Abstract
Mental disorders represent an increasing personal and financial burden and yet treatment development has stagnated in recent decades. Current disease classifications do not reflect psychobiological mechanisms of psychopathology, nor the complex interplay of genetic and environmental factors, likely contributing to this stagnation. Ten years ago, the longitudinal IMAGEN study was designed to comprehensively incorporate neuroimaging, genetics, and environmental factors to investigate the neural basis of reinforcement-related behaviour in normal adolescent development and psychopathology. In this article, we describe how insights into the psychobiological mechanisms of clinically relevant symptoms obtained by innovative integrative methodologies applied in IMAGEN have informed our current and future research aims. These aims include the identification of symptom groups that are based on shared psychobiological mechanisms and the development of markers that predict disease course and treatment response in clinical groups. These improvements in precision medicine will be achieved, in part, by employing novel methodological tools that refine the biological systems we target. We will also implement our approach in low- and medium-income countries to understand how distinct environmental, socio-economic and cultural conditions influence the development of psychopathology. Together, IMAGEN and related initiatives strive to reduce the burden of mental disorders by developing precision medicine approaches globally.
Introduction
Mental disorders account for 22.9% of years lived with disability1 and are an increasing financial public burden, costing Europe €798 billion2 and the USA $2.5 trillion in 20103. Improving how we identify at-risk individuals and subsequently treat them could reduce such costs. However, current treatments are based on categorical diagnoses and have not advanced in part due to pharmaceutical companies disinvesting from mental disorders due to the high cost of clinical trial failures where candidate drugs targeted symptoms and not the underlying psychobiological mechanisms4. Furthermore, successful preclinical findings from animal models have not translated to humans5, 6. Therefore, there is a therapeutic stagnation that urgently needs addressing.
While traditional psychiatric nosology considers mental disorders as distinct from one another, an alternative notion is that mental disorders might constitute an end of one or more quantitative behaviour traits. This suggests that traditional psychiatric diagnoses can be deconstructed into behavioural domains that reflect specific underlying neural processes. However, current psychiatric diagnoses do not take into account underlying psychobiological processes but, rather, rely on patient self-report symptom information and behavioural observation. It would, therefore, be beneficial to develop effective treatments for specific populations of patients according to the relevant pathophysiology of their symptoms7, an approach called ‘precision medicine’ gaining traction in other areas of medicine such as cancer8.
Until recently there have not been large enough datasets that simultaneously acquire data across different behavioural and biological domains over time. Some ongoing longitudinal cohort studies are prospective birth cohorts, such as the Avon Longitudinal Study of Parents and Children (ALSPAC; http://www.bristol.ac.uk/alspac/) study and the Generation R study (https://generationr.nl/researchers/), enabling researchers to identify early life, including pre- and perinatal, influences on physical, behavioural, and cognitive development. Longitudinal studies following twins, such as the Twins Early Development Study (TEDS; https://www.teds.ac.uk/) and its sister study, the Environmental Risk (E-Risk; http://eriskstudy.com/) Longitudinal Twin Study, are disentangling the influence of genetics and the environment on behavioural development. And while participants in these large studies are richly characterized, the IMAGEN study was one of the first in which participants undergo repeated biological sample collection, including neuroimaging, genotyping, DNA methylation, and gene expression, as well as behavioural and cognitive characterization. The Adolescent Brain Cognitive Development (ABCD; https://abcdstudy.org/) study, which was conceptually inspired by IMAGEN, has recently recruited more than 12,000 9-10 year olds to be followed for ten years. During this time the participants will be repeatedly assessed on their physical and mental health, neuroimaging, cognition, substance use, culture/environment, and biospecimens obtained for DNA, hormones, as well as substance use and toxin exposures. Such large longitudinal multimodal studies are well placed to identify psychobiological markers of mental disorders that could be used to stratify patients above and beyond traditional symptom classification.
How IMAGEN investigates the development of mental disorders
Common mental disorders, such as internalizing, externalizing, and thought disorders emerge in late adolescence and early adulthood, with half of the lifetime burden emerging by the mid-teens and 75% by the mid-20s. This suggests a need to identify biological markers earlier in the course of the disease process. As adolescence is a period of much social, physical, and neurobiological change, it offers a prime opportunity to study the psychobiological and environmental factors that might engender the development of mental disorders.
Such was the rationale for the creation of IMAGEN, a longitudinal neuroimaging-genetics study of adolescents with a focus on reinforcement behaviours (i.e., impulsivity, reward sensitivity, and emotional processing) in normal development and psychopathology9. Over 2,000 participants were initially assessed at age 14 (Baseline) and followed up at ages 16 (Follow-up 1: n=1700), 19 (Follow-up 2: n=1500; see Table 1 for mental health disorder prevalence), and 22 (Follow-up 3, ongoing: n>1300). The neuroimaging battery, standardised across study centres and held constant across time, consists of a high-resolution structural scan (T1 MPRAGE), three functional MRI (fMRI) scans (Monetary Incentive Delay (MID), Stop Signal Task (SST) and Emotional Faces), resting state fMRI, and 32-direction diffusion tensor imaging (DTI), with neurite orientation dispersion and density imaging (NODDI) added at Follow-up 3. Blood was collected at Baseline, Follow-up 2, and Follow-up 3 to support genome-wide (GWAS), epigenome-wide (EWAS), RNA, proteomic and metabolomic analyses. Clinical and self-report measures acquired information on personality, familial psychopathology history, substance use, cognitive function, stress, and family dynamics. Follow-ups 2 and 3 incorporated additional measures to capture potential environmental moderators (e.g., childhood trauma, urbanicity) of reinforcement behaviours and neural processes. The breadth of data acquired across IMAGEN participants’ adolescence has placed the study in a unique position to understand the interplay of factors influencing the development of common mental disorders.
Table 1. Counts and percentage of mental health disorders* in IMAGEN at age 19 (n=1525).
| Mental disorder symptom | Count and percentage |
|---|---|
| Any disorder | 204 (13.4%) |
| Emotional disorder | 168 (11.0%) |
| Generalized anxiety | 42 (2.8%) |
| Major depression | 47 (3.1%) |
| ADHD | 10 (0.7%) |
| Eating disorder | 29 (2.0%) |
| Psychosis | 4 (0.3%) |
assessed using the Development and Well-Being Assessment (DAWBA)
We set out to identify psychobiological mechanisms that predict the development of clinically relevant symptoms in adolescents and also predict disease course in clinical groups. In support of the former, a requisite for good precision medicine, we also want to understand how these aims are impacted in low and medium income countries (LMIC) with distinct environmental and sociocultural conditions, thus ensuring a globally viable approach. We have begun addressing these aims by 1) developing novel methods that support the analysis of large multivariate datasets; 2) taking full advantage of the multimodal longitudinal design to see how data at age 14 predict clinically relevant symptoms at later ages; 3) establishing a comparable study of clinical groups through which we can see if markers identified in (2) are still applicable in patients; and 4) harmonizing assessment protocols in a number of global LMIC adolescent cohorts. In this prospective review we discuss the achievements over the past 10 years and ongoing initiatives to reduce the burden of mental disorders through precision medicine.
Why it is important to identify psychobiological mechanisms of mental disorders
A major hurdle to elucidating the psychobiological basis of psychiatric disorders is the heterogeneity in symptom presentation. Therefore, to better understand such phenotypic complexity, we must move from dichotomous disease classifications to ones that reflect underlying biological mechanisms – which may show similarities across traditionally distinct symptom groups. Of particular relevance are individual differences in quantitative psychological traits related to reinforcement behaviors such as impulsivity, reward sensitivity, and emotional reactivity. These behaviors and their neurobiological processes are essential for the survival of the human species. However, when these processes deviate from normal they can lead to pervasive problems such as substance abuse disorders, affective disorders, and externalizing disorders9–18. It was therefore essential that we study these neurobiological processes and resulting behaviors in a normative sample during the neurodevelopmental period when the relevant brain areas are maturing.
To date, investigation of the IMAGEN dataset has yielded significant progress in the identification of psychobiological mechanisms of reinforcement behaviours; there are over 100 IMAGEN publications identifying the neural and genetic influences on mental health disorder symptoms. To illustrate this, we have selected studies that are multimodal (i.e., utilize multiple phenotypes including behaviour, MRI, and genetics) and/or are either translational (i.e., incorporate pre-clinical models) or transdiagnostic (i.e., investigate biological mechanisms across/within diagnostic boundaries) in their exploration of the psychobiological underpinnings of mental disorders. The findings presented in this Perspectives article not only highlight the methodological and scientific achievements of the IMAGEN and other cohort studies but also provide context for discussing how their application has clinical relevance.
Methods to elucidate neural mechanisms of mental disorders
Alterations in reinforcement-related neurobiological systems could help explain the development of mental health disorders but with the large amounts of data being generated in rich phenotypic cohort studies such as IMAGEN, standard statistical approaches (e.g., linear regression) are neither robust nor flexible enough. As described below, we have implemented methods that enable simultaneous multivariate examination of behavioral data with high-dimensional data (i.e., neurobiological and genetic data), as well as data-driven dimension reduction (variable selection) that maximizes statistical power to detect meaningful relationships between neurobiological, behavioral, and genetic data.
Employing novel methodological tools might improve precision medicine by refining the biological systems we target. Through simultaneous integration of large amounts of data (i.e., whole-brain neuroimaging and whole-genome sequencing) we can maximize the likelihood of identifying key mechanistic players. Methods such as factor analysis and adapted voxel-wise weighted correlated network analysis (WCNA18) can characterize networks of interdependent brain regions, as opposed to identifying single regions of interest as per the traditional fMRI mass univariate approach. Using WCNA we identified a coordinated network of 21 brain areas active during reward anticipation and found that activation was differentially associated with externalizing behaviors: activation in the striatum was related to hyperactivity symptoms whereas activation in the occipital cortex was related to alcohol consumption19. These seemingly incongruent findings are some of the first indications that coordinated activity of regions within a network can manifest as varied, but related and sometimes comorbid, psychopathologies.
Disorders associated with deficits in reinforcement behaviours might arise via common neural mechanisms (helping explain the symptomatic comorbidity that exists across disorders) or distinct neural mechanisms (accounting for some of the phenotypic heterogeneity between some disorders). We found that impulsivity-related neural networks explain common and unique variance across externalizing disorders. Exploratory factor analysis on the Stop Signal Task found reduced activation in successful inhibition networks was related to substance misuse whereas reduced activation in stop fail networks was only related to ADHD symptoms, suggesting distinct inhibitory control mechanisms contribute to these disorders. The same factor analysis-derived impulsivity networks were entered into a series of structural equation models along with externalizing symptoms: increased activation during successful inhibition (presupplementary motor area and precentral gyrus) was common to ADHD, conduct disorder (CD), and substance use but low activation during failed inhibition (frontal cortices) was only related to ADHD/CD17. The identification of shared neural mechanisms of impulsivity in ADHD and CD suggests therapies targeting these networks could improve multiple externalizing mental disorders. These findings necessitate incorporation of longitudinal data to see if the same neural signatures predict these symptoms in late adolescence/early adult adulthood.
Genetic impact on neural mechanisms of mental disorders
The aforementioned relationships between neurobiology and mental disorder symptoms may be partly due to genetically-induced neurobiological differences in brain structure and function20, 21. Using genome-wide complex trait analysis22, 23, a method that enables the simultaneous inclusion of single nucleotide polymorphisms (SNPs) across the genome rather than the numerous univariate tests of a standard GWAS, we found that 44-54% of the variation in intracranial volume, total brain volume, and hippocampal volume is explained by the additive effect of single nucleotide polymorphisms across the genome24. This method may prove more comprehensive than traditional GWAS studies where single SNPs account for 0.5% or less of the variance in these measures. When pooled with 30,000 datasets from ENIGMA, a global imaging genetics consortium, we found that individual genetic variants explained 0.52% of variance in putamen volume25. The SNP with the strongest effect on putamen volume was subsequently linked to ADHD scores26.
Reinforcement-related brain function is also heritable27 and we find that both the genetics and neurobiology of reward sensitivity are linked to mental disorders. The most consistent findings involve GTPase proteins, which influence synaptic signalling/signal transduction (e.g., through regulation of vesicle transport, actin cytoskeleton dynamics, and dendritic spine plasticity28, 29). Associated genes, such as synaptic signalling gene Ras suppressor 130, ras-specific guanine-nucleotide releasing factor 231,32, ADP-ribosylation factor 633, and Kalirin34 have been found to affect alcohol consumption as well as ventral striatum function in both animal models and IMAGEN participants. The next empirical step would be to test whether these biological mechanisms relate to drinking behaviour in patients with alcohol use disorder.
Our translational results indeed suggest conserved modulatory roles of genetics and neurobiology on behaviour across species. Mielenz et al 35 reported that EF hand domain containing 2 (EFhd2) knockout mice had increased alcohol consumption and low-anxiety phenotype. This was corroborated in the IMAGEN sample where the rs112146896 SNP had a positive association with lifetime drinking and a negative association with anxiety symptoms. Such translational approaches are essential as they may improve the likelihood that clinical trials show therapeutic efficacy in humans by incorporating genetics, potentially reducing the number of treatment non-responders.
Sex differences in neural mechanisms of mental disorders
While genetics can shed light on the heritability of mental disorders, linking them to measures of neurobiological structure and function could suggest new therapy targets. But mechanistic understanding does not stop there, as our research suggests neurobiological mechanisms vary according to demographics such as sex. A SNP in the kinectin-encoding KTN1 gene (associated with organelle motility and associated with putamen volume25) was found to interact with sex such that stronger putamen activation during reward anticipation was seen in girls with more copies of the C allele26. Furthermore, stronger putamen activation was correlated with less severe ADHD symptoms. In another study, we combined data from three independent cohorts and found that in boys who used cannabis, higher polygenic risk scores were associated with lower cortical thickness. Another sex-dependent finding emerged in our work examining neural activation modules during reward anticipation19. Of 21 modules identified, striatal node activation was negatively correlated with hyperactivity in boys. Notably, through GWAS analysis we found that vacuolar protein-sorting associated protein 4A was negatively associated with striatal node activation and positively associated with hyperactivity in boys. These findings highlight how sex plays a key role in understanding the relations between genetic and neurobiological mechanisms of psychiatric disorders, certainly when we consider the known sex differences in mental disorder prevalence36. Unfortunately, sex differences are often ignored in mental disorder clinical trials research37. These data advocate that future mental health research investigate interindividual factors that affect treatment efficacy, as it may be sensitive to not only sex38 but also differences in their environment.
Environmental impact on neural mechanisms of mental disorders
Adverse environmental experiences such as neighbourhood deprivation39, air pollution40, childhood maltreatment41, and even common stressful life events42 are associated with higher rates of mental disorders in young people but for many years the neurobiological underpinnings of such relationships were unknown. The advent of neuroimaging has enabled researchers to identify structural neural correlates of maltreatment43, 44, and urban upbringing45; generally, these early life stressors are associated with smaller brain structures in adolescence and adulthood. However, few studies have incorporated mental disorder-relevant behavior, neuroimaging, and environmental variables. For example, peer victimization has been shown to be a contributor to mental health disorders46, 47, even above and beyond that of family background and genetic risk48, but if and how the brain moderates these relationships was unknown. In IMAGEN we showed for the first time that peer victimization results in increased generalized anxiety in part by affecting the rate of structural brain development across adolescence49. Early interventions to reduce these exposures could mitigate the changes in brain structure underlying the development of mental disorders.
In addition to brain structure we find that brain function is also impacted by one’s environment. Fourteen-year old IMAGEN participants with high levels of conduct or ADHD symptoms had stronger activation in the amygdala, an area crucial for emotional processing and response, during emotional face processing only when they had also experienced a high number of stressful life events in the previous year – an interaction not observed in internalizing symptoms50. The phenotypic richness of the IMAGEN study is helping unpack the relationship between environmental risk factors, neurobiology, and mental health.
The most commonly studied environmental influences, such as trauma and stress, concern an individual’s life events and are generally captured via self-report. Satellite technology is providing a uniquely objective window into the past and how the evolution of one’s physical environment also impacts their mental health. Urbanization is one of the greatest modern environmental challenges and indices such as ground level population, vegetation, and night light can be quantified using remote sensing satellite data. We have started to explore how urban living relates to brain structure, function, and symptoms of depression in adolescents51. In addition to risk factors, such as urbanicity, for the development of mental disorders, satellite data is also showing promise in identifying factors that might convey resilience, such as green space52,53 against them.
Using longitudinal data to develop prediction and developmental trajectory models of mental disorders
In order to get an accurate handle on risk and resilience factors for developing mental disorders or begin to develop therapies and study their behavioural impact/effects over time, we first need to understand how potential psychobiological mechanisms related to these behaviors develop over time. While the cross-sectional IMAGEN papers have generated novel insights into the genetic and neural networks that characterize psychopathology-relevant behaviours in early adolescence, for most of the identified neurobehavioral mechanisms the question remains: do these mechanisms of psychopathology symptoms at age 14 predict clinically relevant behavior in late adolescence/early adulthood? Whilst few studies incorporating age 19 data have been published, data from age 14 has successfully predicted psychopathology symptoms at age 16. Reduced activity in the ventral striatum during reward anticipation predicted problematic drug use in novelty seeking adolescents54 as well as the transition to subthreshold or clinical levels of depression55. While the latter finding suggests a neural mechanism across the risk spectrum for depression, together the results provide good evidence that a shared neural mechanism could help identify individuals at risk for developing these comorbid diagnoses56.
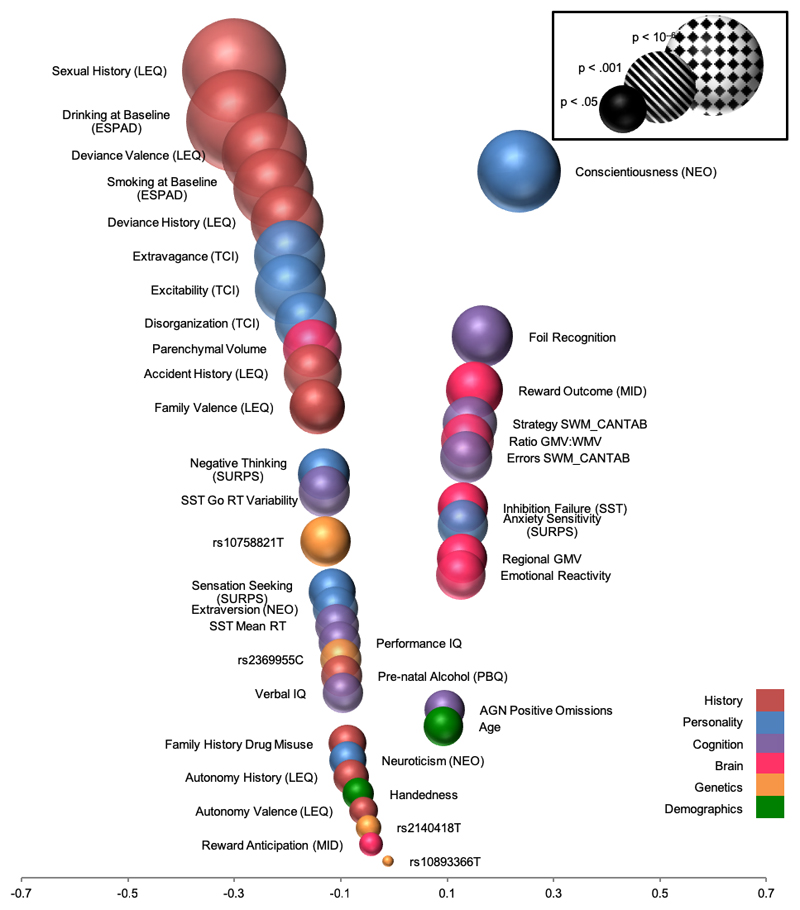
Using a machine learning approach we generated a multivariate model that identified 30 variables from 6 domains (personal history, personality, genetics, brain, cognition, demographics) that correctly predicted 66% of binge drinkers and 73% of future alcohol binge drinkers57 with personal history, personality, and neural domains being the most predictive. This multivariate prediction profile serves as a methodological benchmark and highlights how the incorporation of variables across many domains outperforms a univariate approach, explaining more variance than any single measure. Developing a sparse model in an informed way by selecting the best predictors (i.e., personality, personal history, brain structure) (Figure 1) would be cheaper to assess than the full model and could be more widely distributed. However, we aim to improve prediction accuracy by refining our methodological approaches and applying the predictive classifiers to clinical data – a vital step to translating identified neurobehavioral mechanisms to the intended patient target group(s).
Figure 1.
Classification accuracy of variables from six domains predicting age 16 binge drinking using data from age 14. Position on the x-axis represents the correlation between each variable and binge drinking. Based on data from Whelan et al 2014 52. CANTAB = Cambridge Neuropsychological Test Automated Battery; ESPAD = European School Survey Project on Alcohol and Other Drugs; GMV = gray matter volume; LEQ = Life Events Questionnaire; MID = fMRI Monetary Incentive Delay task; NEO = NEO Personality Inventory; PBQ = Pregnancy and Birth Questionnaire; RT = reaction time; SURPS = Substance Use Risk Profile Scale; SST = fMRI Stop Signal Task; SWM = Spatial Working Memory; TCI = Temperament and Character Inventory.
In addition to identifying psychopathology predictors we aim to establish longitudinal trajectories of neurobehavioral development during adolescence and young adulthood that relates to reinforcement-related psychopathology. In a recent study we identified a groups of adolescents who were chronically victimized by their peers across adolescence49. By age 19 these individuals had higher rates of anxiety, depression, and hyperactivity. The novel finding was that the relationship between chronic peer victimization and generalized anxiety was partially mediated by decreases in the volumes of the left caudate and putamen. These data suggest that the experience of chronic peer victimization during adolescence might induce psychopathology-relevant deviations from normative brain development. Availability of neuroimaging data from age 22 will enable linkage of peer victimization trajectories with trajectory curves of adolescent brain development. Examining longitudinal genetic, epigenetic, and environmental data will help us elucidate which factors influence the behavioral/neural trajectories and perhaps convey risk for, or resilience58–61 against, the development of brain network configurations associated with mental disorders.
Exploration of IMAGEN-derived biological mechanisms in clinical samples
With each longitudinal follow-up, IMAGEN will continue to further our understanding of "healthy" adolescent brain and behavioural development. However, as IMAGEN participants were school-recruited, and not recruited because they had, or were at high risk for, mental disorders, clinical phenotypes are limited in the normative sample. While most mental disorders have their peak incidence during the second and third decades of life as measured by first psychiatric treatment62, half of the lifetime psychopathological burden of most mental disorders can be detected by the mid-teens and 75% by the mid-20s63. This illustrates that there is a time period of several years between the occurrence of first symptoms and the first contact with psychiatric services (i.e., patients often present at a point when severe psychopathology has developed, which gravely impairs daily functioning). Furthermore, obstacles to early diagnoses are the facts that early behavioral symptoms of later mental disorders are often unspecific and/or reversible. Therefore, the identification of widely applicable early biological markers, which specifically predict the development or remission of mental disorders and allow for targeted early interventions in adolescence/early adulthood, is imperative.
To achieve this we are recruiting 800 patients (aged 19-25) with major depression, alcohol use disorder, and psychosis, as well as controls, in the STRATIFY project. Furthermore, by harmonizing the STRATIFY protocol to the IMAGEN protocol, we will be able to use IMAGEN to identify a group of individuals at the extreme end of a clinically-relevant phenotype at age 19 or 22 and determine whether the biological signatures in those individuals correspond to signatures identified in the clinical samples. We hypothesize that comorbidity among these disorders is driven largely by alterations in neurobehavioral reinforcement processes. Crucially, STRATIFY will also support backwards translation of the clinically-derived biological markers to the IMAGEN sample, determining whether there is predictive mechanistic overlap from age 14. This would subsequently allow for the development of early interventions in adolescence that target specific neurobehavioral phenotypes, thereby facilitating more efficient and cost-effective public health programs.
Going global: studying mental health in varied cultural and environmental contexts
It is naïve to assume that mental health therapies and programs will be universally effective. Inherent in the quest for precision medicine is the understanding of how cultural and environmental contexts influence the development of psychopathology64. Until recently, large-scale neuroimaging genetics studies have been relegated to the West, confounding the generalizability to other countries and cultures. To address this knowledge gap, the IMAGEN study has aligned itself with other global neuroimaging-genetics adolescent cohorts, an effort called the Global Imaging Genetics of Adolescents (GIGA). It will enable comparative research on brain development and behavior in different cultures, environments and ethnic groups, both in industrialized nations and low- and medium-income countries (LMIC). A great burden of mental disorders exists in LMIC with environmental and sociocultural factors distinct from High Income Countries (HIC). For example, in many HIC planned urbanization results in improved availability and quality of infrastructure. In contrast, LMIC often experience rapid urbanization with lack of planned infrastructure leading to increased poverty and environmental diversity, as well as increased rates of anxiety, depression, and substance abuse. Environmental factors related to urbanization are the most rapidly growing cause for mental illness.
While Europe and North America are among the most urbanized regions, India will contain 404 million urban dwellers by 202065. Furthermore, alcohol use disorder, with a prevalence of 17% (www.nhp.gov.in/healthlyliving/alcohol-use-disorder) and a high comorbidity with other externalizing disorders66, comprises a large share of India’s mental health burden and will likely increase as people endure the stresses of urbanization67. As part of GIGA, the c-VEDA (Consortium on the Vulnerability to Externalizing Disorders and Addictions) project, a joint venture between the United Kingdom and 6 centers in India, will recruit 10,000 participants (age 6-23 years) over four years, 1500 of whom will undergo an MRI scan. The c-VEDA protocol significantly overlaps with IMAGEN but has added relevant domains (e.g., neurotoxins, air pollution, nutrition). The study has an accelerated longitudinal design to facilitate better understanding of brain growth and maturation trajectories, as well as identify the environmental and biological vulnerabilities to mental health problems.
The GIGA data from IMAGEN and c-VEDA are being enhanced by several cohorts in China and North America. Beijing Normal University, Tianjin Medical University, Peking University and Fudan University will contribute behavioral, neuroimaging, and genetic data from ~15000 healthy children and adolescents. The U.S.-based Adolescent Brain Cognitive Development (ABCD) project is following almost 12,000 individuals from ages 9 to 19. Central to the mission of GIGA, we are harmonizing the protocols among the cohorts, covering domains of environment/early life exposures, substance use, personality, and psychopathology (see Table 2). The multivariate analysis tools we have developed in IMAGEN can be applied to the GIGA cohorts, enabling mega analyses as well as comparative studies across cohorts, cultures, and geographical regions.
Table 2. Shared assessment domains for the GIGA cohorts.
| Domain | Assessment | |
|---|---|---|
| Sociodemographic information | ||
| MRI | ||
| Resting-state fMRI | ||
| Structural MRI (T1) | ||
| Diffusion Tensor Imaging (DTI) | ||
| Anthropometrics | ||
| BMI | ||
| Biological Information | ||
| Saliva | ||
| Blood | ||
| Environment/Early life exposures | ||
| Socioeconomic information | ||
| Urbanization | ||
| Pre-/peri-natal information | ||
| Childhood maltreatment | ||
| Family/community violence | ||
| Life events | ||
| Parenting style | ||
| Mobile phone use | ||
| Temperament/Personality | ||
| Inhibition/impulsivity | ||
| Reward sensitivity | ||
| Mental health disorder symptoms | ||
| Substance use | ||
| Anxiety | ||
| Depression | ||
| Conduct disorder | ||
| ADHD | ||
| Psychosis | ||
| Neuropsychology | ||
| Cognitive ability/IQ | ||
| Emotional attention | ||
Pertinent questions that can be addressed using multiple cohorts include, but are not limited to: 1) Are there common biological mechanisms (neural constructs, genetics) underlying mental health disorders across cultural and ethnic backgrounds? That is, do neural constructs related to mental health disorder symptoms identified in the Western European IMAGEN cohort hold in China, India, and the USA? 2) Are such mechanisms differentially modulated according to unique cultural or environmental contexts? To help answer these questions we need globally applicable quantitative environmental measures acquired over time. While GIGA has strived to harmonize assessments across the cohorts, they are not all directly comparable. Another way to acquire globally applicable measures is through remote sensing satellite data, which have been recorded continuously since the 1970’s and can, therefore, provide suitable indices of the evolution of the natural and urban environments. From the satellite data we can extract environmental indices unique to an individual and then relate them to biological and behavioral data. Recently completed work explored whether brain differences related to urbanicity were similar or distinct between two of the GIGA cohorts51, the Chinese-based CHIMGEN study and IMAGEN.
Conclusions and Impact
The current state of psychiatric diagnoses and treatment is based on symptoms presumed to be identical in all individuals with a particular diagnosis, but this generalized approach is not feasible. Up to a third of individuals do not respond well, if at all, to pharmacotherapy, with mixed results for limited research into non-pharmacological interventions68. Findings from the IMAGEN study suggest the reason for such differential responses to therapy lies in the heterogeneity of the biological factors underlying psychopathology-relevant symptoms. To date our research efforts have identified functional and structural neural networks, often stratified by genetics, which are common and distinct among psychiatric symptom clusters and traditional diagnostic disorders.
Despite the academic advances made thus far, IMAGEN cannot address all mental disorder mechanism research questions. One consequence of IMAGEN recruiting a 14-year old population cohort is that we cannot identify psychobiological mechanisms of disorders with an earlier presentation, such as autism spectrum disorder or early onset anxiety disorders, eating disorders, etc. Furthermore, potential participants were excluded if they had been previously treated for bipolar disorder or schizophrenia. IMAGEN is not suited to examine certain environmental influences such as nutrition or hazardous exposures as participant families were of a relatively homogeneous socioeconomic Western European background. Lastly, due to a 25% attrition rate longitudinal multivariate research efforts may result in smaller sample sizes and reduced power for some statistical analyses. These are just a few reasons highlighting the importance of large diverse studies such as those comprising the GIGA consortium. The GIGA consortium is also a good model for a future international cooperative based on STRATIFY in order to enrich the dataset and augment patient numbers per diagnosis.
Precision medicine aims to harness knowledge gained from studies like IMAGEN in order to match the right patient to the right therapy. We need to understand not only the psychobiological underpinnings of symptoms but also how a person’s environment factors into the development of mental disorders. Protocol harmonization across international neuroimaging-genetics studies will enable us to validate the mechanistic findings from the IMAGEN project in different cultural and environmental contexts. In support of this we are developing novel analysis methods that can be applied to other large multivariate datasets to generate parsimonious predictor profiles of mental disorders. Ultimately, our approaches will advance precision medicine in mental health by allowing accurate patient stratification. By doing so, pharmacological, behavioral, and environmental interventions could be developed and implemented at an earlier stage in the disease process, improving quality of life and reducing countries' mounting fiscal burdens.
Acknowledgments
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (PR-ST-0416-10004), BRIDGET (JPND: BRain Imaging, cognition Dementia and next generation GEnomics) (MR/N027558/1), the FP7 projects IMAGEMEND(602450; IMAging GEnetics for MENtal Disorders) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grant 'c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the Swedish Research Council FORMAS, the Medical Research Council, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL 01EE1406A, 01EE1406B), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-2, SFB 940/2), the Medical Research Foundation and Medical research council (grant MR/R00465X/1), the Human Brain Project (HBP SGA 2). Further support was provided by grants from: ANR (project AF12-NEUR0008-01 - WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence.
Footnotes
Conflict of Interest
Dr. Banaschewski has served as an advisor or consultant to Actelion, Hexal Pharma,Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Lundbeck, Medice, Neurim Pharmaceuticals, Novartis, Pfizer, and Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, and Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire and Viforpharma; he received royalities from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press; the present work is unrelated to these relationships. Dr. Barker has received honoraria from General Electric Healthcare for teaching on scanner programming courses and acts as a consultant for IXICO. The other authors report no biomedical financial interests or potential conflicts of interest.
Disclosures
Dr. Banaschewski served in an advisory or consultancy role for Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Shire. He received conference support or speaker’s fee by Lilly, Medice, Novartis and Shire. He has been involved in clinical trials conducted by Shire & Viforpharma. He received royalities from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press. The present work is unrelated to the above grants and relationships. Dr. Barker has received honoraria from General Electric Healthcare for teaching on scanner programming courses and acts as a consultant for IXICO. The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 2.Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(10):718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Trautmann S, Rehm J, Wittchen HU. The economic costs of mental disorders: Do our societies react appropriately to the burden of mental disorders? EMBO Rep. 2016;17(9):1245–1249. doi: 10.15252/embr.201642951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pangalos MN, Schechter LE, Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat Rev Drug Discov. 2007;6(7):521–532. doi: 10.1038/nrd2094. [DOI] [PubMed] [Google Scholar]
- 5.Perry CJ, Lawrence AJ. Hurdles in Basic Science Translation. Front Pharmacol. 2017;8:478. doi: 10.3389/fphar.2017.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Doef TF, Zaragoza Domingo S, Jacobs GE, Drevets WC, Marston HM, Nathan PJ, et al. New approaches in psychiatric drug development. Eur Neuropsychopharmacol. 2018;28(9):983–993. doi: 10.1016/j.euroneuro.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Schumann G, Binder EB, Holte A, de Kloet ER, Oedegaard KJ, Robbins TW, et al. Stratified medicine for mental disorders. Eur Neuropsychopharmacol. 2014;24(1):5–50. doi: 10.1016/j.euroneuro.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Friedman AA, Letai A, Fisher DE, Flaherty KT. Precision medicine for cancer with next-generation functional diagnostics. Nat Rev Cancer. 2015;15(12):747–756. doi: 10.1038/nrc4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15(12):1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 10.Wong CC, Schumann G. Review. Genetics of addictions: strategies for addressing heterogeneity and polygenicity of substance use disorders. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3213–3222. doi: 10.1098/rstb.2008.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien BS, Frick PJ. Reward dominance: associations with anxiety, conduct problems, and psychopathy in children. J Abnorm Child Psychol. 1996;24(2):223–240. doi: 10.1007/BF01441486. [DOI] [PubMed] [Google Scholar]
- 12.Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- 13.Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Olson SL, Schilling EM, Bates JE. Measurement of impulsivity: construct coherence, longitudinal stability, and relationship with externalizing problems in middle childhood and adolescence. J Abnorm Child Psychol. 1999;27(2):151–165. doi: 10.1023/a:1021915615677. [DOI] [PubMed] [Google Scholar]
- 15.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26(4):379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15(6):920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- 17.Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde AL, et al. Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am J Psychiatry. 2014;171(12):1310–1319. doi: 10.1176/appi.ajp.2014.13111499. [DOI] [PubMed] [Google Scholar]
- 18.Mumford JA, Horvath S, Oldham MC, Langfelder P, Geschwind DH, Poldrack RA. Detecting network modules in fMRI time series: a weighted network analysis approach. Neuroimage. 2010;52(4):1465–1476. doi: 10.1016/j.neuroimage.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia T, Macare C, Desrivieres S, Gonzalez DA, Tao C, Ji X, et al. Neural basis of reward anticipation and its genetic determinants. Proc Natl Acad Sci U S A. 2016;113(14):3879–3884. doi: 10.1073/pnas.1503252113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63(9):852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, et al. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010;49(1):42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toro R, Poline JB, Huguet G, Loth E, Frouin V, Banaschewski T, et al. Genomic architecture of human neuroanatomical diversity. Mol Psychiatry. 2015;20(8):1011–1016. doi: 10.1038/mp.2014.99. [DOI] [PubMed] [Google Scholar]
- 25.Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivieres S, Jahanshad N, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015;520(7546):224–229. doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu B, Jia T, Macare C, Banaschewski T, Bokde ALW, Bromberg U, et al. Impact of a Common Genetic Variation Associated With Putamen Volume on Neural Mechanisms of Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(5):436–444 e434. doi: 10.1016/j.jaac.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Silverman MH, Krueger RF, Iacono WG, Malone SM, Hunt RH, Thomas KM. Quantifying familial influences on brain activation during the monetary incentive delay task: an adolescent monozygotic twin study. Biol Psychol. 2014;103:7–14. doi: 10.1016/j.biopsycho.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 29.Hall A. The cellular functions of small GTP-binding proteins. Science. 1990;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- 30.Ojelade SA, Jia T, Rodan AR, Chenyang T, Kadrmas JL, Cattrell A, et al. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc Natl Acad Sci U S A. 2015;112(30):E4085–4093. doi: 10.1073/pnas.1417222112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, et al. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc Natl Acad Sci U S A. 2011;108(17):7119–7124. doi: 10.1073/pnas.1017288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stacey D, Bilbao A, Maroteaux M, Jia T, Easton AC, Longueville S, et al. RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release. Proc Natl Acad Sci U S A. 2012;109(51):21128–21133. doi: 10.1073/pnas.1211844110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez DA, Jia T, Pinzon JH, Acevedo SF, Ojelade SA, Xu B, et al. The Arf6 activator Efa6/PSD3 confers regional specificity and modulates ethanol consumption in Drosophila and humans. Mol Psychiatry. 2018;23(3):621–628. doi: 10.1038/mp.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pena-Oliver Y, Carvalho FM, Sanchez-Roige S, Quinlan EB, Jia T, Walker-Tilley T, et al. Mouse and Human Genetic Analyses Associate Kalirin with Ventral Striatal Activation during Impulsivity and with Alcohol Misuse. Front Genet. 2016;7:52. doi: 10.3389/fgene.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mielenz D, Reichel M, Jia T, Quinlan EB, Stockl T, Mettang M, et al. EFhd2/Swiprosin-1 is a common genetic determinator for sensation-seeking/low anxiety and alcohol addiction. Mol Psychiatry. 2018;23(5):1303–1319. doi: 10.1038/mp.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, et al. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66(7):785–795. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howard LM, Ehrlich AM, Gamlen F, Oram S. Gender-neutral mental health research is sex and gender biased. Lancet Psychiatry. 2017;4(1):9–11. doi: 10.1016/S2215-0366(16)30209-7. [DOI] [PubMed] [Google Scholar]
- 38.Riecher-Rossler A. Sex and gender differences in mental disorders. Lancet Psychiatry. 2017;4(1):8–9. doi: 10.1016/S2215-0366(16)30348-0. [DOI] [PubMed] [Google Scholar]
- 39.Caspi A, Taylor A, Moffitt TE, Plomin R. Neighborhood deprivation affects children's mental health: environmental risks identified in a genetic design. Psychol Sci. 2000;11(4):338–42. doi: 10.1111/1467-9280.00267. [DOI] [PubMed] [Google Scholar]
- 40.Roberts S, Arseneault L, Barratt B, Beevers S, Danese A, Odgers CL, et al. Exploration of NO2 and PM2.5 air pollution and mental health problems using high-resolution data in London-based children from a UK longitudinal cohort study. Psychiatry Res. 2019;272:8–17. doi: 10.1016/j.psychres.2018.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lansford JE, Dodge KA, Pettit GS, Bates JE, Crozier J, Kaplow J. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Arch Pediatr Adolesc Med. 2002;156(8):824–830. doi: 10.1001/archpedi.156.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Low NC, Dugas E, O'Loughlin E, Rodriguez D, Contreras G, Chaiton M, et al. Common stressfu life events and difficulties are associated with mental health symptos and substance use in young adolescents. BMC Psychiatry. 2012;12:116–125. doi: 10.1186/1471-244X-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiatry. 2014;171(8):854–63. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- 44.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by structural and functional structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 45.Haddad L, Schäfer A, Streit F, Lederbogen F, Grimm O, Wüst S, et al. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia. Schizophr Bull. 2015;41(1):115–22. doi: 10.1093/schbul/sbu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arseneault L, Bowes L, Shakoor S. Bullying victimization in youths and mental health problems: 'much ado about nothing'? Psychol Med. 2010;40(5):717–29. doi: 10.1017/S0033291709991383. [DOI] [PubMed] [Google Scholar]
- 47.Singham T, Viding E, Schoeler T, Arseneault L, Ronald A, Cecil CM, et al. Concurrent and longitudinal contribution of exposure to bullyig in childhood to mental health: the role of vulnerability and resilience. JAMA Psychiatry. 2017;74(11):1112–1119. doi: 10.1001/jamapsychiatry.2017.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer JD, Moffitt TE, Arseneault L, Danese A, Fisher HL, Houts R, et al. Adolescent victimization and early-adult psychopathology: approaching causal inference using a longitudinal twin study to rule out noncausal explanations. Clin Psychol Sci. 2018;6(3):352–371. doi: 10.1177/2167702617741381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinlan EB, Barker ED, Luo Q, Banaschewski T, Bokde ALW, Bromberg U, et al. Peer victimization and its impact on adolescent brain development and psychopathology. Mol Psychiatry. 2018 doi: 10.1038/s41380-018-0297-9. [DOI] [PubMed] [Google Scholar]
- 50.Quinlan EB, Cattrell A, Jia T, Artiges E, Banaschewski T, Barker G, et al. Psychosocial stress and brain function in adolescent psychopathology. Am J Psychiatry. 2017;174(8):785–794. doi: 10.1176/appi.ajp.2017.16040464. [DOI] [PubMed] [Google Scholar]
- 51.Xu J, Liu X, Ing A, Li Q, Qin W, Guo L, et al. Satellite imaging of global urbanicity relates to adolescent brain development and behavior. Lancet. In review. [Google Scholar]
- 52.Engemann K, Pedersen CV, Arge L, Tsirogiannis C, Mortensen PB, Svenning J-C. Residential green space in childhood is associated with lower risk of psychiatric disorders from adolescence into adulthood. PNAS. 2019;116(11):5188–5193. doi: 10.1073/pnas.1807504116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Berg AE, Maas J, Verheij RA, Groenewegen PP. Green space as a buffer between stressful life events and health. Soc Sci Med. 2010;70(8):1203–1210. doi: 10.1016/j.socscimed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Buchel C, Peters J, Banaschewski T, Bokde AL, Bromberg U, Conrod PJ, et al. Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nat Commun. 2017;8 doi: 10.1038/ncomms14140. 14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, et al. The Brain's Response to Reward Anticipation and Depression in Adolescence: Dimensionality, Specificity, and Longitudinal Predictions in a Community-Based Sample. Am J Psychiatry. 2015;172(12):1215–1223. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Curr Opin Psychiatry. 2008;21(1):14–18. doi: 10.1097/YCO.0b013e3282f32408. [DOI] [PubMed] [Google Scholar]
- 57.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burt KB, Whelan R, Conrod PJ, Banaschewski T, Barker GJ, Bokde AL, et al. Structural brain correlates of adolescent resilience. J Child Psychol Psychiatry. 2016;57(11):1287–1296. doi: 10.1111/jcpp.12552. [DOI] [PubMed] [Google Scholar]
- 59.Galinowski A, Miranda R, Lemaitre H, Paillere Martinot ML, Artiges E, Vulser H, et al. Resilience and corpus callosum microstructure in adolescence. Psychol Med. 2015;45(11):2285–2294. doi: 10.1017/S0033291715000239. [DOI] [PubMed] [Google Scholar]
- 60.Crush E, Areseneault L, Moffitt TE, Danese A, Caspi A, Jaffee SR, et al. Protective factors for psychotic experience amongst adolescents exposed to multiple forms of victimization. J Psychiatr Res. 2018;104:32–38. doi: 10.1016/j.jpsychires.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowes L, Maughan B, Caspi A, Moffitt TE, Areseneault L. Families promote emotional and behavioural resilience to bullying: evidence of an environmental effect. J Child Psychol Psychiatry. 2010;51(7):809–17. doi: 10.1111/j.1469-7610.2010.02216.x. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen CB, Mors O, Bertelsen A, Waltoft BL, Agerbo E, McGrath JJ, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry. 2014;71(5):573–581. doi: 10.1001/jamapsychiatry.2014.16. [DOI] [PubMed] [Google Scholar]
- 63.Kessler RC, Angermeyer M, Anthony JC, de Graaf R, Demyttenaere K, Gasquet I, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Institute. World Psychiatry. 2007;6(3):168–176. [PMC free article] [PubMed] [Google Scholar]
- 64.Schumann G, Benegal V, Yu C, Tao S, Jernigan T, Heinz A, et al. Precision medicine and global mental health. Lancet Glob Health. 2019;7(1):e32. doi: 10.1016/S2214-109X(18)30406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malhotra S, Kohli A, Kapoor M, Pradhan B. Incidence of childhood psychiatric disorders in India. Indian J Psychiatry. 2009;51(2):101–107. doi: 10.4103/0019-5545.49449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ganesh S, Kandasamy A, Sahayaraj US, Benegal V. Adult Attention Deficit Hyperactivity Disorder in Patients with Substance Use Disorders: A Study from Southern India. Indian J Psychol Med. 2017;39(1):59–62. doi: 10.4103/0253-7176.198945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srivastava K. Urbanization and mental health. Ind Psychiatry J. 2009;18(2):75–76. doi: 10.4103/0972-6748.64028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlaepfer TE, Agren H, Monteleone P, Gasto C, Pitchot W, Rouillon F, et al. The hidden third: improving outcome in treatment-resistant depression. J Psychopharmacol. 2012;26(5):587–602. doi: 10.1177/0269881111431748. [DOI] [PubMed] [Google Scholar]