Highlights
-
•
CBS and PSP patients show heterogeneous language profiles.
-
•
Patients with nfvPPA profile show the typical nfvPPA hypometabolic pattern.
-
•
Parietal hypometabolism characterizes CBS cases with undefined language deficits.
-
•
Frontal hypometabolism characterizes PSP cases with undefined language deficits.
-
•
Patients without language deficit show a predominant right hemisphere involvement.
Keywords: Positron Emission Tomography, FDG-PET, Corticobasal syndrome, Progressive supranuclear palsy, Non-fluent variant of primary progressive aphasia, nfv-PPA
Abstract
Purpose
To assess the clinical-metabolic correlates of language impairment in a large sample of patients clinically diagnosed as corticobasal syndrome (CBS) and progressive supranuclear palsy syndrome (PSPs).
Methods
We included 70 patients fulfilling current criteria for CBS (n = 33) or PSPs (n = 37). All subjects underwent clinical-neuropsychological and FDG-PET assessments at the time of diagnosis. The whole patient's cohort was grouped into three subgroups according to the language characteristics, i.e., (a) nfv-PPA; (b) subtle language impairments, LANG-; (c) no language deficits, NOL-. FDG-PET data were analysed using an optimized voxel-based SPM method at the single-subject and group levels in order to evaluate specific hypometabolic patterns and regional dysfunctional FDG-PET commonalities in subgroups.
Results
21 patients had a nfvPPA diagnosis (i.e., nfv-PPA/CBS = 12 and nfv-PPA/PSPs = 9), while 20 patients had a subtle language impairment LANG- (i.e., CBS = 12 and PSPs = 8), not fulfilling the criteria for a nfv-PPA diagnosis. The remaining sample (i.e., 9/33 CBS and 20/37 PSPs patients) did not show any language deficit. FDG-PET results in individuals with a nfv-PPA diagnosis were consistent with the typical nfv-PPA pattern of hypometabolism (i.e., left fronto-insular and superior medial frontal cortex involvement), both in CBS and PSPs. The LANG-CBS and LANG-PSPs subjects had different FDG-PET hypometabolic patterns involving, respectively, parietal and frontal regions. As expected, NOL-CBS and NOL-PSPs showed a predominant right hemisphere involvement, with selective functional metabolic signatures typical of the two syndromes.
Conclusions
Language impairments, fulfilling the nfv-PPA criteria, are associated with both CBS and PSPs clinical presentations early in the disease course. Subtle language deficits may be present in an additional proportion of patients not fulfilling the nfv-PPA criteria. The topography of brain hypometabolism is a major dysfunctional signature of language deficits in CBS and PSPs clinical phenotypes.
1. Introduction
Progressive supranuclear palsy (PSP) and corticobasal (CBS) syndromes are two distinct sporadic adult-onset neurodegenerative conditions included within the so-called “atypical parkinsonisms” (Horvath et al., 2013). Such disorders usually pose a challenge for clinicians, as they are difficult to differentiate from each other, or even from Parkinson's Disease (PD) due to clinical heterogeneity (Boeve, 2011; Respondek et al., 2014). Although some unifying features (i.e., predominance of axial rigidity and bradykinesia with early falls; rare presence of classic, pill-rolling rest tremor; poor or transient levodopa response) can differentiate these patients from typical PD, the large clinical and pathological overlap has led to the concept of a PSP-CBS spectrum (Armstrong et al., 2013; Boxer et al., 2017; Coyle-Gilchrist et al., 2016; Hoglinger et al., 2017).
The recent literature supports a wide spectrum of potential phenotypes in autopsy-confirmed PSP cases. The classic Richardson's syndrome (i.e., postural instability and falls with vertical ocular motor dysfunction) is present in only 24% of cases (Respondek et al., 2014). In view of this, the new clinical diagnostic criteria for PSP focus on the assessment of core clinical features (ocular motor dysfunction, postural instability, akinesia and cognitive dysfunction) to define a number of combinations of symptoms leading to several PSP-related clinical syndromes (Hoglinger et al., 2017). Among the various PSP variants, a speech/language clinical presentation is considered, including the nonfluent/agrammatic variant of primary progressive aphasia (nfv-PPA) as a possible first clinical presentation of PSP (Hoglinger et al., 2017). Comparably, CBS phenotypes may include predominant cognitive (e.g., agnosia, apraxia), motor (e.g., bradykinesia or myoclonus) or language disorders (e.g., anomia, speech apraxia, and/or agrammatism) (Armstrong et al., 2013). Different types and degrees of language impairment characterize the cognitive profiles of CBS patients. Motor speech and grammar disorders are reported as an early presentation in about 40% of subjects (Burrell et al., 2014; Oliveira et al., 2017). In addition, a clinical diagnosis of nfv-PPA according to current criteria (Gorno-Tempini et al., 2011) may occur years before the onset of a full-blown CBS phenotype (Cerami et al., 2017; Santos-Santos et al., 2016).
FDG-PET imaging, with the implementation of semi-quantitative methods for the analysis of FDG-PET uptake, has been recently suggested as useful imaging biomarker supporting the clinical classification of atypical parkinsonisms associated with dementia (Walker et al., 2018). The brain metabolic profile of CBS and PSP (Caminiti et al., 2017; Niccolini and Politis, 2016), as well as the hypometabolism characterizing CBS and PSP with nfv-PPA (Cerami et al., 2017) have been previously described. The FDG-PET pattern in PSP is characterized by hypometabolism in bilateral medial and dorsolateral frontal regions, as well as in subcortical structures (i.e., midbrain and thalamus). CBS is characterized by asymmetric hypometabolism contralateral to the most affected side, including the parietal, the sensorimotor and the premotor cortex.
In the case of CBS and PSP with nfv-PPA, patients show a left lateralized hypometabolic pattern with a selective involvement of the left inferior frontal gyrus (Cerami et al., 2017).
Current FDG-PET literature mainly reported specific information about the functional metabolic features of CBS and PSP cases in relation to motor symptomatology (see Niccolini and Politis, 2016, for review). To the best of our knowledge, brain metabolic changes associated to language profiles in CBS and PSP syndrome have not been fully identified. In this study, we specifically aimed at evaluating the correlations of FDG-PET patterns of hypometabolism with language impairment in a large sample of patients fulfilling clinical criteria for CBS and PSPs (Armstrong et al., 2013). We hypothesized the presence of a common FDG-PET pattern in CBS and PSP subjects with nfv-PPA phenotype, and of different trajectories of brain hypometabolism in CBS and PSP patients with subtle language impairment not fulfilling the criteria for PPA (Gorno-Tempini et al., 2011).
2. Materials and methods
2.1. Participants
The retrospective sample includes subjects with atypical parkinsonism selected from the database of the Neurology Centers for Cognitive Disorders of San Raffaele Hospital (Milan, Italy) referred to the Nuclear Medicine Unit of San Raffaele Hospital for an FDG-PET scan in the years between 2012 and 2016. Clinical information (medical history, neurological examination and neuropsychological assessment) at the time of the diagnosis was obtained by neurologists expert in dementia who reached a diagnostic classification fulfilling criteria for CBS and PSP syndromes according to Armstrong et al. (2013), confirmed at 2-year follow-up. The FDG-PET study was performed as part of a research study and was not used by the clinicians for diagnostic purposes.
Seventy cases were classified as CBS (n = 33; 18 females; age = 70.4 ± 6.2 years; disease duration = 36.8 ± 21.1 months; raw MMSE score: 21.1 ± 6.2) or PSPs (n = 37; 23 females; age = 69.9 ± 7.3 years; disease duration = 39.1 ± 20.5 months; raw MMSE score: 24.5 ± 4.5). See Table 1 for demographic and clinical details on patients sample and supplementary Table s1 for the frequency of motor, cognitive and behavioral symptoms required for the CBS or PSPs classification (Armstrong et al., 2013).
Table 1.
Demographic, clinical and cognitive features of the whole sample.
| CBS n = 33 | PSPs n = 37 | Statistics | |
|---|---|---|---|
| Female/Male ratio | 18/15 | 23/14 | X2(1) = 0.4, p = 0.52 |
| Age in years (mean ± st.dev.) | 70.4 ± 6.2 | 69.9 ± 7.3 | t(68) = 0.3, p = 0.78 |
| Age range in years | 54–79 | 53–86 | – |
| Years of education (mean ± st.dev.) | 11.4 ± 5.2 | 10.3 ± 4.3 | t(68) = 0.9, p = 0.33 |
| Time from symptoms’ onset in months (mean ± st.dev.) | 36.8 ± 21.1 | 39.1 ± 20.5 | t(68) = −0.5, p = 0.64 |
| CDR global score (median [range]) | 0.5 [0.5–1] | 0.5 [0.5–1] | – |
| MMSE raw score (mean ± st.dev.) | 21.1 ± 6.2 | 24.5 ± 4.5 | t(68) = −2.5, p = 0.014 |
| Motor speech disorder (% of impaired patients) | 33% | 65% | X2(1) = 6.9, p = 0.008 |
| Agrammatism (% of patients) | 48% | 41% | X2(1) = 0.4, p = 0.50 |
| Single word comprehension (% of impaired patients) | 6% | 8% | X2(1) = 0.1, p = 0.74 |
| Sentence comprehension (% of impaired patients) | 60% | 41% | X2(1) = 2.8, p = 0.094 |
| Working memory (% of impaired patients) | 74% | 53% | X2(1) = 3.3, p = 0.071 |
| Verbal long-term memory immediate recall (% of impaired patients) | 67% | 44% | X2(1) = 3.6, p = 0.056 |
| Verbal long-term memory delayed recall (% of impaired patients) | 63% | 36% | X2(1) = 4.9, p = 0.028 |
| Visuo-spatial long term memory (% of impaired patients) | 67% | 54% | X2(1) = 1.3, p = 0.25 |
| Visuo-constructive abilities (% of impaired patients) | 93% | 75% | X2(1) = 4.4, p = 0.036 |
| Ideomotor apraxia (% of impaired patients) | 58% | 31% | X2(1) = 5.1, p = 0.024 |
| Executive functions (% of impaired patients) | 81% | 69% | X2(1) = 1.3, p = 0.26 |
CBS = corticobasal syndrome; PSPs = progressive supranuclear palsy syndrome; CDR = clinical dementia rating scale; MMSE = mini-mental status examination.
All subjects, or their informants/caregivers, gave written informed consent to the FDG-PET scan acquisition and for the use of imaging data for research purposes. The retrospective clinical study was approved by the Ethical Committee of San Raffaele Hospital.
2.2. Neuropsychological examination
At the time of diagnosis, all patients underwent an evaluation of the global cognitive status (i.e., Mini Mental State Examination), and a neuropsychological battery assessing memory (i.e., Digit Span Forward, immediate and delayed recall of Rey Auditory Verbal Learning (RAVLT), recall of copy of the Rey–Osterrieth complex figure (ROCF)), attention and executive functions (i.e., Attentional Matrices, Raven Colored Progressive Matrices; Digit Span backward), and visuo-spatial abilities (i.e., ROCF copy).
Language examination included a clinical assessment of connected speech production, noting the presence of speech apraxia and articulation difficulties, of anomia and circumlocutions, and of agrammatism; the naming and the word-picture matching subtests of the CAGI battery for the assessment of semantic memory; phonemic and semantic controlled associations; the repetition subtest of the Italian version of the Aachener Aphasia Test (AAT); the Token test; the sentence comprehension subtests from the “Batteria per l'Analisi dei Deficit Afasici” (BADA); phonemic (P-F-L)) and semantic (animals-fruits-cars) verbal fluency.
Diagnosis of primary progressive aphasia (PPA) was based on current diagnostic criteria (Gorno-Tempini et al., 2011). Nfv-PPA diagnosis was based on the presence of a slow and hesitant speech with articulation impairment and distortions and word finding pauses, impaired sentence comprehension and naming, with spared single words comprehension and non-verbal semantics. Subjects with subtle language dysfunctions, but not fulfilling PPA criteria were classified as LANG-CBS or LANG-PSPs, respectively. Patients without any language deficits were classified as NOL-CBS or NOL-PSPs.
2.3. Statistical analyses
Demographic, clinical and neuropsychological variables were compared among subgroups (i.e., nfv-PPA, LANG- and NOL) within the CBS and PSPs global sample. Gender distribution was compared using the χ2 test, while one-way ANOVA was used to compare demographic variables. Patient cognitive performances were classified as impaired or unimpaired according to age- and education corrected normative values. The binary variables obtained were then compared with the χ2 or Fisher exact tests in the different groups. Statistical analyses were performed utilizing the IBM SPSS Statistics for Windows version 20.0 (Armonk, NY IBM Corp.) with α set at 0.05.
2.4. FDG-PET imaging acquisition and processing
FDG-PET scans were performed at baseline in each case. The analysis was performed at the Nuclear Medicine Unit, San Raffaele Hospital (Milan, Italy) by physicians expert in FDG-PET imaging. FDG-PET acquisitions were done according to the guidelines of the European Association of Nuclear Medicine (EANM), following standardized procedures (Morbelli et al., 2012). Before radiopharmaceutical injection of FDG (185–250 Mbq: usually, 5-8 mCi via a venous cannula), subjects were fasted for at least 6 h and their blood glucose level was <120 mg/dL. All images were acquired with a Discovery STE (GE Medical Systems, Milwaukee, WI) multi-ring PET tomography (PET-CT) system (time interval between injection and scan start = 45 min; scan duration = 15 min). Images were reconstructed using an ordered subset expectation maximization (OSEM) algorithm. Each PET phase was corrected for attenuation with CT data of the corresponding phase. For each PET scan 47 transaxial tomographic slices of 4.25 mm, re-oriented into the coronal and the sagittal planes, were obtained. The emission images were then reconstructed using a filtered back-projection, using the software provided by the manufacturers.
Image processing was performed using SPM statistical parametric mapping (http://www.fil.ion.ucl.ac.uk/spm/software) according to standardized and validated procedures (Della Rosa et al., 2014; Perani et al., 2016; Perani et al., 2014) implementing a standardized SPM FDG dementia-specific template for spatial normalization (Della Rosa et al., 2014). Images were normalized and smoothed with a 8-mm Full-Width-Half-Maximum (FWHM) Gaussian-kernel, proportional scaling was used to remove inter-subject global variation in PET intensities, and each FDG-PET scan was then tested for relative “hypometabolism” by means of a two-sample t-test implemented in SPM in a comparison with a normal FDG-PET image database (n = 112) on a voxel-by-voxel basis, using age as covariate (Perani et al., 2014).
Cerebral regions showing significant hypometabolism were localized using SPM Anatomy toolbox v2.0. The threshold was set at p = 0.05, FWE-corrected for multiple comparisons at the voxel level. Only clusters containing more than 100 voxels were deemed to be significant.
In order to evaluate the regional differences in the patterns of FDG-PET hypometabolism for the CBS and PSPs cases sub-grouped according to the language profiles (nfvPPA, LANG- and NOL), we computed whole-brain group analyses including the cases belonging to each subgroup for both CBS and PSPs, using a one-sample t-test with the contrast images resulting from each first-order “single-subject” analysis. The p-value was set at p < 0.001 uncorrected with a cluster extent k = 100, FWE-corrected at the cluster level.
3. Results
3.1. Clinical-neuropsychological classification of the sample
Demographic variables did not differ between CBS and PSPs groups. As expected, the CBS group showed a significantly reduced global cognitive status in comparison to PSPs. CBS patients, compared to PSPs , showed a cognitive profile characterized by more severe deficits in multiple cognitive domains, (i.e., delayed verbal long-term memory, visuo-constructive abilities, ideomotor apraxia) (Table 1).
CBS and PSPs groups significantly differed in their language impairment profiles (X2(1) = 5.2, p = 0.023) (See Tables 2 and 3). 12/33 CBS and 9/37 PSPs patients fulfilled criteria for nfv-PPA (Gorno-Tempini et al., 2011) (thus defined as nfv-PPA/CBS and nfv-PPA/PSPs). In addition, 12/33 CBS and 8/37 PSPs showed subtle language dysfunction not fulfilling PPA criteria (thus defined as LANG-CBS and LANG-PSPs). The remaining sample (i.e., 9/33 CBS and 20/37 PSPs patients) did not show any language deficits and was thus classified as NOL-CBS or NOL-PSPs.
Table 2.
Clinical-neuropsychological features of the CBS sample stratified according to the language profile.
| nfv-PPA/CBS n = 12 | LANG-CBS n = 12 | NOL-CBS n = 9 | Statistics | |
|---|---|---|---|---|
| Female/Male ratio | 10/2 | 4/8 | 4/5 | X2(2)=6.6, p = 0.051* |
| Age in years (mean ± st.dev.) | 69±7.2 | 71.9 ± 5.5 | 70.1 ± 5.9 | F(2,32) = 0.6, p = 0.54 |
| Age range in years | 54–77 | 61–79 | 58–77 | – |
| Years of education (mean ± st.dev.) | 11.4 ± 5.5 | 11.9 ± 5.4 | 10.7 ± 4.9 | F(2,32) = 0.1, p = 0.892 |
| Time from symptoms’ onset in months | 37.1 ± 22.3 | 43.9 ± 21.6 | 27 ± 16.4 | F(2,32) = 1.7, p = 0.194 |
| CDR global score (median [range]) | 0.5 [0.5–1] | 0.75 [0.5–1] | 0.5 [0.5–0.5] | – |
| MMSE raw score (mean±st.dev.) | 20.7 ± 6.3 | 18±5.4 | 25.4 ± 5.2 | F(2,32) = 4.3, p = 0.023 Lang-CBS < NOL-CBS |
| Motor speech disorder (% of impaired patients) | 50% | 27% | 0% | X2(2) = 5.72, p = 0.048* |
| Presence of agrammatism (% of patients) | 100% | 33% | 0% | X2(2) = 22.3, p < 0.001* |
| Single words comprehension (% of impaired patients) | 8% | 8% | 0% | X2(2) = 0.8, p = 1.00* |
| Sentences comprehension (% of impaired patients) | 91% | 75% | 0% | X2(2) = 19.7, p < 0.001* |
| Working memory (% of impaired patients) | 91% | 83% | 37% | X2(2) = 7.7, p = 0.036* |
| Verbal long-term memory immediate recall (% of impaired patients) | 63% | 75% | 63% | X2(2) = 0.5, p = 0.794* |
| Verbal long-term memory delayed recall (% of impaired patients) | 60% | 83% | 37% | X2(2)=4.4, p = 0.124* |
| Visuo-spatial long term memory (% of impaired patients) | 45% | 83% | 75% | X2(2) = 4.1, p = 0.179* |
| Visuo-constructive abilities (% of impaired patients) | 92% | 92% | 100% | X2(2) = 0.7, p = 1.00* |
| Ideomotor apraxia (% of impaired patients) | 33% | 58% | 88% | X2(2) = 6.5, p = 0.033* |
| Executive functions (% of impaired patients) | 92% | 100% | 37% | X2(2) = 13.7, p = 0.002* |
CBS = corticobasal syndrome; nfv-PPA = non fluent variant of primary progressive aphasia; LANG-CBS = CBS with language disorders; NOL-CBS = CBS without language disorders; CDR = clinical dementia rating scale; MMSE = mini-mental status examination.
Exact Fisher test.
Table 3.
Clinical-neuropsychological features of the PSPs sample stratified according to the language profile.
| nfv-PPA/PSPs n = 9 | LANG-PSPs n = 8 | NOL-PSPs n = 20 | Statistics | |
|---|---|---|---|---|
| Female/Male ratio | 6/3 | 5/3 | 12/8 | X2(2) = 1.2, p = 0.94 |
| Age in years (mean ± st.dev.) | 71.2 ± 6.3 | 74.3 ± 7.7 | 67.6 ± 7.1 | F(2,36) = 2.7, p = 0.078 |
| Age range in years | 62–78 | 60–86 | 53–82 | – |
| Years of education (mean ± st.dev.) | 8 ± 4.3 | 8.9 ± 4.6 | 11.8 ± 3.8 | F(2,36) = 3.3, p = 0.05 |
| Time from symptoms’ onset in months (mean ± st.dev.) | 33.1 ± 10.9 | 33.5 ± 18.5 | 23.5 ± 5.3 | F(2,36) = 1.3, p = 0.28 |
| CDR global score (median [range]) | 0.5 [0.5–1] | 0.75 [0.5–1] | 0.5 [0.5–0.5] | – |
| MMSE raw score (mean ± st.dev.) | 23.5 ± 4 | 20.4 ± 5.7 | 26.3 ± 3.2 | F(2,36) = 6.01, p = 0.006 Lang-PSP < Pure PSP |
| Motor speech disorder (% of impaired patients) | 67% | 37% | 0% | X2(2) = 16.4, p < p < 0.001* |
| Presence of agrammatism (% of patients) | 100% | 75% | 0% | X2(2) = 30.8, p < 0.001* |
| Single word comprehension (% of impaired patients) | 22% | 13% | 0% | X2(2) = 4.4, p = 0.88* |
| Sentence comprehension (% of impaired patients) | 55% | 75% | 20% | X2(2) = 8.2, p = 0.017* |
| Working memory (% of impaired patients) | 50% | 75% | 45% | X2(2) = 2.1, p = 0.437* |
| Verbal long-term memory immediate recall (% of impaired patients) | 50% | 75% | 30% | X2(2) = 4.8, p = 0.083* |
| Verbal long-term memory delayed recall (% of impaired patients) | 37% | 75% | 20% | X2(2) = 7.5, p = 0.026* |
| Visuo-spatial long term memory (% of impaired patients) | 44% | 100% | 40% | X2(2) = 8.7, p = 0.010* |
| Visuo-constructive abilities (% of impaired patients) | 67% | 100% | 70% | X2(2)=2.8, p = 0.26* |
| Ideomotor apraxia (% of impaired patients) | 22% | 71% | 20% | X2(2) = 6.8, p = 0.047* |
| Executive functions (% of impaired patients) | 63% | 100% | 60% | X2(2) = 4.7, p = 0.126* |
PSPs = progressive supranuclear palsy syndrome; nfv-PPA = non fluent variant of primary progressive aphasia; LANG-PSP = PSPs with language disorders; NOL-PSPs = PSPs without language disorders; CDR = clinical dementia rating scale; MMSE = mini-mental status examination.
Exact Fisher test.
Compared to NOL- subjects, LANG-CBS were characterized by anomia and verbal circumlocutions, deficits in sentence comprehension, and executive functions. LANG-PSPs showed severe anomia and verbal circumlocutions, agrammatism and deficits in sentence comprehension, verbal and visuo-spatial long term memory visuo-constructive abilities and executive functions.
3.2. FDG-PET imaging findings
3.2.1. Single-subject analyses
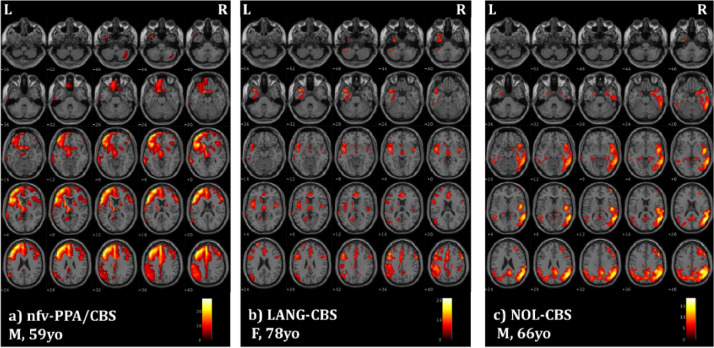
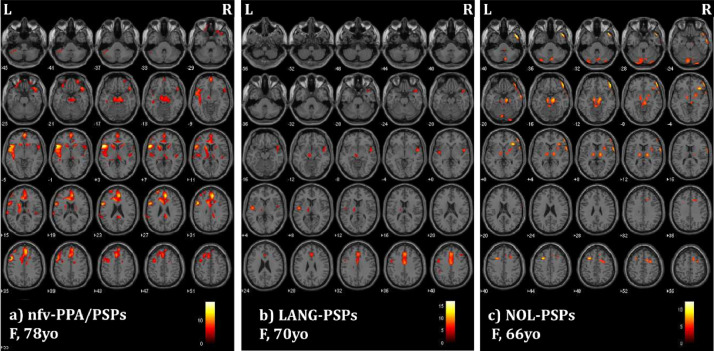
At the single-subject level, CBS patients showed heterogeneous patterns of left- or right-side brain hypometabolism, involving dorsolateral and ventromedial prefrontal cortex, superior and inferior parietal lobuli, posterior cingulate cortex and precuneus. Basal ganglia (i.e., thalamus and caudate nucleus) were involved in approximately half of the sample. PSPs patients showed a consistent pattern of reduced glucose metabolism in the mesencephalic and thalamic regions often associated with prefrontal medial cortex hypometabolism. Patients fulfilling criteria for nfv-PPA showed a hypometabolic pattern lateralized on the left side (Figs. 1(a) and 2(a)) with a selective involvement of the left inferior frontal gyrus. LANG-CBS and LANG-PSPs showed a bilateral pattern of brain hypometabolism, less severe than the patients with nfv-PPA (Figs. 1(b) and 2(b)). Finally, CBS and PSPs patients without language symptoms were characterized by a bilateral or right-lateralized hypometabolic pattern (Figs. 1(c) and 2(c)).
Fig. 1.
SPM maps of significant FDG-PET hypometabolism at the single-subject level in examples of CBS subjects with different language profiles: (a) nfv-PPA (nfv-PPA/CBS); (b) language disorders not fulfilling PPA criteria (LANG-CBS); (c) no language disorders (i.e., NOL-CBS). Images are shown in neurological convention; the colour bars represent t values. M: male F: female, yo: years old, L: left, R: right.
Fig. 2.
SPM maps of significant FDG-PET hypometabolism at the single-subject level in examples of PSPs subjects characterized by different language profiles: (a) nfv-PPA (nfv-PPA/PSPs); (b) language disorders not fulfilling PPA criteria (LANG-PSPs); (c) no language disorders (i.e., NOL-PSPs). Images are shown in neurological convention; the colour bars represent t values. M: male F: female, yo: years old L: left, R: right.
See Figs. 1 and 2 for single-subject FDG-PET patterns details.
3.2.2. Whole-brain group analyses
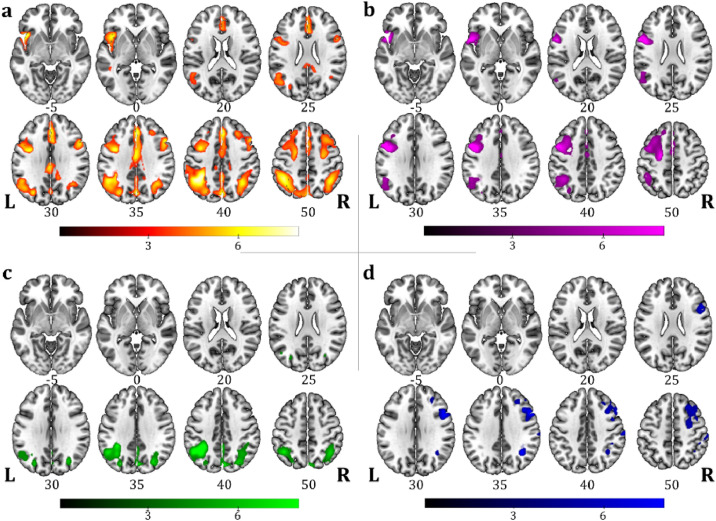
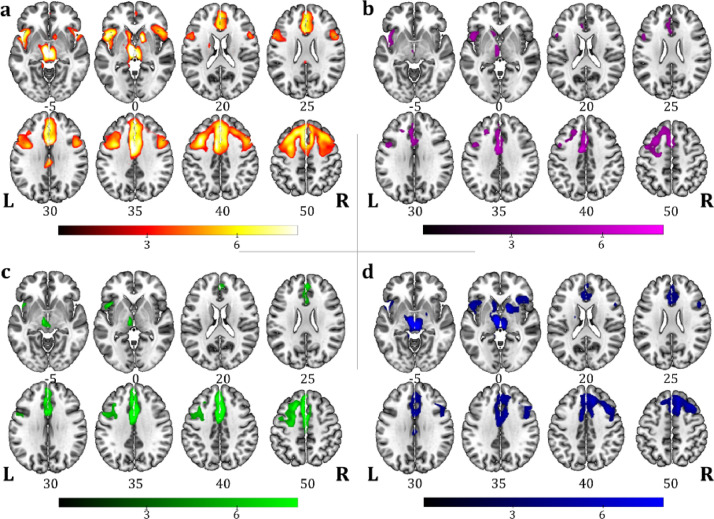
One-sample SPM group analysis of FDG-PET data performed in the whole CBS sample (n = 33) showed an extended and bilateral parietal hypometabolic pattern with additional involvement of the left inferior frontal gyrus (Fig. 3(a)). In PSPs sample (n = 37), the group analysis showed hypometabolism in the midbrain, the thalamus, the caudate, the anterior cingulate cortex, and the inferior, medial and superior frontal gyri bilaterally (Fig. 4(a)).
Fig. 3.
One-sample SPM FDG-PET group analysis showing the hypometabolism regional commonalities in (a) all CBS sample (n = 33), and in patient subgroups according to language profiles: (b) nfv-PPA/CBS (n = 12), (c) LANG-CBS (n = 12), (d) NOL-CBS (n = 9). Images are shown in neurological convention; the colour bars represent t values.
Fig. 4.
One-sample SPM FDG-PET group analysis showing the hypometabolic regional commonalities in (a) the whole PSPs sample (n = 37); and in patient subgroups according to language profiles: (b) nfv-PPA/PSPs (n = 9), (c) LANG-PSPs (n = 8), (d) NOL-PSPs (n = 20). Images are shown in neurological convention; the colour bars represent t values.
Both the nfv-PPA/CBS and the nfv-PPA/PSPs subgroups were characterized by a left-side hypometabolic pattern involving the inferior frontal gyrus and the supplementary motor cortex, with additional features typical of CBS (i.e., inferior parietal cortex) or PSPs (i.e., midbrain and thalamus) (Figure 3b and 4b). A less extended, left-sided hypometabolic pattern characterized the LANG-CBS and LANG-PSPs with a specific involvement of the angular gyrus, the precuneus and the inferior parietal cortex in LANG-CBS and of the prefrontal, the anterior cingulate cortex and the thalamus in LANG-PSPs (Figure 3c and 4c). NOL-CBS and NOL-PSPs subgroups showed a selective right hemisphere hypometabolic pattern involving the prefrontal and parietal cortices in NOL-CBS and the prefrontal cortex, the caudate, the midbrain and the thalamus in NOL-PSPs (Figure 3d and 4d).
4. Discussion
In this study, we explored the functional correlates of language impairments in patients with CBS and PSPs phenotypical presentation, providing evidence of specific hypometabolic signatures in patient subgroups according to the language profile. To this aim, we used at the single-subject and group levels a semi-quantitative optimized procedure for the analysis of FDG-PET data, which has been proved to be a reliable functional biomarker to support the differential diagnosis of neurodegenerative dementia conditions and atypical parkinsonism (Walker et al., 2018; Caminiti et al., 2017; Perani et al., 2016).
As expected, and in line with literature evidence (Armstrong et al., 2013; Hoglinger et al., 2017), 30% of the overall sample satisfied the nfv-PPA criteria (Gorno-Tempini et al., 2011). Namely, these patients showed agrammatism, anomia and verbal circumlocution, and significant deficits in sentence comprehension, with spared single-word comprehension and object knowledge. Nfv-PPA phenotype can characterize patients that will later develop full-blown CBS and PSPs, even before the clinical onset of motor symptoms (Armstrong et al., 2013; Hoglinger et al., 2017). In a previous study by our group, we provided evidence of a two-year progression to CBS and PSPs phenotypes in a consistent number of nfv-PPA cases (i.e., 58% and 16% respectively) (Cerami et al., 2017). Notably, semi-quantitative analysis of FDG-PET data at the time of nfv-PPA diagnosis was already supportive for a diagnosis of atypical parkinsonism (Cerami et al., 2017).
Here, group analyses demonstrated that nfv-PPA/CBS and nfv-PPA/PSPs subgroups share a significant common left-sided dysfunctional pattern involving the inferior frontal gyrus and the supplementary motor cortex, which is a major driver of the same language phenotype, compatible with nfv-PPA.
Twenty-nine per cent of the overall sample showed subtle language dysfunctions not fulfilling PPA criteria. The relevance of language impairments in the clinical picture of atypical parkinsonisms has been previously underlined, particularly in CBS (McMonagle et al., 2006). In our sample, impairments in LANG-CBS and LANG-PSPs patients were characterized mainly by difficulties in both verbal production (e.g., increased anomic behavior and use of circumlocutions) and sentence comprehension. Notably, these two subgroups showed largely different regional distribution at FDG-PET hypometabolic maps. In the case of LANG-PSPs, the whole-brain group analysis revealed a focal left-sided hypometabolic pattern, mainly involving the prefrontal and anterior cingulate cortices and the thalamus. Considering this left-sided dysfunctional distribution, it is a possibility that some of these patients may lately progress to nfv-PPA, according to a pathology-driven progression of the neurodegenerative process. It is of note, however, that this group showed worse cognitive dysfunctions (see Table 3). Longitudinal prospective studies are needed to clarify this issue.
On the other side, LANG-CBS patients shared language and functional metabolic features with the lv-PPA phenotype (Gorno-Tempini et al., 2011). Whole-brain group analysis in LANG-CBS showed a widespread bilateral parietal hypometabolism particularly in the left inferior parietal gyrus, which is the functional metabolic signature of lv-PPA cases (Gorno-Tempini et al., 2011). LANG-CBS cases showed impaired complex sentence repetition at the neuropsychological evaluation. This is the main feature of lv-PPA phenotype, that can be explained in terms of dysfunction in the phonological loop (Gorno-Tempini et al., 2011, 2008). Notably, lv-PPA is actually considered as one of the atypical presentation of Alzheimer's disease (AD) (Dubois et al., 2014), and AD is one of the possible pathology substrates of CBS phenotype (Shelley et al., 2009; Josephs et al., 2010). It is possible that some of these LANG-CBS subjects had a undetected lv-PPA diagnosis, perhaps due to the limitations of clinical assessment of spontaneous speech.
Finally, NOL-CBS and NOL-PSPs showed a significant right-lateralized pattern of brain hypometabolism with a selective sparing of the left brain regions involved in the language networks. This result once more confirms the presence of specific trajectories of brain degeneration, selectively sparing language functions in some cases.
In this large sample of CBS and PSPs patients, we showed that about one third of subjects presented a nfv-PPA profile, one third showed language disorders not fulfilling PPA criteria, and the last third did not present language disorders. These differences in language profiles were reflected by significant heterogeneities in functional metabolic signatures, possibly driven by different neuropathological substrates (Pardini et al., 2019). In fact, while underlying corticobasal pathology has been associated to asymmetric hypometabolism in fronto-parietal regions and basal ganglia, CBS patients with underlying AD pathology show a more marked reduction of glucose metabolism in lateral parietal and temporal regions, together with the posterior cingulate cortex (Pardini et al., 2019). Moreover, in CBS with language disorders, nfv-PPA phenotype is mainly linked to an underlying tau pathology (Josephs and Duffy, 2008), while impaired sentence repetition is more frequently associated to a positivity to AD amyloid biomarkers (Burrell et al., 2013).
In conclusion, our findings support the need of a better characterization of CBS and PSPs populations by means of imaging markers aiding physicians in clinical settings. The supportive role of FDG-PET findings in atypical parkinsonisms is matter of discussion (Walker et al., 2018). FDG-PET studies are often affected by methodological shortcomings, such as a defective definition of critical outcomes, inadequate gold standard and no head-to-head comparison with appropriate reference standards (Walker et al., 2018). The lack of pathological confirmation is an undeniable limitation of many clinical investigations, including the present study. Nonetheless, the use of clear clinical criteria for the diagnosis, a follow-up for the confirmation of the diagnostic classification, and a validated semi-quantitative analysis of PET imaging data at the single-subject level can be expected to result in improved accuracy. The differential diagnosis of atypical parkinsonism, particularly in the early stage of the disease, might be misled by the phenotypical overlap in motor, cognitive and behavioral symptoms. Moreover, differences in underlying pathologies, even in combination, may drive the disease trajectories in specific ways (Hu et al., 2009). The use of in vivo imaging biomarkers with the implementation of semiquantitative single-subject analysis certainly adds crucial information, particularly in presence of language disturbances, highlighting the presence of individual metabolic changes (see Fig. 1(b) and (c) in comparison to group analysis). Given the evidence coming from neuropathological studies showing coexisting pathology findings in CBS series (e.g., AD, CBD or PSP plus cerebrovascular or Lewy body disease; or AD plus CBD or PSP (Pardini et al., 2019)), individual changes in brain metabolism driven by multiple pathologies are lost at the group level (Pardini et al., 2019). Further combined biomarker studies (e.g., FDG-PET and amyloid-PET or CSF) exploring language disorders in CBS and PSPs will certainly help to clarify the relationship between symptom combination and underlying pathology.
Finally, an in-depth evaluation of language features through ad-hoc neuropsychological tools (e.g., the Screening for Aphasia in NeuroDegeneration (Catricala et al., 2017)) may also help in subtype classification. Unfortunately, the retrospective nature of this study did not allow to clarify this issue. Notwithstanding this limitation, our results strongly support the usefulness for a detailed language assessment in the neuropsychological evaluation of CBS and PSP patients.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102009.
Appendix. Supplementary materials
References
- Horvath J., Burkhard P.R., Bouras C., Kovari E. Etiologies of parkinsonism in a century-long autopsy-based cohort. Brain Pathol. 2013;23:28–33. doi: 10.1111/j.1750-3639.2012.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve B.F. The multiple phenotypes of corticobasal syndrome and corticobasal degeneration: implications for further study. J. Mol. Neurosci. 2011;45:350–353. doi: 10.1007/s12031-011-9624-1. [DOI] [PubMed] [Google Scholar]
- Respondek G., Stamelou M., Kurz C., Ferguson L.W., Rajput A., Chiu W.Z. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov. Disord. 2014;29:1758–1766. doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- Armstrong M.J., Litvan I., Lang A.E., Bak T.H., Bhatia K.P., Borroni B. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer A.L., Yu J.T., Golbe L.I., Litvan I., Lang A.E., Hoglinger G.U. Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 2017;16:552–563. doi: 10.1016/S1474-4422(17)30157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle-Gilchrist I.T., Dick K.M., Patterson K., Vazquez Rodriquez P., Wehmann E., Wilcox A. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger G.U., Respondek G., Stamelou M., Kurz C., Josephs K.A., Lang A.E. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov. Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell J.R., Hodges J.R., Rowe J.B. Cognition in corticobasal syndrome and progressive supranuclear palsy: a review. Mov. Disord. 2014;29:684–693. doi: 10.1002/mds.25872. [DOI] [PubMed] [Google Scholar]
- Oliveira L.M., Barcellos I., Teive H.A.G., Munhoz R.P. Cognitive dysfunction in corticobasal degeneration. Arq. Neuropsiquiatr. 2017;75:570–579. doi: 10.1590/0004-282X20170077. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami C., Dodich A., Greco L., Iannaccone S., Magnani G., Marcone A. The role of single-subject brain metabolic patterns in the early differential diagnosis of primary progressive aphasias and in prediction of progression to dementia. J. Alzheimers Dis. 2017;55:183–197. doi: 10.3233/JAD-160682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Santos M.A., Mandelli M.L., Binney R.J., Ogar J., Wilson S.M., Henry M.L. Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurol. 2016;73:733–742. doi: 10.1001/jamaneurol.2016.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Z., Gandolfo F., Orini S., Garibotto V., Agosta F., Arbizu J. Clinical utility of FDG pet in Parkinson's disease and atypical parkinsonism associated with dementia. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:1534–1545. doi: 10.1007/s00259-018-4031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti S.P., Alongi P., Majno L., Volonte M.A., Cerami C., Gianolli L. Evaluation of an optimized [(18) F]fluoro-deoxy-glucose positron emission tomography voxel-wise method to early support differential diagnosis in atypical Parkinsonian disorders. Eur. J. Neurol. 2017;24 doi: 10.1111/ene.13269. 687-e26. [DOI] [PubMed] [Google Scholar]
- Niccolini F., Politis M. A systematic review of lessons learned from pet molecular imaging research in atypical Parkinsonism. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:2244–2254. doi: 10.1007/s00259-016-3464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbelli S., Drzezga A., Perneczky R., Frisoni G.B., Caroli A., van Berckel B.N. Resting metabolic connectivity in prodromal alzheimer's disease. A European alzheimer disease consortium (EADC) project. Neurobiol. Aging. 2012;33:2533–2550. doi: 10.1016/j.neurobiolaging.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Della Rosa P.A., Cerami C., Gallivanone F., Prestia A., Caroli A., Castiglioni I. A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014;12:575–593. doi: 10.1007/s12021-014-9235-4. [DOI] [PubMed] [Google Scholar]
- Perani D., Cerami C., Caminiti S.P., Santangelo R., Coppi E., Ferrari L. Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:499–508. doi: 10.1007/s00259-015-3170-y. [DOI] [PubMed] [Google Scholar]
- Perani D., Della Rosa P.A., Cerami C., Gallivanone F., Fallanca F., Vanoli E.G. Validation of an optimized SPM procedure for FDG-pet in dementia diagnosis in a clinical setting. Neuroimage Clin. 2014;6:445–454. doi: 10.1016/j.nicl.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMonagle P., Blair M., Kertesz A. Corticobasal degeneration and progressive aphasia. Neurology. 2006;67:1444–1451. doi: 10.1212/01.wnl.0000240215.43492.01. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Brambati S.M., Ginex V., Ogar J., Dronkers N.F., Marcone A. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Shelley B.P., Hodges J.R., Kipps C.M., Xuereb J.H., Bak T.H. Is the pathology of corticobasal syndrome predictable in life? Mov Disord. 2009;24:1593–1599. doi: 10.1002/mds.22558. [DOI] [PubMed] [Google Scholar]
- Josephs K.A., Whitwell J.L., Boeve B.F., Knopman D.S., Petersen R.C., Hu W.T. Anatomical differences between CBS-corticobasal degeneration and CBS-Alzheimer's disease. Mov. Disord. 2010;25:1246–1252. doi: 10.1002/mds.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini M., Huey E.D., Spina S., Kreisl W.C., Morbelli S., Wassermann E.M. FDG-PET patterns associated with underlying pathology in corticobasal syndrome. Neurology. 2019;92:e1121–e1e35. doi: 10.1212/WNL.0000000000007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K.A., Duffy J.R. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr. Opin. Neurol. 2008;21:688–692. doi: 10.1097/WCO.0b013e3283168ddd. [DOI] [PubMed] [Google Scholar]
- Burrell J.R., Hornberger M., Villemagne V.L., Rowe C.C., Hodges J.R. Clinical profile of pib-positive corticobasal syndrome. PLoS One. 2013;8:e61025. doi: 10.1371/journal.pone.0061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W.T., Rippon G.W., Boeve B.F., Knopman D.S., Petersen R.C., Parisi J.E. Alzheimer's disease and corticobasal degeneration presenting as corticobasal syndrome. Mov. Disord. 2009;24:1375–1379. doi: 10.1002/mds.22574. [DOI] [PubMed] [Google Scholar]
- Catricala E., Gobbi E., Battista P., Miozzo A., Polito C., Boschi V. SAND: a screening for aphasia in neurodegeneration. Development and normative data. Neurol. Sci. 2017;38:1469–1483. doi: 10.1007/s10072-017-3001-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.