Graphical abstract
Keywords: Jackfruit, Seeds, Hemolysis, Brine shrimp, T47D, HT29, B16F10
Abstract
Background
Plants and their parts are a part of life in many Brazilian communities, as observed in the jackfruit. The jackfruit seeds are consumed, usually, as roasted, boiled, steamed, and are eaten as a snack.
Objective
The present study was carried out to identify the Artocarpus heterophyllus seeds toxicity and cytotoxic activity.
Methods
The extracts were tested in toxicity assays like, brine shrimp lethality assay, hemolysis assay, and effect of seed extracts on T47D, TH29 and B16F10 cancer cell lines, and in acute and subchronic toxicity in mice.
Results
Artocarpus heterophyllus seed presents no toxic effects in brine shrimp, no hemolytic activity, and was effective in cancer cell lines like T47D, TH29 and B16F10. IC50 obtained from extracts was 46.67 μg/ml of chloroform extract in T47D cells, 23.42 μg/ml of ethanolic extract in HT29 cells, and 74.31 μg/ml of ethyl acetic extract in B16F10 cells. Ethanolic extract presented zero lethality index and was able to reduce the level of glycemia in females (32.3%) in the subchronic test.
Conclusions
With this results we can conclude that Artocarpus heterophyllus seeds presents no toxicity, and is very effective in determinated cancer cell lines, requiring further studies to validate their use as active natural product against cancer cells.
1. Introduction
Artocarpus heterophyllus (Moraceae) is an evergreen fruit tree belonging to the Artocarpus genus named as jackfruit, found in tropical and subtropical regions. Previous studies suggested that preparations from Artocarpus heterophyllus was rich in phenolic compounds, flavonoids, stilbenoids, and arylbenzofurans [1] and possessed wide medicinal uses including antioxidant, anti-inflammatory, antibacterial, antifungal, antineoplasic, and hypoglycemic effects [2].
Jackfruit are composed of several berries of yellow pulp and brown seeds encased in a hard shell and are rich in carbohydrates, complex B vitamins and minerals. However, only 15–20% of the fruit is used as food, which can be cooked, baked or roasted on coals [3]. Jackfruit seeds are from 2 to 4 cm long, and a fruit can contain from 100 to 500 seeds, representing 8–15% of the total fruit weight. These seeds usually are consumed roasted, boiled, steamed, and are eaten as a snack. The addition of jackfruit seed flour in the preparation of biscuits, sweets and breads has been investigated as an alternative use of this by-product [[4], [5], [6]]. However, the fresh seeds have short shelf-life.
According to the World Health Organization [7] the number of people worldwide who will die of cancer will increase to 12.0 million in 2030. Among, techniques used for cancer control are those based on the use of anticancer agents, which unfortunately offer limited results. Consequently, the development of new drugs presents an important role in cancer control, and is greatly desired. In this area, plants are considered to be very promising, since they have been important sources of substances with several therapeutic uses. Therefore, this work aimed to investigate the cytotoxicity potential of Artocarpus heterophyllus seed extracts on tumoral cell lines. In parallel, the in vitro and in vivo toxicity effects of seed extracts were evaluated.
2. Materials and methods
2.1. Reagents
Methanol, dimethyl sulfoxide (DMSO), polisorbate, formaldehyde,sodium chloride (NaCl), potassium chloride (KCl), and calcium chloride (CaCl2) were purchased from Vetec Química Fina Ltda (Rio de Janeiro, Brazil). Glutamine,RPMI 1640 medium, fetal bovine serum, penicillin,streptomycin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), and Triton X-100 wereobtained from Sigma-Aldrich (Germany). Carbon dioxide (CO2) was from White Martins (Sao Paulo, Brazil) and doxorubicin (Doxolem) from Zodiac Produtos Farmacêuticos S/A (São Paulo, Brazil).
2.2. Plant materials and extraction procedures
Seeds of Artocarpus heterophyllus were collected in Arapongas − Brazil, in September 2014. The material was identified by the botanist José Tadeu Weidlich Motta of the Municipal Botanical Museum of Curitiba, an exsiccate were deposited under the number 397798. The use of this native species was authorized by the Brazilian Institute for the Environment and Renewable Natural Resources (IBAMA).
After drying in the shade, 80 g of seeds were submitted to ethanol extraction, following by liquid–liquid partition with hexane, chloroform and ethyl acetate, using Sohxlet, as described previously [[8], [9]]. After the solvent evaporation under reduced pressure at 40 °C, the hexane extract (HE, 0.53 g), ethyl acetate extract (EAE, 1.89 g), chloroform extract (CHLE, 0.3 g), and 0.11 g of ethanol extract (EE) were obtained.
A qualitative phytochemical screening for chemical constituents was realized on the ethanolic extract. Alkaloids, flavonoids, carbohydrates, terpenes, and tannins [10] were evidenced by colorimetric, and precipitation reactions. Total flavonoids content was carried by the method of Chang et al. [11]. To 0.5 ml of seed extrac added 1.5 ml of methanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water, it remained at room temperature for 30 min, the absorbance of the reaction mixture was measured at 415 nm on U/V visible spectrophotometer. The results were expressed as mg of rutin equivalents.
2.3. Brine shrimp lethality assay
The assay was performed according to Meyer et al. [12] with modifications. Cysts of Artemia salina L. (200 mg) were incubated in 400 ml artificial sea water (38 g marine salts dissolved in 1000 ml purified water). The system was kept under constant stirring and aeration at 30 °C for 48 h for hatching. After hatching, 10 nauplii of A. saline were transferred to tubes containing different concentrations of the substances for evaluation (HE, EAE, CHLE, EE in concentrations: 10 μg/ml; 100 μ/ml and 1000 μg/ml), which were solubilized in artificial sea water with 0.5% Tween® 80 (m/v) added. Quinidine sulfate was used as a positive control at the same concentrations as the samples, and artificial sea water containing 0.5% Tween® 80 (m/v) was used as a negative control. After 24 h, the live and dead nauplii were counted. Live larvae were counted, transformed into percentage mortality, and corrected according to the Abbott’s formula [12]:
| mortality (%) = [(dead larvae − dead larvae caused by the negative control)/(100 − dead larvae caused by the negative control)] × 100%. |
The Probit method [12] was used to determine the LC50 and LC90 values, as well as the corresponding 95% confidence intervals and chi square values for the assays with A. salina, using the SPSS Statistical Software Package version 19.0.
2.4. Hemolysis assay
For hemolytic activity testing in vitro, was used the technique proposed by Banerjee [13] adapted by Henneberg [14], employing sheep erythrocytes solution in phosphate buffer (PBS) (erythrocytes pope 2% in PBS). The erythrocytes pope was obtained from homogenized sheep blood (Newprov®), washed with ice cold phosphate buffer and centrifuged at 3000 rpm for 5 min, at least three times. The extracts and fractions were tested at concentrations of 100, 250, 500 and 1000 μg/ml in triplicate. Centrifuge tubes were placed in 200 μl of diluted extract in PBS and 200 of 2% RBC solution. To carry out the controls, samples were replaced by PBS for the negative control (baseline hemolysis) and distilled water for the positive control (total hemolysis), respectively. Samples were incubated in an oven for 3 h at 37 °C. After incubation, the micro tubes were centrifuged for 5 min at 3000 rpm. The supernatants were checked for the presence or absence of total hemolysis formation (clear solution, red and deposit of erythrocytes). For quantification of hemolysis supernatants were pipetted into an 96 well plate and the absorbance read at 540 nm (Multiskan FC – Thermo Scientific). The hemolysis was calculated based on absorbance values of total hemolysis tube, whose value is considered 100%.
The hemolysis rate was calculated as follows:
| Hemolysis (%) = [(Sample Absorbance − Basal Absorbance)/(Absorbance Total − Basal Absorbance)] × 100 |
Data were subjected to analysis variance (ANOVA) (α = 0.05) and when required Tukey's test with a significance level of 5% (α = 0.05) for mean comparison.
2.5. Effect of seed extracts on cancer cell lines
The cytotoxicity assay was used cell lines T47D (breast cancer), HT29 (colon cancer), B16F10 (low wall melanoma) and L929 (normal cell lines). The cytotoxic potential was assessed using the MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) assay [15]. Cells (103 cells/well) were seeded in RPMI 1640 medium (Sigma Chemical Co.) supplemented with 10% fetal bovine serum (FBS) in 96-well plates and incubated in a humidified atmosphere with 5% CO2 at 37 °C, for 24 h to full accession to the surface. Then the culture medium was replaced by fresh medium supplemented with different concentrations of extracts and fractions (1, 10, 100 and 1000 μg/ml). The cells were then incubated at 37 °C for 48 h and after this time, about 10 μl of a stock solution of 5 mg/ml MTT in PBS was added to each well containing the cells and incubated again for 4 h.
Then the cell free supernatant was aspirated from each well and 100 μl of DMSO was added to dissolve the resulting dark blue crystals of formazan MTT reduction and then homogenized in plate shaker. The extent of reduction of MTT formazan in the cell was measured on microliter plate reader at 600 nm. As positive control was used doxorubicin (0.01 to 5 mg/ml). The dose which inhibits 50% of cell growth (IC50) in μg/ml was determined graphically program for graphics and data analysis (Graph Pad Prism 6). The experiment was carried out in triplicates.
2.6. Animals
Adult mice (male and female) weighing 25–35 g were purchased from the Animal Department of Federal University of Parana (Parana, Brazil) and randomized to treatment groups. Efforts were made to minimize the number of animals used and the suffering of the animals selected. All experimental procedures were performed under the guidelines of the Experimental Laboratory Animal Committee of Federal University of Parana and were in strict accordance with the principles and guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocols used in this work were evaluated and approved by the Experimental Laboratory Animal Committee of Federal University of Parana (Protocol 2016-917).
2.7. Hippocratic screening (test of general activities) and acute oral toxicity
In order to study any possible toxic effect or changes on the general behavior of conscious animals, female Swiss albino mice (Mus musculus) (n = 6) were fasted overnight (12 h) with free access to water prior to administration of single oral or intraperitoneal doses of EE, HE, CHLE and EAE (0.005, 0.05, 0.5 and 5 g/kg). The signs and symptoms associated after the EE, HE, CHLE and EAE administration were observed in freely moving animals at 0, 30, 60, 120, 180, 240 and 300 min afterwards and there after daily up to7 days. At the end of the period the acute toxicity for each administration route was expressed by the required doseing/kg body weight to cause death in 50% of the animals tested (LD50) [16].
2.8. Subacute toxicity
Twenty four healthy Swiss albino mice (Mus musculus; 12 male, 12 female), with age ∼60 days, were used in this study. The animals were divided into two groups of 6 mice each with water and food ad libitum. One male and one female group were treated with EE, and the other two groups were used as controls. The control groups, male and female, received physiological saline daily, via gavage, during 21 days. The other two groups of mice were administered daily doses of 50 mg EE/kg body weight per animal, by gavage, during 21 days. The animals were daily observed, and on days 1, 7 and 21 the animals were weighed and blood was collected from the tail to check the glycemia. The analyzed data was expressed as the mean ± SD (standard deviation).
The statistical analysis was performed by a one-way analysis of variance (ANOVA) and F-values were presented only if p < 0.05. Post-hoc analysis was performed when appropriate using the Tukey’s.
3. Results and discussion
The qualitative phytochemical analysis of the ethanolic extract revealed the presence of different chemical groups. Strong positive reactions were registered for flavonoids, carbohydrates, volatile acid sterols, and tannins. Whereas, alkaloids and terpenes were not detected. The amount of flavonoids found in the ethanolic extract was 1.20 mg of rutin equivalents/g. This class of compounds is important because both they are essential components to the humans and animal diet (flavonoids cannot be synthesized by humans and animals) and possess a therapeutic potential, including antitumoral activity. Most of the pharmacological effects can be explained by the phenolic compounds including flavonoids, stilbenoids, arylbenzofurons present in all plant parts and Jacalin, a lectin present in seeds [1].
We initiated to evaluate the cytotoxic properties of Artocarpus heterophyllus seeds extracts using a range of assays to provide evidence of her low toxicity and potent anticancer abilities. Brine shrimp lethality is the simplest bioassay useful for screening large number of extracts in the drug discovery process. All the extracts were tested for lethality toward brine shrimp, revealing LC50 values lower than 100 μg/ml only for one sample (Table 1), HE being the most toxic to larvae (LC50 of 50.14 μg/ml).
Table 1.
Effect of the different concentration CEE (ethanolic extract), HE (hexanic extract), CHLE (chloroform extract) and EAE (ethyl acetate extract) of Artocarpus heterophyllus seeds in mortality and LC50 A. saline.
| Mortality/Concentration μg/mL | |||||
|---|---|---|---|---|---|
| 10 | 100 | 1000 | LC50 | (LCL–UCL) | |
| CEE | 0,3ns | 0,3ns | 9a | 389,17 | 350–400 |
| HE | 8a | 10a | 10a | 50,14 | 35,3–75,5 |
| CHLE | 0ns | 2ns | 6.3a | 1000 | – |
| EAE | 0,3ns | 4a | 10a | 138,22 | 121–160 |
NSA treatment differ significantly not mean.
LC50 = lethal concentration that kills 50% of the exposed organisms.
UCL = 95% upper confidence limit.
LCL = 95% lower confidence limit.
No mortality was observed in the control group.
A different treatment significantly medium (P < 0.05) by Tukey test.
The brine shrimp lethality assay has been widely used to screen toxicity and the level of toxicity of pesticides, dental materials, crude plant extracts and fractions, secondary metabolites and nanoparticles [17]. Meyer [11] has reported that extracts obtained from natural products which have LC50 ≤ 1.0 mg/mL are known to possess toxic effects.
In vitro hemolysis test is used routinely in toxicity studies of medicinal plants and livestock showing positive interest [18]. Although phytochemical studies demonstrate the presence of compounds such as terpenes, flavons, phenolic and steroidal substances [1], in vitro toxicity testing of this seeds extracts did not show hemolytic activity of the statement. The acceptable hemolysis rate shown by all A. heterophyllus seeds extracts denotes its non-hemolytic property up to 100 μg/ml. So the Artocarpus heterophyllus seeds extracts may be considered as biosafe for internal use, and can be considered for development of suitable formulation.
Cytotoxicity of different fractions of seeds of Artocarpus heterophyllus extract were evaluated on the human breast cancer (T47D), colon cancer (HT29), low wall melanoma cancer (B16F10) and normal (L929) cell lines by MTT assay and IC50 were demonstrated in Table 2. Extracts from seeds of Artocarpus heterophyllus, were active against tumoral cells, but showed no cytotoxic effects in L929, a normal cell line. A IC50 determination which the lowest value was obtained for EE (against HT-29), CHLE (against T47D) and EAE (against B16F10). These three extracts presented IC50 values less than 80 μg/ml (23.42, 46.67 and 74.31 μg/ml respectivelly) (Table 2).
Table 2.
IC50 of Artocarpus heterophyllus seeds extracts CEE (ethanolic extract), HE (hexanic extract), CHLE (chloroform extract) and EAE (ethyl acetate extract) against three tumor cell lines: T47D (human breast); HT-29 (colon) and B16F10 (murine skin).
| IC50 (CI 95%) μg/ml values | ||||
|---|---|---|---|---|
| T47D | HT-29 | B16F10 | L929 | |
| Doxo | 0.1116 | 0.036 | 0.3182 | 0.5500 |
| CEE | 156.2 | 23.42 | 102.9 | >1000 |
| HE | >250.00 | 129.5 | 85.49 | >1000 |
| CHLE | 46.67 | 74.80 | 88.85 | >1000 |
| EAE | 52.39 | 123.2 | 74.31 | >1000 |
IC50 (CI 95%) (μg/ml): 50% inhibitory concentration and its 95% confidence interval in μg/ml (three replicates per treatment).
The Artocarpus species are rich in phenolic compounds including flavonoids, stilbenoids and arylbenzofurons. The chemical constituents of Artocarpus species have earlier been reviewed [19].
The jacalin is a single major protein representing more than 50% of the proteins of the jackfruit crude seed extract [20]. An other protein isolated from Artocarpus heterophyllus seeds is artocarpin. KM+/artocarpin carbohydrate binding properties revealed that it is a polyspecific lectin that reacts with wide range of monosaccharides [21]. The artocarpin is made up of a single polypeptide chain of 159 amino acids sharing 52% identity with the jacalin sequences. Jacalin is a glycosylated while artocarpin is not [22].
Studies have also shown that artocarpin exhibited potent cytotoxic activity on cultured human T47D breast cancer cells in vitro. Incubation of the cells with graded doses of artocarpin (5.7, 11.5, 20, and 28.7 mM) for 24 h resulted in a concentration dependent cytotoxic effects [23]. The IC50 was calculated and observed to be 12.6 μg/ml. Artocarpin caused concentration-dependent apoptosis and mechanistic studies showed that it was mediated by the activation of caspase 3 and caspase 8 but not caspase 9 or caspase 10 [23]. Together these observations clearly indicate that the isoprenoid-substituted flavonoids of jackfruit possess cytotoxic effects and that artocarpin is a potential candidate [23]. Among the results from the activity of the extracts on cell line T47D, the chloroform extract was the one with a lower IC50. The positive results obtained in T47D likely due to the presence of Artocarpin substance, which previously had alone brought this action. The IC50 of the fraction has relatively low compared to the IC50 of the isolated compound, suggesting possible synergism between compounds.
The antiproliferative effect of Artocarpus heterophyllus seeds extracts was too evaluated using HT29 cell line and all samples inhibited cancer cell growth, specially ethanolic extract. According Serra et al. [24], the bioactive effect can be related to the highest total phenolic content and composition in phenolic acids. These compounds exert their bioactivity of protection due to its antioxidant capacity. Polyphenols can wipe out the spread of harmful free radicals to the body, which causes cellular lipid peroxidation by transferring an equivalent electron radical. The antioxidant capacity seemed to be also responsible for this effect, and this result suggests that the compounds responsible for the scavenging of peroxyl radicals and inhibition capacity of hydroxyl radicals in juices may contribute to the inhibition of colon cancer cell growth. In that context, according Burci et al. [25], ethanolic extract was the most effective in oxidation inhibition and peroxide radical scavenging.
Added Isoprenoid-substituted flavonoids isolated from Moraceae plants have shown cytotoxic activity in some cancer cell lines, including artelastin, artelast o chromene, artelasticin in MCF-7 (human breast cancer), TK-10 (human renal cancer) and UACC-62 (human melanoma) cells, and artelast o xanthone and artonol A in A549 (human lung cancer), Hep3 B (human hepatocellular cancer), HT-29 (human colorectal cancer) and MCF-7 (human breast cancer) cells [[23], [26]]. Compounds with an isoprenoid-substituted moiety have been considered as possible candidates for chemotherapy based on the observed cytotoxicity against B16 melanoma cells when compared to other tested compounds: namely, non isoprenoid-substituted flavonoids [27], inserted in the group of isoprenoid-substituted we found Artocarpin. Polyphenolic flavones with additional prenyl substituents could be useful as skin whitening agents as remedies for hyperpigmentation [28]. The isolated isoprenoid-substituted flavonoids were more active than vinblastine, carmustine, and 5-fluorourasil, which are known as anticancer agents.
Artocarpus heterophyllus seed extracts were first investigated for acute toxicity in mice, and a single oral administration of EE, HE, CHLE and EAE at the doses of 0.005, 0.05 and 0.5 g/kg did not produce any visible signs or symptoms of toxicity in the treated animals. However, the oral treatment of animals with EE (5 g/kg) produced respiratory alterations, pilomotor erection, body tremors and increase urinary flow, starting 10 min after the administration of the fraction and lasting up to 4 h. The treatment of animals with EE, at doses of 0.005, 0.05 and 0.5 g/kg by intraperitoneal route, also did not show signs of toxicity. Similarly, the dose of 5 g/kg, i.p. of EE caused body tremors starting 5 min after the administration. The acute study demonstrated that there were no adverse changes or mortality in animals following Artocarpus heterophyllus seed extracts administration.
Extract from the seeds are supposed to be helpful in digestion, and fresh extract from seeds are also useful in the treatment of diarrhea and dysentery [29]. The decoction of seeds is supposed to help in digestion while ripe fruits may be used as a natural laxative [30]. Starch extract from the seed is supposed to relieve biliousness, while the roasted seeds are considered to be aphrodisiac [31].
Endeavors should be directed at encouraging the food industry to process the good qualities of jackfruit and seeds for exporting of both canned and finished foods. This will directly help the growers and also increase their financial resources. The food industry should also attempt at optimizing the quality and develop novel finished products. Attempts should also be on the part of policymakers and the governmental agencies to support the integrated agricultural procedures involving jackfruit trees by providing necessary guidance and assistance to the growers as well as offering a minimal procurement price, especially to benefit the marginal farmers.
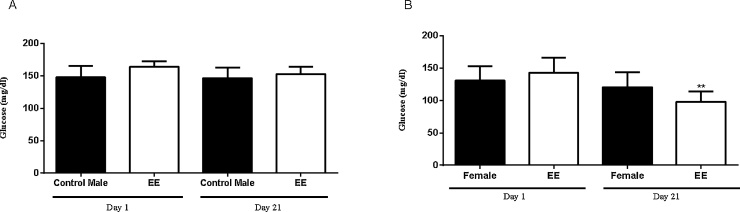
The subacute use of EE resulted in a significant reduction in blood glucose levels (32.3%) in the female group, while the tendency to reduce glucose (6.8%) was tenuous in the male group (Fig. 1). The reason for this discrepancy is not known considering the health status of the mice used in this experiment.
Fig. 1.
Levels of blood glucose in mice exposed to sub-chronic treatment with EE. (A) male and (B) female groups. (*) Values based on p < 0.05.
Several studies show a hypoglycaemic activity of Artocarpus heterophyllus leaves and fruits in streptozotocin induced diabetes in rats. Artocarpus heterophyllus is generally recommended by traditional medical practitioners as a classical treatment for diabetes mellitus. Significant reduction of blood glucose (49%) through the administration of flavonoid fraction of the leaf in the alloxan induced diabetic rats has been reported [32]. Similarly, in other investigation conducted [33] the hypoglycemic effect of Artocarpus heterophyllus leaves have proved a noteworthy reduction in the blood glucose and total cholesterol. Barik et al. [34] have revealed the potent antidiabetic activity of Artocarpus heterophyllus fruits and its remarkable impact in the increase of glucose tolerance. Furthermore, investigation in the leaves of Artocarpus heterophyllus have classically indicated its efficiency in the attenuation of glycosylation of hemoglobin, enhancement in the transport of glucose across cells, stimulation of insulin release and inhibition of cholesterol biosynthetic enzymes [35]. To date there is only one study carried out with extracts obtained from the seeds of Artocarpus heterophyllus, and it points significant and superior effect of the antiglycation property of extract obtained from pressurized hot water extraction of Artocarpus heterophyllus seeds [36], and now our study that corroborated with the above mentioned scientific documentation. There was no significant change in weight between the control group and the EE group.
The use of various underexploited food materials in product development is on the increase, probably due to growth in human population with resultant hike in prices of foods. Toxicological implications of such products and their phytoconstituents on consumers health are rarely investigated [37]. Interactions between constituents in the food materials during processing and their adverse effect cannot be ruled out. In the last years many plants and their different morphological parts as seeds have been studied for its antioxidant, antitumor and toxic capacity against the continuous use by humans [[38], [39]]. There are promising results regarding seed safety, in addition to the increasing demonstration of the amount of benefits they present when used.
4. Conclusion
The results of cytotoxic activity tests indicate that the extracts of Artocarpus heterophyllus seeds could be useful in the search for new agents for antitumoral treatments, and it did present a little specific toxicity, as suggested by results obtained with brine shrimp lethality and hemolysis assays. It presented low toxicity in the acute toxicity test, with zero lethality. Even during the subchronic study there was no incidence of toxicity, however, it showed a marked decrease in plasma glucose levels. Further studies are necessary for chemical characterization of the active substances and more extensive biological evaluations.
Acknowledgements
We would like to thank CAPES, CNPQ and Fundação Araucária for financial support of the project.
Contributor Information
Lígia Moura Burci, Email: ligia.burci@gmail.com.
Marilis Dallarmi Miguel, Email: marilisdmiguel@ufpr.br.
References
- 1.Baliga M., Haniadka R., Dsouza J., Bhat H. Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus Lam (jackfruit): a review. Food Res. Int. 2011;44:1800–1811. [Google Scholar]
- 2.Jagtap U.B., Bapat V.A. Artocarpus: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2010;129(2):142–166. doi: 10.1016/j.jep.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 3.Silva W., Pereira J., Carvalho C.W., Ferrua F.Q. Determinação da cor, imagem superficial topográfica e ângulo de contato de biofilmes de diferentes fontes de amido. Ciênc agrotec. 2007;31:154–163. [Google Scholar]
- 4.Aldana D., Gómez B., Oca M., Ayerdi S., Meraz F., Pérez L. Isolation and characterization of Mexican jackfruit (Artocarpus heterophyllus L.) seeds starch in two mature stages. Starch. 2011;63:364–372. [Google Scholar]
- 5.Bobbio F.O., El-Dash A.A., Bobbio P.A., Rodrigues L.R. Isolation and characterization of the physicochemical properties of the starch of jackfruit seeds (Artocarpus heterorphyllus) Cereal Chem. 1978;55:505–511. [Google Scholar]
- 6.Mukprasirt A., Sajjaanantakul K. Physico-chemical properties of flour and starch from jackfruit seeds (Artocarpus heterophyllus Lam.) compared with modified starches. J. Food Sci. Technol. 2004;39:271–276. [Google Scholar]
- 7.World Health Organization − WHO . 2009. Programmes and Projects: Cancer. http://www.who.int/cancer/en/. (Accessed 6 June 2017) [Google Scholar]
- 8.Andrade C.A., Costa C.K., Bora K., Miguel M.D., Miguel O.G., Kerber V.A. Determinação do conteúdo fenólico e avaliação da atividade antioxidante de Acacia podalyriifolia A. Cunn. ex G. Don, Leguminosae-mimosoideae. Rev. Bras. Farmacogn. 2007;17:231–235. [Google Scholar]
- 9.Carvalho J.L.S., Cunico M.M., Dias J.F.G., Miguel M.D., Miguel O.G. Term-stability of extractive processes from Nasturtium officinale R Br., brassicaceae for Soxhlet modified system. Quim. Nova. 2009;32(4):1031–1035. [Google Scholar]
- 10.Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction: a review. Int. Pharm. Sci. 2011;1:98–106. [Google Scholar]
- 11.Chang C., Yang M., Wen H., Chern J. Estimation of total flavonoids content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 12.Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobsen L.B., Nichols D.E., McLaughlin J.L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. [PubMed] [Google Scholar]
- 13.Banerjee A., Kunwar A., Mishra B., Priyadarsini K.I. Concentration dependent antioxidant/pro- oxidant activity of curcumin studies from AAPH induced hemolysis of RBCs. Chem. Biol. Interact. 2008;174:134–139. doi: 10.1016/j.cbi.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Henneberg R., Otuki M.F., Furman A.E.F., Hermann P., Nascimento A.J., Leonart M.S.S. Protective effect of flavonoids against reactive oxygen species production in sickle cell anemia patients treated with hydroxy urea. Rev. Bras. Hematol. Hemoter. 2013;35:52–55. doi: 10.5581/1516-8484.20130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 16.Litchfield J.T., Wilcoxon F., Jr A simplified method of evaluating dose effect experiments. J. Pharm. Exp. Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 17.Hamidi M.R., Jovanova B., Panovska T.K. ToxicМlogical evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014;60(1):9–18. [Google Scholar]
- 18.Pequeno N.F., Soto-Blanco B. Toxicidade in vitro de plantas tóxicas: avaliação do teste de ação hemolítica. Acta Sci. Vet. 2006;34:45–48. [Google Scholar]
- 19.Hakim E.H., Achmad S.A., Juliawaty L.D., Makmur L., Syah Y.M., Aimi N., Kitajima M., Ghisalberti E.L. Prenylated flavonoids and related compounds of the Indonesian Artocarpus (Moraceae) J. Nat. Med. 2006;60:161–184. doi: 10.1007/s11418-006-0048-0. [DOI] [PubMed] [Google Scholar]
- 20.Kabir S., Aebersold R., Daar A.S. Identification of a novel 4 kD a immunoglobulin-a-binding peptide obtained by the limited proteolysis of jacalin. Biochim. Biophys. Acm. 1993;1161:194–200. doi: 10.1016/0167-4838(93)90213-b. [DOI] [PubMed] [Google Scholar]
- 21.Barre A., Peumans W.J., Rossignol M., Borderies G., Culerrier R., Els J.M., Damme V., Rougé P. Artocarpin is a polyspecific jacalin-related lectin with a monosaccharide preference for mannose. Biochimie. 2004;86:685–691. doi: 10.1016/j.biochi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Rosa J.C., De Oliveira P.S.L., Garratt R., Beltramini L., Resing K., Roque-Barreira M.C. KM+, a mannose-binding lectin from Artocarpus integrifolia: amino acid sequence, predicted tertiary structure, carbohydrate recognition, and analysis of the b-prism fold. Protein Sci. 1999;8:13–24. doi: 10.1110/ps.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arung E.T., Wicaksono B.D., Handoko Y.A., Kusuma I.W., Shimizu K., Yulia D., Sandra F. Cytotoxic effect of artocarpin on T47D cells. J. Nat. Med. 2010;64:423–429. doi: 10.1007/s11418-010-0425-6. [DOI] [PubMed] [Google Scholar]
- 24.Serra A., Duarte R., Bronze M., Duarte C. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011;125:318–325. [Google Scholar]
- 25.Burci L.M., Silva C.B., Oliveira M., Dalarmi L., Zanin S.M.W., Miguel O.G., Dias J.F.G., Miguel M.D. Determination of antioxidant, radical scavenging activity and total phenolic compounds of Artocarpus heterophyllus (Jackfuit) seeds extracts. J. Med. Plants Res. 2015;40:1013–1020. [Google Scholar]
- 26.Dabros W., Nikiforuk A., Kordowiak A.M. The influence of bis(kojato)-oxovanadium (IV) on viability and proliferation of rat hepatoma cell line H 35-19. ELSO Proceedings. 2004 Abstract no. 653, p. 299. [Google Scholar]
- 27.Ijichi H., Chytil A., Gorska A.E., Aakre M.E., Fujitani Y., Fujitani S., Wright C.V.E., Moses H.L. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor- signaling in cooperation with active Kras expression. Genes. Dev. 2006;22(20):3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anonymous . Southampton Centre for Underutilised Crops Printe dat RPM Print and Design; Chichester, England, UK: 2006. Jackfruit Artocarpus Heterophyllus. [Google Scholar]
- 29.Hossain M.K., Nath T.K. In: Vozzo J.A., editor. vol. 721. Department of Agriculture Forest Service; Washington, DC: 2002. (Artocarpus Heterophyllus Lam. In Tropical Tree Seed Manual: Agriculture Handbook). [Google Scholar]
- 30.Morton J.F. Resources System Inc; 1987. Fruits of Warm Climates Creative; pp. 58–64. [Google Scholar]
- 31.Khan M.R., Omoloso A.D., Kihara M. Antibacterial activity of Artocarpus heterophyllus. Fitoter. 2003;74:501–505. doi: 10.1016/s0367-326x(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 32.Chandrika U.G., Wedage W.S., Wickramasinghe S.M.D.N., Fernando W.S. Hypoglycaemic action of the flavonoid fraction of Artocarpus heterophyllus leaf. Afr. J. Tradit. 2006;3:42–50. [Google Scholar]
- 33.Shahin N., Alam S., Ali M. Pharmacognostical standardisation and antidiabetic activity of Artocarpus heterophyllus leaves Lam. Int. J. Drug Dev. Res. 2012;4:346–352. [Google Scholar]
- 34.Barik R., Jain S., Sharma R., Kumar P., Bhatt D. Effect of fruit extract of Artocarpus heterophyllus in alloxan induced diabetic rats. Int. J. Pharm. Sci. Res. 2010;11:59–64. [Google Scholar]
- 35.Chackrewarthy S., Thabrew M.I., Weerasuriya M.K.B., Jayasekera S. Evaluation of the hypoglycemic and hypolipidemic effects of an ethylacetate fraction of Artocarpus heterophyllus (jak) leaves in streptozotocin-induced diabetic rats. Pharmacogn. Mag. 2010;23:186–190. doi: 10.4103/0973-1296.66933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deve A.S., Kumar S., Kumaresan K., Rapheal V.S. Extraction process optimization of polyphenols from Indian Citrus sinensis –as novel antiglycative agents in the management of diabetes mellitus. J. Diabetes Metab. Dis. 2014;13:11. doi: 10.1186/2251-6581-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saad B., Azaizeh H., Abu Hijleh G., Said O. Safety of traditional arab herbal medicine: a review evidence of complementary. Altern. Med. 2006;3:433–439. doi: 10.1093/ecam/nel058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Hak H.N.G., Moustafa A.R.A., Mansour S.R. Toxic effect of Moringa peregrina seeds on histological and biochemical analyses of adult male Albino rats. Toxicol. Rep. 2017;12(5):38–45. doi: 10.1016/j.toxrep.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Memariani T., Hosseini T., Mohammadi A., Ghorbani M., Shakeri A., Spandidos D.A., Tsatsakis A.M., Shahsavand S. Evaluation of the cytotoxic effects of Cyperus longus extract, fractions and its essential oil on the PC3 and MCF7 cancer cell lines. Oncol. Lett. 2016;11(2):1353–1360. doi: 10.3892/ol.2015.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]