to disease-related changes.
In terms of study limitations, the residual reserve index ultimately summarizes the effects of unknown variables that influence memory function, as noted previously. Although our latent variable modeling approach minimizes the influence of measurement error, the residual memory index may still capture error and unknown variables that do not truly reflect cognitive reserve (Reed et al. 2010). Our incorporation of measures of central nervous system integrity was also limited by a) what was available in the study, and b) what is currently measurable in the field. Although we included common neuroimaging variables of brain structure into our models, measures of disease-specific pathology (e.g., amyloid, phosphorylated tau) were not available. Moreover, proteinopathies, vascular changes, and synaptic changes that are not measurable in-vivo at this time may underlie the observed heterogeneity in memory performance; these unmeasured pathological factors are likely subsumed under the residual reserve component in this study, which remains an important conceptual issue to consider when interpreting the meaning of residual-defined reserve outcomes. An additional limitation of the study is that our residual-defined dynamic measure of cognitive reserve does not address underlying mechanisms of reserve. As noted previously, direct measures of reserve - and by extension, mechanisms of reserve - have historically been challenging to implement. Functional imaging methods have been proposed as a possible means of assessing cognitive reserve directly, although these approaches are still tied to specific methodologies, as noted in the recent whitepaper (Stern 2018). While the delineation of processes that explain why some individuals are able to adapt to pathology is critically important, these mechanisms are outside the scope of our study. Finally, given considerations related to sample size and number of MRI measurements, we were limited in our ability to appraise non-linear trends in reserve trajectories; as such, our latent variable models assumed a linear change in both the residual reserve index and the cognitive outcomes, in the presence of modeled practice effects.
Of note, we elected to focus on episodic memory as the primary indicator of reserve. The decision to use episodic memory to define our reserve index was based on several factors, including prior literature on cognitive reserve (Reed et al. 2010; Stern et al. 1999; Zahodne et al. 2015; Zahodne et al. 2013), as well as its sensitivity to aging and Alzheimer’s disease (Bondi et al. 2014; Busse et al. 2006; Driscoll et al. 2003; Ewers et al. 2010). Prior studies suggest that the operationalization of reserve may be applied to non-memory domains, with comparable findings (Reed et al. 2010; Reed et al. 2011). Nonetheless, an imperative for future research will be to further refine the generalizability and practicality of the measurement of reserve. Whether based on memory or non-memory domains, the residual reserve index score is dependent upon the characteristics of the study participant sample and the measures that are available, which limits its current applicability to clinical settings. For any given person, the same cognitive test and MRI results may yield a different residual index score in a different sample used to derive the regression model and if different methods are used to measure cognition and brain status. Consequently, there is a need for standardization of measures used to define reserve and for calibration of the measurement model for reserve in a suitably diverse sample that represents the diverse target population of older adults. Future work of this nature will be critical for establishing practical and clinically useful, dynamic measurement of cognitive reserve.
The current study has multiple strengths, including the use of latent variable modeling to measure residual reserve in a longitudinal design. By leveraging a residual reserve approach, we were able to operationally define, measure, and track changes in reserve over time. The application of the residual reserve approach to a longitudinal framework further allowed us to capitalize on multiple time points and thereby more precisely estimate cognitive slopes. Importantly, a long-term implication of this residual reserve approach is that we may ultimately be able to use this method to identify and better understand mechanisms for cognitive reserve in late life. An additional strength of the study was the minimization of circularity in our design. The measures used to define reserve (i.e., SENAS memory, MRI measures of central nervous system integrity) were not used to define clinical status or non-memory cognitive change. Diagnostic status (i.e., normal aging, MCI, dementia) was based on a separate neuropsychological evaluation, with no overlapping cognitive measures. Moreover, in the context of correlated cognitive domains, circularity was further minimized by appraising the moderating effects of reserve on the relationship between brain atrophy and cognitive decline; given that reserve was defined as residual memory performance not explained by brain atrophy and demographics, its modifying effects on the association between longitudinal brain changes and non-memory decline are all the more striking. Finally, our sample was composed of ethnically diverse participants and included individuals with a range of cognitive impairment levels at baseline, both of which are critical to the generalization and applicability of study finding to a wider aging population.
In summary, our study suggests that cognitive reserve changes dynamically over time, is associated with change in clinical diagnostic status, and has modifying effects on the association between brain atrophy and cognitive decline. Importantly, the effects of gray matter atrophy on cognitive trajectories were unmasked by rapidly declining reserve, whereas maintenance of high reserve over time —or less rapidly declining reserve—exerted a protective buffer against the effects of changing brain status on cognitive decline. Our findings underscore the mutable nature of cognitive reserve and suggest that dynamic changes in reserve may have meaningful implications for the progression of clinical diagnostic status and individual cognitive trajectories.
Supplementary Material
Bettcher et al. Highlights.
Cognitive reserve changes dynamically over time
Changes in reserve were associated with progression of clinical diagnosis
Rapid depletion of reserve was associated with faster decline in non-memory function
Depletion of reserve unmasked the effects of brain atrophy on cognitive decline
Maintenance of reserve buffered against the negative effects of brain atrophy
Acknowledgements.
We would like to acknowledge the devotion of the participants in this study who volunteered their time for comprehensive annual evaluations and repeated MRI scans. Many staff of the UC Davis Alzheimer’s Disease Center made this study a reality. Esther Lara supervised all aspects of study implementation from participant recruitment through retention over time leading to successful longitudinal follow-up.
Disclosures.
This work was supported by multiple grants from the National Institute on Aging (NIA) (P30 AG10129, R01 AG021028, and R01 AG047827, C DeCarli, PI; R01 AG10220, D Mungas, PI; R01/RF1 AG031563, B Reed/D Mungas, PI; R01 AG031252, S Tomaszewski Farias, PI; R01 AG051170, R Jones, PI; R01 AG058772, B. Bettcher, PI). None of the authors have financial or personal conflicts of interest related to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike H 1987. “Factor Analysis and AIC.” Psychometrika 52: 317–32. [Google Scholar]
- Aljabar P, a Heckemann R, a Hammers JV Hajnal, and Rueckert D. 2009. “Multi-Atlas Based Segmentation of Brain Images: Atlas Selection and Its Effect on Accuracy.” Neuroimage 46: 726–38. 10.1016/j.neuroimage.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Aljabar P, Heckemann R, Hammers A, Hajnal JV, and Rueckert D. 2007. “Classifier Selection Strategies for Label Fusion Using Large Atlas Databases.” Med Image Comput Comput Assist Interv 10 (Pt 1): 523–31. [DOI] [PubMed] [Google Scholar]
- Ashburner J, and Friston KJ. 2000. “Voxel-Based Morphometry–the Methods.” Neuroimage 11 (6 Pt 1): 805–21. 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Association, American Psychiatric. 1987. Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised. Washington, DC: American Psychiatric Association. [Google Scholar]
- Blom G 1958. Statistical Estimates and Transformed Beta-Variables. New York: Wiley. [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, et al. 2014. “Neuropsychological Criteria for Mild Cognitive Impairment Improves Diagnostic Precision, Biomarker Associations, and Progression Rates.” Journal of Alzheimer’s Disease : JAD 42 (1): 275–89. 10.3233/JAD-140276 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle Patricia A., Wilson Robert S., Yu Lei, Barr Alasdair M., Honer William G., Schneider Julie A., and Bennett David A.. 2013. “Much of Late Life Cognitive Decline Is Not Due to Common Neurodegenerative Pathologies.” Annals of Neurology 74 (3): 478–89. 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster PW, Melrose RJ, Marquine MJ, Johnson JK, Napoles A, MacKay-Brandt A, Farias S, Reed B, and Mungas D. 2014. “Life Experience and Demographic Influences on Cognitive Function in Older Adults.” Neuropsychology 28 (6): 846–58. https://doi.org/2014-24826-001 [pii] 10.1037/neu0000098 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse A, Hensel A, Guhne U, Angermeyer MC, and Riedel-Heller SG. 2006. “Mild Cognitive Impairment: Long-Term Course of Four Clinical Subtypes.” Neurology 67 (12): 2176–85. https://doi.org/67/12/2176 [pii]. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, and Jagust W. 2005. “Anatomical Mapping of White Matter Hyperintensities (WMH) Exploring the Relationships Between Periventricular WMH, Deep WMH, and Total WMH Burden.” Stroke 36: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, and Sutherland RJ. 2003. “The Aging Hippocampus: Cognitive, Biochemical and Structural Findings.” Cerebral Cortex (New York, N.Y.: 1991) 13 (12): 1344–51. [DOI] [PubMed] [Google Scholar]
- Early DR, Widaman KF, Harvey D, Beckett L, Park LQ, Farias ST, Reed BR, Decarli C, and Mungas D. 2013. “Demographic Predictors of Cognitive Change in Ethnically Diverse Older Persons.” Psychology and Aging 28 (3): 633–45. 10.1037/a0031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR, Feldman HH, et al. 2010. “Prediction of Conversion from Mild Cognitive Impairment to Alzheimer’s Disease Dementia Based Upon Biomarkers and Neuropsychological Test Performance.” Neurobiology of Aging 2010/December/17 https://doi.org/S0197-4580(10)00464-1 [pii] 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Gavett B, Harvey D, Farias ST, Olichney J, Beckett L, DeCarli C, and Mungas D. 2018. “Brain Volume Change and Cognitive Trajectories in Aging.” Neuropsychology 32 (4): 436–49. 10.1037/neu0000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Knaack A, Singh B, Lloyd E, Wu E, Carmichael O, and DeCarli C. 2013. “Combining Boundary-Based Methods with Tensor-Based Morphometry in the Measurement of Longitudinal Brain Change.” Medical Imaging, IEEE Transactions on 32 (2): 223–36. 10.1109/tmi.2012.2220153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Singh B, Harvey D, Carmichael O, and DeCarli C. 2012. “Adaptive Image Segmentation for Robust Measurement of Longitudinal Brain Tissue Change.” Conf Proc IEEE Eng Med Biol Soc 2012: 5319–22. 10.1109/EMBC.2012.6347195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher Evan. 2014. “Using Prior Information to Enhance Sensitivity of Longitudinal Brain Change Computation” In Frontiers of Medical Imaging, edited by Chen CH, 63–81. World Scientific. [Google Scholar]
- Fletcher Evan, Villeneuve Sylvia, Maillard Pauline, Harvey Danielle, Reed Bruce, Jagust William, and Decarli Charles. 2016. “Beta-Amyloid, Hippocampal Atrophy and Their Relation to Longitudinal Brain Change in Cognitively Normal Individuals.” Neurobiology of Aging 40: 173–80. 10.1016/j.neurobiolaging.2016.01.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni Giovanni B., and Jack Clifford R.. 2011. “Harmonization of Magnetic Resonance-Based Manual Hippocampal Segmentation: A Mandatory Step for Wide Clinical Use.” Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 7 (2): 171–74. 10.1016/j.jalz.2010.06.007. [DOI] [PubMed] [Google Scholar]
- ———. 2015. “HarP: The EADC-ADNI Harmonized Protocol for Manual Hippocampal Segmentation. A Standard of Reference from a Global Working Group.” Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 11 (2): 107–10. 10.1016/j.jalz.2014.05.1761. [DOI] [PubMed] [Google Scholar]
- Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, and Mungas D. 2010. “Recruitment of a Community-Based Cohort for Research on Diversity and Risk of Dementia.” Alzheimer’s Disease and Associated Disorders 24 (3): 234–41. 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, McLaren DG, Mormino EC, Gifford KA, Libon DJ, Jefferson AL, and Initiative Alzheimer’s Disease Neuroimaging. 2016. “Asymptomatic Alzheimer Disease: Defining Resilience.” Neurology 87 (23): 2443–50. 10.1212/WNL.0000000000003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Leow A, Parikshak N, Lee S, Chiang M, Toga A, Jackjr C, Weiner M, and Thompson P. 2008. “Tensor-Based Morphometry as a Neuroimaging Biomarker for Alzheimer’s Disease: An MRI Study of 676 AD, MCI, and Normal Subjects.” Neuroimage 43 (3): 458–69. 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, and Stern Y. 2011. “Conceptual and Measurement Challenges in Research on Cognitive Reserve.” Journal of the International Neuropsychological Society 17 (4): 593–601. https://doi.org/S1355617710001748 [pii] 10.1017/S1355617710001748 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Beiser A, Machulda MM, Fields J, Roberts RO, Pankratz VS, Aakre J, et al. 2015. “Spectrum of Cognition Short of Dementia: Framingham Heart Study and Mayo Clinic Study of Aging.” Neurology 85 (19): 1712–21. 10.1212/WNL.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, and Fox P. 2001. “Regional Spatial Normalization: Toward and Optimal Target.” Journal of Computer Assisted Tomography 25 (5): 805–16. [DOI] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, and DeCarli C. 2010. “Vascular and Degenerative Processes Differentially Affect Regional Interhemispheric Connections in Normal Aging, Mild Cognitive Impairment, and Alzheimer Disease.” Stroke 41 (8): 1791–7. 10.1161/STROKEAHA.110.582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose RJ, Brewster P, Marquine MJ, MacKay-Brandt A, Reed B, Farias ST, and Mungas D. 2015. “Early Life Development in a Multiethnic Sample and the Relation to Late Life Cognition.” Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 70 (4): 519–31. 10.1093/geronb/gbt126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, et al. 2006. “The Uniform Data Set (UDS): Clinical and Cognitive Variables and Descriptive Data from Alzheimer Disease Centers.” Alzheimer’s Disease and Associated Disorders 20 (4): 210–6. 10.1097/01.wad.0000213865.09806.9200002093-200610000-00007 [pii]. [DOI] [PubMed] [Google Scholar]
- Mungas D, Gavett B, Fletcher E, Farias ST, DeCarli C, and Reed B. 2018. “Education Amplifies Brain Atrophy Effect on Cognitive Decline: Implications for Cognitive Reserve.” Neurobiology of Aging 68 (August): 142–50. 10.1016/j.neurobiolaging.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, and Gonzalez H. 2004. “Spanish and English Neuropsychological Assessment Scales (SENAS): Further Development and Psychometric Characteristics.” Psychol Assess 16 (4): 347–59. 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Haan MN, and Gonzalez H. 2005. “Spanish and English Neuropsychological Assessment Scales: Relationship to Demographics, Language, Cognition, and Independent Function.” Neuropsychology 19 (4): 466–75. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Marshall SC, and Gonzalez HM. 2000. “Development of Psychometrically Matched English and Spanish Language Neuropsychological Tests for Older Persons.” Neuropsychology 14 (2): 209–23. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Tomaszewski Farias S, and DeCarli C. 2005. “Criterion-Referenced Validity of a Neuropsychological Test Battery: Equivalent Performance in Elderly Hispanics and Non-Hispanic Whites.” Journal of the International Neuropsychological Society 11: 620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Widaman KF, Reed BR, and Tomaszewski Farias S. 2011. “Measurement Invariance of Neuropsychological Tests in Diverse Older Persons.” Neuropsychology 25 (2): 260–9. 10.1037/a0021090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, and Muthén BO. 1998. Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nettiksimmons J, DeCarli C, Landau S, Beckett L, and Initiative Alzheimer’s Disease Neuroimaging. 2014. “Biological Heterogeneity in ADNI Amnestic Mild Cognitive Impairment.” Alzheimers Dement 10 (5): 511–21 e1. 10.1016/j.jalz.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Rudow G, et al. 2009. “Neuropathologic Studies of the Baltimore Longitudinal Study of Aging (BLSA).” J Alzheimers Dis 18 (3): 665–75. 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, et al. 2009. “Neuropathology of Nondemented Aging: Presumptive Evidence for Preclinical Alzheimer Disease.” Neurobiology of Aging 30 (7): 1026–36. 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Dowling M, Tomaszewski Farias S, Sonnen J, Strauss M, Schneider JA, Bennett DA, and Mungas D. 2011. “Cognitive Activities During Adulthood Are More Important Than Education in Building Reserve.” Journal of the International Neuropsychological Society 17 (4): 615–24. https://doi.org/S1355617711000014 [pii] 10.1017/S1355617711000014 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, Hinton L, and DeCarli C. 2010. “Measuring Cognitive Reserve Based on the Decomposition of Episodic Memory Variance.” Brain 133 (Pt 8): 2196–2209. 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert Daniel, Aljabar Paul, Heckemann Rolf A., Hajnal Joseph V., and Hammers Alexander. 2006. “Diffeomorphic Registration Using B-Splines.” In, edited by Larsen R, Nielsen M, and Sporring J, 4191:702–9. Lecture Notes in Computer Science-MICCAI; Springer-Verlag. [DOI] [PubMed] [Google Scholar]
- Satz P, Cole MA, Hardy DJ, and Rassovsky Y. 2011. “Brain and Cognitive Reserve: Mediator(s) and Construct Validity, a Critique.” J Clin Exp Neuropsychol 33 (1): 121–30. 10.1080/13803395.2010.493151. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Albert SM, Manly JJ, and Stern Y. 2006. “Education and Rates of Cognitive Decline in Incident Alzheimer’s Disease.” Journal of Neurology, Neurosurgery and Psychiatry 77 (3): 308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G 1978. “Estimating the Dimension of a Model.” Annals of Statistics 6: 461–64. [Google Scholar]
- Sclove SL 1987. “Application of Model-Selection Criteria to Some Problems in Multivariate Analysis.” Psychometrika 52: 333–43. [Google Scholar]
- Stern Y 2009. “Cognitive Reserve.” Neuropsychologia 47 (10): 2015–28. 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, and Tsai WY. 1999. “Rate of Memory Decline in AD Is Related to Education and Occupation: Cognitive Reserve?” Neurology 53 (9): 1942–7. [DOI] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, et al. 2018. “Whitepaper: Defining and Investigating Cognitive Reserve, Brain Reserve, and Brain Maintenance.” Alzheimers Dement, September 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky Igor, Leow Alex D., Lee Suh, Osher Stanley J., and Thompson Paul M.. 2009. “Comparing Registration Methods for Mapping Brain Change Using Tensor-Based Morphometry.” Medical Image Analysis 13 (5): 679–700. 10.1016/j.media.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Brickman AM, Narkhede A, Griffith EY, Guzman VA, Schupf N, and Stern Y. 2015. “Is Residual Memory Variance a Valid Method for Quantifying Cognitive Reserve? A Longitudinal Application.” Neuropsychologia 77 (October): 260–6. 10.1016/j.neuropsychologia.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Brickman AM, Siedlecki KL, Decarli C, and Stern Y. 2013. “Quantifying Cognitive Reserve in Older Adults by Decomposing Episodic Memory Variance: Replication and Extension.” Journal of the International Neuropsychological Society 19 (8): 854–62. 10.1017/S1355617713000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon request subject to establishing a data use agreement.