Graphical abstract

Keywords: Reactive oxygen species, Oxidative stress, Enzymatic antioxidants, Non-enzymatic antioxidants
Highlights
-
•
ROS in low concentration play important roles in signaling pathways.
-
•
The balance between production and suppression of ROS disappears under stress.
-
•
Enhancement of ROS concentration causes oxidative damage.
-
•
Algae use antioxidant enzyme and non-enzyme systems to cope on oxidative damage.
Abstract
Reactive oxygen species (ROS) typically produce in algae and act as secondary messengers in numerous cellular processes. Under abiotic stresses, the balance between production and suppression of ROS disappears and causes increase of ROS. Increasing excessive ROS can cause damage to various cellular components comprising cell membranes, proteins and lipids. Algae have an antioxidant defense system to overcome on oxidative damage. Antioxidant defense mechanisms are of two types, namely enzymatic and non-enzymatic antioxidants. The enzymatic antioxidants include superoxide dismutase, catalase, ascorbate peroxidase and glutathione reductase. The non-enzymatic antioxidants include carotenoids, tocopherol, ascorbic acid, glutathione, flavonoids and phenolic compounds. In this review, we describe the various types of ROS and their production, and antioxidant defense mechanisms for ROS suppression.
1. Introduction
Algae are autotroph organisms that use light and inorganic nutrients. These organisms have valuable compounds include polyunsaturated fatty acids (PUFA), carotenoids, phycobiliproteins, polysaccharides and phycotoxins [1]. Algae exist in dry and aquatic environments, and some have nutritional value and are used as food, and other some have biodiesel application [2]. Aerobic organisms use oxygen to breathe their energy. Electron leak in the electron chains in the plasma membrane, mitochondria, and chloroplasts produce reactive oxygen species (ROS) [3,4].
ROS is very harmful at high concentrations and lead to oxidative damage. Abiotic stresses cause oxidative stress through excessive production of ROS. ROS can react with biomolecules and inactivate or change them and cause to organelle dysfunction, alteration in cell structure and mutagenesis. Algae have antioxidant defense systems for cope on the harmful effects of ROS and maintain them under oxidative stress [[5], [6], [7]].
ROS act as a secondary messenger in biological processes such as response to environmental stresses. The balance between production and suppression of ROS determines its role. Control the level of ROS is vital due to the multifunctional role of ROS for avoid any oxidative damage and not to remove them completely. Antioxidative system including the non-enzymatic and enzymatic antioxidants causes ROS detoxification [8,9].
The present review focused on kinds of ROS, their site of creation, and their role as messenger and inducer of oxidative stress. Further, role of Antioxidative defense system in combating damage posed by overproduced ROS under stresses has been illuminated in detail.
2. Generation of reactive oxygen species
ROS generate in chloroplasts, peroxisomes, cytosol, and mitochondria and are as a by-product of respiration. ROS are produced in thylakoid membranes when the absorption of light is more than the requirement of photosynthetic apparatus. Photosystem I (Psi) is the most main source of ROS production due to the completion of the electron transfer in the thylakoid membrane on its stromal side. PSII caused ROS production when the excitation energy delivery to the PSII reaction center and the electron transport chain between the photosystems limited. ROS activity depends on its type and reaction conditions. For example HO* has a very high chemical activity and is able to oxidize the most organic molecules and H2O2 has moderate activity. The ROS activity decreases in the order HO˖ > 1O2 > H2O2 > O2˖_ in biological environments [10,11].
2.1. Singlet oxygen
Singlet oxygen (1O2) counters with chief biological compounds such as unsaturated fatty acids of membrane lipids and is electrophilic. O2 reacts with singlet or triplet excited state of a pigment molecule and produces 1O2 in photosynthetic tissues. Carotenoids and tocopherols are scavengers of 1O2 [12,13].
2.2. Hydrogen peroxide
Hydrogen Peroxide (H2O2) produces by lessening of oxygen by two electrons. Superoxide dismutase causes the generation of H2O2 by dismutation of O2˖−. H2O2 suppressed by peroxidases and catalases [14].
2.3. Superoxide anion radical
PSI by electron reduction of oxygen causes generation of superoxide anion radical (O2˖−), but PSII produces low amount of O2˖−. Superoxide dismutase detoxifies O2˖- via the dismutation reaction [15].
2.4. Hydroxyl radical
Hydroxyl radical (HO˖) creates of homolytic cleavage of water (H2O HO˖ + H˖). HO• radicals rapidly attack to proteins and lipids and lead to oxidative injury [16].
3. Oxidative damage
Imbalance between pro-oxidant and antioxidant homeostasis augmented ROS and oxidative damage. ROS disrupt cell function by lipid peroxidation, oxidizing proteins and damaging nucleic acids (Fig. 1).
Fig. 1.
Reactive oxygen species (ROS) induce oxidative damage to proteins, lipids and DNA [48].
3.1. Lipid peroxidation
Lipids, as the key components of the membrane, are the primary goal of the ROS and lead to lipid peroxidation by removing hydrogen from the unsaturated chain of fatty acids. Therefore, lipid peroxidation determinates cellular harmful in living organism under oxidative stress. Lipid peroxidation produced wide types of cytotoxic products from polyunsaturated acids such as malondialdehyde (MDA) and aldehydes. MDA is an identifier for oxidative hurt. Chloroplasts have a composite system of membranes rich of polyunsaturated fatty acids in algae, which are main targets for peroxidation [17,18].
3.2. Protein oxidation
ROS cause amino acids oxidation, change of electrical charge of proteins, breakup of peptide chain, cross-linking of proteins and increase susceptibility to proteolysis by proteases. Proteins have sulfur comprising amino acids or thiol groups are the target of ROS. Cysteine and methionine residues are mainly more susceptible to oxidation. Oxidation of methionine residues or sulfhydryl groups lead to conformational alternations, unfolding and degradation proteins. ROS generated disulfide bonds between sulfur-containing amino acids and destroyed the function and structure of proteins. Oxidized proteins lead cellular dysfunction making their deletion necessary. They are normally recognized and degraded by proteasomal complexes. If oxidized proteins are not removed, they collected and change the cell function [19,20].
3.3. DNA oxidation
Hydroxyl radicals attack to the base and saccharide in DNA and cause saccharide fragmentation and strand breaks. Strand break are more destructive and lethal than base damage in cells. ROS cause alteration in DNA bases for example thymine and guanine residues in DNA can be hydroxylated or degraded. Oxidation of thymine residues stop replication by DNA polymerase and transcription by RNA polymerase [21,22].
4. Antioxidative defense system in algae
Algae applied two defensive strategies for cope on ROS before they can damage to cellular difference components (Fig. 2). The first system comprise antioxidant enzymes (high molecular weight) such as superoxide dismutases, glutathione reductase, catalases, ascorbate peroxidase and non-enzymatic (low molecular weight) that comprise ascorbate, flavonoids, carotenoids, glutathione, tocopherols and phenols. The second mechanism is repair enzymes that repair and remove damaged macromolecules. The enzymatic antioxidants are important in the detoxification of the destructive effects of formed ROS by electron transport (O2 •−, H2O2, HO•), but the non-enzymatic antioxidants are more effective in the prevention of ROS prodction by the excitation energy transfer (1O2) [[23], [24], [25]].
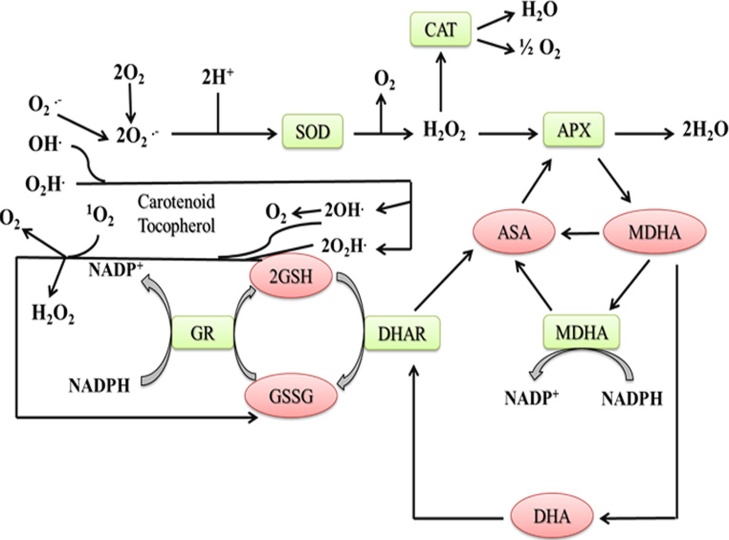
Fig. 2.
Enzymatic and non-enzymatic antioxidants in algae. ASC, Ascorbate; APX, Ascorbate peroxidase; CAT, Catalase; DHA, Dehydroascorbate; GSH, Glutathione; GR, Glutathione reductase; GSSG, glutathione disulfide; MDHA, Monodehydroascorbate; SOD, Superoxide dismutase; DHA, Dehydroascorbate reductase [49].
4.1. Enzymatic components of antioxidative defense system
4.1.1. Superoxide dismutase
Superoxide dismutase (SOD) is a metalloprotein and first line of defense against oxidative stress that catalyzes the dismutation of superoxide radicals into oxygen and hydrogen peroxide. SOD include three isoforms that determined by their metal center cofactors: Cu/ZnSOD found in the thylakoid membranes and cytosol of higher plants, certain dinoflagellates and charophycean green algae, MnSOD located in mitochondria, and FeSOD found in the chloroplast stroma. The SOD activity enhanced by abiotic stresses and acts a defense tool [[26], [27], [28]].
4.1.2. Catalase
Catalase (CAT) is tetrameric enzyme contain heme that has a main role to convert H2O2 into H2O and O2. CAT has three groups. Manganese catalases exist in prokaryotes. Catalase peroxidases act as both catalases and peroxidases and have been found in prokaryotes and some eukaryotes. Classical catalases (cat) include heme groups and covert H2O2 to H2O and O2 in a two-step process. First, one molecule of H2O2 is reduced to water and the Fe3+ of the catalase is altered to cat(Fe[V]O). Second, the cat(Fe[V]O) is converted back to Fe3+ while another molecule of H2O2 is reduced to H2O and O2 [29].
4.1.3. Ascorbate peroxidase
Ascorbate peroxidase (APX) is a heme enzyme and converts the H2O2 into H2O through ascorbate as electron donor. APX is existed in plants and algae. Two cytosolic APX (cAPX) isoenzymes have been showed in the red algae Galdieria partita and G. sulphuraria [30,31].
4.1.4. Glutathione reductase and glutathione peroxidase
Glutathione reductase (GR) is a flavoprotein oxidoreductase that exists in eukaryotes and prokaryotes. GR is a great enzyme of the ascorbate-glutathione cycle and have a necessary function in defense system against ROS by maintain the reduced status of GSH. Like Ascorbate peroxidase, Glutathione peroxidase decomposes H2O2 to H2O by GSH [32,33].
4.2. Nonenzymatic components of antioxidative defense system
4.2.1. Glutathione
The Tripeptide glutathione is the major low molecular weight thiol and formed of glutamate, cysteine and glycine. Reduced glutathione by oxidizing itself into oxidized glutathione via ascorbate-glutathione cycle causes the regeneration of ascorbate. Glutathione involves in adjust redox potential for amino acids and proteins, scavenging oxidative damage, non-specific reductant, substrate/cofactor for enzyme-catalyzed reactions, reconstruction of protein disulfide bonds and suppression of H2O2 and organic peroxides (ROOH) [34,35].
4.2.2. Ascorbate
Ascorbic acid (ascorbate, vitamin C) is a water-soluble antioxidant that acts as substrate for ascorbate peroxidase, electron donor for •OH radical, good scavenger, reducing antioxidant and donating its electrons to ROS. Ascorbate suppresses H2O2 thought ascorbate-glutathione cycle. [36].
4.2.3. Carotenoids
Carotenoids are isoprenoid and lipophilic compounds and are colored yellow, orange or red that 750 kinds of carotenoid are exist in plants and microorganisms. Carotenoids include carotenes such as lycopene, α- carotene and β-carotene, and xanthophylls with oxygen as hydroxyl groups (e.g. lutein), as oxy-groups (e.g. canthaxanthin) or as a combination of both of them (e.g. astaxanthin). Some of xanthophylls (violaxanthin, antheraxanthin, zeaxanthin, neoxanthin, lutein, loroxanthin, astaxanthin and canthaxanthin) produced by microalgae whereas others (diatoxanthin, diadinoxanthin and fucoxanthin) also synthesized by brown algae. Carotenoids have antioxidant characterizes and cause maintain of cells against free radicals, inhibition of lipid peroxidation, increase the stability of the photosynthetic apparatus and protection of integrity membranes [[37], [38], [39]].
4.2.4. Tocopherols
Tocopherols (vitamin E) have an aromatic ring and a long hydrocarbon chain. Tocopherols play in oxidationreduction reactions by the aromatic ring. Tocopherols destroy reactive types of oxygen and protect unsaturated fatty acids from oxidation. Among the four tocopherols (α-, β, ϒ and ϐ-tocopherols), α-tocopherol is a key antioxidant because it can suppress 1O2, reduce O2. − and finish lipid peroxidation reaction [40].
4.2.5. Phenolic compounds
Phenolic compounds include more than 8000 compounds and divided into 10 various groups according to their chemical structure. Phenolic compounds are as main natural antioxidants through single electron transfer and hydrogen atom transfer. Many studies showed that the phenolic content in microalgae is lower than or equal to the minimum amounts founded in terrestrial plants. Also, types of flavonoids, such as isoflavones, flavanones, flavonols, and dihydrochalcones produced in microalgae. Microalgae synthesized complex phenolic compounds, so identify of phenolic compounds are required in microalgae. Marine algae have many polyphenols such as phlorotannins that have antioxidant activities. Phenolic are involved in defense mechanisms against environmental stresses [[41], [42], [43]].
5. ROS signaling
ROS in low concentration have significant roles in the defense against infection, the cell signaling and the apoptosis. O2 −, OH˖ and H2O2 have more signaling capacity because they can transfer of membranes via aquaporins [44]. The transduction of H2O2- based signals is centered on sulfur chemistry, with the main player being the reversible oxidative modification of cellular sulfur-containing groups (e.g., cysteine residues and thioredoxin), which in turn results in disturbances of metabolism and signaling pathways in microorganisms. H2O2 oxidized cysteine thiol groups of phosphatases in mitogen activated protein kinase (MAPK) pathways, which act in signaling response for various environmental stimuli. Late studies showed moreover change of gene expression, ROS have an essential role for resistance under oxidative stress by post-translational changes. Some of mitogen-activated protein kinase family includes extracellular signal-regulated kinases, JNK, and p38 play a role in cellular processes including proliferation, differentiation, and apoptosis regulated by ROS [[45], [46], [47]].
6. Conclusion
ROS have two divergent functions in algae; in low concentration they are as signaling molecules that regulate cellular processes, whereas they cause damage to cellular components in high concentration. The balance between production and suppression of ROS disappears under stress condition. In order to avoid the oxidative damage, algae have a complex antioxidative defense system include of enzymatic and non- enzymatic components.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Markou G., Nerantzis E. Microalgae for high-value compounds and biofuels production: are view with focus on cultivation under stress conditions. Biotechnol. Adv. 2013;8:1532–1542. doi: 10.1016/j.biotechadv.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Chu W.L., Yuen Y.S., Wong C.Y., Teoh M.L., Phang S.M. Isolation and culture of microalgae from the windmill Island region, Antarctica. Kuala Lumpur, Malaysia; 2001. pp. 53–59. August (2001) [Google Scholar]
- 3.Alscher R.G., Donahue J.L., Cramer C.L. Reactive oxygen species and antioxidants: relationships in green cells. Physiol. Plant. 1997;100:224–233. [Google Scholar]
- 4.Foyer C.H. Oxygen metabolism and electron transport in photosynthesis. In: Scandalios J., editor. Molecular Biology of Free Radical Scavenging Systems. Cold Spring Harbor Laboratory Press; New York, NY: 1997. pp. 587–621. [Google Scholar]
- 5.Halliwell B., Gutteridge J. Oxford Science Publications; New York: 1999. Free Radicals in Biology and Medicine. pp.;936. [Google Scholar]
- 6.Lim S.N., Cheung P.C.K., Ooi V.E.C., Ang P.O. Evaluation of antioxidative activity of extracts from brown seaweed, Sargassum siliquastrum. J. Agric. Food Chem. 2002;50:3862–3866. doi: 10.1021/jf020096b. [DOI] [PubMed] [Google Scholar]
- 7.Vranová E., Inzé D., Van Breusegem F. Signal transduction during oxidative stress. J. Exp. Bot. 2002;53:1227–1236. [PubMed] [Google Scholar]
- 8.Desikan R., Mackerness S.A.H., Hancock S.J.T., Neill J. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001;127:159–172. doi: 10.1104/pp.127.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noctor G., Foyer C.H. Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 10.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 12.Schweitzer C., Schmidt R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003;103:1685–1757. doi: 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- 13.Triantaphylidès C., Krischke M., Hoeberichts F.A., Ksas B., Gresser G., Havaux M. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008;148:960–968. doi: 10.1104/pp.108.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruzzi M., Sartori E., Moscatelli A., Khudyakov I.V., Turro N.J. Time-resolved EPR study of singlet oxygen in the gas phase. J. Phys. Chem. A. 2013;117:5232–5240. doi: 10.1021/jp403648d. [DOI] [PubMed] [Google Scholar]
- 15.Pospíšil P. Production of reactive oxygen species by Photosystem II. Biochim. Biophys. Acta. 2009;1787:1151–1160. doi: 10.1016/j.bbabio.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg N., Manchanda G. ROS generation in plants: boon or bane. Plant Biosyst. - Int. J. Deal. With All Asp. Plant Biol. 2009;143:8–96. [Google Scholar]
- 18.Halliwell B., Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993;57:7155–7245. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 19.Kelly F.J., Mudway I.S. Protein oxidation at the air-lung interface. Amino Acids. 2003;25:375–396. doi: 10.1007/s00726-003-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyras L., Cairns N.J., Jenner A., Jenner P., Halliwell B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J. Neurochem. 1997;68:2061–2069. doi: 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- 21.Breimer L.H., Lindahl T. Excision of oxidized thymine from DNA. J. Biol. Chem. 1984;259:5543–5548. [PubMed] [Google Scholar]
- 22.Martinez A., Kolter R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J. Bacteriol. 1997;179:5188–5194. doi: 10.1128/jb.179.16.5188-5194.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pikula K.S., Zakharenko A.M., Aruoja V., Golokhvast K.S., Tsatsakis A.M. Oxidative stress and its biomarkers in microalgal ecotoxicology–A minireview. Curr. Opin. Toxicol. 2019;13:8–15. [Google Scholar]
- 24.Jahan S., Yusoff I.B., Alias Y.B., Bakar A.F. Reviews of the toxicity behavior of five potential engineered nanomaterials (ENMs) into the aquatic ecosystem. Toxicol. Rep. 2017;4:211–220. doi: 10.1016/j.toxrep.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia T., Kovochich M., Liong M., Madler L., Gilbert B., Shi H.B., Yeh J.I., Zink J.I., Nel A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 27.Davies K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 28.Mallick N., Mohn F.H. Reactive oxygen species: response of algal cells. J. Plant Physiol. 2000;157:183–193. [Google Scholar]
- 29.Mate M.J., Zamocky M., Nykyri L.M., Herzog C., Alzari P.M., Betzel C., Koller F., Fita I. Structure of catalase-A from Saccharomyces cerevisiae. J. Mol. Biol. 1999;286:135–149. doi: 10.1006/jmbi.1998.2453. [DOI] [PubMed] [Google Scholar]
- 30.Oesterhelt C., Vogelbein S., Shrestha R.P., Stanke M., Weber A.P. The genome of the thermoacidophilic red microalga Galdieria sulphuraria encodes a small family of secreted class III peroxidases that might be involved in cell wall modification. Planta. 2008;227:353–362. doi: 10.1007/s00425-007-0622-z. [DOI] [PubMed] [Google Scholar]
- 31.Sano S., Ueda M., Kitajima S., Takeda T., Shigeoka S., Kurano N., Miyachi S., Miyake C., Yokota A. Characterization of ascorbate peroxidases from unicellular red alga Galdieria partita. Plant Cell Physiol. 2001;42:433–440. doi: 10.1093/pcp/pce054. [DOI] [PubMed] [Google Scholar]
- 32.Edwards E.A., Rawsthorne S., Mullineaux P.M. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.) Planta. 1990;180:278–284. doi: 10.1007/BF00194008. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Puertas M.C., Corpas F.J., Sandalio L.M., Leterrier M., Rodriguez Serrano M., Rio L.A.D. Glutathione reductase from pea leaves: response to abiotic stress and characterization of the peroxisomal isozyme. New Phytol. 2006;170:43–52. doi: 10.1111/j.1469-8137.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 34.Foyer C.H., Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1997;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B., Gutteridge J.M. 3rd ed. Oxford University Press; Midsomer Norton, Avon, England: 1999. Free Radicals in Biology and Medicine. [Google Scholar]
- 36.Wheeler G.L., Jones M.A., Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- 37.Di Pietro N., Di Tomo P., Pandolfi A. Carotenoids in cardiovascular disease prevention. JSM Atheroscler. 2016;1:1–13. [Google Scholar]
- 38.Katsumata T., Ishibashi T., Kyle D. A sub-chronic toxicity evaluation of a natural astaxanthin-rich carotenoid extract of Paracoccus carotinifaciens in rats. Toxicol. Rep. 2014;1:582–588. doi: 10.1016/j.toxrep.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasso S., Pohnert G., Lohr M., Mittag M., Hertweck C. Microalgae in the postgenomic era: a blooming reservoir for new natural products. FEMS Microbiol. Rev. 2012;36:761–785. doi: 10.1111/j.1574-6976.2011.00304.x. [DOI] [PubMed] [Google Scholar]
- 40.Takenaka Y., Miki M., Yasuda H., Mino M. The effect of a-tocopherol as an antioxidant on the oxidation of membrane protein thiols induced by free radicals generated in different sites. Arch. Biochem. Biophys. 1991;285:344–351. doi: 10.1016/0003-9861(91)90370-x. [DOI] [PubMed] [Google Scholar]
- 41.Cornish M.L., Garbary D.J. Antioxidants from macroalgae: poten-tial applications in human health and nutrition. Algae. 2010;25:155–171. [Google Scholar]
- 42.de la Coba F., Aguilera J., Figueroa F.L., de Galvez M.V., Herrera E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009;21:161–169. [Google Scholar]
- 43.Jahnke L.S. Massive carotenoid accumulation in Dunaliella bardawil induced by ultraviolet-A radiation. J. Photochem. Photobiol. 1999;48:68–74. [Google Scholar]
- 44.Reczek C.R., Chandel N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lushchak V.I. Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C. 2011;153:175–190. doi: 10.1016/j.cbpc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Morano K.A., Grant C.M., Moye-Rowley W.S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics. 2012;190:1157–1195. doi: 10.1534/genetics.111.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veal E.A., Day A.M., Morgan B.A. Hydrogen peroxide sensing and signaling. Mol. Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012 [Google Scholar]
- 49.Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]