Graphical abstract

Keywords: M. rosenbergii, GSI, ESI, 60Co, Egg count, Egg hatching
Highlights
-
•
Identified the effect of 60Co on reproductive physiology of prawn.
-
•
GSI and ESI decreased in irradiated groups.
-
•
Self regulating mechanisms could be the reason for preventing the prawn from the lethality.
Abstract
The present study was designed to evaluate the effect of acute level of 60Co gamma radiation on fecundity of freshwater prawn Macrobrachium rosenbergii. Prawns were exposed to four different dose levels (3, 30, 300 and 3000 mGy) and their reproductive disturbances i.e Gonadosomatic Index (GSI), Egg Clutch somatic Index (ESI), Egg Counts and Egg hatching rates were calculated. The experimental group showed significant reduction in GSI (0.47 ± 0.01) & ESI (1.22 ± 0.08) after exposure to 60Co gamma radiation. Egg Count (3713 ± 21) and Egg hatching rates (3798 ± 11) were significantly reduced in all irradiated groups. The number of dead larva increased with the increasing level of doses. With the increase in dosage level, the gonad and egg clutch weight were decreased which likely lead to reduced number of eggs. Our results proved that even low level of ionizing radiation (60Co) affects the fecundities of freshwater crustacean M. rosenbergii.
1. Introduction
Nowadays, various international directives (ERICA and PROTECT) show interest on studying the impact of radioactive emissions on the environment, particularly the ionizing radiation impact on non-human biota of both aquatic and terrestrial organisms, to develop the protective measures against the radioactive pollution [1,2]. The emergence of ionizing radiation into the environment is by two major sources such as natural and anthropogenic. Nuclear weapons testing, nuclear disasters and permitted discharges from nuclear reprocessing plants are the three major sources of anthropogenic radionuclides in the environment [3]. Releasing of radioactive waste from nuclear facilities [4] either accidently or intentionally makes the aquatic ecosystem as a sink for the radionuclides [5]. However, a very few attempt has only been made in this aspect [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]], (Table 1, Table 2). Reproduction, the most sensitive endpoint of radiation exposure in non-human biota [17], is considered as the eco-toxicological and environmental risk assessment studies [18,19]. Hence, an attempt was made to analyze the impact of cobalt 60 gamma (ionizing) radiation on fresh water crustaceans Macrobrachium rosenbergii egg production and its further development in low dose level (mGy). M.rosenbergii, a native prawn species of Southeast Asian countries [20], constitutes a major species of fishery in River Cauvery (Tamil Nadu, India), an important perennial river of South India [21].
Table 1.
List of Gamma Radiation (60Co) studies in non-human biota by various researchers (www.fredrica-online.org; IAEA, 2002).
| Species (Common name) | Dose Rate (Gy) | Radiation Effect | References |
|---|---|---|---|
| Pisces | |||
| Esox lucius L. (Pike) | 0, 2 | Reproduction (delayed hatching), anomaly | [6] |
| Cyprinus carpio (Carp) | 5, 7.5, 10, 15, 20, 40, 80, 120, 160 | Reproduction | [7] |
| Oncorhynchus tshawytscha (Chinook salmon) | 0, 0.41, 0.95, 2.3, 3.5, 8.1, 17, 42 | Reproduction (retardation of Gonadal differentiation) | [8] |
| Salmo gairdnerii (Rainbow trout) | 0.25, 0.5, 1, 2, 4 | Reproduction (embryo abnormalities) | [9] |
| Tinca tinca L. (Tench) | 0, 0.04,0.25, 0.5, 1, 2.5, 40.25, 40.5, 41, 42.5 | Reproduction (reduced survival rate in larvae). | [10] |
| Crustaceans | |||
| Physa heterostopha (Aquatic Snail) | 1, 10, 25 | Reproduction and fecundity | [11] |
| Mercenaria mercenaria (Clams) | 0.0, 0.25, 2.5, 28, 1020 | Growth and Survival monitored | [12] |
| Mytilus edulis (Mussel) | 0.9 or 2.0μGy | Observation of Cilia beating monitored and behavioural response also | [13] |
| Biomphalarica qlabrata (Snail) | 0, 2.5, 10, 20 | Counted off spring | [14] |
Table 2.
Rationale for establishing a derived consideration reference level (DCRL) for a hypothetical reference animal or plant.
| Dose rate interval (mGy/ day) | Observations (hypothetical) | Concern |
|---|---|---|
| 1000–10,000 | Significant mortality | High to very high |
| 100–1000 | Population disturbance from prolonged exposure | High if prolonged exposure |
| 10–100 | Effects on morbidity | Increasing |
| 1–10 | Some reproductive disturbance | DCRL band |
| 0.1–1 | No effects observed | Very low to low |
| 0.01–0.1 | Close to or within natural background, no observation of effects | Nil to very low |
Source: Table 2 from ICRP 108 publication (ICRP, 2008b).
2. Materials and methods
2.1. Experimental prawn & irradiation
Live prawns were purchased from the Dhanalakshmi Prawn Farm, Cuddalore, Tamil Nadu (India) and acclimatized under controlled condition using indoor fiber water tanks (1.5 × 1.0 m) with proper aeration. The setup was maintained in the Environmental Research Laboratory, Jamal Mohamed College, Tiruchirappalli (Tamil Nadu, India) and maintained in a 12:12 h (light/dark) photoperiod. Prawns were fed with boiled and chopped goat liver ad libitum every day.
Pre-moulted females and mature male prawns were irradiated using Theratron phoenix (P-33) tele cobalt unit (Canada) having specification in the dose rate of 360 mGy/min in a 60Co radionuclide source located in GVN Cancer Cure Research Center and Hospital, Tiruchirappalli, Tamilnadu, India. The experimental animals were placed in the polypropylene boxes (0.25 × 0.05 m (L × B × H) of capacity 1.5 L water). The experiment consisted of five groups of six prawns each (Three replicates i.e. n = 18). Lethal dosage of 60Co gamma radiation for M. rosenbergii was first studied and analyzed by SPSS tool - probit analysis. One group was considered as the control and the other four groups were exposed to 3, 30, 300 and 3000 mGy each and were observed for the next 96 h.
2.2. Morphometric assessment
The individual weight of each prawn was measured in grams (gm) using a digital balance (DENVER). The sex of each specimen was determined by the visual observation of the base of the fifth pair of pereiopods [22]. The different stages of ovarian development were classified based on the color, size and outline of the ovary [23].
2.3. Assessment of organo somatic index
At the end of the experiment, the weight of each prawn was measured. The prawns (2 pairs in each group) were randomly selected and cut open in the mid-dorsal line and their adjoining tissues were removed [24]. The gonads of both control and irradiated group of prawns were collected and weighed for Gonado Somatic Index [25,26]. The egg clutches were removed for the Egg clutch somatic index [27].
Calculation of GSI and ESI:
2.4. Egg counting & hatching rate
Egg clusters were removed 7 days after spawning from both control and the irradiated broods. Eggs were carefully removed from the brood pouch by following the standard procedures [28]. Eggs were counted manually by using the magnifying glass and observed under the stereomicroscope [29]. The eggs were incubated in vitro in a fiber glass tank at a salinity of 8ppt with moderate aeration. The hatching rate was calculated from the number of eggs in a brood from the number of larvae that hatched out. The number of live larvae and that of the dead eggs were observed after 24 h after hatching [30,27].
2.5. Statistical analyses
The obtained values were expressed as the mean ± standard deviation (SD). Differences between groups were assessed by one-way analysis of variance using the SPSS software package for windows (version 16.0). A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Lethal dose
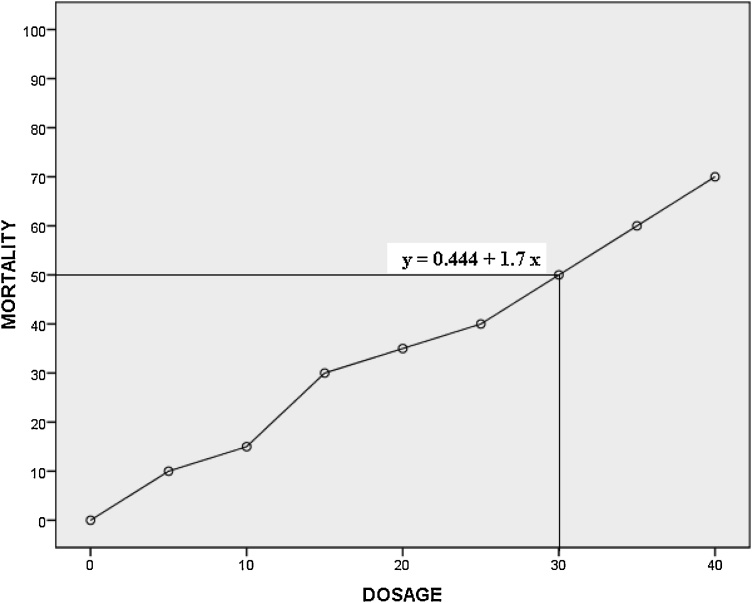
Lethal dose of M. rosenbergii was determined using the probit analysis and LD50 of gamma irradiated M. rosenbergii was identified at 30 Gy (Fig. 1).
Fig. 1.
Probit analysis Graph showing LD50 in M.rosenbergii.
3.2. GSI and ESI
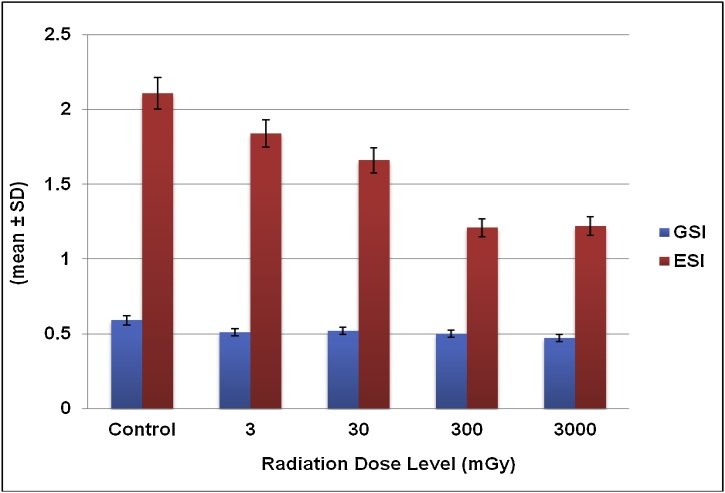
Yellowish or bright orange coloured eggs were observed in the mature females where the brood pouch was located in the cephalothorax, which was visible through the carapace. The gonado somatic index of the control group (Table 3) was 0.59 ± 0.02. A radiation stress which developed in prawns, reduced the GSI in all the irradiated groups to 0.51 ± 0.01, 0.52 ± 0.03, 0.5 ± 0.01 and 0.47 ± 0.01 respectively (Fig. 2).
Table 3.
Gonado somatic and Egg clutch Somatic indices of Macrobrachium rosenbergii exposed with 60Co gamma radiation (n = 18).
| Dose level (mGy) | Mean ± SD |
|
|---|---|---|
| GSI | ESI | |
| Control | 0.59 ± 0.02 | 2.11 ± 0.14 |
| 3 | 0.51 ± 0.01 | 1.84 ± 0.01 |
| 30 | 0.52 ± 0.03 | 1.66 ± 0.06 |
| 300 | 0.50 ± 0.01 | 1.21 ± 0.05 |
| 3000 | 0.47 ± 0.01 | 1.22 ± 0.08 |
Fig. 2.
Gonado somatic and Egg clutch somatic indices of M. rosenbergii exposed with 60Co gamma radiation.
The collected egg clutches were placed in a filter paper to avoid moisture content for few seconds, and then it was weighed and the values were entered. The obtained value for the control group was 2.11 ± 0.14 (Table 3). The ESI range had significantly decreased to 1.84 ± 0.01, 1.66 ± 0.06, 1.21 ± 0.05 and 1.22 ± 0.08 in 3, 30, 300, 3000 mGy respectively (Fig. 2).
3.3. Egg counting rate and hatching rate
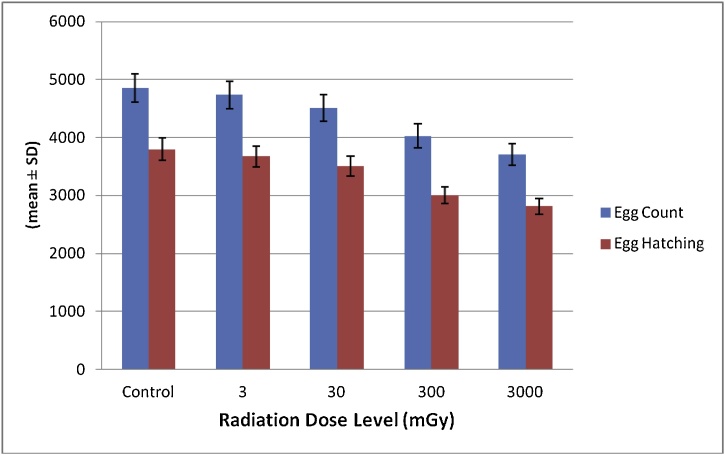
The collected eggs were observed using a stereomicroscope along with water (8ppt). The number of eggs in all three replicates, and after hatching the live post larvae’s (PL) were counted and recorded (Table 4). The dead larvae were removed from the tank. In the control group, 4850 ± 23 eggs were observed. Irradiated groups showed decreased number of eggs when compared to that of the control. The least number of eggs were found in 3000 mGy of the irradiated group as of 3713 ± 21 eggs (Fig. 3). In the control group, 3798 ± 11 juveniles were counted. The number of dead ones had significantly increased with increased doses. In 3000 mGy, 2813 ± 25 PL was counted (Fig. 3).
Table 4.
Total egg count and hatched egg count in control and 60Co gamma irradiated groups M. rosenbergii (n = 18).
| Dose (mGy) | No. of. Egg Count (Mean ± SD) | No. of. Egg Hatched (Mean ± SD) |
|---|---|---|
| Control | 4850 ± 23 | 3798 ± 11 |
| 3 | 4739 ± 15 | 3672 ± 32 |
| 30 | 4511 ± 35 | 3503 ± 12 |
| 300 | 4030 ± 56 | 3005 ± 40 |
| 3000 | 3713 ± 21 | 2813 ± 25 |
Fig. 3.
Egg counting and hatching rate of control and 60Co gamma irradiated prawn M. rosenbergii.
4. Discussion
In the crustaceans, the female reproductive outputs were considered as the typical endpoints including the production of new eggs [31] as well as the hatchability of eggs [32,33]. Organosomatic indices reflected the status of organ systems and their changes by various environmental factors and stressors [34]. Reduced gonado somatic index was observed in irradiated groups due to the stress which was developed by the 60Co gamma ionizing radiation.
[35] estimated the normal fecundity of M. rosenbergii by counting the number of eggs on the pleopods where it ranged from 20,000 to 70,000 eggs and their mean number of eggs per female was around 4500 eggs which was similar to our control results. Infrared (IR) radiation treated female M. rosenbergii (0, 5, 10, 15 Gy) and P. japonicus (20 Gy) showed 100% and 52.2% (relative to non-irradiated females) reduced fecundity [36].
The reduced fecundity observed in the crustacean P. japonicus was due to the deleterious effect of ionizing radiation [37,38]. A significantly reduced fecundity was observed in the female brine shrimp (Artemia sp.) exposed to IR (1–5 Gy) and the absence of oocytes production after the exposure to 10 Gy (Squire, 1970). Larvae Hatch Fecundity (LHF) is the number of larvae released from the egg mass following the incubation [39], which was reduced in the irradiated prawn than that of the control.
Several recent reports and international bodies were grappling with the problems of regulating exposure of biota [[40], [41], [42], [43], [44]] and the most fundamental issue was due to the lack of adequate scientific data with concern to low dose exposure effects.
Different organizations such as USDOE, NCRP, IAEA, ICRP and UNSCEAR had published many data about the benchmark values regarding to the environmental radiation protection in various countries [45]. The FREDERICA database contains over 30,000 data entries from a number of international radiation effects whereas the directives contains data on chronic dose ranges of 0 – >10,000μGy/hr [46].
There is a lacuna in acute ionizing radiation studies in freshwater crustacean species. Hence, this work and its related other studies [47,48] provides the necessary information about the biological effect of ionizing radiation to develop the permissible dose levels. Similar kind of acute radiation studies were performed in fresh water O. mossambicus [49] by the same team, hence we suggested having in other freshwater species also.
5. Conclusion
The study proved that even the minimal dose of 3 mGy of 60Co gamma irradiation is sufficient to reduce the egg production, growth and hatching rate of M. rosenbergii. Hence, it is suggested that the outputs from the nuclear industries, hospitals and research institutes should be kept below 3 mGy of 60Co gamma irradiation.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
Authors were thankful to Dr. A.K. Khaja Nazeemudeen Sahib, Secretary and Correspondent, Dr. S. Ismail Mohideen, Principal and Dr. I. Joseph Antony Jerald, Head, P.G. and Research Department of Zoology, Jamal Mohamed College (Autonomous), Tiruchirappalli for Institutional support. Thanks are due to the Bhabha Atomic Research Center, Department of Atomic Energy, Mumbai for funding the project. The Director, GVN Cancer Cure Research Centre and Hospital, Tiruchirappalli is remembered with gratitude for technical support.
References
- 1.Howard B.J., Beresford N.A., Andersson P., Brown J.E., Copplestone D., Beaugelin-Seiller K., Whitehouse P. Protection of the environment from ionising radiation in a regulatory context an overview of the coordinated action project. J. Radiol. Prot. 2010;30(2):195. doi: 10.1088/0952-4746/30/2/S01. [DOI] [PubMed] [Google Scholar]
- 2.Larsson C.M. An overview of the ERICA integrated approach to the assessment and management of environmental risks from ionizing contaminants. J. Environ. Radioact. 2008;99(9):1364–1370. doi: 10.1016/j.jenvrad.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Aarkrog A. Input of anthropogenic radionuclides into the World Ocean. Deep Sea Res. Part II: Top. Stud. Oceanogr. 2003;50(17):2597–2606. [Google Scholar]
- 4.Burton J.D. Radioactive nuclides in the marine environment. In: Riley J.P., Skirrow G., editors. Chemical Oceanography. 2nd ed. Academic Press; NewYork: 1975. [Google Scholar]
- 5.Avery S.V. Fate of caesium in the environment: distribution between the abiotic and biotic components of aquatic and terrestrial ecosystems. J. Environ. Radioact. 1996;30(2):139–171. [Google Scholar]
- 6.Kulikov N.V. Radiosensitivity of roe of pike (Esox lucius L) during fertilization and early cleavage. Radiobiology. 1970;10(5):768–770. [PubMed] [Google Scholar]
- 7.Frank M.L., Blaylock B.G. University of Tennessee; 1971. Effects of Acute Ionizing Radiation on Carp (Cyprinuscarpio L.) Embryos; pp. 1–48. M.Sc. Thesis. [Google Scholar]
- 8.Bonham K., Donaldson L.R. Sex ratios and retardation of gonadal development in chronically gamma-irradiated chinook salmon smolts. Trans. Am. Fish. Soc. 1972;101(3):428–434. [Google Scholar]
- 9.McGregor J.F., Newcombe H.B. Decreased risk of embryo mortality following low doses of radiation to trout sperm. Radiat. Res. 1972;52:536–544. [PubMed] [Google Scholar]
- 10.Kulikov N.V., Timofeeva N.A., Al’shits L.K. Decrease in the radiosensitivity of tench embryos (Tinca tinca L.) as a result of preliminary irradiation. Radiobiology. 1969;9:637–639. [PubMed] [Google Scholar]
- 11.Cooley J.L., Nelson D.J. University of Tennessee; 1970. Effects of Chronic Irradiation and Temperature on Populations of the Aquatic Snail Physa heterostropha; pp. 1–71. PhD Thesis. [Google Scholar]
- 12.Baptist J.P., Wolfe D.A., Colby D.R. Effects of chronic gamma radiation on the growth and survival of juvenile clams (Mercenaria mercenaria) and scallops (Argopecten irradians) Health Phys. 1976;30:79–83. doi: 10.1097/00004032-197601000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Karpenko A.A., Ivanovsky A., Yu A. Effect of very low doses of gamma radiation on motility of gill ciliated epithelia of Mytilus edulis. Radiat. Res. 1993;133:108–110. [PubMed] [Google Scholar]
- 14.Tallarico L.F., Okazaki K., Kawano T., Pereira C.A.B., Nakano E. Dominant lethal effect of Co-60 gamma radiation in Biomphalaria glabrata (SAY, 1818) Mutat. Res. 2004;561:139–145. doi: 10.1016/j.mrgentox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar T., Alam M.M., Parvin N., Fardous Z., Chowdhury A.Z., Hossain S., Haque M.E., Biswas N. Assessment of heavy metals contamination and human health risk in shrimp collected from different farms and rivers at Khulna-Satkhira region, Bangladesh. Toxicol. Rep. 2016;3:346–350. doi: 10.1016/j.toxrep.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezemonye L.I., Adebayo P.O., Enuneku A.A., Tongo I., Ogbomida E. Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River, Nigeria. Toxicol. Rep. 2019;6:1–9. doi: 10.1016/j.toxrep.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNSCEAR . Report to the General Assembly with Scientific Annexes; New York: United Nations: 2008. United Nations Scientific Committee on Effects of Atomic Radiation, Sources and Effects of Ionizing Radiation. [Google Scholar]
- 18.Anderson S.L., Wild G.C. Linking genotoxic responses and reproductive success in ecotoxicology. Environ. Health Perspect. 1994;102(Suppl. 12):9. doi: 10.1289/ehp.94102s129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dallas L.J., Keith-Roach M., Lyons B.P., Jha A.N. Assessing the impact of ionizing radiation on aquatic invertebrates: a critical review. Radiat. Res. 2012;177(5):693–716. doi: 10.1667/rr2687.1. [DOI] [PubMed] [Google Scholar]
- 20.New M.B., Valenti W.C. Blackwell Science Ltd.; United Kingdom: 2000. Freshwater Prawn Culture: the Farming of Macrobrachium rosenbergii; p. 443. [Google Scholar]
- 21.Bhavan P.S., Geraldine P. Biochemical stress responses in tissues of the prawn Macrobrachium malcolmsonii on exposure to endosulfan. Pesticide Biochem. Physiol. 2001;70:27–41. [Google Scholar]
- 22.Hart A., Ansa E., Sekibo I. Sex ratio, sexual dimorphism and fecundity in pond reared Niger River prawn, Macrobrachium felicinum (HOLTHIUS 1949) Zoologist. 2003;2(1):56–61. [Google Scholar]
- 23.Chang E.S., Shih T.W. Reproductive cycle of ovarian development and vitellogenin profile in the freshwater prawn, Macrobrachium rosenbergii. Invertbr. Reprod. Dev. 1995;27:11–20. [Google Scholar]
- 24.Shanju S., Geraldine P. Quantitative Protein profile of three Macrobrachium species during Reproductive cycle. Asian J. Anim. Vet. Adv. 2011;6(7):731–737. [Google Scholar]
- 25.King M. Vol. 341. Blackwell Science Ltd.; London: 1995. (Fisheries Biology Assessment and Management Fishing News Books). [Google Scholar]
- 26.Shanju S., Geraldine P. Yolk protein profiles of three Macrobrachium species during reproductive cycle. Indian J. Biochem. Biophys. 2005;42:258–261. [PubMed] [Google Scholar]
- 27.Shailender M., Krishna P.V., Babu Ch. Suresh, Srikanth B. Reproductive performance and offspring quality of giant freshwater prawn, Macrobrachium rosenbergii broodstock from different regions. World J. Fish Mar. Sci. 2012;4(6):629–636. [Google Scholar]
- 28.Kurup B.M., Kuriakose B. Fecundity indices in an indigenous endangered carp Labeo dussumeiri. Fish. Tech. 1994;31(1):8–11. [Google Scholar]
- 29.Rashid A., Kamruzzaman M., Ferdous Z., Shamimul Alam M., Begum R.A., Shahzahan R. Allele frequency distributions on polymorphic esterase loci in experimental populations of three Macrobrachium species. J. Curr. Res. Sci. 2013;1(5):356–363. [Google Scholar]
- 30.Soundarapandian P. Breeding behavior and effect of salinity and osmolarity on incubation and hatching of M. malcolmsonii (H. Miline Edwards) under laboratory conditions. Int. J. Zool. Res. 2008;4(1):81–84. [Google Scholar]
- 31.Won E.J., Lee J.S. Gamma radiation induces growth retardation, impaired egg production, and oxidative stress in the marine copepod Paracyclopina nana. Aquat. Toxicol. 2014;150:17–26. doi: 10.1016/j.aquatox.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki T. Sensitivity of Artemia eggs to the (irradiation: I. Hatchability of encysted dry eggs. J. Radiat. Res. 1964;5(1):69–75. doi: 10.1269/jrr.5.69. [DOI] [PubMed] [Google Scholar]
- 33.Sellars M.J., Degnan B.M., Carrington L.E., Preston N.P. The effects of ionising radiation on the reproductive capacity of adult Penaeus (Marsupenaeus) Japonicus (Bate) Aquaculture. 2005;250(1):194–200. [Google Scholar]
- 34.Goede R.W., Barton B.A. Organismic indices and an autopsy-based assessment as indicators of health and condition of fish. Am. Fish. Soc. Symp. 1990;8:93–108. [Google Scholar]
- 35.Rao K.J. Emerging technology on the seed, production of M. rosenbergii. In: Thomas P.C., editor. Current and Emerging Trends in Aquaculture. Daya Publishing House; New Delhi: 1998. pp. 164–169. [Google Scholar]
- 36.Lee N. Louisiana State University; Louisiana, USA: 2000. Effects of Cobalt-60 Gamma Irradiation on the Malaysian Prawn Macrobrachium rosenbergii. Ph.D. Thesis. [Google Scholar]
- 37.Cordeli E., Fresegna A.M., Leter G., Eleuteri P., Spano M., Villani P. Evaluation of DNA damage in different stages of mouse spermatogenesis after testicular X irradiation. Radiat. Res. 2003;160:443–451. doi: 10.1667/rr3053. [DOI] [PubMed] [Google Scholar]
- 38.Coates P.J., Lorimore A.A., Wright E.G. Damaging and protective cell signalling in the untargeted effects of ionizing radiation. Mutat. Res. 2004;568:5–20. doi: 10.1016/j.mrfmmm.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 39.Abowei J.F.N., George A.D.I., Davies A.O. Mortality, exploitation rate and recruitment pattern of Callinectes amnicola (De Rochebrune, 1883) from Okpoka Creek, Niger Delta, Nigeria. Asian J. Agric. Sci. 2010;2(1):27–34. [Google Scholar]
- 40.Amiro B.D. Radiological dose conversion factors for genericnon-human biota used for screening potential ecologicalimpacts. J. Environ. Radiol. 1997;35:37–51. [Google Scholar]
- 41.Hinton T., Brechignac F. In: The Scientific Basis for Radiologicalprotection of the Environment. Brechignac F., Howard B.J., editors. IRSN Series; Paris: 2004. [Google Scholar]
- 42.Borretzen P., Brown J., Strand P., Johansson E., Ramstedt M., Avila R., Prohl G., Ulanovsky A., Copplestone D. The ERICA assessment tool. In: Strand P., Borretzen P., Jolle T., editors. Osteras; 2005. pp. 43–46. [Google Scholar]
- 43.International Commission on Radiological Protection (ICRP) Publication 91. A framework for assessing the impact ofionising radiation on non-human species. Ann. ICRP. 2005;33(3):201–270. [Google Scholar]
- 44.International Commission on Radiological Protection (ICRP) Publication 103. New recommendations of the ICRP. Ann. ICRP. 2008 doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Andersson P., Beaugelin-Seiller K., Beresford N., Copplestone D., Della Vedova C., Garnier-Laplace J., Whitehouse P. PROTECT Deliverable 5. EC Contract No.36 425 (FI6R) Lancaster Centre for Ecology and Hydrology; 2008. Numerical benchmarks for protecting biota against radiation in the environment: proposed levels, underlying reasoning and recommendations. [Google Scholar]
- 46.Copplestone D., Hingston J., Real A. The development and purpose of the FREDERICA radiation effects database. J. Environ. Radioact. 2008;99(9):1456–1463. doi: 10.1016/j.jenvrad.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Stalin A., Broos K.V., Sadiq Bukhari A., Syed Mohamed H.E., Singhal R.K., Venu-babu P. Effects of 60Co gamma irradiation on behavior and gill histoarchitecture of giant fresh water prawn M. rosenbergii (DEMAN) Ecotoxicol. Environ. Saf. 2013;92:155–160. doi: 10.1016/j.ecoenv.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Stalin A., Broos K.V., Sadiq Bukhari A., Syed Mohamed H.E., Singhal R.K., Venu-Babu P. Morphological and histological studies on freshwater prawn M. rosenbergii (de man) irradiated with 60Co gamma radiation. Aquat. Toxicol. 2013;144:36–49. doi: 10.1016/j.aquatox.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Sadiq Bukhari A., Syed Mohamed H.E., Broos K.V., Stalin A., Singhal R.K., Venubabu P. Histological variations in liver of freshwater fish Oreochromis mossambicus exposed to 60Co gamma irradiation. J. Environ. Radioact. 2012;113:57–62. doi: 10.1016/j.jenvrad.2012.04.011. [DOI] [PubMed] [Google Scholar]