Abstract
Background
Studies in adults and children suggested that starting antiretroviral therapy (ART) soon after infection positively influences early events in HIV infection raising the possibility that remission may be achieved in some.
Methods
We designed an analytic treatment interruption (ATI) trial to test the hypothesis that a sizable minority of HIV-infected neonates who initiated ART <14 days of birth and maintained on ART would be able to maintain viral suppression when ART was withdrawn. To yield the target cohort for this trial, 73 HIV-infected neonates identified at one hospital in Johannesburg, South Africa, were initiated on ART <14 days of birth and maintained on ART tracking viral load (VL) decline and immune recovery (clinicaltrials.gov # NCT02431975).
Findings
Three HIV-infected infants (4.1%) died and nine (12.3%) were lost to follow-up before 48 weeks of age. Of those surviving on study, 52.5% attained and sustained VL <50 copies/ml and half of these sustained CD4+ T-cell percentage >30% which were the primary entry criteria for the ATI trial. Proportions achieving ATI eligibility criteria were similar in the 46 infants starting ART <48 h (19.6%) to 27 infants starting 2–14 days (25.9%) (p = 0.567).
Interpretation
Very early ART on its own, using regimens available when the trial was designed, is insufficient to attain minimum entry criteria needed to justify our trial of ART interruption. Decisions about how quickly to start ART should be based on optimizing standard clinical outcomes rather than with the expectation that remission can be attained.
Funding
NICHD/NIAID (U01HD080441), South African Research Chairs Initiative of DST and NRF (South Africa).
Keywords: HIV, Neonate, Antiretroviral therapy, Remission
Research in context.
Evidence before this study
Our study was designed shortly after the report of the infant in Mississippi who started antiretroviral treatment (ART) within 30 h of birth and who was able to maintain viral suppression off treatment for over two years. This case report was consistent with studies of ART started soon after primary HIV infection in adults that had observed that some individuals can achieve periods of post-treatment viral control when ART is withdrawn (remission). Taken together with knowledge from developmental biology of the neonatal immune profile, we hypothesized that starting and maintaining effective treatment in early life would lead to protection of critical immune processes and establishment of smaller viral reservoirs, leading to remission in a sizable minority (>20%). Adequate viral suppression and immune competence while ART is maintained is generally taken as minimum criteria for ART interruption trials. We conducted a PubMed search, with no language restrictions, including the following search terms in combination: HIV, neonate, newborn, children, perinatal, antiretroviral therapy, cure, remission, treatment interruption, to identify articles pertinent to our study through May 6, 2019.
Added value of this study
We started ART within 14 days of birth among 73 HIV-infected neonates identified within 48 h of birth at one hospital in Johannesburg, South Africa. Of those surviving on study, 52.5% attained and sustained viral load <50 copies/ml and half of these sustained CD4+ T-cell percentage >30%. Proportions achieving these virological and immunological endpoints were similar in the 46 infants who started ART <48 h to 27 infants starting 2–14 days.
Implications of all available evidence
Very early ART on its own, using the regimens available at the time our study was designed, is unlikely to be sufficient to achieve remission in a sizable minority of infants. The rare cases of perinatally-infected children who have been able to achieve prolonged periods of remission also suggest that early treatment may interact with host and viral factors to achieve remission but ART on its own may not be sufficient. Early initiation of more potent antiretroviral regimens, long-acting formulations and/or alternative interventions, such as broadly-neutralizing antibodies, need to be investigated to enable more rapid and sustained viral control and immune recovery as a stepping stone to achieve remission in perinatally-infected infants.
Alt-text: Unlabelled box
1. Introduction
Studies in adults given antiretroviral therapy (ART) soon after primary infection have observed that some individuals can achieve periods of post-treatment viral control when ART is withdrawn (remission) [1], [2], [3], [4], [5], [6]. The report of the infant in Mississippi who started treatment within 30 h of birth and who was able to maintain viral suppression off treatment for over two years raised the tantalizing possibility that similar results might be observed in other early treated infants [7]. ART started close to birth is not only close in time to acquisition of infection but is also being given during a developmentally-critical time period when the immune system is transitioning to a more mature form and is at its most quiescent [8,9]. We hypothesized that starting and maintaining effective treatment in early life would lead to protection of critical immune parameters and establishment of smaller viral reservoirs, leading to remission in a sizable minority (>20%).
To test the hypothesis, we designed an analytic treatment interruption (ATI) trial. Interruption of treatment is currently the only known approach to test whether periods of remission can be achieved. Here we describe the cohort of HIV-infected neonates identified within 48 h of birth, started on ART within 14 days and maintained on ART, that we intended as the target population for this trial. We describe the proportions of these early-treated, HIV-infected children who meet virologic and immunologic eligibility criteria for entry into the ATI trial.
2. Methods
2.1. Study design
We designed an ATI trial to test the hypothesis that a sizable minority (>20%) of HIV-infected neonates started on ART within 14 days of birth and maintained on ART for at least two years would be able to sustain viral suppression after ART withdrawal. To yield the target population for the ATI trial, we established clinical protocols at Rahima Moosa Mother and Child Hospital (RMMCH), Johannesburg, South Africa, a) to provide HIV diagnostic testing at birth to identify HIV-infected neonates with a positive result within 48 h of birth and b) to initiate ART within 14 days of birth and maintain ART to at least two years of age (clinicaltrials.gov # NCT02431975). RMMCH is a centrally-located, large urban public tertiary hospital and one of the teaching facilities of the University of the Witwatersrand. Children who met virologic and immunologic endpoints would be eligible for entry into the ATI trial. The protocols were approved by the Institutional Review Boards (IRB) of the University of the Witwatersrand and Columbia University. Written informed consent was obtained from mothers for their own and their infants’ participation. In addition to IRB approval, the ATI trial protocol was developed in consultation with a Data Safety and Monitoring Board (DSMB) who met annually and monitored progress of the target cohorts’ potential to enter the trial.
2.2. Participants
When the study was initially proposed, we hypothesized that ART would need to be initiated within 48 h of birth to have the later beneficial outcomes. For practical purposes, in order to start ART <48 h of birth, a point-of-care neonatal diagnosis program was established that used Xpert HIV-1 Qual (Cepheid, Sunnyvale, CA) [10]. The site already had a routine birth testing program sending samples to the national laboratory for diagnosis (HIV-1 total nucleic acid (TNA) COBAS TaqMan HIV-1 Qualitative Test Version 2·0 Roche Molecular Systems, Inc., Branchburg, NJ). As part of the routine program, all HIV-exposed neonates have blood collected by venipuncture after birth prior to discharge. When staff capacity permitted, one of the vials from this blood collection was tested on-site using Xpert. Positive Xpert results were re-run with residual sample from the same blood collection. Neonates with two positive Xpert results were eligible for immediate on-site ART initiation. The on-site testing protocol was intended to yield a cohort enriched with infants initiating ART <48 h after birth although inclusion criteria for the ATI trial required only ART initiation <14 days. Two clinical protocols were in place to recruit infants intended for the trial. These protocols included Xpert co-tested neonates as well as infants with positive or indeterminate results from the routine diagnosis program not co-tested with Xpert. The latter neonates were recalled to the site for ART initiation once routine results were available.
2.3. Procedures
The initial ART regimen consisted of nevirapine, lamivudine and zidovudine. Nevirapine was changed to lopinavir-ritonavir no sooner than 42 weeks post-menstrual age taking into account patient readiness. Drugs were given in liquid form twice daily. Either stavudine or abacavir were given in the event of zidovudine toxicity and abacavir substituted for zidovudine once infants were ≥3 months of age. Cotrimoxazole was started at 4–6 weeks of age. Routine infant prophylaxis was one dose of nevirapine as soon as possible after birth and daily nevirapine for 6 weeks after discharge. Infants considered high risk had twice-daily zidovudine added. For infected neonates identified before discharge, prophylaxis was discontinued and the treatment regimen initiated. For the infected neonates recalled to the site, prophylaxis was discontinued once ART was initiated.
ART was initiated based on results of the first round of diagnostic testing. To confirm diagnosis, a second blood sample was collected prior to the first ART dose. For those co-tested with Xpert, confirmatory testing was usually done on the same day as initial testing. The qualitative HIV-1 TNA diagnostic PCR was repeated and viral load (VL) done (quantitative HIV-1 RNA COBAS AmpliPrep/COBAS TaqMan HIV-1 test, version 2·0, Roche Molecular Systems, Inc., Branchburg, NJ). If subsequent tests did not confirm HIV diagnosis, infants were excluded from eligibility for the ATI trial and were managed based on the profile of their results.
ART was continued for a minimum of 104 weeks. Infants were followed on one of two protocols. The preferred protocol repeated VL tests at 1, 2 and 4 weeks of age, every 4 weeks to 24 weeks and then every 8 weeks to 104 weeks. The alternative protocol, repeated VL at 4, 8, 12, 16, and 24 weeks then every 12 weeks to 104 weeks. Diagnostic HIV PCR tests were repeated at 24, 48, 72 and 104 weeks. CD4+ T-cell count and percentage (TruCount Method, BD Biosciences, Germany) was measured at enrolment, 24, 48, 72 and 104 weeks. Complete blood counts were done at 4, 12, 24, 48, 72 and 104 weeks. Alanine aminotransferase (ALT) was measured at 16 weeks. Results were graded using the Division of AIDS toxicity tables [11]. All grade 3 and 4 abnormalities were repeated. A maternal blood sample was collected at enrolment for VL, CD4+ T-cell count and complete blood count. Antenatal information and maternal HIV treatment history was collected. Children were assessed for clinical progression at every visit and anthropometric data were collected.
2.4. Sample size
Power calculations determined the need for 40 children to enter the ATI trial. Initially, it was anticipated that 60 early-treated children would be sufficient to obtain this number based on the assumption that ~80% would become eligible for the interruption trial and ~85% of these would remain in care. As outcome data accumulated, it was clear that the cohorts of early-treated infants would need to be larger and enrolment continued. A DSMB review in May 2018 concluded that the numbers of early-treated children recruited would not be able to attain a target population of sufficient size to enter the ATI trial as originally designed. Further enrolment into the early-treatment protocols ended shortly thereafter.
2.5. Outcomes
Here we report the proportions of children in the early-treated cohorts who met the primary virologic and immunologic endpoints for consideration for entry into the ATI trial. The primary virologic endpoint was VL <400 copies/ml by 24 weeks after ART initiation and <50 copies/ml by 48 weeks of age and no confirmed VL (i.e. two consecutive measurements) >50 copies/ml after suppression was attained. The primary immunologic endpoint was a CD4+ T-cell percentage >30% by 24 weeks which was sustained through follow-up.
2.6. Statistical analysis
The analysis included children with confirmed HIV infection (first sample indicating HIV-infection collected <48 h) and ART initiated within 14 days born between March 1, 2015 (start of prospective enrolment into the earliest study protocol) and September 30, 2017 (to allow at least 48 weeks of follow-up data on all children). Follow-up data accrued through September 2018 or truncated at 104 weeks of age were utilized. The primary analysis describes proportions meeting eligibility criteria for the ATI trial. Binomial proportion confidence intervals were reported under 0.05 significance level. If cell counts for proportions were >10, Wald-type confidence intervals were reported; for counts ≤10, Clopper-Pearson estimation method was used. Secondary analyses compared proportions stratified by whether ART was initiated before or after 48 h of birth. Proportions were compared with Fisher's exact tests and continuous variables were compared with t-tests, if normally-distributed, in which case the means were shown, and with Wilcoxon signed-rank tests otherwise, in which case the medians were shown. Statistical analyses were conducted in SAS version 9.4 (Cary, NC).
3. Results
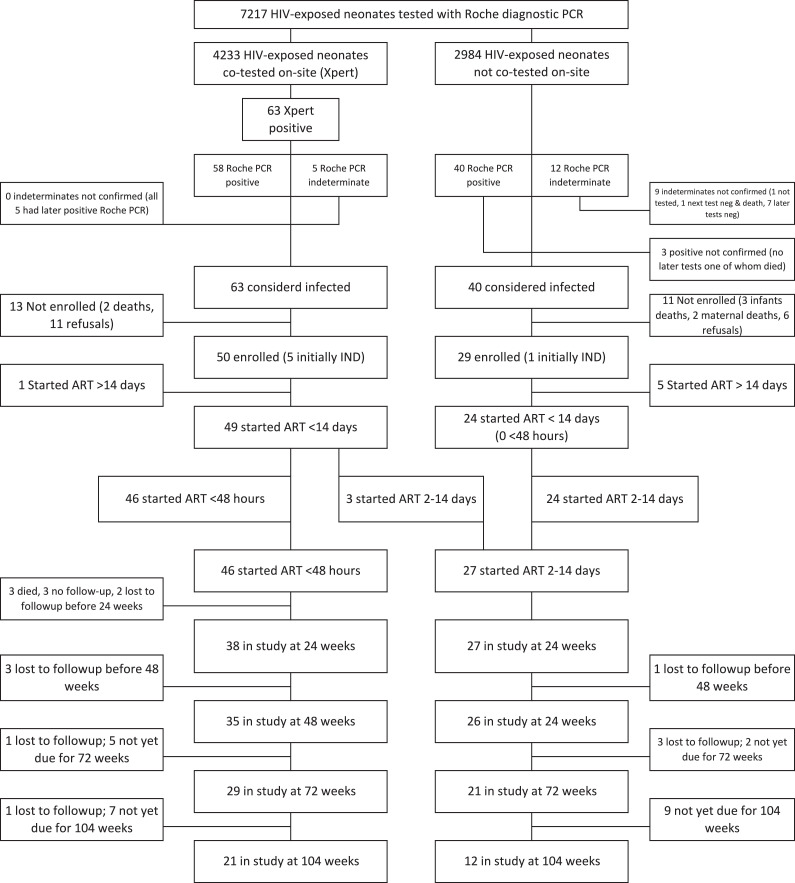
Between March 1, 2015 and September 30, 2017, 7217 HIV-exposed neonates were tested with the routine diagnostic HIV PCR among which 4233 (59%) were co-tested with Xpert yielding 115 neonates with an initial positive or indeterminate result. From these, 79 confirmed infected infants were enrolled and 73 of these started ART within 14 days of birth (Fig. 1).
Fig. 1.
Flow diagram of screening to identify potential participants, their disposition prior to enrollment and follow-up after enrollment.
Among 46 infants who started ART <48 h of birth, the screening blood sample was collected at a median of 13.0 h (interquartile range (IQR) 9.0–15.8; range 2.0–31.1 h) after birth; all but one after having received a dose of nevirapine as prophylaxis (median time of blood draw 3.3 h after prophylaxis [IQR 2.5–4.3; range 0.5–9.0 h]). The routine diagnostic PCR run on the same sample was positive in 41 and indeterminate in five (all turned positive on subsequent PCR tests). The second sample was drawn a median of 5.9 h (IQR 5.0–22.6) after the first sample a median of 22.6 h (IQR 18.3–30.7) after birth. The median baseline VL was 35,071 copies/ml (IQR 5370–267,000; range 60–2445,950 copies/ml) (Table 1).
Table 1.
Baseline characteristics of 73 HIV-infected neonates initiating antiretroviral therapy (ART) within 14 days of life at Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa between March 1, 2015 and September 30, 2017.
| Characteristic | Total (N = 73) | Initiated ART <48 h (N = 46) | Initiated ART 2–14 days (N = 27) | p-value |
|---|---|---|---|---|
| Sex, N (%) | ||||
| Male | 39 (53.4) | 26 (56.5) | 13 (48.2) | 0.628 |
| Female | 34 (46.6) | 20 (43.5) | 14 (51.9) | |
| Birth Weight (grams), Range | 905–4150 | 1860–4150 | 905–3890 | |
| Birth Weight (grams), Mean (SD) | 2820 (602) | 2954 (477) | 2592 (723) | 0.026 |
| Birth Weight (grams), N (%) | ||||
| <2500 | 16 (21.9) | 6 (13.0) | 10 (37.0) | 0.075 |
| 2500–3000 | 29 (39.7) | 20 (43.5) | 9 (33.3) | |
| ≥3000 | 28 (38.4) | 20 (43.5) | 8 (29.6) | |
| Gestational age by Ballard (weeks), N (%) | ||||
| ≥37 weeks (term) | 63 (86.3) | 44 (95.6) | 19 (70.4) | 0.004 |
| <37 weeks (pre-term) | 10 (13.7) | 2 (4.4) | 8 (29.6) | |
| Mode of delivery, N (%) | ||||
| Vaginal | 55 (75.3) | 38 (82.6) | 17 (63.0) | 0.091 |
| Caesarean | 18 (24.7) | 8 (17.4) | 10 (37.0) | |
| Ever breastfed, N (%) | ||||
| Yes | 57 (78.1) | 39 (84.8) | 18 (66.7) | 0.085 |
| No | 16 (21.9) | 7 (15.2) | 9 (33.3) | |
| Infant prophylaxis, N (%) | ||||
| None | 2 (2.7) | 1 (2.2) | 1 (3.7) | |
| Nevirapine only | 67 (91.8) | 45 (97.8) | 22 (81.5) | 0.016 |
| Nevirapine + Zidovudine | 4 (5.5) | 0 (0.0) | 4 (14.8) | |
| Pre-treatment Viral load (copies/ml), Median (IQR) | 31,445 (5355–290,807) | 35,071 (5370–267,000) | 12,335 (1124–454,790) | 0.780 |
| Age when pre-treatment Viral load measured (days), Median (IQR) | 1 (1–4) | 1 (1–1) | 7 (4–8) | <0.001 |
| Pre-treatment Viral load (copies/ml), N (%) | ||||
| <400 | 9 (13.0) | 5 (10.9) | 4 (17.4) | 0.271 |
| 400–1000 | 4 (5.8) | 3 (6.5) | 1 (4.4) | |
| 1000–10,000 | 10 (14.5) | 6 (13.0) | 4 (17.4) | |
| 10,000–100,000 | 20 (29.0) | 14 (30.4) | 6 (26.1) | |
| 100,000–1000,000 | 18 (26.1) | 15 (32.6) | 3 (13.0) | |
| ≥1000,000 | 8 (11.6) | 3 (6.5) | 5 (21.7) | |
| Pre-treatment CD4+ T-cell count (cells/mm3), Median (IQR) | 1823 (1474–2372) | 1819 (1474–2442) | 1875 (1182–2372) | 0.926 |
| Pre-treatment CD4+ T-cell percentage (%), Median (IQR) | 40.6 (32.2–49.8) | 39.9 (32.2–48.8) | 47.4 (30.9–51.8) | 0.354 |
| Pre-treatment CD4+ T-cell percentage (%), N (%) | ||||
| <25 (severe) | 6 (9.8) | 3 (7.1) | 3 (15.8) | 0.296 |
| 25–30 (advanced) | 6 (9.8) | 6 (14.3) | 0 (0.0) | |
| 30–35 (mild) | 9 (14.8) | 6 (14.3) | 3 (15.8) | |
| >35 (none or not significant) | 40 (65.6) | 27 (64.3) | 13 (68.4) | |
| Mother age (years), Mean (SD) | 28.4 (6.0) | 28.4 (5.7) | 28.4 (6.7) | 0.976 |
| Maternal antiretroviral therapy (ART) category, N (%) | ||||
| ART started before pregnancy and continued | 12 (16.4) | 6 (13.0) | 6 (22.2) | 0.276 |
| ART started during pregnancy, ≥12 weeks | 20 (27.4) | 15 (32.6) | 5 (18.5) | |
| ART started during pregnancy, <12 weeks | 25 (34.3) | 14 (30.4) | 11 (40.7) | |
| ART started during pregnancy, unknown time | 1 (1.4) | 0 (0.0) | 1 (3.7) | |
| No ART up until delivery | 15 (20.6) | 11 (23.9) | 4 (14.8) | |
| Maternal ART regimen at delivery, N (%) | ||||
| Efavirenz-based | 56 (76.7) | 35 (76.1) | 21 (77.8) | 0.134 |
| Lopinavir-ritonavir-based | 2 (2.7) | 0 (0.0) | 2 (7.4) | |
| No ART before delivery | 15 (20.6) | 11 (23.9) | 4 (14.8) | |
| Maternal Viral load closest to birth (copies/ml), Median (IQR) | 38,459 (1760–104,538) | 31,433 (1292–104,538) | 50,400 (1760–125,515) | 0.547 |
| Maternal CD4+ T-cell count closest to birth (cells/mm3), Median (IQR) | 327 (207–567) | 357 (231–603) | 259 (128–480) | 0.097 |
| Maternal CD4+ T-cell count closest to birth (cells/mm3), N (%) | ||||
| <200 | 17 (23.3) | 8 (17.4) | 9 (33.3) | 0.500 |
| 200–349 | 23 (31.5) | 15 (32.6) | 8 (29.6) | |
| 350–499 | 13 (17.8) | 9 (19.6) | 4 (14.8) | |
| >500 | 20 (27.4) | 14 (30.4) | 6 (22.2) |
Denominators are as shown.
Abbreviations: Antiretroviral treatment (ART), Standard deviation (SD), Inter-quartile range (IQR).
Among 27 infants who started ART >48 h and <14 days of age (2–14 days), the screening blood sample was collected a median of 13.0 h (IQR 7.1–24.0; range 0.9–60.0 h) after birth; in 66.7% of cases after having received a dose of nevirapine (median time of blood draw 4.8 h after prophylaxis [IQR 2.8–6.3; range 2.0–7.8 h]). All infants, except one, received at least one dose of nevirapine prophylaxis and were prescribed daily nevirapine on discharge; four of these were prescribed zidovudine as well. Three infants were co-tested with Xpert but did not initiate ART on-site at the time and 24 infants had not been co-tested with Xpert on-site and had to be recalled. One of the 27 infants had an indeterminate result on initial screening (Fig. 1). The median baseline VL was 12,335 copies/ml (IQR 1124-454,790; range 102–8630,000 copies/ml) (Table 1).
Among 46 neonates initiating ART <48 h of birth, nevirapine, lamivudine and zidovudine was initiated at a median of 24 h after birth (IQR 18.1–30.5; range 8.1–48.0 h). ART initiation was a median of 6.4 h (IQR 5.6-22.2; range 4.9–34.8 h) after the blood draw to identify infection. Among 27 neonates initiating ART 2–14 days of birth, the median age at ART initiation was 6 days (IQR 4–8 days). All, except one, initiated treatment with nevirapine, lamivudine and zidovudine (Table 2).
Table 2.
Timing of antiretroviral therapy (ART) initiation and ART modifications among 73 infants identified as HIV-infected within 48 h of life.
| Characteristic | Total (N = 73) | Initiated ART <48 h (N = 46) | Initiated ART 2–14 days (N = 27) | p-value |
|---|---|---|---|---|
| Age at ART start in days of life | ||||
| Range | 0–14 | 0–2 | 2–14 | |
| Mean (SD) | 3.0 (3.2) | 1.0 (0.5) | 6.4 (2.9) | |
| Median (IQR) | 1.0 (1.0–5.0) | 1.0 (1.0–1.0) | 6.0 (4.0–8.0) | |
| Age at ART start in hours since birth | ||||
| Range | 8.1–48.0* | |||
| Mean (SD) | 25.1 (10.5) | |||
| Median (IQR) | 24.0 (18.1–30.5) | |||
| Initial regimen, N (%) | ||||
| Nevirapine/Lamivudine/Zidovudine§ | 72 (98.6) | 46 (100.0) | 26 (96.3) | 0.370 |
| Lopinavir-ritonavir/Lamivudine/Zidovudine | 1 (1.4) | 0 (0.0) | 1 (3.7) | |
| Lopinavir-ritonavir status in 48 weeks of follow-up, N (%) | ||||
| Lost before age to switch | 3 (4.1) | 3 (6.5) | 0 (0.0) | 0.410 |
| Initiated on lopinavir-ritonavir | 1 (1.4) | 0 (0.0) | 1 (3.7) | |
| Switched to lopinavir-ritonavir in first 28 days | 34 (46.6) | 21 (45.7) | 13 (48.2) | |
| Switched to lopinavir-ritonavir after 28 days | 35 (48.9) | 22 (47.8) | 13 (48.2) | |
| If switched in first 28 days, age switched (days) | ||||
| Range | 14–32 | 14–30 | 18–32 | |
| Median (IQR) | 21 (17–29) | 18 (16–24) | 28 (21–30) | |
| If switched after 28 days, age switched (days) | ||||
| Range | 29–132 | 29–71 | 34–132 | |
| Median (IQR) | 44 (33–61) | 35 (31–47) | 57 (45–80) | |
| Lopinavir-ritonavir switch in relation to estimated post-menstrual age (PMA), N (%) | ||||
| No follow-up | 3 (4.1) | 3 (6.5) | 0 (0.0) | 0.491 |
| Initiated on lopinavir-ritonavir | 1 (1.4) | 0 (0.0) | 1 (3.7) | |
| Before 42 weeks PMA | 2 (2.7) | 1 (2.2) | 1 (3.7) | |
| 42 weeks PMA | 25 (34.3) | 16 (34.8) | 9 (33.3) | |
| After 42 weeks PMA | 42 (57.5) | 26 (56.5) | 16 (59.3) | |
| Zidovudine changed to abacavir in 48 weeks of follow-up, N | 33 | 21 | 12 | |
| If switched to abacavir, median age switched in days (IQR) | 169 (125–198) | 149 (125–186) | 182 (130–260) | |
| Zidovudine changed to stavudine in 48 weeks of follow-up, N | 4 | 2 | 2 |
Exact hours known in 41/45. In the other 4 known to be <48 h but exact numbers of hours not recorded.
One child received one dose of lopinavir-ritonavir in error at the start of treatment but then continued with nevirapine.
Abbreviations: Antiretroviral treatment (ART), Standard deviation (SD), Inter-quartile range (IQR), Post-menstrual age (PMA).
Nevirapine was changed to lopinavir-ritonavir at a median of 30 days (IQR 18–36; range 14–71 days) in neonates who started ART <48 h and 33 days (IQR 28–57; range 18–132 days) in neonates who started ART 2–14 days of life. In two infants this was sooner than the recommended 42 weeks, 34.3% at 42 weeks, and 57.5% > 42 weeks (Table 2). Two children with Grade 3 neutropenia substituted stavudine for zidovudine at 14 and 74 days of age (both in the 2–14 days group). One child with Grade 3 low hemoglobin substituted stavudine for zidovudine at 66 days of age (2–14 days group). All other Grade 3 and 4 laboratory abnormalities resolved on repeat testing without a change in regimen (Supplementary Table 1).
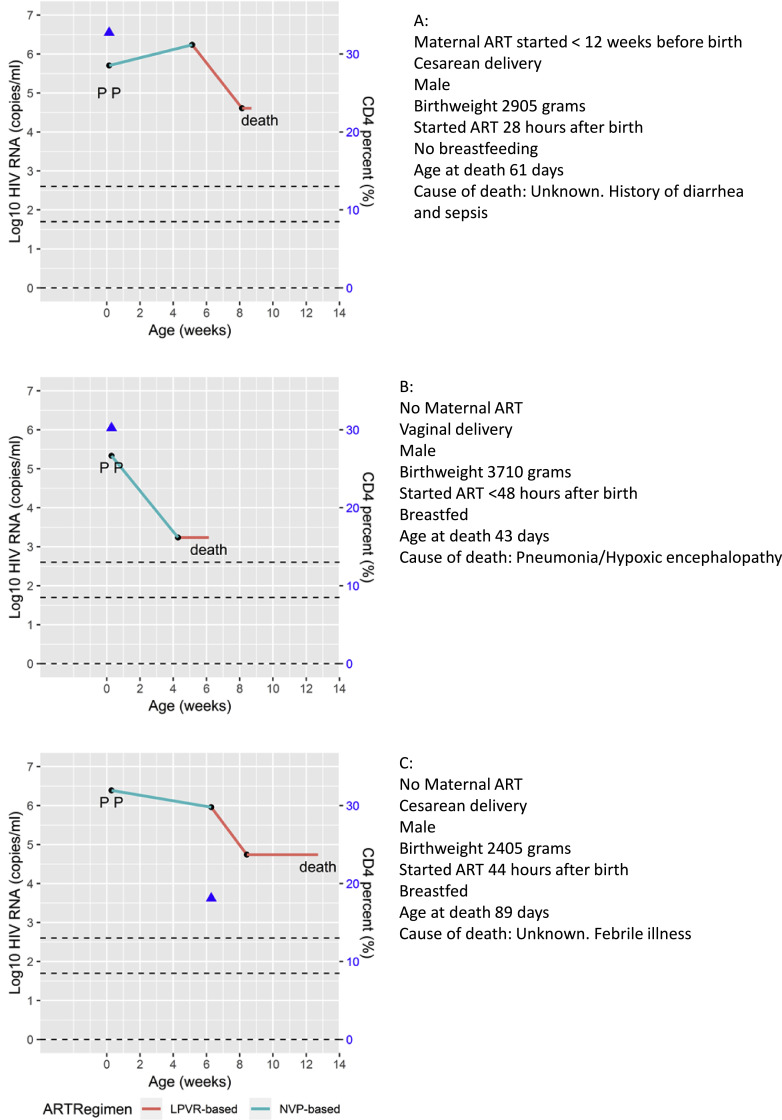
Three infants died at 43, 61 and 89 days of age. These three infants had started ART at <48 (exact time unknown), 28, and 44 h respectively. All three were male born at term, one was low birth weight, and two were cesarian section deliveries. All had high baseline VL and had transitioned to the lopinavir-ritonavir regimen, but none attained viral suppression before death (Fig. 2).
Fig. 2.
Viral load and other characteristics of the three study participants who died on study. P indicates the timing of positive diagnostic PCR tests.
Nine infants (12.3%) were lost to follow-up before 48 weeks of age: 8/46 who started ART <48 h and 1/27 who started 2–14 days. Taking into account the three deaths, survival on study was lower in those who started <48 h (35/46) compared to 2–14 days (26/27) (p = 0.026).
Overall 63.0% (46/73) of children achieved <50 copies/ml by 48 weeks of age (45/46 achieved <400 copies/ml by 24 weeks) and 32/73 (43.8%) sustained VL <50 copies/ml without a confirmed measurement above this threshold to 48 weeks or last follow-up visit (32/61 [52.5%] of those remaining in follow-up). Most infants (56/61; 91.8%) had CD4+ T-cell percentage >30% during follow-up with 46/61 (75.4%) starting above this threshold. Sixteen (26.2%) of 61 children remaining in follow-up met both the virologic and immunologic criteria. There were no significant differences in the proportions meeting these benchmarks by age at ART initiation (Table 3).
Table 3.
Proportion of 73 HIV-infected infants starting antiretroviral therapy (ART) within 14 days of birth who met virologic and immunologic eligibility criteria for the analytic treatment interruption study by age at ART initiation.
| Characteristic | Total |
Initiated ART <48 h |
Initiated ART 2 to 14 days |
p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % of total (95% CI) N = 73 | % of those in follow-up (95% CI) N = 61 | N | % of total (95% CI) N = 46 | % of those in follow-up (95% CI) N = 35 | N | % of total (95% CI) N = 27 | % of those in follow-up (95% CI) N = 26 | ||
| Deaths | 3 | 4.1 (0.9–11.5) | 3 | 6.5 (1.4–17.9) | 0 | 0 (0–12.8) | 0.291* | |||
| Loss to Follow-up before 48 weeks | 9 | 12.3 (5.8–22.1) | 8 | 17.4 (7.8–31.4) | 1 | 3.7 (0–19.0) | 0.141* | |||
| Meets virologic criteria | ||||||||||
| No: VL not <50 copies/mL by 48 weeks | 15 | 20.6 | 6 | 13.0 | 9 | 33.3 | ||||
| No: VL <50 copies/mL achieved but confirmed rebound >50 copies/mL | 14 | 19.2 | 11 | 23.9 | 3 | 11.1 | ||||
| Yes: VL achieved and sustained <50 copies/mL | 32 | 43.8 (32.5–55.2) | 52.5 (40.0–65.0) | 18 | 39.1 (25.0–53.2) | 51.4 (34.9–68.0) | 14 | 51.9 (33.0–70.7) | 53.9 (34.7–73.0) | 0.335* |
| Meets immunologic criteria | ||||||||||
| No | 35 | 19 | 16 | |||||||
| Yes: CD4+ T-cell% sustained >30% | 26 | 42.6 (30.2–55.0) | 16 | 45.7 (29.2–62.2) | 10 | 38.5 (19.8–57.2) | 0.610§ | |||
| Virologic and immunologic criteria | ||||||||||
| Meets both | 16 | 21.9 (12.4–31.4) | 26.2 (15.2–37.3) | 9 | 19.6 (9.4–33.9) | 25.7 (12.5–43.3) | 7 | 25.9 (11.1–46.3) | 26.9 (11.6–47.8) | 1.000§ 0.567* |
| Meets virologic but not immunologic | 16 | 21.9 | 26.2 | 9 | 19.6 | 25.7 | 7 | 25.9 | 26.9 | |
| Meets immunologic but not virologic | 10 | 13.7 | 16.4 | 7 | 15.2 | 20.0 | 3 | 11.1 | 11.5 | |
| Meets neither | 19 | 26.0 | 31.2 | 10 | 21.7 | 28.6 | 9 | 33.3 | 34.6 | |
Fisher Exact test results among all children (N = 73) comparing proportions between two ART initiation groups.
Fisher Exact test results among surviving children (N = 61) comparing proportions between two ART initiation groups
Abbreviations: Antiretroviral treatment (ART), Confidence intervals (CI), Viral load (VL).
The proportion of children with sustained CD4+ T-cell percentage >30% was not significantly higher in those who met virologic criteria (16/32 [50.0%]) versus those who did not (10/29 [34.5%]) (p = 0.30). The proportion with sustained CD4+ T-cell count >1000 cells/mm3 was also not significantly higher in children who met virologic criteria (29/32 [90.6%]) versus those who did not (22/29 [75.9%]) (p = 0.17).
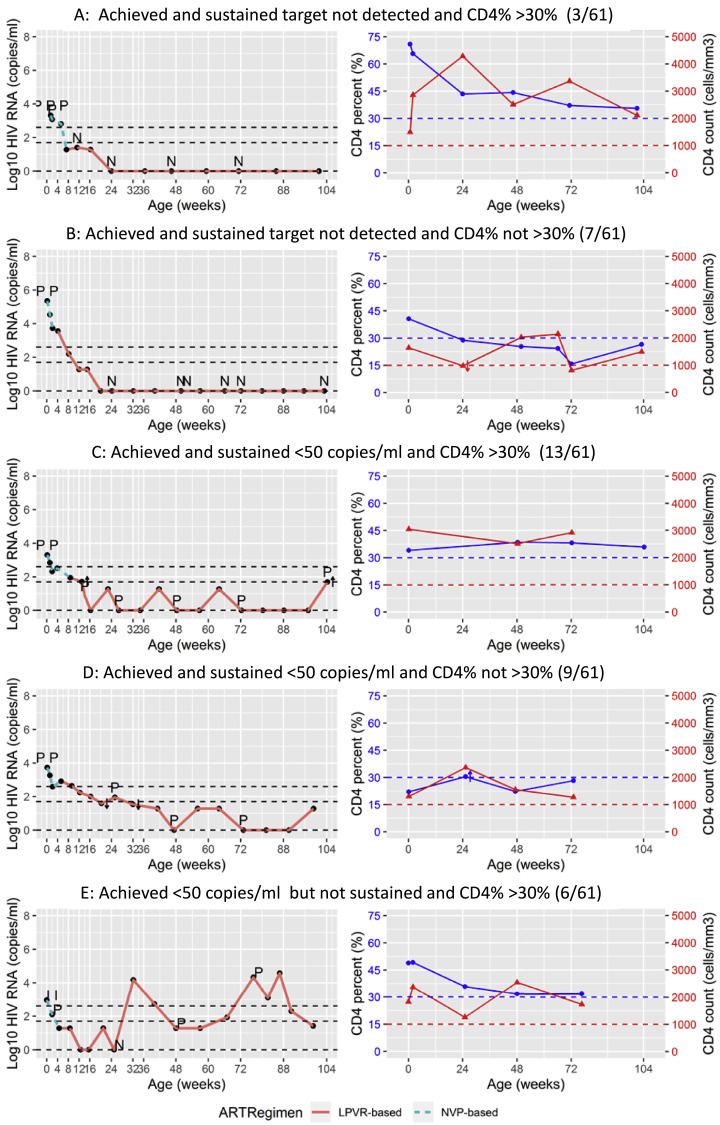
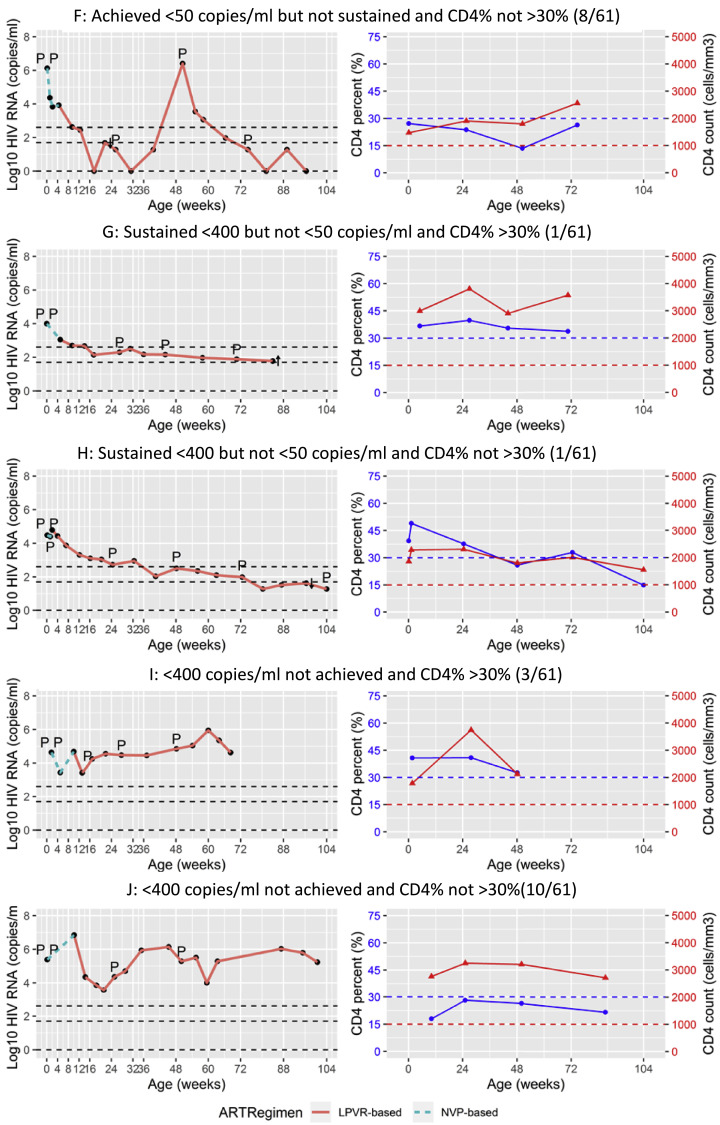
Applying more stringent virologic criteria, 10/61 (16.4%) children remaining in follow-up achieved and sustained VL of target not detected (TND) without any rebounds above this level. Six of these 10 children were also ever diagnostic PCR negative (Table 4). Three of 10 met the study immunologic criteria. Individual plots of VL and CD4 response of representative children are shown in Fig. 3.
Table 4.
Proportion of 61 HIV-infected infants starting antiretroviral therapy (ART) within 14 days of birth surviving in follow-up who met more stringent virologic endpoints by age at ART initiation.
| Characteristic | Total |
Initiated ART <48 h |
Initiated ART 2 to 14 days |
p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % of 61 in follow-up (95% CI) | n/N (%) in sub-group | N | % of 35 in follow-up (95% CI) | n/N (%) in sub-group | N | % of 26 in follow-up (95% CI) | n/N (%) in sub-group | ||
| Did not achieve “Target not detected” (TND) by 48 weeks | 25 | 41.0 | 13 | 37.1 | 12 | 46.2 | ||||
| Achieved TND then any rebound | 26 | 42.6 | 16 | 45.7 | 10 | 38.5 | ||||
| Sustained TND | 10 | 16.4 (8.2–28.1) | 6 | 17.1 (6.6–33.7) | 4 | 11.1 (4.4–34.9) | 1.000 | |||
| Ever PCR negative | 14 | 23.0 (13.2–35.5) | 10 | 28.6 (14.6–46.3) | 4 | 15.4 (4.4–34.9) | 0.357 | |||
| Non-transient PCR negative | 9 | 14.8 (7.0–26.2) | 6 | 17.1 (6.6–33.7) | 3 | 11.5 (2.5–30.2) | 0.720 | |||
| Non-transient PCR negative in achieved & sustained <50 copies/ml group | 9 | 9/32 (28.1) | 6 | 6/18 (33.3) | 3 | 3/14 (21.4) | ||||
| Non-transient PCR negative in achieved & sustained TND group | 5 | 5/10 (50.0) | 4 | 4/6 (66.7) | 1 | 1/4 (25.0) | ||||
Abbreviations: Antiretroviral treatment (ART), Confidence intervals (CI), Target not detected (TND), polymerase chain reaction (PCR).
Fig. 3.
Individual plots of viral load and CD4+ T-cell response to antiretroviral therapy of ten children in the study. P indicates the timing of positive, I indeterminate and N negative diagnostic PCR tests. A: Children who achieved and sustained target not detected and sustained CD4% >30% (3/61) B: Children who achieved and sustained target not detected but did not sustain CD4% >30% (7/61) C: Children who achieved and sustained <50 copies/ml and sustained CD4% >30% (13/61) D: Children who achieved and sustained <50 copies/ml but did not sustain CD4% >30% (9/61) E: Children who achieved <50 copies/ml but did not sustain this level (rebounded) and sustained CD4% >30% (6/61) F: Children who achieved <50 copies/ml but did not sustain this level (rebounded) and did not sustain CD4% >30% (8/61) G: Children who achieved and sustained <400 copies/ml but did not reach <50 copies/ml and sustained CD4% >30% (1/61) H: Children who achieved and sustained <400 copies/ml but did not reach <50 copies/ml but did not sustain CD4% >30% (1/61) I: Children who did not achieve <400 copies/ml (failure) but sustained CD4% >30% (3/61) J: Children who did not achieve <400 copies/ml (failure) and did not sustain CD4% >30% (10/61).
4. Discussion
The study was designed at a time when optimism about the potential of early ART in neonates to facilitate remission was high. We observed that less than a quarter of confirmed HIV-infected early-treated neonates remained in follow-up and met the virologic and immunologic criteria predefined as minimum thresholds to consider ART interruption. About half attained the virologic criteria (sustained VL <50 copies/ml); and the eligible proportion was further reduced when the immunologic criteria (sustained CD4+ T-cell percentage >30%) was applied. We concluded that too few infants met the entry criteria for the ATI trial, as we had originally designed it, to justify its implementation. Alternate designs of trials may still be scientifically meaningful for this population or subsets thereof. For example, the IMPAACT P1115 protocol defined highly stringent virologic entry criteria including sustained absence of all detectable virus [12].
While the protocol allowed initiation of ART within 14 days of birth, it was designed to encourage ART initiation within 48 h of birth. An on-site, point-of-care, diagnostic testing program was established to facilitate ART initiation before discharge after birth. On-site testing was challenging and coverage of testing only slightly exceeded 50% [10]. Lack of full testing coverage provided an excellent opportunity to compare infants starting ART <48 h to those starting slightly later. While these comparisons are vulnerable to potential confounders, as timing of ART initiation was not assigned by study design, the major reason for the time of ART initiation was based on the reach of the testing program. There was a slight excess of preterm and low birth weight infants in the later treated group most likely due to operational challenges ensuring adequate testing coverage of higher risk infants admitted to the hospital neonatal unit. We would have expected this to bias towards worse outcomes in the later treated group. Nevertheless, we observed no significant differences in the protocol-specified virologic and immunologic endpoints by the timing of ART initiation.
Lack of apparent benefits of very early ART on viral suppression may, at first glance, appear to contradict previous studies [13]. However, prior studies considered the timing of ART in much wider age bands than examined here [14]. Moreover, maternal ART was in place before birth in almost 80% of the participants, and all infants received nevirapine prophylaxis at birth regardless of the time of ART initiation. Drug resistance is unlikely to explain the findings as all were switched to a ritonavir-lopinavir-based regimen. While studies of acutely infected adults have the advantage of being able to classify into Feibig stages and, depending on behavioural factors, potentially even know the exact date of infection, knowledge of the precise timing of infection in infants is not possible. Our study was confined to presumed intrauterine-infected neonates; who generally have a worse prognosis than infants with intrapartum or post-natally-acquired infection [15]. Although intrauterine infection is thought to mainly occur late in pregnancy [16], age is only a rough proxy for duration of infection and high maternal ART coverage may have led to over-representation of intrauterine infections occurring earlier in pregnancy. Thus, even ART started within hours of birth may be too late in relation to the true timing of infection. Among birth-tested infants, intrauterine-acquired infection predominates in the era of effective antiretroviral prevention of transmission, lack of benefit in this group is a major limitation of this strategy.
Viral suppression rates, especially to more stringent cut-offs than required by our protocol were lower than expected. This is most likely explained by the significant challenges of adequate adherence with ART in neonates and infants. Most of the study participants’ caregivers live in impoverished economic circumstances with complex social problems and experience a high degree of HIV-related stigma. This social profile is likely representative of transmitters in an era of high maternal ART coverage. Moreover, there are major practical difficulties of sustaining adherence with twice-daily, poorly-palatable liquids for infants.
Mortality was only observed in those initiating ART <48 h of birth, however sample size was small and attrition before ART initiation may counter-balance this apparent excess.
Almost half of the early-treated neonates did not attain the protocol-specified immunologic criteria required for entry into the ATI trial; even among those who attained the protocol-specified virologic criteria. Discordance in virologic and immunologic response to ART in children has been described [17], [18], [19]. Reported frequency of discordance in other cohorts is lower than we observed but differences in definitions makes comparisons difficult [17], [18], [19]. Unless liberal cut-offs on CD4+ T-cell count had been used, an immunologic endpoint based on count rather than percentage would not have been meaningfully different.
Two recent case reports of long-term remission are of interest, although, in both cases ART was not started in the neonatal period as in our study [20,21]. Given that critical host and viral factors very likely contributed to remission in these cases, they support the findings from our study that early treatment on its own may not be sufficient. Moreover, in the one case, where a denominator could be ascertained, remission was attained in only one child out of more than 200 children who initiated ART reasonably early and interrupted ART [21]. The apparent rarity of post-treatment control supports our conclusion that very early ART on its own, with routinely-available regimens, is unlikely to lead to remission in a sizable minority of early-treated infants.
The major limitation of our study is that early antiretroviral therapy for infants is entirely reliant on maternal adherence. Although we supported adherence through an experienced, multi-disciplinary team, the challenges of sustained adherence likely contribute to the results. A second limitation is that we advised use of antiretroviral regimens and formulations that we considered best for this age group available in this setting at the time the study was designed. Other alternatives, including raltegravir which was only approved for use in this age group when the study was almost complete [22], may be more effective. Although raltegravir addresses palatability issues associated with lopinavir-ritonavir, it adds a layer of complexity around preparation for neonates and young infants. Thus raltegravir offers only minor respite from the adherence challenges which likely drive the poor response to early ART. Further research on possible long-acting formulations is needed. The virological and immunological outcomes occurred less frequently than expected necessitating longer recruitment. However, there were no other deviations from the original protocols.
Decisions about how quickly to start ART should be based on expectations about the capacity of ART to achieve standard clinical outcomes i.e. sustained viral suppression, and reduced morbidity and mortality rather than with the expectation that remission might be attained. Early initiation of more potent antiretroviral regimens or long-acting formulations that can address adherence challenges and/or alternative interventions, such as broadly neutralizing antibodies, need to be investigated to enable more rapid and sustained viral control and immune recovery as a stepping stone to achieving remission in this population.
Declaration of Competing Interest
Drs. Kuhn, Technau and Coovadia report receiving grants from NIH.
Dr. Abrams and Patel report receiving grants from NICHD.
Dr. Burke reports receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institute of Allergy and Infectious Disease, National Institutes of Health.
Dr. Tiemessen reports receiving grants from the NIH and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation.
The other authors have nothing to disclose.
Acknowledgments
Acknowledgements
We would like to thank the members of the Data Safety and Monitoring Board including Drs.
Jintanat Ananworanich, Marc Bulterys, Mark Cotton, Jane Lindsey, Mike McCune, and Andrew Prendergast. We would also like to thank other members of the study team: Drs. Martie Conradie, Ndileka Mbete, Pamela Murnane and Devasena Gnanashanmugam. The study was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institute of Allergy and Infectious Disease, National Institutes of Health (U01HD080441), USAID/PEPfAR, the South African National HIV Programme, and South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa. Cepheid provided cartridges at discounted prices and loan of machines and technical support. We gratefully acknowledge the infants and families who participated in the study as well as the hard-working study team.
Role of the funding source
Representatives from the NIH were involved in the design of the study, in oversight of its implementation and contributed to the writing of this manuscript. None of the other funders played any role in the writing of this manuscript or the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.100241.
Appendix. Supplementary materials
References
- 1.Saez-Cirion A., Bacchus C., Hocqueloux L., Avettand-Fenoel V., Girault I., Lecuroux C. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS visconti study. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stohr W., Fidler S., McClure M., Weber J., Cooper D., Ramjee G. Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy. PLoS ONE. 2013;8(10):e78287. doi: 10.1371/journal.pone.0078287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams J.P., Hurst J., Stohr W., Robinson N., Brown H., Fisher M. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife. 2014;3:e03821. doi: 10.7554/eLife.03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodi S., Meyer L., Kelleher A.D., Rosinska M., Ghosn J., Sannes M. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch Intern Med. 2012;172(16):1252–1255. doi: 10.1001/archinternmed.2012.2719. [DOI] [PubMed] [Google Scholar]
- 5.Steingrover R., Pogany K., Fernandez Garcia E., Jurriaans S., Brinkman K., Schuitemaker H. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS. 2008;22(13):1583–1588. doi: 10.1097/QAD.0b013e328305bd77. [DOI] [PubMed] [Google Scholar]
- 6.Namazi G., Fajnzylber J.M., Aga E., Bosch R.J., Acosta E.P., Sharaf R. The control of hiv after antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis. 2018;218(12):1954–1963. doi: 10.1093/infdis/jiy479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persaud D., Gay H., Ziemniak C., Chen Y.H., Piatak M., Jr., Chun T.W. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobin N.H., Aldrovandi G.M. Are infants unique in their ability to be “functionally cured” of HIV-1? Curr HIV/AIDS Rep. 2014;11(1):1–10. doi: 10.1007/s11904-013-0189-1. [DOI] [PubMed] [Google Scholar]
- 9.Goulder P.J., Lewin S.R., Leitman E.M. Paediatric hiv infection: the potential for cure. Nat Rev Immunol. 2016;16(4):259–271. doi: 10.1038/nri.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Technau K.G., Kuhn L., Coovadia A., Murnane P.M., Sherman G. Xpert HIV-1 point-of-care test for neonatal diagnosis of hiv in the birth testing programme of a maternity hospital: a field evaluation study. The Lancet HIV. 2017;4(10):e442–e4e8. doi: 10.1016/S2352-3018(17)30097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avettand-Fenoel V., Hocqueloux L., Ghosn J., Cheret A., Frange P., Melard A., Viard J.P., Rouzioux C. Total HIV-1 DNA, a marker of viral reservoir dynamics with clinical implications. Clin Microbiol Rev. 2016;29(4):859–880. doi: 10.1128/CMR.00015-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IMPAACT P1115 protocol: very early intensive treatment of HIV-infected infants to achieve remission: a phase I/II proof of concept study. 2018; (accessed 12 November 2019).
- 13.Violari A., Cotton M.F., Gibb D.M., Babiker A.G., Steyn J., Madhi S.A. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiau S., Abrams E.J., Arpadi S.M., Kuhn L. Early antiretroviral therapy in HIV-infected infants: can it lead to HIV remission? The Lancet HIV. 2018;5(5):e250–e2e8. doi: 10.1016/S2352-3018(18)30012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marston M., Becquet R., Zaba B., Moulton L.H., Gray G., Coovadia H. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40(2):385–396. doi: 10.1093/ije/dyq255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouzioux C., Costagliola D., Burgard M., Blanche S., Mayaux M.J., Griscelli C., Valleron A.J. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. The HIV infection in newborns french collaborative study group. Am J Epidemiol. 1995;142(12):1330–1337. doi: 10.1093/oxfordjournals.aje.a117601. [DOI] [PubMed] [Google Scholar]
- 17.Kelly C., Gaskell K.M., Richardson M., Klein N., Garner P., MacPherson P. Discordant immune response with antiretroviral therapy in HIV-1: a systematic review of clinical outcomes. PLoS ONE. 2016;11(6) doi: 10.1371/journal.pone.0156099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krogstad P., Patel K., Karalius B., Hazra R., Abzug M.J., Oleske J. Incomplete immune reconstitution despite virologic suppression in HIV-1 infected children and adolescents. Aids. 2015;29(6):683–693. doi: 10.1097/QAD.0000000000000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) study group in EuroCoord Prevalence and clinical outcomes of poor immune response despite virologically suppressive antiretroviral therapy among children and adolescents with HIV in Europe and Thailand: cohort study. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz253. An official publication of the Infectious Diseases Society of America Mar 28: pii.ciz253. [DOI] [PubMed] [Google Scholar]
- 20.Frange P., Faye A., Avettand-Fenoel V., Bellaton E., Descamps D., Angin M. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. The Lancet HIV. 2016;3(1):e49–e54. doi: 10.1016/S2352-3018(15)00232-5. [DOI] [PubMed] [Google Scholar]
- 21.Violari A., Cotton M.F., Kuhn L., Schramm D.B., Paximadis M., Loubser S., Tiemessen C.T. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun. 2019;10(1):412. doi: 10.1038/s41467-019-08311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke D.F., Penazzato M., Capparelli E., Cressey T.R., Siberry G., Sugandhi N., Mirochnick M. Prevention and treatment of HIV infection in neonates: evidence base for existing WHO dosing recommendations and implementation considerations. Expert Rev Clin Pharmacol. 2018;11(1):83–93. doi: 10.1080/17512433.2018.1393331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.