Abstract
The aim of this study was to evaluate the Candida species, predisposing factors, antifungal treatment approaches, and clinical outcomes of invasive Candida infections (ICIs) in a tertiary pediatric intensive care unit (PICU). A retrospective study was performed from January 2008 to January 2019 including 102 children with ICIs who were admitted to a university hospital in southeastern Turkey. Positive blood cultures were detected in 43 (42.1%) patients, and positive urine cultures were detected in 59 (57.8%). According to our results, Candida albicans (42.2%) was the most common species for all isolates followed by C. parapsilosis (17.6%). In our patient population, non- albicans Candida species were dominant (57.8%) in all isolates. The most common facilitating factor in our study was the use of mechanical ventilator support (87.3%). The mortality rate of our patients with ICIs was 13.7%. Candida albicans was found to have the highest mortality rate among all Candida species (30.7%). When we compared patients with C. albicans and those with non- albicans Candida species in terms of risk factors, we detected a significant difference between the two groups for total parenteral nutrition use ( p = 0.027). Fluconazole was the most preferred (58.8%) treatment option in our PICU for ICIs. Our results showed an increased trend in micafungin use in recent years. ICIs are a significant problem due to the high mortality and morbidity rates in critically ill pediatric patients in PICUs. In recent years, an increase in Candida infections caused by non- albicans Candida species has been reported. Multicenter prospective studies are needed to determine the risk factors for ICIs.
Keywords: pediatric, invasive candidiasis, non- albicans Candida, pediatric intensive care unit
Introduction
Infections caused by Candida species, which are the normal flora elements of the body, can range from simple mucocutaneous disease to invasive Candida infection (ICI). 1 ICIs caused by Candida species are significant causes of nosocomial infections and mortality, especially in critically ill children and neonates. 2 The high-risk group for ICI includes pediatric patients with hematological–oncological malignancies undergoing chemotherapy or bone marrow transplantation, those with congenital or acquired immunodeficiency states, and those who need immunosuppressive therapy due to various reasons. 3 Candida infections are the third most common cause of healthcare-associated bloodstream infections and the second most common cause of central catheter associated bloodstream infections in children. 4
ICIs have increased in the last two decades as a result of the improvement in intensive care units and the increased use of invasive procedures. Broad-spectrum antibiotics, central venous catheters (CVCs), parenteral nutrition, renal replacement therapy, neutropenia, malignancy, and immunosuppressive treatments are the most reported facilitating factors for ICI in intensive care units. 5
Although C. albicans is the most common isolated species for ICI, the incidence of non- albicans types has increased in recent years. Candida glabrata , C. parapsilosis , C. krusei , and C. tropicalis , in particular, are the non- albicans Candida species reported with increasing frequencies. 6 7 Candida infections are a major cause of morbidity and mortality in critically ill children in PICUs, and the reported mortality rates are different in pediatric studies. 8 9 10
In this study, we aimed to determine the isolated Candida species, identify predisposing factors and antifungal treatment approaches, and investigate the demographic characteristics and clinical outcomes of patients with ICI in a tertiary pediatric intensive care unit (PICU) during an 11-year period.
Materials and Methods
This study is a retrospective review conducted at a university hospital in Adana, Turkey. Patients diagnosed with invasive candidiasis according to the surveillance criteria of Center for Disease Control and Prevention (CDC) were included in this study.
In accordance with the CDC surveillance criteria, blood, urine, tracheal aspirate, cerebrospinal fluid, and pleural fluid cultures were evaluated retrospectively. The presence of at least one type of Candida in the blood culture and the presence of an infection such as concomitant fever, hypothermia, leukocytosis, elevation in acute phase reactants, tachycardia, and hypotension were evaluated as candidemia. 11
Diagnosis of symptomatic urinary tract infection was made in the case of urinary tract infection, fever > 38°C, and at least one of the signs and symptoms of urgency, frequency, dysuria or suprapubic tenderness, suprapubic susceptibility, and isolation of Candida species in the urine culture. 11
In patients with new or progressive infiltration on chest X-ray, the presence of two or more of the findings of fever > 38°C or hypothermia, leukocytosis or leukopenia, purulent secretion, and Candida isolation in tracheal aspirate culture was defined as pneumonia. 11
The records of patients were examined retrospectively, and all patients' clinical and laboratory findings, age, gender, underlying disease, Candida species isolated from blood culture, urine culture and tracheal aspirate culture, antifungal treatments, duration of treatment, length of stay in hospital and intensive care unit, mechanical ventilator requirement, and mortality information were recorded. In patients with more than one episode, only the first episode was included. Risk factors for ICI included the presence of CVC, permanent catheter and urinary catheter, trauma, neutropenia, total parenteral nutrition (TPN), steroid treatment, hemodialysis, recent surgery, and broad-spectrum antibiotic treatment.
Microscopic morphology of Candida species was examined in agar–agar with corn flour and 1% Tween using the API ID 32 C yeast identification kit (bioMérieux, Marcy L'Etoile, France).
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences, version 21.0 (SPSS Inc., Chicago, Illinois, United States). All collected variables were analyzed using descriptive and inferential statistics. Categorical variables are described and reported as counts and percentages. Continuous variables are described and reported as mean ± standard deviation. Patient survival status was the main study outcome variable and was analyzed versus all demographic and clinical characteristics of the patients using univariate and multivariate analyses. A p -value of ≤0.05 was accepted as the significance level for all statistical tests.
Ethical Approval
The study was reviewed and approved by the Ethics Committee of the Medical Faculty of the University (2019:84).
Informed Consent
Since the study was conducted retrospectively, family consent could not be obtained.
Results
During the 11-year period, a total of 102 patients with ICI were included, of whom 58.8% of the patients were males. The mean age was 52.80 ± 58.58 months. Table 1 summarizes the underlying diagnoses of the patients. Positive blood culture was detected in 43 (42.1%) patients, and positive urine culture was detected in 59 (57.8%). Nine different Candida species were detected in blood and urine cultures. Candida -associated nosocomial pneumonia was not detected in any patients. Among the patients with Candida infections, C. albicans was isolated from 43 (42.2%) patients, C. parapsilosis from 18 (17.6%), C. tropicalis from 14 (13.7%), C. glabrata from 8 (7.8%), C. famata from 7 (6.9%), C. krusei from 1 (%1), C. lusitaniae from 1 (1%), and C. guilliermondii from 1 (1%), and other nonidentified species were isolated from 9 (8.8%) patients. The Candida species that was the most commonly isolated from blood culture was C. albicans ( 37.2%) followed by C. parapsilosis (34.8%). The Candida that was the most commonly isolated from urine culture was C. albicans (44.1%) (similar to blood culture) followed by C. tropicalis (15.2%). For all isolates, non- albicans Candida species were dominant (57.8%) in our patient population. The frequency of isolated Candida species according to years is shown in Table 2 . The frequency of ICI in our PICU according to years is shown in Fig. 1 .
Table 1. Underlying diagnosis of patients with ICIs.
| n = 102 | n (%) |
|---|---|
| Neurologic disease | 29 (28.5) |
| Metabolic disorders | 15 (14.7) |
| Postoperative cardiac surgery | 9 (8.8) |
| Major noncardiac surgery | 9 (8.8) |
| Infectious diseases | 18 (17.6) |
| Trauma | 2 (1.9) |
| Hematological–oncological malignancies/chemotherapy or allogenic bone marrow transplantation | 12 (11.8) |
| Congenital or acquired immunodeficiency | 8 (7.9) |
Abbreviation: ICI, invasive Candida infection.
Table 2. Isolated Candida species according to years .
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida albicans | 11 | 5 | 1 | 2 | 2 | 1 | 3 | 4 | 6 | 3 | 5 |
| Candida tropicalis | 4 | 1 | 4 | 1 | 2 | 1 | 1 | ||||
| Candida parapsilosis | 4 | 5 | 3 | 2 | 1 | 2 | 1 | ||||
| Candida glabrata | 2 | 2 | 2 | 1 | 1 | ||||||
| Candida crusei | 1 | ||||||||||
| Candida lusitaniae | 1 | ||||||||||
| Candida famata | 1 | 1 | 2 | 3 | |||||||
| Candida guilliermondii | 1 | ||||||||||
| Nonidentified species | 1 | 2 | 1 | 2 | 3 | ||||||
| Total | 22 | 14 | 5 | 9 | 8 | 4 | 8 | 8 | 7 | 8 | 9 |
Fig. 1.
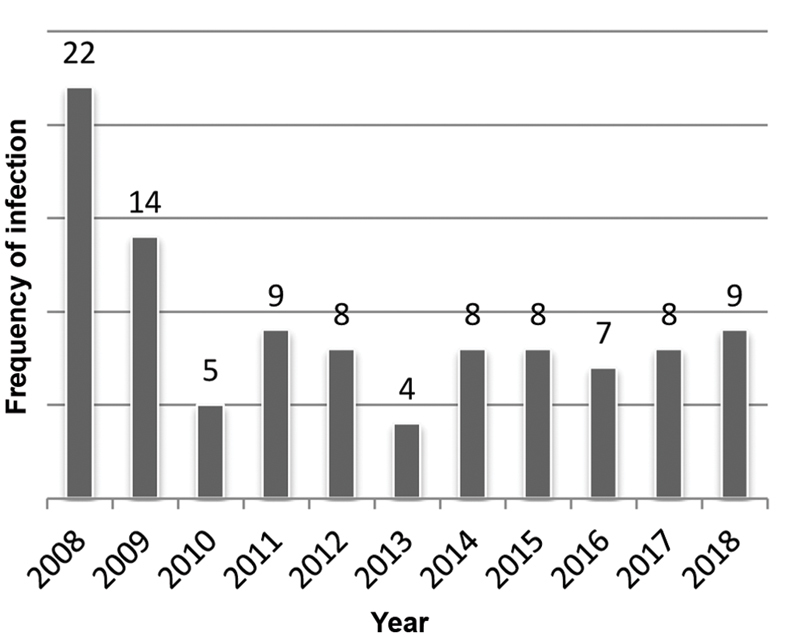
Invasive Candida infection frequency according to years.
The reported risk factors of ICI were the presence of a CVC in 81 (79.4%) patients, the presence of a permanent catheter in 10 (9.8%), TPN in 16 (15.7%), neutropenia in 17 (16.7%), steroid treatment in 46 (45.1%), requirement of mechanical ventilators in 89 (87.3%), wide-spectrum antibiotic use in 72 (70.6%), hemodiafiltration in 8 (7.8%), surgery in 38 (37.3%), and Foley catheter in 77 (75.5%).
The mortality rate of our patients with ICI was 13.7%. Candida albicans was found to have the highest mortality rate among all Candida species (30.7%). We compared the surviving and nonsurviving groups in terms of risk factors but did not detect any significant difference between the two groups ( Table 3 ). We compared patients with C. albicans and those with non- albicans Candida species in terms of risk factors and detected a higher TPN use rate in the C. albicans group (25.6%) compared with the non- albicans group (8.5%). There was a significant difference between the two groups in terms of TPN use ( p = 0.027).
Table 3. Comparison of predisposing factors for survived and nonsurvived groups.
|
Nonsurvived group,
n
(%)
14 (13.7) |
Survived group,
n
(%)
88 (86.2) |
p -Value | |
|---|---|---|---|
| Sex | 1 | ||
| Female | 6 (42.9) | 36 (40.9) | |
| Male | 8 (57.1) | 52 (59.1) | |
| Central venous catheter | 0.290 | ||
| Yes | 13 (92.9) | 68 (77.3) | |
| No | 1 (7.1) | 20 (22.7) | |
| Permanent catheter | 0.350 | ||
| Yes | 0 | 10 (11.4) | |
| No | 14 (100) | 78 (88.69 | |
| Foley catheter | 0.179 | ||
| Yes | 13 (92.9) | 64 (72.7) | |
| No | 1 (7.1) | 24 (27.3) | |
| Neutropenia | 0.244 | ||
| Yes | 4 (28.6) | 13 (14.8) | |
| No | 10 (71.4) | 75 (85.2) | |
| Total parenteral nutrition | 0.042 | ||
| Yes | 5 (35.7) | 11 (12.5) | |
| No | 9 (64.3) | 77 (87.5) | |
| Mechanical ventilation | 0.206 | ||
| Yes | 14 (100) | 75 (85.2) | |
| No | 0 | 13 (14.8) | |
| Broad-spectrum antibiotics | 0.223 | ||
| Yes | 12 (85.7) | 60 (68.2) | |
| No | 2 (14.3) | 28 (31.8) | |
| Hemodialysis | 0.302 | ||
| Yes | 2 (14.3) | 6 (6.8) | |
| No | 12 (85.7) | 82 (93.2) | |
| Steroid treatment | 0.776 | ||
| Yes | 7 (50) | 39 (44.3) | |
| No | 7 (50) | 49 (55.7) | |
| Surgery | 0.562 | ||
| Yes | 4 (28.6) | 34 (38.6) | |
| No | 10 (71.4) | 54 (61.4) | |
| Trauma | 0.257 | ||
| Yes | 1 (7.1) | 1 (1.1) | |
| No | 13 (92.9) | 87 (98.9) |
Fluconazole was the most preferred (58.8%) treatment option for ICI in our PICU. We applied combination therapy (triazole + echinocandin) in 12 (11.8%) patients. Our results showed an increased trend in use of micafungin in recent years. We detected that we used micafungin for all patients with candiduria in 2018.
Discussion
More than 30 species of Candida have been reported to cause ICI. 12 13 Candida species are the second most common cause of central catheter-associated bloodstream infections in hospitalized patients. 14 Fungal infections have an increasing mortality rate in children with malignancies and stem cell transplants. 15 16 Fungi are the second most common pathogen associated with sepsis in children. 14
In our study, we evaluated patients with ICI in a tertiary university hospital in Southeastern Turkey. The study group included a total of 102 patients during an 11-year period. According to our results, C. albicans (42.2%) was the most common species for all isolates, followed by C. parapsilosis (17.6%). When we separately evaluated blood and urine cultures, we found the second most commonly detected Candida types to be C. parapsilosis in bloodstream infections and C. tropicalis in urinary tract infections. When all isolates were evaluated, our results showed that non- albicans Candida types were dominant, with a 57.8% frequency. In a 5-year retrospective pediatric study in Saudi Arabia, Almoosa et al 17 reported the most common isolated Candida species to be C. albicans , similar to our study. However, both the study of Almoosa et al 17 and the results of another pediatric multicenter study showed a collective predominance for non- albicans Candida species, in parallel with our study. 18 In another single-center retrospective pediatric study including 226 patients aged between 6 months and 18 years, they found the most common isolate to be C. albicans followed by C. parapsilosis , similar to our results. 19 In an adult study by Omrani et al, 20 it was reported that C. albicans was the most common species followed by C. tropicalis . In our country, Sütçü et al 21 evaluated 54 pediatric and neonatal patients aged between 0 and 18 years and reported the most commonly isolated agent to be C. albicans , but the collective results of the study showed that C. albicans and non- albicans species were equal. Noni et al in their retrospective study including 178 pediatric cases from three neonatal intensive care units, one PICU, two hematology–oncology units, and one bone marrow transplantation unit in Greece reported that the most isolated species were C. albicans and C. parapsilosis . 22 In a study performed on 102 children with nosocomial candidemia in our country between 1997 and 2005, non- albicans Candida species were the most common species. 23 In another single-center study performed on children with malignancy and nosocomial candidemia in our country between 2007 and 2013, non- albicans candidemia was determined in 81.4% of 135 candidemia episodes. 24 Öncü et al 25 reported that C. parapsilosis was the most common non- albicans Candida species in both neonates and children.
Candida infections are a major cause of morbidity and mortality in critically ill children in PICUs. 26 Despite improvements in disease prevention and antifungal therapy options, reported mortality rates vary between 7.7 and 26% in different pediatric studies. 8 9 10 Mortality is highest, especially in infants. In a study on infants with positive urine and blood or positive urine and cerebrospinal fluid, the mortality rate was found as 57%. 27 In a study including pediatric and neonatal cases, the mortality rate was 43.8% in patients staying in intensive care units. 17 In an 8-year retrospective adult study, a mortality rate of 50% was reported. Data from Prospective Antifungal Therapy records from 25 tertiary medical centers from the United States and Canada including 2,496 adult non- albicans Candida cases showed a high mortality rate ranging from 46 to 75%. In a pediatric study, C. parapsilosis was found to have the highest mortality rate among all Candida species. 17 In our study, the mortality rate was 13.7%, and C. albicans was found to have the highest mortality rate among all Candida species (30.7%).
In the literature, the most common involvements of ICI are blood and urinary tract. Acar et al reported a Candida -associated bloodstream infection frequency of 42.9% and a urinary tract infection frequency of 37.1% in adult patients in the intensive care unit. 28 Two adult studies in our country reported that the most common involvement of ICI was urinary infection. 29 30 In our study, bloodstream and urinary infections were the first two involvements of ICI, in parallel with the information in the literature.
The most frequently defined risk factors for ICI are broad-spectrum antibiotic use, central venous catheterization, TPN, renal replacement therapy, neutropenia, malignancy, and use of immunosuppressive agents. 5 TPN and mechanical ventilation (MV), in particular, are the two specific defined risk factors for pediatric ICI. 31 32 In a previous study, gender, prolonged intensive care unit stay, and mechanical ventilatory support were defined as major risk factors for invasive candidiasis and were reported to be associated with high mortality rates and poor clinical outcomes. 17 In another study, potential risk factors were reported as the presence of CVC (97.8%), urinary catheter (73.3%), MV (64.4%), and broad-spectrum antibiotic treatment (95.6%). 33 A previous study reported that Candidemia was found to be related to vascular catheters in 58% of newborns and 70% of children. In this study, the main risk factors were prematurity and staying in ICU in newborns, and hematological malignancy and neutropenia in children. 34 Sütçü et al 21 reported the most common facilitating factor for ICI to be the use of broad-spectrum antibiotics in their study group. The most common facilitating factor in our study was the use of mechanical ventilator support. We compared surviving and nonsurviving groups according to risk factors of ICI but could not detect any significant differences between the two groups.
The normal gastrointestinal barrier serves an important function in preventing candidiasis. 25 The destruction of this flora by a major surgery or TPN use is a potential risk factor for ICI. Our results showed a statistically significant difference between the C. albicans and non- albicans groups in terms of TPN use.
Triazoles are the most commonly preferred antifungal agents due to high oral bioavailability and safety of use. 21 Recent guidelines for the management of ICI in neonates and pediatric patients suggest the use of echinocandins, fluconazole, and amphotericin B. 35 Echinocandins are first-line agents for the treatment of IC in children. Amphotericin B is an alternative treatment option when isolates are resistant to azoles or echinocandins. 36 In our study, fluconazole was the most commonly preferred treatment option, and echinocandins were the second most commonly preferred antifungal treatment option in 21 (20.5%) patients (caspofungin in 9, micafungin in 12). Our results showed an increasing trend in micafungin use, especially in the year 2018.
To the best of our knowledge, our study is one of the largest pediatric studies on ICI in a PICU in our country but has some potential limitations. First, it was a single-center study. The second limitation was the retrospective design of the study. The third limitation was a lack of data on antifungal susceptibilities and treatment times. Future prospective multicenter studies with larger isolate numbers are needed to contribute to the literature.
In conclusion, ICI is a common cause of nosocomial infections in PICUs and is still a significant problem due to its high mortality and morbidity rates in pediatric patients, especially those with malignancies and immunosuppressive status. Candida albicans is the most common isolate among all Candida species. Our results showed a dominance of non- albicans Candida species, and the most commonly preferred treatment option in our unit was fluconazole. Furthermore, we detected an increasing use of echinocandins, particularly micafungin, in recent years. There are some studies on pediatric invasive candidiasis in Turkey, but multicenter prospective studies are needed to describe the clinical and epidemiological characteristics of pediatric patients with ICI and to obtain healthier data.
Footnotes
Conflict of Interest None declared.
References
- 1.Pappas P G, Kauffman C A, Andes D et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(05):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfaller M A, Diekema D J. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(01):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaoutis T. Candidemia in children. Curr Med Res Opin. 2010;26(07):1761–1768. doi: 10.1185/03007995.2010.487796. [DOI] [PubMed] [Google Scholar]
- 4.Mesini A, Bandettini R, Caviglia I et al. Candida infections in paediatrics: results from a prospective single-centre study in a tertiary care children's hospital. Mycoses. 2017;60(02):118–123. doi: 10.1111/myc.12570. [DOI] [PubMed] [Google Scholar]
- 5.Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5(01):161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orasch C, Mertz D, Garbino J et al. Fluconazole non-susceptible breakthrough candidemia after prolonged low-dose prophylaxis: a prospective FUNGINOS study. J Infect. 2018;76(05):489–495. doi: 10.1016/j.jinf.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Harrington R, Kindermann S L, Hou Q, Taylor R J, Azie N, Horn D L. Candidemia and invasive candidiasis among hospitalized neonates and pediatric patients. Curr Med Res Opin. 2017;33(10):1803–1812. doi: 10.1080/03007995.2017.1354824. [DOI] [PubMed] [Google Scholar]
- 8.Montagna M T, Lovero G, Borghi E et al. Candidemia in intensive care unit: a nationwide prospective observational survey (GISIA-3 study) and review of the European literature from 2000 through 2013. Eur Rev Med Pharmacol Sci. 2014;18(05):661–674. [PubMed] [Google Scholar]
- 9.Tragiannidis A, Fegeler W, Rellensmann G et al. Candidaemia in a European paediatric university hospital: a 10-year observational study. Clin Microbiol Infect. 2012;18(02):E27–E30. doi: 10.1111/j.1469-0691.2011.03720.x. [DOI] [PubMed] [Google Scholar]
- 10.Guery B P, Arendrup M C, Auzinger G et al. Management of invasive candidiasis and candidemia in adult non-neutropenic intensive care unit patients: part I. Epidemiology and diagnosis. Intensive Care Med. 2009;35(01):55–62. doi: 10.1007/s00134-008-1338-7. [DOI] [PubMed] [Google Scholar]
- 11.Horan T C, Andrus M, Dudeck M A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(05):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller M A, Messer S A, Hollis R J et al. Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location in the United States in 2001 to 2007 . J Clin Microbiol. 2009;47(10):3185–3190. doi: 10.1128/JCM.00946-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller M A, Diekema D J. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42(10):4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson R S, Carcillo J A, Linde-Zwirble W T, Clermont G, Lidicker J, Angus D C. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(05):695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 15.Pagano L, Caira M, Candoni A et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(08):1068–1075. [PubMed] [Google Scholar]
- 16.Profumo R J. Pediatric liver transplant recipients: mortality analysis over 20 years. J Insur Med. 2006;38(01):3–8. [PubMed] [Google Scholar]
- 17.Almooosa Z, Ahmed G Y, Omran A et al. Invasive candidiasis in pediatric patients at King Fahad Medical City in Central Saudi Arabia. A 5-year retrospective study. Saudi Med J. 2017;38(11):1118–1124. doi: 10.15537/smj.2017.11.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palazzi D L, Arrieta A, Castagnola E et al. Candida speciation, antifungal treatment and adverse events in pediatric invasive candidiasis: results from 441 infections in a prospective, multi-national study. Pediatr Infect Dis J. 2014;33(12):1294–1296. doi: 10.1097/INF.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 19.Dutta A, Palazzi D L. Candida non-albicans versus Candida albicans fungemia in the non-neonatal pediatric population . Pediatr Infect Dis J. 2011;30(08):664–668. doi: 10.1097/INF.0b013e318213da0f. [DOI] [PubMed] [Google Scholar]
- 20.Omrani A S, Makkawy E A, Baig K et al. Ten-year review of invasive Candida infections in a tertiary care center in Saudi Arabia. Saudi Med J. 2014;35(08):821–826. [PubMed] [Google Scholar]
- 21.Sütçü M, Acar M, Genç G E et al. Evaluation of Candida species and antifungal susceptibilities among children with invasive candidiasis . Turk Pediatri Ars. 2017;52(03):145–153. doi: 10.5152/TurkPediatriArs.2017.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noni M, Stathi A, Vaki I, Velegraki A, Zachariadou L, Michos A. Changing epidemiology of invasive candidiasis in children during a 10-year period. J Fungi (Basel) 2019;5(01):19. doi: 10.3390/jof5010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celebi S, Hacimustafaoglu M, Ozdemir O, Ozkaya G. Nosocomial candidaemia in children: results of a 9-year study. Mycoses. 2008;51(03):248–257. doi: 10.1111/j.1439-0507.2007.01464.x. [DOI] [PubMed] [Google Scholar]
- 24.Devrim İ, Demirağ B, Yaman Y et al. A 7-year study of the distribution of nosocomial candidemia in children with cancer. Turk J Pediatr. 2015;57(03):225–229. [PubMed] [Google Scholar]
- 25.Öncü B, Belet N, Emecen A N, Birinci A.Health care-associated invasive Candida infections in childrenMed Mycol2019(e-pub ahead of print). doi: 10.1093/mmy/myz005 [DOI] [PubMed]
- 26.Sievert D M, Ricks P, Edwards J R et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34(01):1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin D K, Jr, Stoll B J, Gantz M G et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126(04):e865–e873. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acar A, Oncül O, Küçükardali Y, Ozyurt M, Haznedaroğlu T, Cavuşlu S. Yoğun bakim ünitelerinde saptanan Candida enfeksiyonlarinin epidemiyolojik özellikleri ve mortaliteye etki eden risk faktörleri. Mikrobiyol Bul. 2008;42(03):451–461. [PubMed] [Google Scholar]
- 29.Çekin Y, Pekintürk N, Çekin A H. Evaluation of species distribution and antifungal resistance of Candida isolates from hospitalized patients. J Clin Anal Med. 2015;6:8–11. [Google Scholar]
- 30.Hazırolan G, Yıldıran D, Baran I, Mumcuoğlu İ, Aksu N. Yatan hasta örneklerinden izole edilen Candida izolatlarının tür dağılımlarının ve antifungal duyarlılık profillerinin değerlendirilmesi. Turk Hij Deney Biyol Derg. 2015;72:17–26. [Google Scholar]
- 31.Stamos J K, Rowley A H. Candidemia in a pediatric population. Clin Infect Dis. 1995;20(03):571–575. doi: 10.1093/clinids/20.3.571. [DOI] [PubMed] [Google Scholar]
- 32.Filioti J, Spiroglou K, Roilides E. Invasive candidiasis in pediatric intensive care patients: epidemiology, risk factors, management, and outcome. Intensive Care Med. 2007;33(07):1272–1283. doi: 10.1007/s00134-007-0672-5. [DOI] [PubMed] [Google Scholar]
- 33.Conde-Rosa A, Amador R, Pérez-Torres D et al. Candidemia distribution, associated risk factors, and attributed mortality at a university-based medical center. P R Health Sci J. 2010;29(01):26–29. [PMC free article] [PubMed] [Google Scholar]
- 34.Blyth C C, Chen S C, Slavin M A et al. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009;123(05):1360–1368. doi: 10.1542/peds.2008-2055. [DOI] [PubMed] [Google Scholar]
- 35.Pappas P G, Kauffman C A, Andes D R et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hope W W, Castagnola E, Groll A H et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp . Clin Microbiol Infect. 2012;18 07:38–52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]


